Transcription of the Envelope Protein by 1-L Protein–RNA Recognition Code Leads to Genes/Proteins That Are Relevant to the SARS-CoV-2 Life Cycle and Pathogenesis
Abstract
:1. Introduction
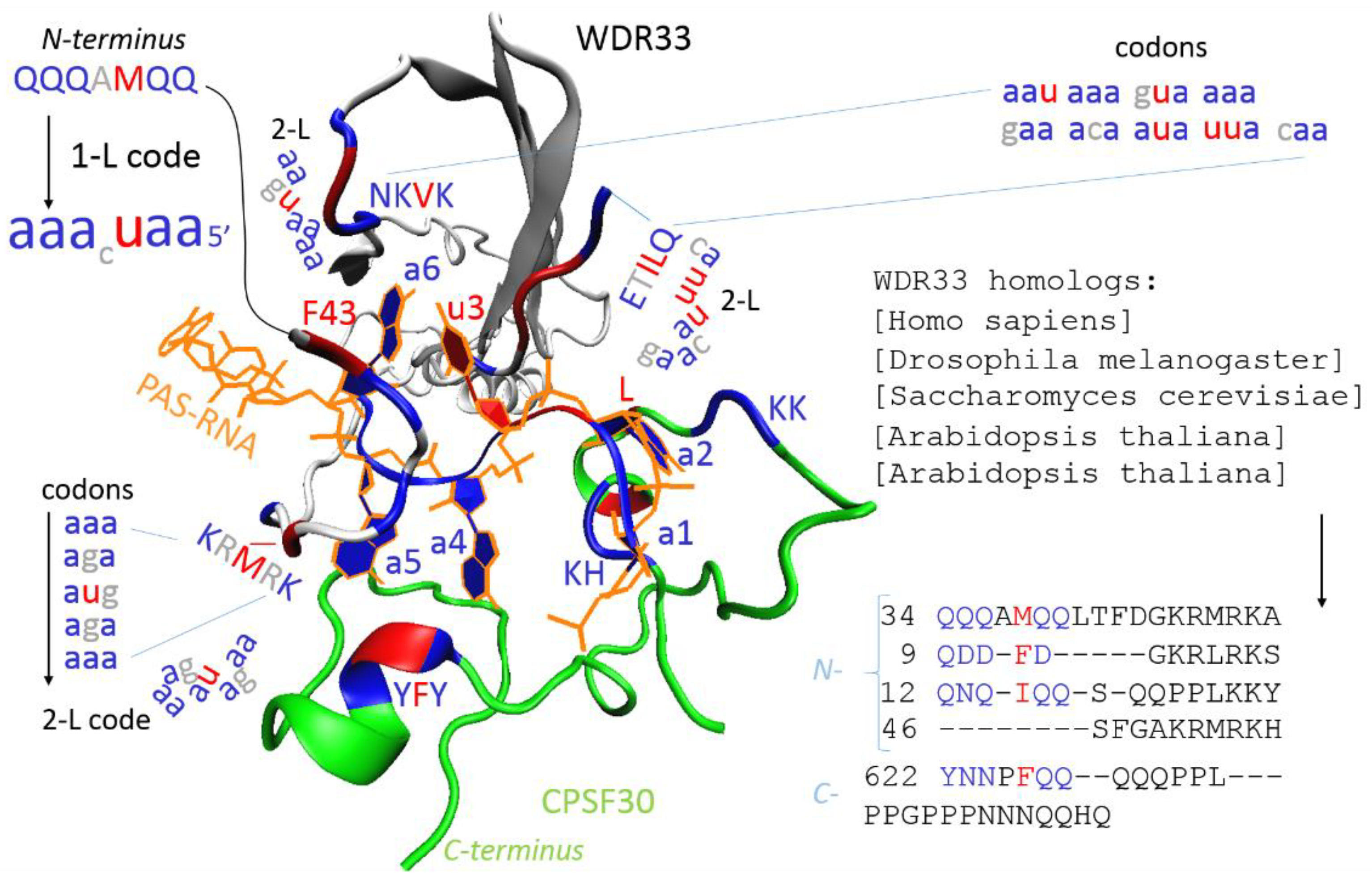
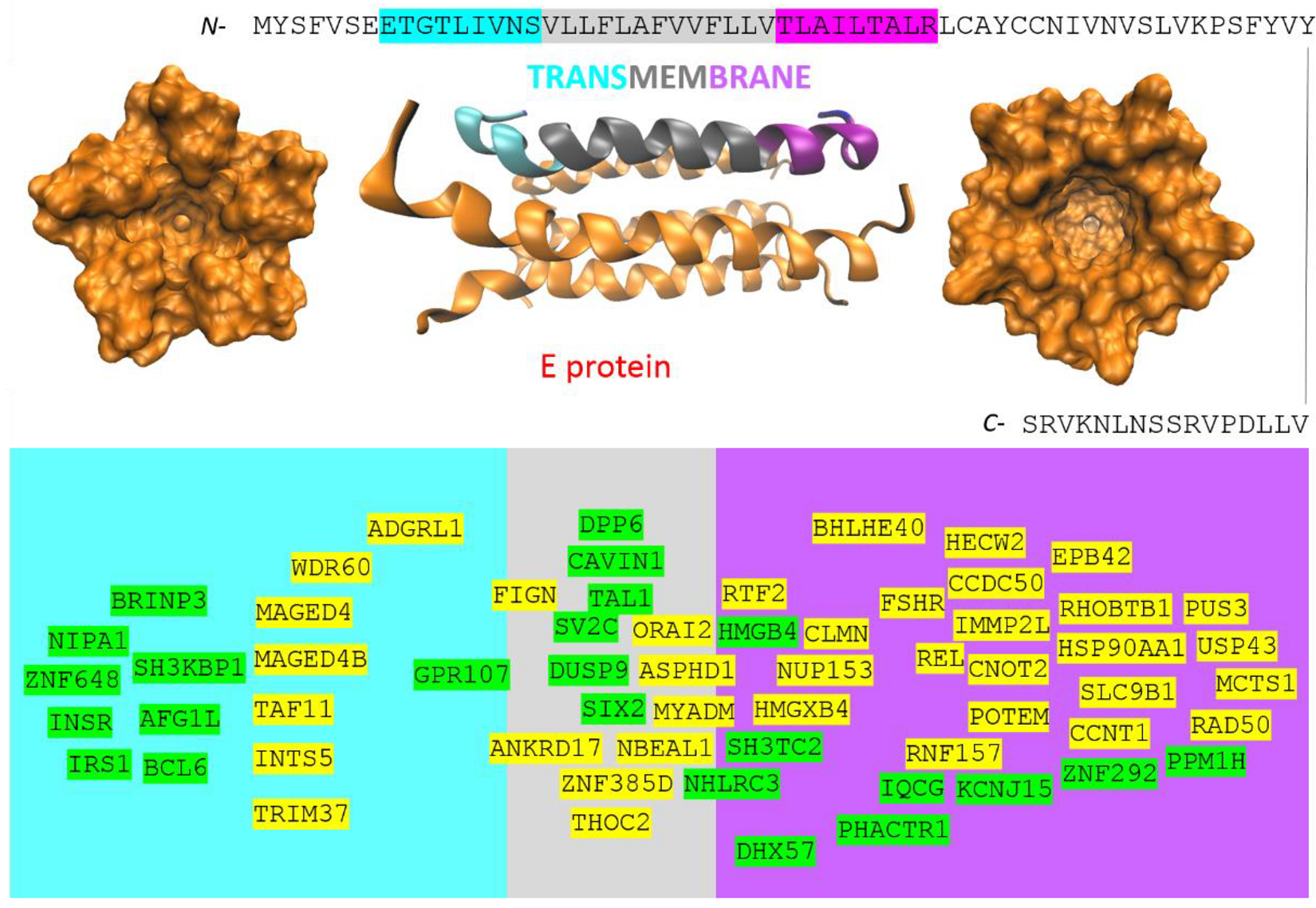
2. Methods
3. Results
4. Discussion
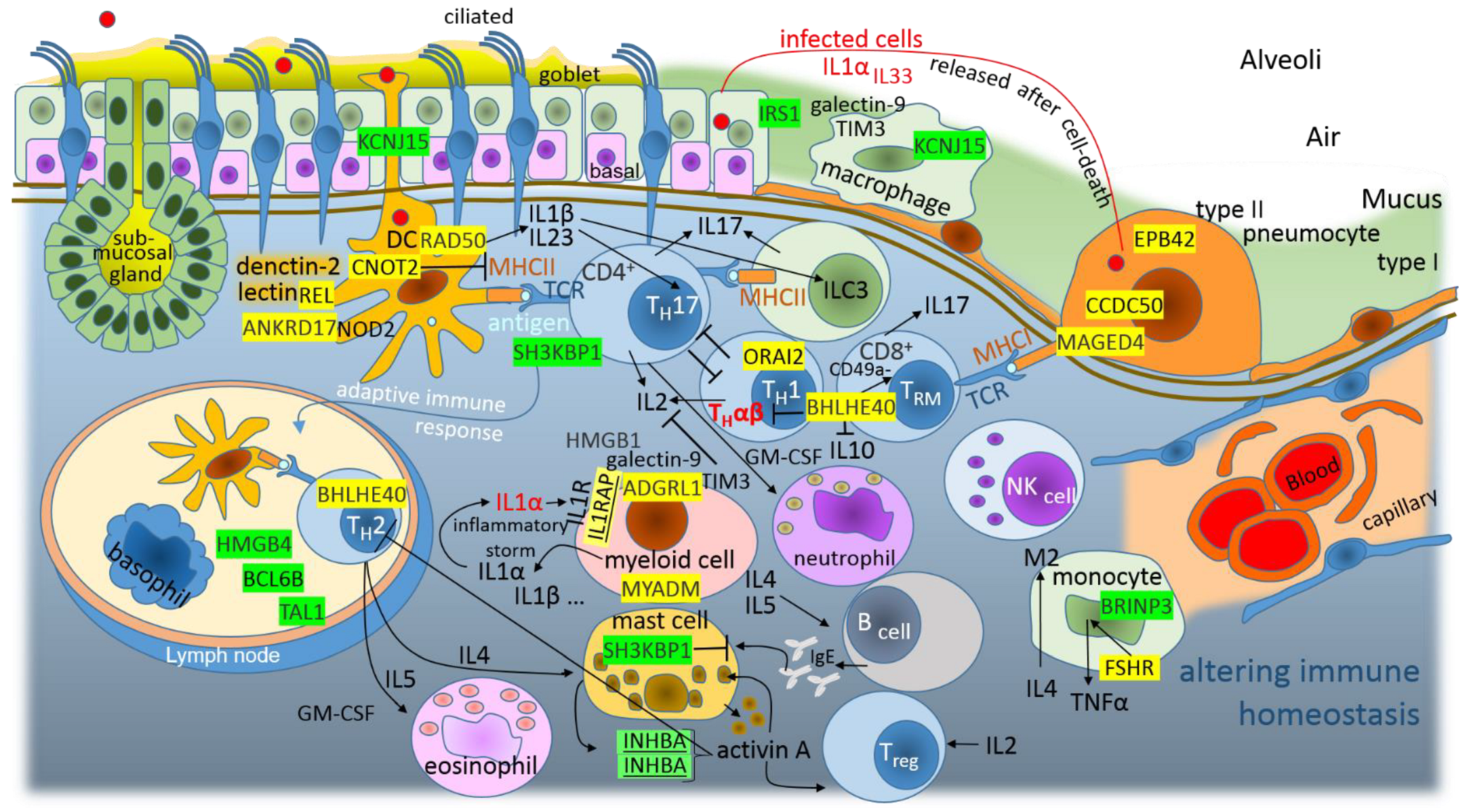
4.1. Altered Immune Homeostasis as a Consequence of SARS-CoV-2 Infection
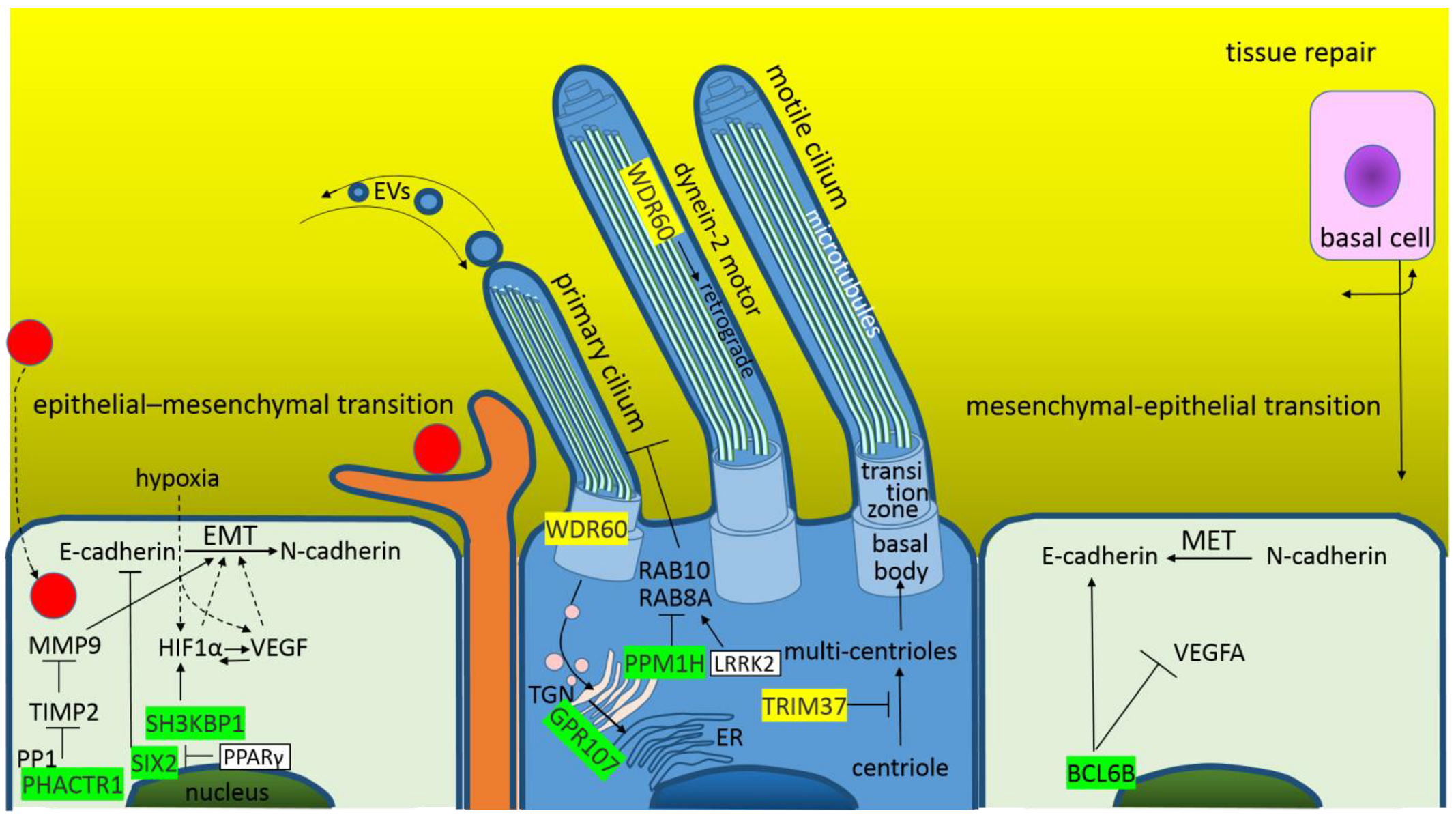
4.2. Altered Homeostasis in the Pulmonary Epithelial Tissue during the SARS-CoV-2 Infection
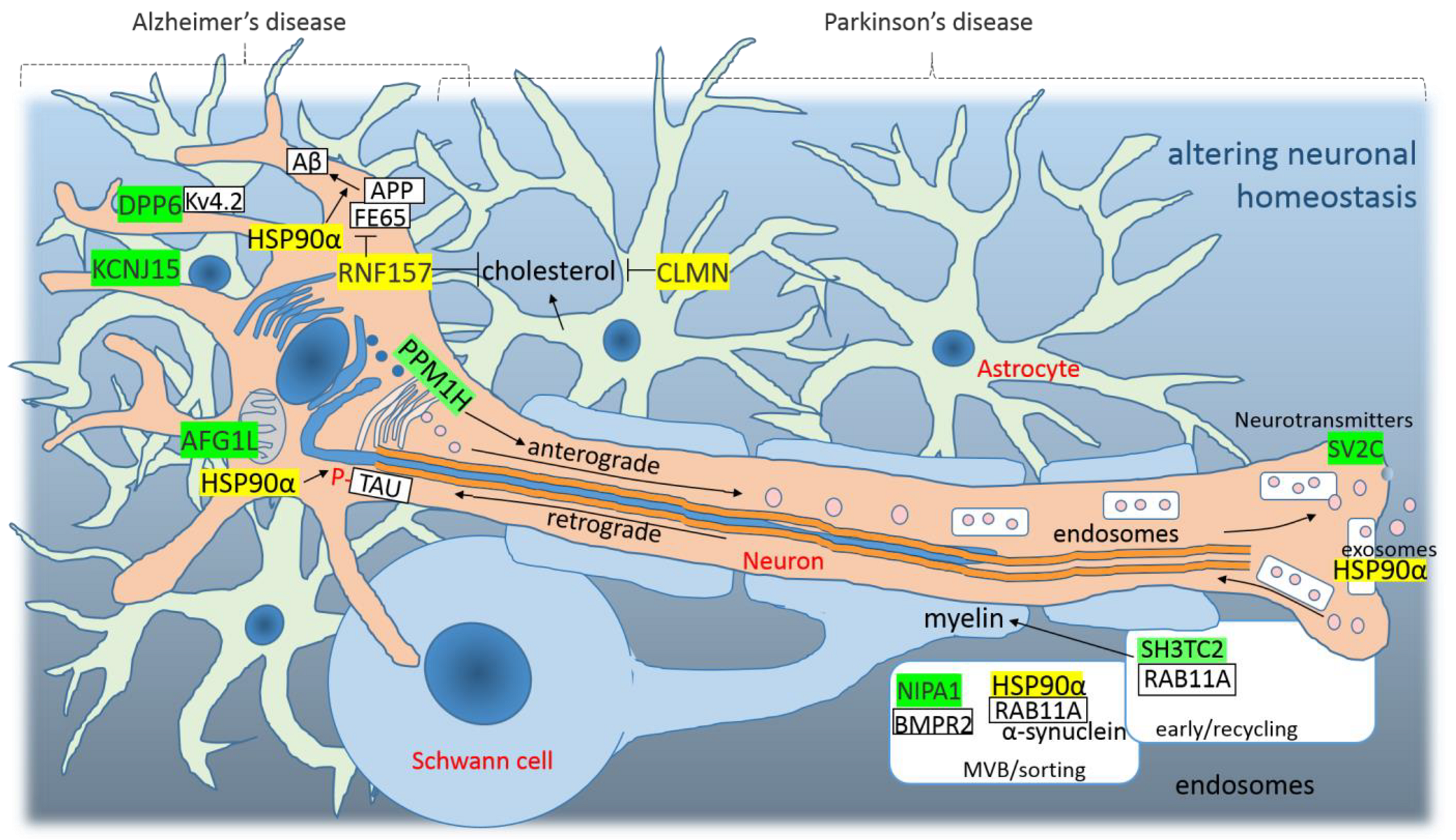
4.3. Altered Neuronal Homeostasis as a Consequence of SARS-CoV-2 Infection
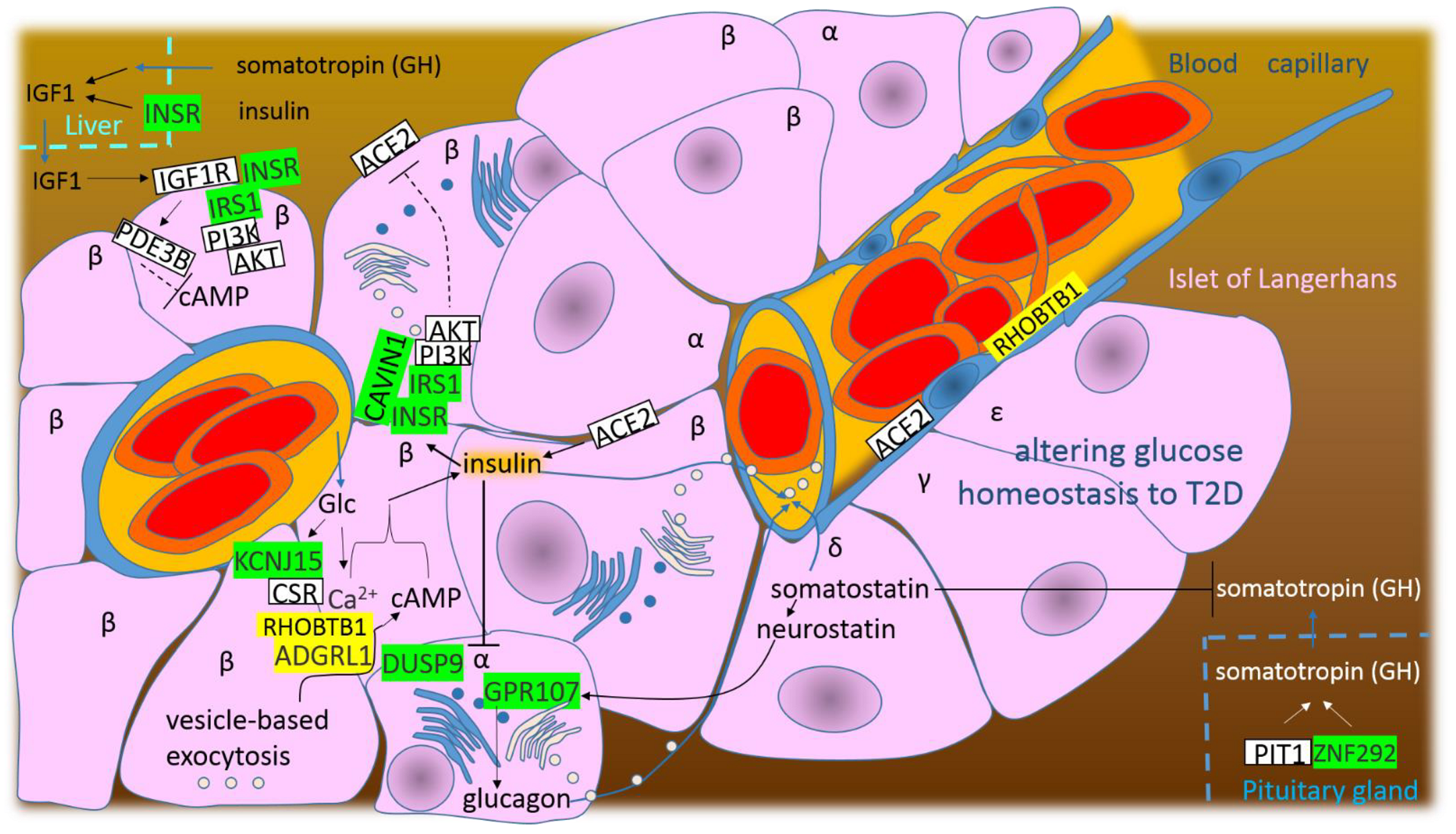
4.4. SARS-CoV-2 Infection Alters Glucose Homeostasis to T2D
4.5. The Host Genes/Proteins Involved in the Life Cycle of SARS-CoV-2
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gebauer, F.; Schwarzl, T.; Valcárcel, J.; Hentze, M.W. RNA-binding proteins in human genetic disease. Nat. Rev. Gen. 2021, 22, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, X.; Gao, L.; You, C. Integrated analysis of the functions and prognostic values of RNA binding proteins in lung squamous cell carcinoma. Front. Genet. 2020, 11, 185. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Li, Z.; Fu, S.; Wang, S.; Liu, Y.; Ma, M.; Yang, X.; Xie, W.; Gong, B.; Sun, T. Development of prognostic signature based on RNA binding proteins related genes analysis in clear cell renal cell carcinoma. Aging 2021, 13, 3926–3944. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Fang, L.; Yang, X.; Zou, Q. Identification of prognosis-related RNA-binding proteins to reveal the role of RNA-binding proteins in the progression and prognosis of colon cancer. Int. J Gen. Med. 2021, 14, 6795–6805. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Yan, Y.; Li, B.; Liu, M.; Liang, Y.; Ye, Y.; Cheng, W.; Li, J.; Jiao, J.; Chang, S. A novel prognostic model for oral squamous cell carcinoma: The functions and prognostic values of RNA-binding proteins. Front. Oncol. 2021, 11, 592614. [Google Scholar] [CrossRef]
- Low, Y.; Asi, Y.; Foti, S.C.; Lashley, T. Heterogeneous nuclear ribonucleoproteins: Implications in neurological diseases. Mol. Neurobiol. 2021, 58, 631–646. [Google Scholar] [CrossRef]
- Nahalka, J. The role of the protein–RNA recognition code in neurodegeneration. Cell. Mol. Life Sci. 2019, 76, 2043–2058. [Google Scholar] [CrossRef]
- Ash, P.; Bieniek, K.; Gendron, T.; Caulfield, T.; Lin, W.; DeJesus-Hernandez, M.; Van Blitterswijk, M.; Jansen-West, K.; Paul, J.; Rademakers, R.; et al. Unconventional translation of C9ORF72 GGGGCC expansion generates insoluble polypeptides specific to c9FTD/ALS. Neuron 2013, 77, 639–646. [Google Scholar] [CrossRef] [Green Version]
- Nahalka, J. Protein-RNA recognition: Cracking the code. J. Theor. Biol. 2014, 343, 9–15. [Google Scholar] [CrossRef]
- Gruber, A.J.; Zavolan, M. Alternative cleavage and polyadenylation in health and disease. Nat. Rev. Gen. 2019, 20, 599–614. [Google Scholar] [CrossRef]
- Sun, Y.; Hamilton, K.; Tong, L. Recent molecular insights into canonical pre-mRNA 3′-end processing. Transcription 2020, 11, 83–96. [Google Scholar] [CrossRef]
- Nahalka, J. Quantification of peptide bond types in human proteome indicates how DNA codons were assembled at prebiotic conditions. J. Proteom. Bioinform. 2011, 4, 153–159. [Google Scholar] [CrossRef] [Green Version]
- Nahalka, J. Theoretical analysis of S, M and N structural proteins by the protein–RNA recognition code leads to Genes/proteins that are relevant to the SARS-CoV-2 life cycle and pathogenesis. Front. Genet. 2021, 12, 1897. [Google Scholar] [CrossRef]
- Schoeman, D.; Fielding, B.C. Is there a link between the pathogenic human coronavirus envelope protein and immunopathology? A review of the literature. Front. Microbiol. 2020, 11, 2086. [Google Scholar] [CrossRef]
- Xia, B.; Shen, X.; He, Y.; Pan, X.; Liu, F.; Wang, Y.; Yang, F.; Fang, S.; Wu, Y.; Duan, Z.; et al. SARS-CoV-2 envelope protein causes acute respiratory distress syndrome (ARDS)-like pathological damages and constitutes an antiviral target. Cell Res. 2021, 31, 847–860. [Google Scholar] [CrossRef]
- Mandala, V.S.; McKay, M.J.; Shcherbakov, A.A.; Dregni, A.J.; Kolocouris, A.; Hong, M. Structure and drug binding of the SARS-CoV-2 envelope protein transmembrane domain in lipid bilayers. Nat. Struct. Mol. Biol. 2020, 27, 1202–1208. [Google Scholar] [CrossRef]
- Cao, Y.; Yang, R.; Lee, I.; Zhang, W.; Sun, J.; Wang, W.; Meng, X. Characterization of the SARS-CoV-2 E protein: Sequence, structure, viroporin, and inhibitors. Protein Sci. 2021, 30, 1114–1130. [Google Scholar] [CrossRef]
- Schoeman, D.; Fielding, B.C. Coronavirus envelope protein: Current knowledge. Virol. J. 2019, 16, 69. [Google Scholar] [CrossRef] [Green Version]
- Lebedeva, S.; Jens, M.; Theil, K.; Schwanhäusser, B.; Selbach, M.; Landthaler, M.; Rajewsky, N. Transcriptome-wide analysis of regulatory interactions of the RNA-binding protein HuR. Mol. Cell 2011, 43, 340–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siang, D.T.C.; Lim, Y.C.; Kyaw, A.M.M.; Win, K.N.; Chia, S.Y.; Degirmenci, U.; Hu, X.; Tan, B.C.; Walet, A.C.E.; Sun, L.; et al. The RNA-binding protein HuR is a negative regulator in adipogenesis. Nat. Commun. 2020, 11, 213. [Google Scholar] [CrossRef]
