Novel Therapeutic Effects in Rat Spinal Cord Injuries: Recovery of the Definitive and Early Spinal Cord Injury by the Administration of Pentadecapeptide BPC 157 Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Drugs
2.3. Surgery and Spinal Cord Injury
2.3.1. Early Post-Injury Course, Immediate Effect
2.3.2. Delayed Post-Injury Course, Immediate Effect
2.3.3. Delayed Post-Injury Course, Long-Term Effect
2.4. Clinical Evaluation
2.5. Histology
2.6. Spinal Cord Volume Presentation Proportional with the Change in the Spinal Cord Surface Area
2.7. Gene Expression Analysis
2.8. Statistical Analyses
3. Results
3.1. Clinical Examinations
3.1.1. Tail Motor Function Score
3.1.2. Tail Spasticity
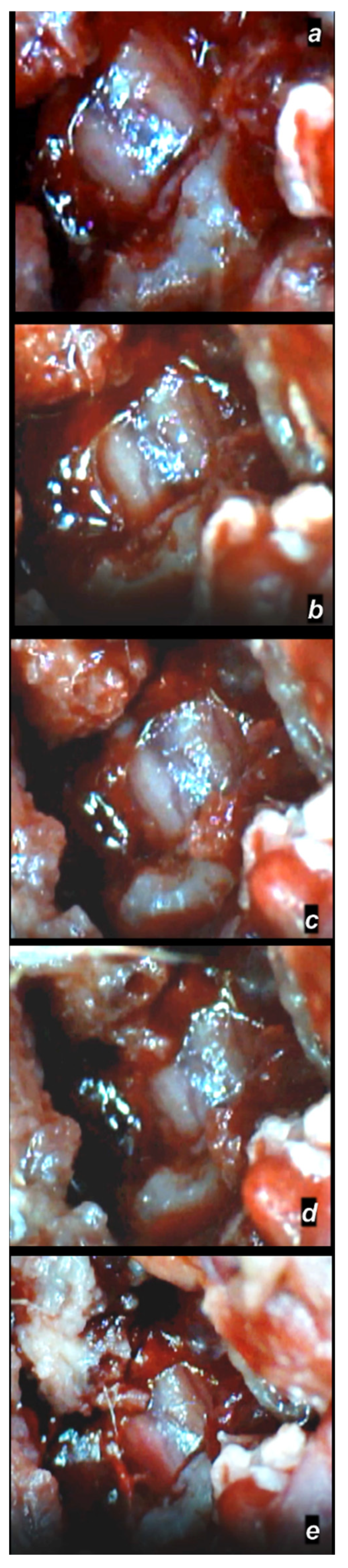
3.1.3. Gross Assessment of the Spinal Cord Injury Hematoma and Swelling
Early Post-Injury Course, Immediate Effect
Delayed Post-Injury Course, Immediate Effect
3.2. Microscopy
3.2.1. Early Post-Injury Course, Immediate Effect
3.2.2. Delayed Post-Injury Course, Immediate Effect
3.2.3. Delayed Post-Injury Course, Long-Term Effect
3.3. mRNA Expression
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Perovic, D.; Kolenc, D.; Bilic, V.; Somun, N.; Drmic, D.; Elabjer, E.; Buljat, G.; Seiwerth, S.; Sikiric, P. Stable gastric pentadecapeptide BPC 157 can improve the healing course of spinal cord injury and lead to functional recovery in rats. J. Orthop. Surg. Res. 2019, 14, 199. [Google Scholar] [CrossRef] [PubMed]
- Gwyer, D.; Wragg, N.M.; Wilson, S.L. Gastric pentadecapeptide body protection compound BPC 157 and its role in accelerating musculoskeletal soft tissue healing. Cell Tissue Res. 2019, 377, 153–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, E.A.; Han, Y.M.; An, J.M.; Park, Y.J.; Sikiric, P.; Kim, D.H.; Kwon, K.A.; Kim, Y.J.; Yang, D.; Tchah, H.; et al. BPC157 as potential agent rescuing from cancer cachexia. Curr. Pharm. Des. 2018, 24, 1947–1956. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Lee, H.J.; Sikiric, P.; Hahm, K.B. BPC 157 rescued NSAID-cytotoxicity via stabilizing intestinal permeability and enhancing cytoprotection. Curr. Pharm. Des. 2020, 26, 2971–2981. [Google Scholar] [CrossRef] [PubMed]
- Seiwerth, S.; Brcic, L.; Vuletic, L.B.; Kolenc, D.; Aralica, G.; Misic, M.; Zenko, A.; Drmic, D.; Rucman, R.; Sikiric, P. BPC 157 and blood vessels. Curr. Pharm. Des. 2014, 20, 1121–1125. [Google Scholar] [CrossRef]
- Seiwerth, S.; Rucman, R.; Turkovic, B.; Sever, M.; Klicek, R.; Radic, B.; Drmic, D.; Stupnisek, M.; Misic, M.; Vuletic, L.B.; et al. BPC 157 and standard angiogenic growth factors. Gastrointestinal tract healing, lessons from tendon, ligament, muscle and bone healing. Curr. Pharm. Des. 2018, 24, 1972–1989. [Google Scholar] [CrossRef]
- Sikiric, P.; Drmic, D.; Sever, M.; Klicek, R.; Blagaic, A.B.; Tvrdeic, A.; Kralj, T.; Kovac, K.K.; Vukojevic, J.; Siroglavic, M.; et al. Fistulas healing. Stable gastric pentadecapeptide BPC 157 therapy. Curr. Pharm. Des. 2020, 14, 153–167. [Google Scholar] [CrossRef]
- Sikiric, P.; Hahm, K.B.; Blagaic, A.B.; Tvrdeic, A.; Pavlov, K.H.; Petrovic, A.; Kokot, A.; Gojkovic, S.; Krezic, I.; Drmic, D.; et al. Stable gastric pentadecapeptide BPC 157, Robert’s stomach cytoprotection/adaptive cytoprotection/organoprotection, and Selye’s stress coping response: Progress, achievements, and the future. Gut Liver 2020, 14, 153–167. [Google Scholar] [CrossRef]
- Sikiric, P.; Rucman, R.; Turkovic, B.; Sever, M.; Klicek, R.; Radic, B.; Drmic, D.; Stupnisek, M.; Misic, M.; Vuletic, L.B.; et al. Novel cytoprotective mediator, stable gastric pentadecapeptide BPC 157. Vascular recruitment and gastrointestinal tract healing. Curr. Pharm. Des. 2018, 24, 1990–2001. [Google Scholar] [CrossRef]
- Sikiric, P.; Seiwerth, S.; Brcic, L.; Sever, M.; Klicek, R.; Radic, B.; Drmic, D.; Ilic, S.; Kolenc, D. Revised Robert’s cytoprotection and adaptive cytoprotection and stable gastric pentadecapeptide BPC 157. Possible significance and implications for novel mediator. Curr. Pharm. Des. 2010, 16, 1224–1234. [Google Scholar] [CrossRef]
- Sikiric, P.; Seiwerth, S.; Rucman, R.; Drmic, D.; Stupnisek, M.; Kokot, A.; Sever, M.; Zoricic, I.; Zoricic, Z.; Batelja, L.; et al. Stress in gastrointestinal tract and stable gastric pentadecapeptide BPC 157. Finally, do we have a solution? Curr. Pharm. Des. 2017, 23, 4012–4028. [Google Scholar] [CrossRef] [PubMed]