- Bhattacharyya, S.N.; Habermacher, R.; Martine, U.; Closs, E.I.; Filipowicz, W. Relief of microRNA-mediated translational repression in human cells subjected to stress. Cell 2006, 125, 1111–1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutchison, J.M.; Capone, R.; Luu, D.D.; Shah, K.H.; Hadziselimovic, A.; van Horn, W.D.; Sanders, C.R. Recombinant SARS-CoV-2 envelope protein traffics to the trans-golgi network following amphipol-mediated delivery into human cells. J. Biol. Chem. 2021, 297. [Google Scholar] [CrossRef] [PubMed]
- de Wilde, A.H.; Snijder, E.J.; Kikkert, M.; van Hemert, M.J. Host factors in coronavirus replication. Curr. Top. Microbiol. Immunol. 2018, 419, 1–42. [Google Scholar] [CrossRef] [Green Version]
- Bussani, R.; Schneider, E.; Zentilin, L.; Collesi, C.; Ali, H.; Braga, L.; Volpe, M.C.; Colliva, A.; Zanconati, F.; Berlot, G.; et al. Persistence of viral RNA, pneumocyte syncytia and thrombosis are hallmarks of advanced COVID-19 pathology. EBioMedicine 2020, 61, 103104. [Google Scholar] [CrossRef]
- Braga, L.; Ali, H.; Secco, I.; Chiavacci, E.; Neves, G.; Goldhill, D.; Penn, R.; Jimenez-Guardeño, J.M.; Ortega-Prieto, A.M.; Bussani, R.; et al. Drugs that inhibit TMEM16 proteins block SARS-CoV-2 spike-induced syncytia. Nature 2021, 594, 88–93. [Google Scholar] [CrossRef]
- Castello, A.; Fischer, B.; Frese, C.K.; Horos, R.; Alleaume, A.; Foehr, S.; Curk, T.; Krijgsveld, J.; Hentze, M.W. Comprehensive identification of RNA-binding domains in human cells. Mol. Cell 2016, 63, 696–710. [Google Scholar] [CrossRef] [Green Version]
- Protter, D.S.W.; Rao, B.S.; Van Treeck, B.; Lin, Y.; Mizoue, L.; Rosen, M.K.; Parker, R. Intrinsically disordered regions can contribute promiscuous interactions to RNP granule assembly. Cell Rep. 2018, 22, 1401–1412. [Google Scholar] [CrossRef] [Green Version]
- Annunziato, F.; Romagnani, C.; Romagnani, S. The 3 major types of innate and adaptive cell-mediated effector immunity. J. Allergy Clin. Immunol. 2015, 135, 626–635. [Google Scholar] [CrossRef]
- Hu, W. A framework of all discovered immunological pathways and their roles for four specific types of pathogens and hypersensitivities. Front. Immunol. 2020, 11, 1992. [Google Scholar] [CrossRef]
- Malik, A.; Kanneganti, T. Function and regulation of IL-1α in inflammatory diseases and cancer. Immunol. Rev. 2018, 281, 124–137. [Google Scholar] [CrossRef]
- Roth, S.; Rottach, A.; Lotz-Havla, A.S.; Laux, V.; Muschaweckh, A.; Gersting, S.W.; Muntau, A.C.; Hopfner, K.; Jin, L.; Vanness, K.; et al. Rad50-CARD9 interactions link cytosolic DNA sensing to IL-1β production. Nat. Immunol. 2014, 15, 538–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gringhuis, S.I.; Wevers, B.A.; Kaptein, T.M.; van Capel, T.M.M.; Theelen, B.; Boekhout, T.; de Jong, E.C.; Geijtenbeek, T.B.H. Selective c-rel activation via Malt1 controls anti-fungal TH-17 immunity by dectin-1 and dectin-2. PLoS Pathog. 2011, 7, e1001259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menning, M.; Kufer, T.A. A role for the ankyrin repeat containing protein Ankrd17 in Nod1- and Nod2-mediated inflammatory responses. FEBS Lett. 2013, 587, 2137–2142. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, T.; Hovingh, E.S.; Foerster, E.G.; Abdel-Nour, M.; Philpott, D.J.; Girardin, S.E. NOD1 and NOD2 in inflammation, immunity and disease. Arch. Biochem. Biophys. 2019, 670, 69–81. [Google Scholar] [CrossRef]
- Wang, Y.; Tong, X.; Li, G.; Li, J.; Deng, M.; Ye, X. Ankrd17 positively regulates RIG-I-like receptor (RLR)-mediated immune signaling. Eur. J. Immunol. 2012, 42, 1304–1315. [Google Scholar] [CrossRef]
- Wu, J.; Chen, Z.J. Innate immune sensing and signaling of cytosolic nucleic acids. Annu. Rev. Immunol. 2014, 32, 461–488. [Google Scholar] [CrossRef]
- Laurent, E.M.N.; Yorgos Sofianatos, Y.; Komarova, A.; Gimeno, J.; Payman Samavarchi-Tehrani, P.; Kim, D.; Abdouni, H.; Duhamel, M.; Cassonnet, P.; Knapp, J.J.; et al. Global BioID-based SARS-CoV-2 proteins proximal interactome unveils novel ties between viral polypeptides and host factors involved in multiple COVID19-associated mechanisms. bioRxiv 2020. [Google Scholar] [CrossRef]
- Liu, J.; Yang, L.; Liu, F.; Li, H.; Tang, W.; Tong, X.; Zuo, J. CNOT2 facilitates dengue virus infection via negatively modulating IFN-independent non-canonical JAK/STAT pathway. Biochem. Biophys. Res. Commun. 2019, 515, 403–409. [Google Scholar] [CrossRef]
- Rodríguez-Gil, A.; Ritter, O.; Saul, V.V.; Wilhelm, J.; Yang, C.; Grosschedl, R.; Imai, Y.; Kuba, K.; Kracht, M.; Lienhard Schmitz, M. The CCR4-NOT complex contributes to repression of major histocompatibility complex class II transcription. Sci. Rep. 2017, 7, 3547. [Google Scholar] [CrossRef]
- Abraham, C.; Cho, J. Interleukin-23/Th17 pathways and inflammatory bowel disease. Inflamm. Bowel Dis. 2009, 15, 1090–1100. [Google Scholar] [CrossRef]
- Wang, Q.; Li, N.; Wang, X.; Shen, J.; Hong, X.; Yu, H.; Zhang, Y.; Wan, T.; Zhang, L.; Wang, J.; et al. Membrane protein hMYADM preferentially expressed in myeloid cells is up-regulated during differentiation of stem cells and myeloid leukemia cells. Life Sci. 2007, 80, 420–429. [Google Scholar] [CrossRef]
- Yasinska, I.M.; Gonçalves Silva, I.; Sakhnevych, S.; Gibbs, B.F.; Raap, U.; Fasler-Kan, E.; Sumbayev, V.V. Biochemical mechanisms implemented by human acute myeloid leukemia cells to suppress host immune surveillance. Cell. Mol. Immunol. 2018, 15, 989–991. [Google Scholar] [CrossRef] [Green Version]
- Fang, D.; Zhu, J. Molecular switches for regulating the differentiation of inflammatory and IL-10-producing anti-inflammatory T-helper cells. Cell. Mol. Life Sci. 2020, 77, 289–303. [Google Scholar] [CrossRef]
- Jarjour, N.N.; Bradstreet, T.R.; Schwarzkopf, E.A.; Cook, M.E.; Lai, C.; Ching-Cheng Huang, S.; Taneja, R.; Stappenbeck, T.S.; Van Dyken, S.J.; Urban, J.F.; et al. BHLHE40 promotes TH2 cell-mediated antihelminth immunity and reveals cooperative CSF2RB family cytokines. J. Immunol. 2020, 204, 923–932. [Google Scholar] [CrossRef]
- Li, C.; Zhu, B.; Son, Y.M.; Wang, Z.; Jiang, L.; Xiang, M.; Ye, Z.; Beckermann, K.E.; Wu, Y.; Jenkins, J.W.; et al. The transcription factor Bhlhe40 programs mitochondrial regulation of resident CD8+ T cell fitness and functionality. Immunity 2019, 51, 491–507.e7. [Google Scholar] [CrossRef]
- Goplen, N.P.; Wu, Y.; Son, Y.M.; Li, C.; Wang, Z.; Cheon, I.S.; Jiang, L.; Zhu, B.; Ayasoufi, K.; Chini, E.N.; et al. Tissue-resident CD8+ T cells drive age-associated chronic lung sequelae after viral pneumonia. Sci. Immunol. 2020, 5, eabc4557. [Google Scholar] [CrossRef]
- Kühn, R.; Löhler, J.; Rennick, D.; Rajewsky, K.; Müller, W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell 1993, 75, 263–274. [Google Scholar] [CrossRef]
- Kong, M.S.; Hashimoto-Tane, A.; Kawashima, Y.; Sakuma, M.; Yokosuka, T.; Kometani, K.; Onishi, R.; Carpino, N.; Ohara, O.; Kurosaki, T.; et al. Inhibition of T cell activation and function by the adaptor protein CIN85. Sci. Signal. 2019, 12, eaav4373. [Google Scholar] [CrossRef]
- Wong, L.E.; Bhatt, A.; Erdmann, P.S.; Hou, Z.; Maier, J.; Pirkuliyeva, S.; Engelke, M.; Becker, S.; Plitzko, J.; Wienands, J.; et al. Tripartite phase separation of two signal effectors with vesicles priming B cell responsiveness. Nat. Commun. 2020, 11, 848. [Google Scholar] [CrossRef]
- Molfetta, R.; Belleudi, F.; Peruzzi, G.; Morrone, S.; Leone, L.; Dikic, I.; Piccoli, M.; Frati, L.; Torrisi, M.R.; Santoni, A.; et al. CIN85 regulates the ligand-dependent endocytosis of the IgE receptor: A new molecular mechanism to dampen mast cell function. J. Immunol. 2005, 175, 4208–4216. [Google Scholar] [CrossRef] [Green Version]
- Smith, P.J.; Levine, A.P.; Dunne, J.; Guilhamon, P.; Turmaine, M.; Sewell, G.W.; O’Shea, N.R.; Vega, R.; Paterson, J.C.; Oukrif, D.; et al. Mucosal transcriptomics implicates under expression of BRINP3 in the pathogenesis of ulcerative colitis. Inflamm. Bowel Dis. 2014, 20, 1802–1812. [Google Scholar] [CrossRef] [Green Version]
- Casado, P.L.; Aguiar, D.P.; Costa, L.C.; Fonseca, M.A.; Vieira, T.C.S.; Alvim-Pereira, C.C.K.; Alvim-Pereira, F.; Deeley, K.; Granjeiro, J.M.; Trevilatto, P.C.; et al. Different contribution of BRINP3 gene in chronic periodontitis and peri-implantitis: A cross-sectional study. BMC Oral Health 2015, 15, 33. [Google Scholar] [CrossRef] [Green Version]
- Robinson, L.J.; Tourkova, I.; Wang, Y.; Sharrow, A.C.; Landau, M.S.; Yaroslavskiy, B.B.; Sun, L.; Zaidi, M.; Blair, H.C. FSH-receptor isoforms and FSH-dependent gene transcription in human monocytes and osteoclasts. Biochem. Biophys. Res. Commun. 2010, 394, 12–17. [Google Scholar] [CrossRef] [Green Version]
- Sato, J.; Kinugasa, M.; Satomi-Kobayashi, S.; Hatakeyama, K.; Knox, A.J.; Asada, Y.; Wierman, M.E.; Hirata, K.; Rikitake, Y. Family with sequence similarity 5, member C (FAM5C) increases leukocyte adhesion molecules in vascular endothelial cells: Implication in vascular inflammation. PLoS ONE 2014, 9, e107236. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, K.; Zhu, K.; Sun, Y.; Hegyi, B.; Zeng, Q.; Murphy, C.J.; Small, J.V.; Chen-Izu, Y.; Izumiya, Y.; Penninger, J.M.; et al. KCNJ15/Kir4.2 couples with polyamines to sense weak extracellular electric fields in galvanotaxis. Nat. Commun. 2015, 6, 8532. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhang, W. Tim 3 regulates the ability of macrophages to counter lipopolysaccharide induced pulmonary epithelial barrier dysfunction via the PI3K/Akt pathway in epithelial cells. Mol. Med. Rep. 2020, 22, 534–542. [Google Scholar] [CrossRef]
- Mankelow, T.J.; Satchwell, T.J.; Burton, N.M. Refined views of multi-protein complexes in the erythrocyte membrane. Blood Cells Mol. Dis. 2012, 49, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Oldenborg, P.; Zheleznyak, A.; Fang, Y.; Lagenaur, C.F.; Gresham, H.D.; Lindberg, F.P. Role of CD47 as a marker of self on red blood cells. Science 2000, 288, 2051–2054. [Google Scholar] [CrossRef]
- Lemke, G. How macrophages deal with death. Nat. Rev. Immunol. 2019, 19, 539–549. [Google Scholar] [CrossRef]
- Andersson, U.; Ottestad, W.; Tracey, K.J. Extracellular HMGB1: A therapeutic target in severe pulmonary inflammation including COVID-19? Mol. Med. 2020, 26, 42. [Google Scholar] [CrossRef]
- Catena, R.; Escoffier, E.; Caron, C.; Khochbin, S.; Martianov, I.; Davidson, I. HMGB4, a novel member of the HMGB family, is preferentially expressed in the mouse testis and localizes to the basal pole of elongating spermatids 1. Biol. Reprod. 2009, 80, 358–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, W.; Lin, L.; Wang, L.; Yang, L.; Ye, G.; Zeng, Q.; Cheng, J.; Xie, Y.; Chen, M.; Luo, Q. Inhibition of Bcl6b promotes gastric cancer by amplifying inflammation in mice. Cell. Commun. Signal. 2019, 17, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manders, P.M.; Hunter, P.J.; Telaranta, A.I.; Carr, J.M.; Marshall, J.L.; Carrasco, M.; Murakami, Y.; Palmowski, M.J.; Cerundolo, V.; Kaech, S.M.; et al. BCL6b mediates the enhanced magnitude of the secondary response of memory CD8 + T lymphocytes. Proc. Natl. Acad. Sci. USA 2005, 102, 7418–7425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porcher, C.; Swat, W.; Rockwell, K.; Fujiwara, Y.; Alt, F.W.; Orkin, S.H. The T cell leukemia oncoprotein SCL/tal-1 is essential for development of all hematopoietic lineages. Cell 1996, 86, 47–57. [Google Scholar] [CrossRef] [Green Version]