- Sikiric, P.; Seiwerth, S.; Rucman, R.; Kolenc, D.; Vuletic, L.B.; Drmic, D.; Grgic, T.; Strbe, S.; Zukanovic, G.; Crvenkovic, D.; et al. Brain-gut axis and pentadecapeptide BPC 157: Theoretical and practical implications. Curr. Neuropharmacol. 2016, 14, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Sikiric, P.; Seiwerth, S.; Rucman, R.; Turkovic, B.; Rokotov, D.S.; Brcic, L.; Sever, M.; Klicek, R.; Radic, B.; Drmic, D.; et al. Stable gastric pentadecapeptide BPC 157-NO-system relation. Curr. Pharm. Des. 2014, 20, 1126–1135. [Google Scholar] [CrossRef] [PubMed]
- Sikiric, P.; Seiwerth, S.; Rucman, R.; Turkovic, B.; Rokotov, D.S.; Brcic, L.; Sever, M.; Klicek, R.; Radic, B.; Drmic, D.; et al. Toxicity by NSAIDs. Counteraction by stable gastric pentadecapeptide BPC 157. Curr. Pharm. Des. 2013, 19, 76–83. [Google Scholar]
- Sikiric, P.; Seiwerth, S.; Rucman, R.; Turkovic, B.; Brcic, L.; Sever, M.; Klicek, R.; Radic, B.; Drmic, D.; Ilic, S.; et al. Focus on ulcerative colitis: Stable gastric pentadecapeptide BPC 157. Curr. Med. Chem. 2012, 19, 126–132. [Google Scholar] [CrossRef]
- Sikiric, P.; Seiwerth, S.; Rucman, R.; Turkovic, B.; Rokotov, D.S.; Brcic, L.; Sever, M.; Klicek, R.; Radic, B.; Drmic, D.; et al. Stable gastric pentadecapeptide BPC 157: Novel therapy in gastrointestinal tract. Curr. Pharm. Des. 2011, 17, 1612–1632. [Google Scholar] [CrossRef]
- Vukojevic, J.; Milavić, M.; Perović, D.; Ilić, S.; Čilić, A.Z.; Đuran, N.; Štrbe, S.; Zoričić, Z.; Filipčić, I.; Brečić, P.; et al. Pentadecapeptide BPC 157 and the central nervous system. Neural Regen. Res. 2022, 17, 482–487. [Google Scholar] [CrossRef]
- Seiwerth, S.; Milavic, M.; Vukojevic, J.; Gojkovic, S.; Krezic, I.; Vuletic, L.B.; Pavlov, K.H.; Petrovic, A.; Sikiric, S.; Vranes, H.; et al. Stable gastric pentadecapeptide BPC 157 and wound healing. Front. Pharmacol. 2021, 12, 627533. [Google Scholar] [CrossRef]
- Sikiric, P.; Skrtic, A.; Gojkovic, S.; Krezic, I.; Zizek, H.; Lovric, E.; Sikiric, S.; Knezevic, M.; Strbe, S.; Milavic, M.; et al. Gastric pentadecapeptide BPC 157 in cytoprotection to resolve major vessel occlusion disturbances, ischemia-reperfusion injury following Pringle maneuver, and Budd-Chiari syndrome. World J. Gastroenterol. 2022, 28, 23–46. [Google Scholar] [CrossRef]
- Deek, S.A. BPC 157 as potential treatment for COVID-19. Med. Hypotheses 2021, 158, 110736. [Google Scholar] [CrossRef]
- Sikiric, P.; Seiwerth, S.; Grabarevic, Z.; Rucman, R.; Petek, M.; Jagic, V.; Turkovic, B.; Rotkvic, I.; Mise, S.; Zoricic, I.; et al. Beneficial effect of a novel pentadecapeptide BPC 157 on gastric lesions induced by restraint stress, ethanol, indomethacin, and capsaicin neurotoxicity. Dig. Dis. Sci. 1996, 41, 1604–1614. [Google Scholar] [CrossRef] [PubMed]
- Gjurasin, M.; Miklic, P.; Zupancic, B.; Perovic, D.; Zarkovic, K.; Brcic, L.; Kolenc, D.; Radic, B.; Seiwerth, S.; Sikiric, P. Peptide therapy with pentadecapeptide BPC 157 in traumatic nerve injury. Regul. Pept. 2010, 160, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Qu, M.; Duan, R.; Shi, D.; Jin, L.; Gao, J.; Wood, J.D.; Li, J.; Wang, G.D. Cytoprotective mechanism of the novel gastric peptide BPC157 in gastrointestinal tract and cultured enteric neurons and glial cells. Neurosci. Bull. 2019, 35, 167–170. [Google Scholar] [CrossRef]
- Zlatar, M.; Kokot, A.; Vuletic, L.B.; Masnec, S.; Kralj, T.; Perisa, M.M.; Barisic, I.; Radic, B.; Milanovic, K.; Drmic, D.; et al. BPC 157 as a therapy for retinal ischemia induced by retrobulbar application of L-NAME in rats. Front. Pharmacol. 2021, 12, 632295. [Google Scholar] [CrossRef] [PubMed]
- Kralj, T.; Kokot, A.; Zlatar, M.; Masnec, S.; Kasnik Kovac, K.; Milkovic Perisa, M.; Batelja Vuletic, L.; Giljanovic, A.; Strbe, S.; Sikiric, S.; et al. Stable gastric pentadecapeptide BPC 157 therapy of rat glaucoma. Biomedicines 2022, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Tudor, M.; Jandric, I.; Marovic, A.; Gjurasin, M.; Perovic, D.; Radic, B.; Blagaic, A.B.; Kolenc, D.; Brcic, L.; Zarkovic, K.; et al. Traumatic brain injury in mice and pentadecapeptide BPC 157 effect. Regul. Pept. 2010, 160, 26–32. [Google Scholar] [CrossRef]