- Shivdasanl, R.A.; Mayer, E.L.; Orkin, S.H. Absence of blood formation in mice lacking the T-cell leukaemia oncoprotein tal-1/SCL. Nature 1995, 373, 432–434. [Google Scholar] [CrossRef]
- Hou, P.; Yang, K.; Jia, P.; Liu, L.; Lin, Y.; Li, Z.; Li, J.; Chen, S.; Guo, S.; Pan, J.A.; et al. A novel selective autophagy receptor, CCDC50, delivers K63 polyubiquitination-activated RIG-I/MDA5 for degradation during viral infection. Cell Res. 2021, 31, 62–79. [Google Scholar] [CrossRef]
- Coulie, P.G.; Van Den Eynde, B.J.; Van Der Bruggen, P.; Boon, T. Tumour antigens recognized by T lymphocytes: At the core of cancer immunotherapy. Nat. Rev. Cancer 2014, 14, 135–146. [Google Scholar] [CrossRef]
- Axelrod, M.L.; Cook, R.S.; Johnson, D.B.; Balko, J.M. Biological consequences of MHC-II expression by tumor cells in cancer. Clin. Cancer Res. 2019, 25, 2392–2402. [Google Scholar] [CrossRef]
- Krämer, B.F.; Schoor, O.; Krüger, T.; Reichle, C.; Müller, M.; Weinschenk, T.; Hennenlotter, J.; Stenzl, A.; Rammensee, H.; Stevanovic, S. MAGED4-expression in renal cell carcinoma and identification of an HLA-A*25-restricted MHC class I ligand from solid tumor tissue. Cancer Biol. Ther. 2005, 4, 943–948. [Google Scholar] [CrossRef] [Green Version]
- Tilley, A.E.; Walters, M.S.; Shaykhiev, R.; Crystal, R.G. Cilia dysfunction in lung disease. Annu. Rev. Physiol. 2015, 77, 379–406. [Google Scholar] [CrossRef] [Green Version]
- Banyard, J.; Bielenberg, D.R. The role of EMT and MET in cancer dissemination. Connect. Tissue Res. 2015, 56, 403–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jarray, R.; Pavoni, S.; Borriello, L.; Allain, B.; Lopez, N.; Bianco, S.; Liu, W.; Biard, D.; Demange, L.; Hermine, O.; et al. Disruption of phactr-1 pathway triggers pro-inflammatory and pro-atherogenic factors: New insights in atherosclerosis development. Biochimie 2015, 118, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Zhang, L.; Xu, Z.; Chen, H.; Ju, S.; Ding, J.; Guo, Y.; Tian, H. Phosphatase actin regulator-1 (PHACTR-1) knockdown suppresses cell proliferation and migration and promotes cell apoptosis in the bend.3 mouse brain capillary endothelial cell line. Med. Sci. Monit. 2019, 25, 1291–1300. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Yu, X.; Cong, P.; Zhou, Y.; Xu, Y.; Jiang, Y. Six2 promotes non–small cell lung cancer cell stemness via transcriptionally and epigenetically regulating E-cadherin. Cell Prolif. 2019, 52, e12617. [Google Scholar] [CrossRef]
- Kozlova, N.; Mennerich, D.; Samoylenko, A.; Dimova, E.Y.; Koivunen, P.; Biterova, E.; Richter, K.; Hassinen, A.; Kellokumpu, S.; Manninen, A.; et al. The pro-oncogenic adaptor CIN85 acts as an inhibitory binding partner of hypoxia-inducible factor prolyl hydroxylase 2. Cancer Res. 2019, 79, 4042–4056. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Dong, L.; Xu, L.; Chu, E.S.H.; Chen, Y.; Shen, J.; Li, X.; Wong, C.C.; Sung, J.J.Y.; Yu, J. B cell CLL/lymphoma 6 member B inhibits hepatocellular carcinoma metastases in vitro and in mice. Cancer Lett. 2014, 355, 192–200. [Google Scholar] [CrossRef] [Green Version]
- Balestra, F.R.; Domínguez-Calvo, A.; Wolf, B.; Busso, C.; Buff, A.; Averink, T.; Lipsanen-Nyman, M.; Huertas, P.; Ríos, R.M.; Gönczy, P. Trim37 prevents formation of centriolar protein assemblies by regulating centrobin. eLife 2021, 10, e62640. [Google Scholar] [CrossRef]
- Balestra, F.; Strnad, P.; Flückiger, I.; Gönczy, P. Discovering regulators of centriole biogenesis through siRNA-based functional genomics in human cells. Dev. Cell 2013, 25, 555–571. [Google Scholar] [CrossRef] [Green Version]
- Berndsen, K.; Lis, P.; Yeshaw, W.M.; Wawro, P.S.; Nirujogi, R.S.; Wightman, M.; Macartney, T.; Dorward, M.; Knebel, A.; Tonelli, F.; et al. PPM1H phosphatase counteracts LRRK2 signaling by selectively dephosphorylating rab proteins. eLife 2019, 8, e62640. [Google Scholar] [CrossRef]
- Elrick, M.M.; Samson, W.K.; Corbett, J.A.; Salvatori, A.S.; Stein, L.M.; Kolar, G.R.; Naatz, A.; Yosten, G.L.C. Neuronostatin acts via GPR107 to increase cAMP-independent PKA phosphorylation and proglucagon mRNA accumulation in pancreatic α-cells. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 310, R143–R155. [Google Scholar] [CrossRef] [Green Version]
- Luxmi, R.; Kumar, D.; Mains, R.E.; King, S.M.; Eipper, B.A. Cilia-based peptidergic signaling. PLoS Biol. 2019, 17, e3000566. [Google Scholar] [CrossRef]
- Ikegami, K.; Ijaz, F. Current understandings of the relationship between extracellular vesicles and cilia. J. Biochem. 2021, 169, 139–145. [Google Scholar] [CrossRef]
- Akella, J.S.; Barr, M.M. The tubulin code specializes neuronal cilia for extracellular vesicle release. Dev. Neurobiol. 2020, 81, 231–252. [Google Scholar] [CrossRef]
- Tafesse, F.G.; Guimaraes, C.P.; Maruyama, T.; Carette, J.E.; Lory, S.; Brummelkamp, T.R.; Ploegh, H.L. GPR107, a G-protein-coupled receptor essential for intoxication by pseudomonas aeruginosa exotoxin a, localizes to the golgi and is cleaved by furin. J. Biol. Chem. 2014, 289, 24005–24018. [Google Scholar] [CrossRef] [Green Version]
- Toropova, K.; Zalyte, R.; Mukhopadhyay, A.G.; Mladenov, M.; Carter, A.P.; Roberts, A.J. Structure of the dynein-2 complex and its assembly with intraflagellar transport trains. Nat. Struct. Mol. Biol. 2019, 26, 823–829. [Google Scholar] [CrossRef]
- Nakayama, K.; Katoh, Y. Architecture of the IFT ciliary trafficking machinery and interplay between its components. Crit. Rev. Biochem. Mol. Biol. 2020, 55, 179–196. [Google Scholar] [CrossRef]
- Vuolo, L.; Stevenson, N.L.; Heesom, K.J.; Stephens, D.J. Dynein-2 intermediate chains play crucial but distinct roles in primary cilia formation and function. eLife 2018, 7, e39655. [Google Scholar] [CrossRef]
- Yu, Y.; Travaglio, M.; Popovic, R.; Leal, N.S.; Martins, L.M. Alzheimer’s and parkinson’s diseases predict different COVID-19 outcomes: A UK biobank study. Geriatrics 2021, 6, 10. [Google Scholar] [CrossRef]
- Rahman, M.A.; Islam, K.; Rahman, S.; Alamin, M. Neurobiochemical cross-talk between COVID-19 and Alzheimer’s disease. Mol. Neurobiol. 2020, 58, 1017–1023. [Google Scholar] [CrossRef]
- Ristori, E.; Donnini, S.; Ziche, M. The homeostatic role of β-amyloid precursor protein in cerebral vasculature. Front. Physiol. 2020, 11, 1056. [Google Scholar] [CrossRef]
- Ristori, E.; Cicaloni, V.; Salvini, L.; Tinti, L.; Tinti, C.; Simons, M.; Corti, F.; Donnini, S.; Ziche, M. Amyloid-β precursor protein APP down-regulation alters actin cytoskeleton-interacting proteins in endothelial cells. Cells 2020, 9, 2506. [Google Scholar] [CrossRef] [PubMed]
- Blair, L.J.; Sabbagh, J.J.; Dickey, C.A. Targeting Hsp90 and its co-chaperones to treat alzheimer’s disease. Expert Opin. Ther. Targets 2014, 18, 1219–1232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matz, A.; Lee, S.; Schwedhelm-Domeyer, N.; Zanini, D.; Holubowska, A.; Kannan, M.; Farnworth, M.; Jahn, O.; Göpfert, M.C.; Stegmüller, J. Regulation of neuronal survival and morphology by the E3 ubiquitin ligase RNF157. Cell Death Differ. 2015, 22, 626–642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fong, L.K.; Yang, M.M.; Chaves, R.D.S.; Reyna, S.M.; Langness, V.F.; Woodruff, G.; Roberts, E.A.; Young, J.E.; Goldstein, L.S.B. Full-length amyloid precursor protein regulates lipoprotein metabolism and amyloid- clearance in human astrocytes. J. Biol. Chem. 2018, 293, 11341–11357. [Google Scholar] [CrossRef] [Green Version]
- Thuringer, D.; Garrido, C. Molecular chaperones in the brain endothelial barrier: Neurotoxicity or neuroprotection? FASEB J. 2019, 33, 11629–11639. [Google Scholar] [CrossRef] [Green Version]
- Pietrzik, C.U.; Yoon, I.; Jaeger, S.; Busse, T.; Weggen, S.; Koo, E.H. FE65 constitutes the functional link between the low-density lipoprotein receptor-related protein and the amyloid precursor protein. J. Neurosci. 2004, 24, 4259–4265. [Google Scholar] [CrossRef] [Green Version]
- Marzinke, M.A.; Clagett-Dame, M. The all-trans retinoic acid (atRA)-regulated gene calmin (clmn) regulates cell cycle exit and neurite outgrowth in murine neuroblastoma (Neuro2a) cells. Exp. Cell Res. 2012, 318, 85–93. [Google Scholar] [CrossRef]
- Barber, M.J.; Mangravite, L.M.; Hyde, C.L.; Chasman, D.I.; Smith, J.D.; McCarty, C.A.; Li, X.; Wilke, R.A.; Rieder, M.J.; Williams, P.T.; et al. Genome-wide association of lipid-lowering response to statins in combined study populations. PLoS ONE 2010, 5, e9763. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Malloy, C.; Tabor, G.T.; Gutzmann, J.J.; Liu, Y.; Abebe, D.; Karlsson, R.; Durell, S.; Cameron, H.A.; Hoffman, D.A. Activity-dependent isomerization of Kv4.2 by Pin1 regulates cognitive flexibility. Nat. Commun. 2020, 11, 1567. [Google Scholar] [CrossRef] [Green Version]
- Cacace, R.; Heeman, B.; Van Mossevelde, S.; De Roeck, A.; Hoogmartens, J.; De Rijk, P.; Gossye, H.; De Vos, K.; De Coster, W.; Strazisar, M.; et al. Loss of DPP6 in neurodegenerative dementia: A genetic player in the dysfunction of neuronal excitability. Acta Neuropathol. 2019, 137, 901–918. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.; Petralia, R.S.; Lake, R.; Wang, Y.; Hoffman, D.A. A novel structure associated with aging is augmented in the DPP6-KO mouse brain. Acta Neuropathol. Commun. 2020, 8, 197. [Google Scholar] [CrossRef]
- Lin, L.; Sun, W.; Throesch, B.; Kung, F.; Decoster, J.T.; Berner, C.J.; Cheney, R.E.; Rudy, B.; Hoffman, D.A. DPP6 regulation of dendritic morphogenesis impacts hippocampal synaptic development. Nat. Commun. 2013, 4, 2270. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Chen, Y.; Mok, K.Y.; Zhao, Q.; Chen, K.; Chen, Y.; Hardy, J.; Li, Y.; Fu, A.K.Y.; Guo, Q.; et al. Identification of genetic risk factors in the chinese population implicates a role of immune system in Alzheimer’s disease pathogenesis. Proc. Natl. Acad. Sci. USA 2018, 115, 1697–1706. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.K.; Muqit, M.M.K. Parkinson’s: A disease of aberrant vesicle trafficking. Annu. Rev. Cell. Dev. Biol. 2020, 36, 237–264. [Google Scholar] [CrossRef]
- Ciruelas, K.; Marcotulli, D.; Bajjalieh, S.M. Synaptic vesicle protein 2: A multi-faceted regulator of secretion. Semin. Cell Dev. Biol. 2019, 95, 130–141. [Google Scholar] [CrossRef]
- Pellett, S.; Tepp, W.H.; Johnson, E.A. Botulinum neurotoxins A, B, C, E, and F preferentially enter cultured human motor neurons compared to other cultured human neuronal populations. FEBS Lett. 2019, 593, 2675–2685. [Google Scholar] [CrossRef]