- Drmic, D.; Kolenc, D.; Ilic, S.; Bauk, L.; Sever, M.; Zenko Sever, A.; Luetic, K.; Suran, J.; Seiwerth, S.; Sikiric, P. Celecoxib-induced gastrointestinal, liver and brain lesions in rats, counteraction by BPC 157 or L-arginine, aggravation by L-NAME. World J. Gastroenterol. 2017, 23, 5304–5312. [Google Scholar] [CrossRef]
- Ilic, S.; Drmic, D.; Zarkovic, K.; Kolenc, D.; Coric, M.; Brcic, L.; Klicek, R.; Radic, B.; Sever, M.; Djuzel, V.; et al. High hepatotoxic dose of paracetamol produces generalized convulsions and brain damage in rats. A counteraction with the stable gastric pentadecapeptide BPC 157 (PL 14736). J. Physiol. Pharmacol. 2010, 61, 241–250. [Google Scholar]
- Ilic, S.; Drmic, D.; Franjic, S.; Kolenc, D.; Coric, M.; Brcic, L.; Klicek, R.; Radic, B.; Sever, M.; Djuzel, V.; et al. Pentadecapeptide BPC 157 and its effects on a NSAID toxicity model: Diclofenac-induced gastrointestinal, liver, and encephalopathy lesions. Life Sci. 2011, 88, 535–542. [Google Scholar] [CrossRef]
- Ilic, S.; Drmic, D.; Zarkovic, K.; Kolenc, D.; Brcic, L.; Radic, B.; Djuzel, V.; Blagaic, A.B.; Romic, Z.; Dzidic, S.; et al. Ibuprofen hepatic encephalopathy, hepatomegaly, gastric lesion and gastric pentadecapeptide BPC 157 in rats. Eur. J. Pharmacol. 2011, 667, 322–329. [Google Scholar] [CrossRef]
- Lojo, N.; Rasic, Z.; Sever, A.Z.; Kolenc, D.; Vukusic, D.; Drmic, D.; Zoricic, I.; Sever, M.; Seiwerth, S.; Sikiric, P. Effects of diclofenac, L-NAME, L-arginine, and pentadecapeptide BPC157 on gastrointestinal, liver, and brain lesions, failed anastomosis, and intestinal adaptation deterioration in 24 h-short-bowel rats. PLoS ONE 2016, 11, e0162590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ilic, S.; Brcic, I.; Mester, M.; Filipovicm, M.; Sever, M.; Klicek, R.; Barisic, I.; Radic, B.; Zoricic, Z.; Bilic, V.; et al. Over-dose insulin and stable gastric pentadecapeptide BPC 157. Attenuated gastric ulcers, seizures, brain lesions, hepatomegaly, fatty liver, breakdown of liver glycogen, profound hypoglycemia and calcification in rats. J. Physiol. Pharmacol. 2009, 60, 107–114. [Google Scholar] [PubMed]
- Klicek, R.; Kolenc, D.; Suran, J.; Drmic, D.; Brcic, L.; Aralica, G.; Sever, M.; Holjevac, J.; Radic, B.; Turudic, T.; et al. Stable gastric pentadecapeptide BPC 157 heals cysteamine-colitis and colon-colon-anastomosis and counteracts cuprizone brain injuries and motor disability. J. Physiol. Pharmacol. 2013, 64, 597–612. [Google Scholar] [PubMed]
- Medvidovic-Grubisic, M.; Stambolija, V.; Kolenc, D.; Katancic, J.; Murselovic, T.; Plestina-Borjan, I.; Strbe, S.; Drmic, D.; Barisic, I.; Sindic, A.; et al. Hypermagnesemia disturbances in rats, NO-related: Pentadecapeptide BPC 157 abrogates, L-NAME and L-arginine worsen. Inflammopharmacology 2017, 25, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Sikiric, P.; Seiwerth, S.; Grabarevic, Z.; Petek, M.; Rucman, R.; Turkovic, B.; Rotkvic, I.; Jagic, V.; Duvnjak, M.; Mise, S.; et al. The beneficial effect of BPC 157, a 15 amino acid peptide BPC fragment, on gastric and duodenal lesions induced by restraint stress, cysteamine and 96% ethanol in rats. A comparative study with H2 receptor antagonists, dopamine promotors and gut peptides. Life Sci. 1994, 54, PL63–PL68. [Google Scholar] [CrossRef]
- Kolovrat, M.; Gojkovic, S.; Krezic, I.; Malekinusic, D.; Vrdoljak, B.; Kasnik Kovac, K.; Kralj, T.; Drmic, D.; Barisic, I.; Horvat Pavlov, K.; et al. Pentadecapeptide BPC 157 resolves Pringle maneuver in rats, both ischemia and reperfusion. World J. Hepatol. 2020, 12, 184–206. [Google Scholar] [CrossRef]
- Gojkovic, S.; Krezic, I.; Vrdoljak, B.; Malekinusic, D.; Barisic, I.; Petrovic, A.; Horvat Pavlov, K.; Kolovrat, M.; Duzel, A.; Knezevic, M.; et al. Pentadecapeptide BPC 157 resolves suprahepatic occlusion of the inferior caval vein, Budd-Chiari syndrome model in rats. World J. Gastrointest. Pathophysiol. 2020, 11, 1–19. [Google Scholar] [CrossRef]
- Vukojevic, J.; Siroglavic, M.; Kasnik, K.; Kralj, T.; Stancic, D.; Kokot, A.; Kolaric, D.; Drmic, D.; Sever, A.Z.; Barisic, I.; et al. Rat inferior caval vein (ICV) ligature and particular new insights with the stable gastric pentadecapeptide BPC 157. Vasc. Pharmacol. 2018, 106, 54–66. [Google Scholar] [CrossRef]