- Dunn, A.R.; Stout, K.A.; Ozawa, M.; Lohr, K.M.; Hoffman, C.A.; Bernstein, A.I.; Li, Y.; Wang, M.; Sgobio, C.; Sastry, N.; et al. Synaptic vesicle glycoprotein 2C (SV2C) modulates dopamine release and is disrupted in parkinson disease. Proc. Natl. Acad. Sci. USA 2017, 114, E2253–E2262. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Zhang, J.; Shi, M.; Quinn, T.; Bradner, J.; Beyer, R.; Chen, S.; Zhang, J. Rab11a and HSP90 regulate recycling of extracellular α-synuclein. J. Neurosci. 2009, 29, 1480–1485. [Google Scholar] [CrossRef] [Green Version]
- Lauwers, E.; Wang, Y.; Gallardo, R.; Van der Kant, R.; Michiels, E.; Swerts, J.; Baatsen, P.; Zaiter, S.S.; McAlpine, S.R.; Gounko, N.V.; et al. Hsp90 mediates membrane deformation and exosome release. Mol. Cell 2018, 71, 689–702.e9. [Google Scholar] [CrossRef] [Green Version]
- Pereira, J.A.; Lebrun-Julien, F.; Suter, U. Molecular mechanisms regulating myelination in the peripheral nervous system. Trends Neurosci. 2012, 35, 123–134. [Google Scholar] [CrossRef]
- Blackstone, C. Cellular pathways of hereditary spastic paraplegia. Annu. Rev. Neurosci. 2012, 35, 25–47. [Google Scholar] [CrossRef] [Green Version]
- Germany, E.M.; Zahayko, N.; Huebsch, M.L.; Fox, J.L.; Prahlad, V.; Khalimonchuk, O. The AAA ATPase Afg1 preserves mitochondrial fidelity and cellular health by maintaining mitochondrial matrix proteostasis. J. Cell Sci. 2018, 131, jcs.219956. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; She, Z.; Cheng, X.; Qin, J.; Zhang, X.; Cai, J.; Lei, F.; Wang, H.; Xie, J.; Wang, W.; et al. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020, 31, 1068–1077. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Bae, J.H.; Kwon, H.; Nauck, M.A. COVID-19 and diabetes mellitus: From pathophysiology to clinical management. Nat. Rev. Endocrionol. 2021, 17, 11–30. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.C.; Shulman, G.I. Mechanisms of insulin action and insulin resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parton, R.G.; McMahon, K.; Wu, Y. Caveolae: Formation, dynamics, and function. Curr. Opin. Cell Biol. 2020, 65, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Lei, J.; Shen, F.; Dai, Y.; Sun, Y.; Liu, Y.; Dai, Y.; Jian, Z.; Wang, S.; Chen, Z.; et al. Cavin1 deficiency causes disorder of hepatic glycogen metabolism and neonatal death by impacting fenestrations in liver sinusoidal endothelial cells. Adv. Sci. 2020, 7, 2000963. [Google Scholar] [CrossRef]
- Röthe, J.; Thor, D.; Winkler, J.; Knierim, A.B.; Binder, C.; Huth, S.; Kraft, R.; Rothemund, S.; Schöneberg, T.; Prömel, S. Involvement of the adhesion GPCRs latrophilins in the regulation of insulin release. Cell Rep. 2019, 26, 1573–1584. [Google Scholar] [CrossRef] [Green Version]
- Mukohda, M.; Fang, S.; Wu, J.; Agbor, L.N.; Nair, A.R.; Ibeawuchi, S.C.; Hu, C.; Liu, X.; Lu, K.; Guo, D.; et al. RhoBTB1 protects against hypertension and arterial stiffness by restraining phosphodiesterase 5 activity. J. Clin. Investig. 2019, 129, 2318–2332. [Google Scholar] [CrossRef] [Green Version]
- Okamoto, K.; Iwasaki, N.; Doi, K.; Noiri, E.; Iwamoto, Y.; Uchigata, Y.; Fujita, T.; Tokunaga, K. Inhibition of glucose-stimulated insulin secretion by KCNJ15, a newly identified susceptibility gene for type 2 diabetes. Diabetes 2012, 61, 1734–1741. [Google Scholar] [CrossRef] [Green Version]
- Lipkin, S.M.; Naar, A.M.; Kalla, K.A.; Sack, R.A.; Rosenfeld, M.G. Identification of a novel zinc finger protein binding a conserved element critical for pit-1-dependent growth hormone gene expression. Genes Dev. 1993, 7, 1674–1687. [Google Scholar] [CrossRef] [Green Version]
- Rotwein, P. Regulation of gene expression by growth hormone. Mol. Cell. Endocrinol. 2020, 507, 110788. [Google Scholar] [CrossRef]
- Zhao, A.Z.; Zhao, H.; Teague, J.; Fujimoto, W.; Beavo, J.A. Attenuation of insulin secretion by insulin-like growth factor 1 is mediated through activation of phosphodiesterase 3B. Proc. Natl. Acad. Sci. USA 1997, 94, 3223–3228. [Google Scholar] [CrossRef] [Green Version]
- Emanuelli, B.; Eberlé, D.; Suzuki, R.; Kahn, C.R. Overexpression of the dual-specificity phosphatase MKP-4/DUSP-9 protects against stress-induced insulin resistance. Proc. Natl. Acad. Sci. USA 2008, 105, 3545–3550. [Google Scholar] [CrossRef] [Green Version]
- Yosten, G.L.C.; Redlinger, L.J.; Samson, W.K. Evidence for an interaction of neuronostatin with the orphan G protein-coupled receptor, GPR107. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 303, R941–R949. [Google Scholar] [CrossRef] [Green Version]
- Walls, A.C.; Park, Y.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 2020, 180, 281–292. [Google Scholar] [CrossRef]
- Batlle, D.; Soler, M.J.; Ye, M. ACE2 and diabetes: ACE of ACEs? Diabetes 2010, 59, 2994–2996. [Google Scholar] [CrossRef] [Green Version]
- Cao, X.; Yang, F.; Xin, Z.; Xie, R.; Yang, J. The ACE2/Ang-(1-7)/Mas axis can inhibit hepatic insulin resistance. Mol. Cell. Endocrinol. 2014, 393, 30–38. [Google Scholar] [CrossRef]
- Klein, S.; Cortese, M.; Winter, S.L.; Wachsmuth-Melm, M.; Neufeldt, C.J.; Cerikan, B.; Stanifer, M.L.; Boulant, S.; Bartenschlager, R.; Chlanda, P. SARS-CoV-2 structure and replication characterized by in situ cryo-electron tomography. Nat. Commun. 2020, 11, 5885. [Google Scholar] [CrossRef]