- Knezevic, M.; Gojkovic, S.; Krezic, I.; Zizek, H.; Vranes, H.; Malekinusic, D.; Vrdoljak, B.; Knezevic, T.; Pavlov, K.H.; Drmic, D.; et al. Complex syndrome of the complete occlusion of the end of the superior mesenteric vein, opposed with the stable gastric pentadecapeptide BPC 157 in rats. Biomedicines 2021, 9, 1029. [Google Scholar] [CrossRef]
- Knezevic, M.; Gojkovic, S.; Krezic, I.; Zizek, H.; Malekinusic, D.; Vrdoljak, B.; Knezevic, T.; Vranes, H.; Drmic, D.; Staroveski, M.; et al. Occluded superior mesenteric artery and vein. Therapy with the stable gastric pentadecapeptide BPC 157. Biomedicines 2021, 9, 792. [Google Scholar] [CrossRef]
- Knezevic, M.; Gojkovic, S.; Krezic, I.; Zizek, H.; Malekinusic, D.; Vrdoljak, B.; Vranes, H.; Knezevic, T.; Barisic, I.; Horvat Pavlov, K.; et al. Occlusion of the superior mesenteric artery in rats reversed by collateral pathways activation: Gastric pentadecapeptide BPC 157 therapy counteracts multiple organ dysfunction syndrome; intracranial, portal and caval hypertension; and aortal hypotension. Biomedicines 2021, 9, 609. [Google Scholar] [CrossRef] [PubMed]
- Gojkovic, S.; Krezic, I.; Vranes, H.; Zizek, H.; Drmic, D.; Pavlov, K.H.; Petrovic, A.; Batelja, L.; Milavic, M.; Sikiric, S.; et al. BPC 157 therapy and the permanent occlusion of the superior sagittal sinus in rat. Vascular recruitment. Biomedicines 2021, 9, 744. [Google Scholar] [CrossRef] [PubMed]
- Strbe, S.; Gojkovic, S.; Krezic, I.; Zizek, H.; Vranes, H.; Barisic, I.; Strinic, D.; Orct, T.; Vukojevic, J.; Ilic, S.; et al. Over-dose lithium toxicity as an occlusive-like syndrome in rats and gastric pentadecapeptide BPC 157. Biomedicines 2021, 9, 1506. [Google Scholar] [CrossRef] [PubMed]
- Gojkovic, S.; Krezic, I.; Vranes, H.; Zizek, H.; Drmic, D.; Batelja Vuletic, L.; Milavic, M.; Sikiric, S.; Stilinovic, I.; Simeon, P.; et al. Robert’s intragastric alcohol-induced gastric lesion model as an escalated general peripheral and central syndrome, counteracted by the stable gastric pentadecapeptide BPC 157. Biomedicines 2021, 9, 1300. [Google Scholar] [CrossRef] [PubMed]
- Tepes, M.; Gojkovic, S.; Krezic, I.; Zizek, H.; Vranes, H.; Madzar, Z.; Santak, G.; Batelja, L.; Milavic, M.; Sikiric, S.; et al. Stable gastric pentadecapeptide BPC 157 therapy for primary abdominal compartment syndrome in rats. Front. Pharmacol. 2021, 12, 718147. [Google Scholar] [CrossRef]
- Barisic, I.; Balenovic, D.; Udovicic, M.; Bardak, D.; Strinic, D.; Milavic, M.; Sikiric, S.; Uzun, S.; Zvianovic Posilovic, G.; Strbe, S.; et al. Stable gastric pentadecapeptide BPC 157 may counteract myocardial infarction induced by isoprenaline in rats. Biomedicines 2022, 10, 265. [Google Scholar] [CrossRef]
- Bakker, N.A.; Veeger, N.J.; Vergeer, R.A.; Groen, R.J. Prognosis after spinal cord and cauda compression in spontaneous spinal epidural hematomas. Neurology 2015, 84, 1894–1903. [Google Scholar] [CrossRef]
- Vukojevic, J.; Vrdoljak, B.; Malekinusic, D.; Siroglavic, M.; Milavić, M.; Kolenc, D.; Boban Blagaic, A.; Batelja, L.; Drmic, D.; Seiverth, S.; et al. The effect of pentadecapeptide BPC 157 on hippocampal ischemia/reperfusion injuries in rats. Brain Behav. 2020, 10, e01726. [Google Scholar] [CrossRef]
- Staresinic, M.; Petrovic, I.; Novinscak, T.; Jukic, I.; Pevec, D.; Suknaic, S.; Kokic, N.; Batelja, L.; Brcic, L.; Boban-Blagaic, A.; et al. Effective therapy of transected quadriceps muscle in rat: Gastric pentadecapeptide BPC 157. J. Orthop. Res. 2006, 24, 1109–1117. [Google Scholar] [CrossRef]
- Novinscak, T.; Brcic, L.; Staresinic, M.; Jukic, I.; Radic, B.; Pevec, D.; Mise, S.; Tomasovic, S.; Brcic, I.; Banic, T.; et al. Gastric pentadecapeptide BPC 157 as an effective therapy for muscle crush injury in the rat. Surg. Today 2008, 38, 716–725. [Google Scholar] [CrossRef]
- Pevec, D.; Novinscak, T.; Brcic, L.; Sipos, K.; Jukic, I.; Staresinic, M.; Mise, S.; Brcic, I.; Kolenc, D.; Klicek, R.; et al. Impact of pentadecapeptide BPC 157 on muscle healing impaired by systemic corticosteroid application. Med. Sci. Monit. 2010, 16, 81–88. [Google Scholar]