- Bindesbøll, C.; Aas, A.; Ogmundsdottir, M.H.; Pankiv, S.; Reine, T.; Zoncu, R.; Simonsen, A. NBEAL1 controls SREBP2 processing and cholesterol metabolism and is a susceptibility locus for coronary artery disease. Sci. Rep. 2020, 10, 4528. [Google Scholar] [CrossRef]
- Luo, J.; Yang, H.; Song, B. Mechanisms and regulation of cholesterol homeostasis. Nat. Rev. Mol. Cell Biol. 2020, 21, 225–245. [Google Scholar] [CrossRef]
- Krishnamoorthy, V.; Khanna, R.; Parnaik, V.K. E3 ubiquitin ligase HECW2 targets PCNA and lamin B1. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 1088–1104. [Google Scholar] [CrossRef]
- Duband-Goulet, I.; Woerner, S.; Gasparini, S.; Attanda, W.; Kondé, E.; Tellier-Lebègue, C.; Craescu, C.T.; Gombault, A.; Roussel, P.; Vadrot, N.; et al. Subcellular localization of SREBP1 depends on its interaction with the C-terminal region of wild-type and disease related A-type lamins. Exp. Cell Res. 2011, 317, 2800–2813. [Google Scholar] [CrossRef] [Green Version]
- Wyler, E.; Mösbauer, K.; Franke, V.; Diag, A.; Gottula, L.T.; Arsiè, R.; Klironomos, F.; Koppstein, D.; Hönzke, K.; Ayoub, S.; et al. Transcriptomic profiling of SARS-CoV-2 infected human cell lines identifies HSP90 as target for COVID-19 therapy. IScience 2021, 24, 102151. [Google Scholar] [CrossRef]
- Sultan, I.; Howard, S.; Tbakhi, A. Drug repositioning suggests a role for the heat shock protein 90 inhibitor geldanamycin in treating COVID-19 infection. Res. Sq. 2020. [Google Scholar] [CrossRef] [Green Version]
- Casarini, L.; Crépieux, P. Molecular mechanisms of action of FSH. Front. Endocrinol. 2019, 10, 305. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Meng, F.; Cao, X.; Du, X.; Bu, G.; Kong, F.; Huang, A.; Zeng, X. FSH promotes fat accumulation by activating PPARγ signaling in surgically castrated, but not immunocastrated, male pigs. Theriogenology 2021, 160, 10–17. [Google Scholar] [CrossRef]
- Huang, J.; Huang, J.; Hu, W.; Zhang, Z. Heat shock protein 90 alpha and 14-3-3η in postmenopausal osteoporotic rats with varying levels of serum FSH. Climacteric 2020, 23, 581–590. [Google Scholar] [CrossRef]
- Chen, C.; Kudo, M.; Rutaganira, F.; Takano, H.; Lee, C.; Atakilit, A.; Robinett, K.S.; Uede, T.; Wolters, P.J.; Shokat, K.M.; et al. Integrin α9β1 in airway smooth muscle suppresses exaggerated airway narrowing. J. Clin. Investig. 2012, 122, 2916–2927. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Liu, X.; Zhang, G.; Zhang, Q.; Chen, H.; Wang, Y.; Fang, F.; Sun, J. Knockdown THOC2 suppresses the proliferation and invasion of melanoma. Bioengineered 2019, 10, 635–645. [Google Scholar] [CrossRef] [Green Version]
- Ji, J.; Tang, B.; Zhu, J.; Gao, Y.; Tu, J.; Yang, W.; Fang, S.; Weng, Q.; Zhao, Z.; Xu, M.; et al. Based on exosome-derived genes for constructing diagnostic, prognostic and recurrence models and predicting therapeutic response for hepatocellular carcinoma. Res. Square 2020. [Google Scholar] [CrossRef]
- Sohn, E.J.; Jung, D.; Lee, J.; Yoon, S.W.; Won, G.; Ko, H.S.; Kim, S. CCR4-NOT2 promotes the differentiation and lipogenesis of 3T3-L1 adipocytes via upregulation of PPARγ, CEBPα and inhibition of P-GSK3α/β and β-catenin. Cell. Physiol. Biochem. 2015, 37, 1881–1889. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Liu, X.; Yang, J.; Li, W.; Liu, S.; Liu, X.; Yang, Z.; Ren, J.; Wang, Y.; Shan, L.; et al. Imbalance of the reciprocally inhibitory loop between the ubiquitin-specific protease USP43 and EGFR/PI3K/AKT drives breast carcinogenesis. Cell Res. 2018, 28, 934–951. [Google Scholar] [CrossRef] [PubMed]
- Sakai, R.; Fukuda, R.; Unida, S.; Aki, M.; Ono, Y.; Endo, A.; Kusumi, S.; Koga, D.; Fukushima, T.; Komada, M.; et al. The integral function of the endocytic recycling compartment is regulated by RFFL-mediated ubiquitylation of Rab11 effectors. J. Cell Sci. 2019, 132, jcs.228007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, E.E.; Horgan, C.P.; McCaffrey, M.W. Rab11 proteins in health and disease. Biochem. Soc. Trans. 2012, 40, 1360–1367. [Google Scholar] [CrossRef] [PubMed]
- Sobajima, T.; Yoshimura, S.; Maeda, T.; Miyata, H.; Miyoshi, E.; Harada, A. The Rab11-binding protein RELCH/KIAA1468 controls intracellular cholesterol distribution. J. Cell Biol. 2018, 217, 1777–1796. [Google Scholar] [CrossRef] [PubMed]
- Kottemann, M.C.; Conti, B.A.; Lach, F.P.; Smogorzewska, A. Removal of RTF2 from stalled replisomes promotes maintenance of genome integrity. Mol. Cell 2018, 69, 24–35. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Wu, F.; Cheng, L.; Zhang, J.; Cha, C.; Chen, L.; Feng, T.; Zhang, J.; Guo, G. A nuclear localization signal is required for the nuclear translocation of fign and its microtubule-severing function. Mol. Med. Rep. 2020, 21, 2367–2374. [Google Scholar] [CrossRef] [Green Version]
- Hu, Z.; Feng, J.; Bo, W.; Wu, R.; Dong, Z.; Liu, Y.; Qiang, L.; Liu, M. Fidgetin regulates cultured astrocyte migration by severing tyrosinated microtubules at the leading edge. Mol. Biol. Cell 2017, 28, 545–553. [Google Scholar] [CrossRef]
- Forsberg, D.; Herlenius, E. Astrocyte networks modulate respiration—Sniffing glue. Respir. Physiol. Neurobiol. 2019, 265, 3–8. [Google Scholar] [CrossRef]
- Rienzo, M.; Casamassimi, A. Integrator complex and transcription regulation: Recent findings and pathophysiology. Biochim. Biophys. Acta Gene Regul. Mech. 2016, 1859, 1269–1280. [Google Scholar] [CrossRef]
- Zaborowska, J.; Isa, N.F.; Murphy, S. P-TEFb goes viral. Bioessays 2016, 38, S75–S85. [Google Scholar] [CrossRef]