- Mihovil, I.; Radic, B.; Brcic, L.; Brcic, I.; Vukoja, I.; Ilic, S.; Boban Blagaic, A.; Seiwerth, S.; Sikiric, P. Beneficial effect of pentadecapeptide BPC 157 on denervated muscle in rats. J. Physiol. Pharmacol. 2009, 60, 69. [Google Scholar]
- Stambolija, V.; Stambolija, T.P.; Holjevac, J.K.; Murselovic, T.; Radonic, J.; Duzel, V.; Duplancic, B.; Uzun, S.; Zivanovic-Posilovic, G.; Kolenc, D.; et al. BPC 157: The counteraction of succinylcholine, hyperkalemia, and arrhythmias. Eur. J. Pharmacol. 2016, 781, 83–91. [Google Scholar] [CrossRef]
- Hsieh, M.J.; Lee, C.H.; Chueh, H.Y.; Chang, G.J.; Huang, H.Y.; Lin, Y.; Pang, J.S. Modulatory effects of BPC 157 on vasomotor tone and the activation of Src-Caveolin-1-endothelial nitric oxide synthase pathway. Sci. Rep. 2020, 10, 17078. [Google Scholar] [CrossRef]
- Chang, C.H.; Tsai, W.C.; Lin, M.S.; Hsu, Y.H.; Pang, J.H.S. The promoting effect of pentadecapeptide BPC 157 on tendon healing involves tendon outgrowth, cell survival, and cell migration. J. Appl. Physiol. 2011, 110, 774–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, C.H.; Tsai, W.C.; Hsu, Y.H.; Pang, J.H.S. Pentadecapeptide BPC 157 enhances the growth hormone receptor expression in tendon fibroblasts. Molecules 2014, 19, 19066–19077. [Google Scholar] [CrossRef] [Green Version]
- Huang, T.; Zhang, K.; Sun, L.; Xue, X.; Zhang, C.; Shu, Z.; Mu, N.; Gu, J.; Zhang, W.; Wang, Y.; et al. Body protective compound-157 enhances alkali-burn wound healing in vivo and promotes proliferation, migration, and angiogenesis in vitro. Drug Des. Dev. Ther. 2015, 9, 2485–2499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsieh, M.J.; Liu, H.T.; Wang, C.N.; Huang, H.Y.; Lin, Y.; Ko, Y.S.; Wang, J.S.; Chang, V.H.; Pang, J.S. Therapeutic potential of pro-angiogenic BPC157 is associated with VEGFR2 activation and up-regulation. J. Mol. Med. 2017, 95, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Tkalcevic, V.I.; Cuzic, S.; Brajsa, K.; Mildner, B.; Bokulic, A.; Situm, K.; Perovic, D.; Glojnaric, I.; Parnham, M.J. Enhancement by PL 14736 of granulation and collagen organization in healing wounds and the potential role of egr-1 expression. Eur. J. Pharmacol. 2007, 570, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Belosic Halle, Z.; Vlainic, J.; Drmic, D.; Strinic, D.; Luetic, K.; Sucic, M.; Medvidovic-Grubisic, M.; Pavelic Turudic, T.; Petrovic, I.; Seiwerth, S.; et al. Class side effects: Decreased pressure in the lower oesophageal and the pyloric sphincters after the administration of dopamine antagonists, neuroleptics, anti-emetics, L-NAME, pentadecapeptide BPC 157 and L-arginine. Inflammopharmacology 2017, 25, 511–522. [Google Scholar] [CrossRef]
- Luetic, K.; Sucic, M.; Vlainic, J.; Halle, Z.B.; Strinic, D.; Vidovic, T.; Luetic, F.; Marusic, M.; Gulic, S.; Pavelic, T.T.; et al. Cyclophosphamide induced stomach and duodenal lesions as a NO-system disturbance in rats: L-NAME, L-arginine, stable gastric pentadecapeptide BPC 157. Inflammopharmacology 2017, 25, 255–264. [Google Scholar] [CrossRef]
- Sucic, M.; Luetic, K.; Jandric, I.; Drmic, D.; Sever, A.Z.; Vuletic, L.B.; Halle, Z.B.; Strinic, D.; Kokot, A.; Seiwerth, R.S.; et al. Therapy of the rat hemorrhagic cystitis induced by cyclophosphamide. Stable gastric pentadecapeptide BPC 157, L-arginine, L-NAME. Eur. J. Pharmacol. 2019, 861, 172593. [Google Scholar] [CrossRef]
- Lozic, M.; Stambolija, V.; Krezic, I.; Dugandzic, A.; Zivanovic-Posilovic, G.; Gojkovic, S.; Kovacevic, J.; Vrdoljak, L.; Mirkovic, I.; Kokot, A.; et al. In relation to NO-system, stable pentadecapeptide BPC 157 counteracts lidocaine-induced adverse effects in rats and depolarisation in vitro. Emerg. Med. Int. 2020, 2020, 6805354. [Google Scholar] [CrossRef]
- Mautes, A.E.; Weinzierl, M.R.; Donovan, F.; Noble, L.J. Vascular events after spinal cord injury: Contribution to secondary pathogenesis. Phys. Ther. 2000, 80, 673–687. [Google Scholar] [CrossRef]
- Losey, P.; Anthony, D.C. Impact of vasculature damage on the outcome of spinal cord injury: A novel collagenase-induced model may give new insights into the mechanisms involved. Neural Regen. Res. 2014, 9, 1783–1786. [Google Scholar] [CrossRef]
- Profyris, C.; Cheema, S.S.; Zang, D.W.; Azari, M.F.; Boyle, K.; Petratos, S. Degenerative and regenerative mechanisms governing spinal cord injury. Neurobiol. Dis. 2004, 15, 415–436. [Google Scholar] [CrossRef]
- Losey, P.; Young, C.; Krimholtz, E.; Bordet, R.; Anthony, D.C. The role of hemorrhage following spinal-cord injury. Brain Res. 2014, 1569, 9–18. [Google Scholar] [CrossRef]
- Fan, H.; Chen, K.; Duan, L.; Wang, Y.Z.; Ju, G. Beneficial effects of early hemostasis on spinal cord injury in the rat. Spinal Cord 2016, 54, 924–932. [Google Scholar] [CrossRef]
- Drmic, D.; Samara, M.; Vidovic, T.; Malekinusic, D.; Antunovic, M.; Vrdoljak, B.; Ruzman, J.; Milkovic Perisa, M.; Horvat Pavlov, K.; Jeyakumar, J.; et al. Counteraction of perforated cecum lesions in rats: Effects of pentadecapeptide BPC 157, L-NAME and L-arginine. World J. Gastroenterol. 2018, 24, 5462–5476. [Google Scholar] [CrossRef]
- Stupnisek, M.; Franjic, S.; Drmic, D.; Hrelec, M.; Kolenc, D.; Radic, B.; Bojic, D.; Vcev, A.; Seiwerth, S.; Sikiric, P. Pentadecapeptide BPC 157 reduces bleeding time and thrombocytopenia after amputation in rats treated with heparin, warfarin or aspirin. Thromb. Res. 2012, 129, 652–659. [Google Scholar] [CrossRef]
- Stupnisek, M.; Kokot, A.; Drmic, D.; Hrelec Patrlj, M.; Zenko Sever, A.; Kolenc, D.; Radic, B.; Suran, J.; Bojic, D.; Vcev, A.; et al. Pentadecapeptide BPC 157 reduces bleeding and thrombocytopenia after amputation in rats treated with heparin, warfarin, L-NAME and L-arginine. PLoS ONE 2015, 10, e0123454. [Google Scholar] [CrossRef] [PubMed]
- Konosic, S.; Petricevic, M.; Ivancan, V.; Konosic, L.; Goluza, E.; Krtalic, B.; Drmic, D.; Stupnisek, M.; Seiwerth, S.; Sikiric, P. Intragastric application of aspirin, clopidogrel, cilostazol, and BPC 157 in rats: Platelet aggregation and blood clot. Oxid. Med. Cell Longev. 2019, 2019, 9084643. [Google Scholar] [CrossRef]
- Marsala, J.; Orendácová, J.; Lukácová, N.; Vanický, I. Traumatic injury of the spinal cord and nitric oxide. Prog. Brain Res. 2007, 161, 171–183. [Google Scholar] [PubMed]
- Zhang, P.; Zhang, L.; Zhu, L.; Chen, F.; Zhou, S.; Tian, T.; Zhang, Y.; Jiang, X.; Li, X.; Zhang, C.; et al. The change tendency of PI3K/Akt pathway after spinal cord injury. Am. J. Transl. Res. 2015, 7, 2223–2232. [Google Scholar] [PubMed]
- Gong, S.; Seng, Z.; Wang, W.; Lv, J.; Dong, Q.; Yan, B.; Peng, L.; He, X. Bosentan protects the spinal cord from ischemia reperfusion injury in rats through vascular endothelial growth factor receptors. Spinal Cord 2015, 53, 19–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graham, Z.A.; Quin, W.; Harlow, L.C.; Ross, N.H.; Bauman, W.A.; Cardozo, C.P. Focal adhesion kinase signaling is decreased 56 days following spinal cord injury in rat gastrocnemius. Spinal Cord 2016, 54, 502–509. [Google Scholar] [CrossRef] [Green Version]
- Kanno, H.; Ozawa, H.; Sekiguchi, A.; Yamaya, S.; Tateda, S.; Yahata, K.; Itoi, E. The role of mTOR signaling pathway in spinal cord injury. Cell Cycle 2012, 11, 3175–3179. [Google Scholar] [CrossRef] [Green Version]
- An, Y.; Li, J.; Liu, Y.; Fan, M. Neuroprotective effect of novel celecoxib derivatives against spinal cord injury via attenuation of COX-2, oxidative stress, apoptosisand inflammation. Bioorg. Chem. 2020, 101, 104044. [Google Scholar] [CrossRef]
- Hoffmann, C. COX-2 in brain and spinal cord implications for therapeutic use. Curr. Med. Chem. 2000, 7, 1113–1120. [Google Scholar] [CrossRef]
- Yu, F.; Sugawara, T.; Maier, C.M.; Hsieh, L.B.; Chan, P.H. Akt/Bad signaling and motor neuron survival after spinal cord injury. Neurobiol. Dis. 2005, 20, 491–499. [Google Scholar] [CrossRef]
- Schucht, P.; Raineteau, O.; Schwab, O.E.; Fouad, K. Anatomical correlates of locomotor recovery following dorsal and ventral lesions of the rat spinal cord. Exp. Neurol. 2002, 176, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Basso, D.M.; Beattie, M.S.; Bresnahan, J.C. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp. Neurol. 1996, 139, 244–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ward, R.E.; Huang, W.; Kostusiak, M.; Pallier, P.N.; Michael-Titus, A.T.; Priestley, J.V. A characterization of white matter pathology following spinal cord compression injury in the rat. Neuroscience 2014, 260, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Hrelec, M.; Klicek, R.; Brcic, L.; Brcic, I.; Cvjetko, I.; Seiwerth, S.; Sikiric, P. Abdominal aorta anastomosis in rats and stable gastric pentadecapeptide BPC 157, prophylaxis and therapy. J. Physiol. Pharmacol. 2019, 60, 161–165. [Google Scholar]