- Patel, A.B.; Greber, B.J.; Nogales, E. Recent insights into the structure of TFIID, its assembly, and its binding to core promoter. Curr. Opin. Struct. Biol. 2020, 61, 17–24. [Google Scholar] [CrossRef]
- Shin, Y.; Chang, Y.; Lee, D.S.W.; Berry, J.; Sanders, D.W.; Ronceray, P.; Wingreen, N.S.; Haataja, M.; Brangwynne, C.P. Liquid nuclear condensates mechanically sense and restructure the genome. Cell 2018, 175, 1481–1491. [Google Scholar] [CrossRef] [Green Version]
- Savastano, A.; Ibáñez de Opakua, A.; Rankovic, M.; Zweckstetter, M. Nucleocapsid protein of SARS-CoV-2 phase separates into RNA-rich polymerase-containing condensates. Nat. Commun. 2020, 11, 6041. [Google Scholar] [CrossRef]
- Vandelli, A.; Monti, M.; Milanetti, E.; Armaos, A.; Rupert, J.; Zacco, E.; Bechara, E.; Delli Ponti, R.; Tartaglia, G.G. Structural analysis of SARS-CoV-2 genome and predictions of the human interactome. Nucleic Acids Res. 2020, 48, 11270–11283. [Google Scholar] [CrossRef]
- Ivics, Z.; Izsvák, Z. Sleeping beauty transposition. Microbiol. Spectr. 2015, 3, 853–874. [Google Scholar] [CrossRef] [Green Version]
- Ying, Y.; Xiao-zhao, L.; Ximiao, H.; Li-quan, Z. Exogenous coronavirus interacts with endogenous retrotransposon in human cells. Front. Cell. Infect. Microbiol. 2021, 11, 609160. [Google Scholar] [CrossRef]
- Devaraj, A.; Singh, M.; Narayanavari, S.; Yong, G.; Wang, J.; Wang, J.; Becker, M.; Walisko, O.; Schorn, A.; Cseresznyés, Z.; et al. HMGXB4 targets sleeping beauty transposition to vertebrate germinal stem cells. bioRxiv 2020. [Google Scholar] [CrossRef]
- Lin, D.H.; Hoelz, A. The structure of the nuclear pore complex (an update). Annu. Rev. Biochem. 2019, 88, 725–783. [Google Scholar] [CrossRef] [Green Version]
- Walther, T.C.; Fornerod, M.; Pickersgill, H.; Goldberg, M.; Allen, T.D.; Mattaj, I.W. The nucleoporin Nup153 is required for nuclear pore basket formation, nuclear pore complex anchoring and import of a subset of nuclear proteins. EMBO J. 2001, 20, 5703–5714. [Google Scholar] [CrossRef] [Green Version]
- Caly, L.; Druce, J.D.; Catton, M.G.; Jans, D.A.; Wagstaff, K.M. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir. Res. 2020, 178, 104787. [Google Scholar] [CrossRef]
- Heath, C.G.; Viphakone, N.; Wilson, S.A. The role of TREX in gene expression and disease. Biochem. J. 2016, 473, 2911–2935. [Google Scholar] [CrossRef] [Green Version]
- Rintala-Dempsey, A.C.; Kothe, U. Eukaryotic stand-alone pseudouridine synthases–RNA modifying enzymes and emerging regulators of gene expression? RNA Biol. 2017, 14, 1185–1196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bekaert, M.; Rousset, J. An extended signal involved in eukaryotic -1 frameshifting operates through modification of the E site tRNA. Mol. Cell 2005, 17, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Lee, J.; Yang, J.; Kim, J.W.; Kim, V.N.; Chang, H. The architecture of SARS-CoV-2 transcriptome. Cell 2020, 181, 914–921. [Google Scholar] [CrossRef] [PubMed]
- Karikó, K.; Muramatsu, H.; Welsh, F.A.; Ludwig, J.; Kato, H.; Akira, S.; Weissman, D. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol. Ther. 2008, 16, 1833–1840. [Google Scholar] [CrossRef] [PubMed]
- Lomakin, I.B.; Dmitriev, S.E.; Steitz, T.A. Crystal structure of the DENR-MCT-1 complex revealed zinc-binding site essential for heterodimer formation. Proc. Natl. Acad. Sci. USA 2019, 116, 528–533. [Google Scholar] [CrossRef] [Green Version]
- Niepmann, M.; Gerresheim, G.K. Hepatitis C virus translation regulation. Int. J. Mol. Sci. 2020, 21, 2328. [Google Scholar] [CrossRef] [Green Version]
- Vangenderen, C.; Harkness, T.A.A.; Arnason, T.G. The role of anaphase promoting complex activation, inhibition and substrates in cancer development and progression. Aging 2020, 12, 15818–15855. [Google Scholar] [CrossRef]
- Yu, B.; Li, C.; Sun, Y.; Wang, D.W. Insulin treatment is associated with increased mortality in patients with COVID-19 and type 2 diabetes. Cell Metab. 2021, 33, 65–77. [Google Scholar] [CrossRef]
- Kamel, W.; Noerenberg, M.; Cerikan, B.; Chen, H.; Järvelin, A.I.; Kammoun, M.; Lee, J.Y.; Shuai, N.; Garcia-Moreno, M.; Andrejeva, A.; et al. Global analysis of protein-RNA interactions in SARS-CoV-2-infected cells reveals key regulators of infection. Mol. Cell 2021, 81, 2851–2867. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nahalka, J. Transcription of the Envelope Protein by 1-L Protein–RNA Recognition Code Leads to Genes/Proteins That Are Relevant to the SARS-CoV-2 Life Cycle and Pathogenesis. Curr. Issues Mol. Biol. 2022, 44, 791-816. https://doi.org/10.3390/cimb44020055
Nahalka J. Transcription of the Envelope Protein by 1-L Protein–RNA Recognition Code Leads to Genes/Proteins That Are Relevant to the SARS-CoV-2 Life Cycle and Pathogenesis. Current Issues in Molecular Biology. 2022; 44(2):791-816. https://doi.org/10.3390/cimb44020055
Chicago/Turabian StyleNahalka, Jozef. 2022. "Transcription of the Envelope Protein by 1-L Protein–RNA Recognition Code Leads to Genes/Proteins That Are Relevant to the SARS-CoV-2 Life Cycle and Pathogenesis" Current Issues in Molecular Biology 44, no. 2: 791-816. https://doi.org/10.3390/cimb44020055
APA StyleNahalka, J. (2022). Transcription of the Envelope Protein by 1-L Protein–RNA Recognition Code Leads to Genes/Proteins That Are Relevant to the SARS-CoV-2 Life Cycle and Pathogenesis. Current Issues in Molecular Biology, 44(2), 791-816. https://doi.org/10.3390/cimb44020055