- Duzel, A.; Vlainic, J.; Antunovic, M.; Malekinusic, D.; Vrdoljak, B.; Samara, M.; Gojkovic, S.; Krezic, I.; Vidovic, T.; Bilic, Z.; et al. Stable gastric pentadecapeptide BPC 157 in the treatment of colitis and ischemia and reperfusion in rats: New insights. World J. Gastroenterol. 2017, 23, 8465–8488. [Google Scholar] [CrossRef]
- Dong, Q.; Sun, L.; Peng, L.; Yan, B.; Lv, J.; Wang, G.; Gong, S. PMX53 protects spinal cord from ischemia-reperfusion injury in rats in the short term. Spinal Cord 2016, 54, 254–258. [Google Scholar] [CrossRef] [Green Version]
- Gong, S.; Seng, Z.; Wang, W.; Lv, J.; Dong, Q.; Yan, B.; Peng, L.; He, X. Bosentan reduces neuronal apoptosis following spinal cord ischemic reperfusion injury. Spinal Cord 2014, 52, 181–185. [Google Scholar] [CrossRef] [Green Version]
- Sámano, C.; Nistri, A. Mechanism of neuroprotection against experimental spinal cord Injury by riluzole or methylprednisolone. Neurochem. Res. 2019, 44, 200–213. [Google Scholar] [CrossRef]
- Liu, N.K.; Xu, X.M. Neuroprotection and its molecular mechanism following spinal cord injury. Neural Regen. Res. 2012, 7, 2051–2062. [Google Scholar]
- Rowland, J.W.; Hawryluk, G.W.; Kwon, B.; Fehlings, M.G. Current status of acute spinal cord injury pathophysiology and emerging therapies: Promise on the horizon. Neurosurg. Focus 2008, 25, E2. [Google Scholar] [CrossRef] [Green Version]
- Barnes, P.J.; Karin, M. Nuclear factor-kappaB: A pivotal transcription factor in chronic inflammatory diseases. N. Engl. J. Med. 1997, 336, 1066–1071. [Google Scholar] [CrossRef] [PubMed]
- Karin, M.; Liu, Z.; Zandi, E. AP-1 function and regulation. Curr. Opin. Cell Biol. 1997, 9, 240–246. [Google Scholar] [CrossRef]
- Xu, J.; Kim, G.M.; Ahmed, S.H.; Xu, J.; Yan, P.; Xu, X.M.; Hsu, C.Y. Glucocorticoid receptor-mediated suppression of activator protein-1 activation and matrix metalloproteinase expression after spinal cord injury. J. Neurosci. 2001, 21, 92–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Fan, G.; Chen, S.; Wu, Y.; Xu, X.M.; Hsu, C.Y. Methylprednisolone inhibition of TNF-alpha expression and NF-kB activation after spinal cord injury in rats. Brain Res. Mol. Brain Res. 1998, 59, 135–142. [Google Scholar] [CrossRef]
- Liu, N.K.; Zhang, Y.P.; Titsworth, W.L.; Jiang, X.; Han, S.; Lu, P.H.; Shields, C.B.; Xu, X.M. A novel role of phospholipase A2 in mediating spinal cord secondary injury. Ann. Neurol. 2006, 59, 606–619. [Google Scholar] [CrossRef]
- Sengelaub, D.R.; Han, Q.; Liu, N.K.; Maczuga, M.A.; Szalavari, V.; Valencia, S.A.; Xu, X.M. Protective effects of estradiol and dihydrotestosterone following spinal cord injury. J. Neurotrauma 2018, 35, 825–841. [Google Scholar] [CrossRef]
- Walker, C.L.; Wu, X.; Liu, N.K.; Xu, X.M. Bisperoxovanadium mediates neuronal protection through Inhibition of PTEN and activation of PI3K/AKT-mTOR signaling after traumatic spinal injuries. J. Neurotrauma 2019, 36, 2676–2687. [Google Scholar] [CrossRef]
- Yuksel, U.; Bakar, B.; Dincel, G.C.; Yildiran, F.A.B.; Ogden, M.; Kisa, U. The Investigation of the Cox-2 selective inhibitor parecoxib effects in spinal cord injury in rat. J. Investig. Surg. 2019, 32, 402–413. [Google Scholar] [CrossRef]
- Park, G.Y.; Christman, J.W. Involvement of cyclooxygenase-2 and prostaglandins in the molecular pathogenesis of inflammatory lung diseases. Am. J. Physiol. Lung. Cell. Mol. Physiol. 2006, 290, 797–805. [Google Scholar] [CrossRef] [Green Version]
- Kopincová, J.; Puzserova, A.; Bernatova, I. Biochemical aspects of nitric oxide synthase feedback regulation by nitric oxide. Interdiscip. Toxicol. 2011, 4, 63–68. [Google Scholar] [CrossRef] [Green Version]
- Funk, C.D. Prostaglandins and leukotrienes: Advances in eicosanoid biology. Science 2001, 294, 1871–1875. [Google Scholar] [CrossRef] [Green Version]
- Saini, R.; Singh, S. Inducible nitric oxide synthase: An asset to neutrophils. J. Leukoc. Biol. 2019, 105, 49–61. [Google Scholar] [CrossRef] [Green Version]
- Conti, A.; Miscusi, M.; Cardali, S.; Germano, A.; Suzuki, H.; Cuzzocrea, S.; Tomasello, F. Nitric oxide in the injured spinal cord: Synthases cross-talk.; oxidative stress and inflammation. Brain Res. Rev. 2007, 54, 205–218. [Google Scholar] [CrossRef]
- Garthwaite, J. Glutamate, nitric oxide, and cell–cell signalling in the nervous system. Trends Neurosci. 1991, 14, 60–67. [Google Scholar] [CrossRef]
- Bilic, Z.; Gojkovic, S.; Kalogjera, L.; Krezic, I.; Malekinusic, D.; Knezevic, M.; Sever, M.; Lojo, N.; Kokot, A.; Kasnik, K.; et al. Novel insight into Robert’s cytoprotection: Complex effect of cytoprotective pentadecapeptide BPC 157 in rats with perforated stomach throughout modulation of nitric oxide-system. Comparison with L-arginine, ranitidine and pantoprazole therapy and L-NG-nitro arginine methyl ester worsening. J. Physiol. Pharmacol. 2021; 72, inpress. [Google Scholar]
- Cheriyan, T.; Ryan, D.J.; Weinreb, J.H.; Cheriyan, J.; Paul, J.C.; Lafage, V.; Kirsch, T.; Errico, T.J. Spinal cord injury models: A review. Spinal Cord 2014, 52, 588–595. [Google Scholar] [CrossRef]
- Nishi, R.A.; Badner, A.; Hooshmand, M.J.; Creasman, D.A.; Liu, H.; Anderson, A.J. The effects of mouse strain and age on a model of unilateral cervical contusion spinal cord injury. PLoS ONE 2020, 15, e0234245. [Google Scholar] [CrossRef]
- Bennett, D.J.; Gorassini, M.; Fouad, K.; Sanelli, L.; Han, Y.; Cheng, J. Spasticity in rats with sacral spinal cord injury. J Neurotrauma 1999, 16, 69–84. [Google Scholar] [CrossRef]
- Rivlin, A.; Tator, C. Effect of duration of acute spinal cord compression in a new acute cord injury model in the rat. Surg. Neurol. 1978, 10, 38–43. [Google Scholar]
- Xu, C.; Sun, L.; Ren, F.; Huang, P.; Tian, Z.; Cui, J.; Zhang, W.; Wang, S.; Zhang, K.; He, L.; et al. Preclinical safety evaluation of body protective compound-157, a potential drug for treating various wounds. Regul. Toxicol. Pharmacol. 2020, 114, 104665. [Google Scholar] [CrossRef]















| Gene Symbol | Synonyms | Gene Name | TaqMan Assay ID | NCBI Reference Sequence | Amplicon Length (bp) |
|---|---|---|---|---|---|
| Actb | Actin, beta | Rn00667869_m1 | NM_031144.3 | 91 | |
| Akt1 | PKB, RAC | AKT serine/threonine kinase 1 | Rn00583646_m1 | NM_033230.2 | 87 |
| Nos1 | nNOS, bNOS | Nitric oxide synthase 1 | Rn00583793_m1 | NM_052799.1 | 65 |
| Nos2 | iNos, Nos2a | Nitric oxide synthase 2 | Rn00561646_m1 | NM_012611.3 | 77 |
| Nos3 | cNOS, eNos | Nitric oxide synthase 3 | Rn02132634_s1 | NM_021838.2 | 117 |
| Mtor | Frap1, RAFT1, RAPT1 | Mechanistic target of rapamycin kinase | Rn00693900_m1 | NM_019906.1 | 70 |
| Prkcg | PKC, Prkc | Protein kinase C, gamma | Rn00440861_m1 | NM_012628.1 | 89 |
| Ptgs2 | COX-2, Cox2, cyclooxygenase 2 | Prostaglandin-endoperoxide synthase 2 | Rn01483828_m1 | NM_017232.3 | 112 |
| Ptk2 | FAK, FADK1, FRNK | Protein tyrosine kinase 2 | Rn01505115_m1 | NM_013081.2 | 81 |
| Vegfa | VEGF-A, VPF | Vascular endothelial growth factor A | Rn01511601_m1 | NM_001110333.2 | 69 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perovic, D.; Milavic, M.; Dokuzovic, S.; Krezic, I.; Gojkovic, S.; Vranes, H.; Bebek, I.; Bilic, V.; Somun, N.; Brizic, I.; et al. Novel Therapeutic Effects in Rat Spinal Cord Injuries: Recovery of the Definitive and Early Spinal Cord Injury by the Administration of Pentadecapeptide BPC 157 Therapy. Curr. Issues Mol. Biol. 2022, 44, 1901-1927. https://doi.org/10.3390/cimb44050130
Perovic D, Milavic M, Dokuzovic S, Krezic I, Gojkovic S, Vranes H, Bebek I, Bilic V, Somun N, Brizic I, et al. Novel Therapeutic Effects in Rat Spinal Cord Injuries: Recovery of the Definitive and Early Spinal Cord Injury by the Administration of Pentadecapeptide BPC 157 Therapy. Current Issues in Molecular Biology. 2022; 44(5):1901-1927. https://doi.org/10.3390/cimb44050130
Chicago/Turabian StylePerovic, Darko, Marija Milavic, Stjepan Dokuzovic, Ivan Krezic, Slaven Gojkovic, Hrvoje Vranes, Igor Bebek, Vide Bilic, Nenad Somun, Ivan Brizic, and et al. 2022. "Novel Therapeutic Effects in Rat Spinal Cord Injuries: Recovery of the Definitive and Early Spinal Cord Injury by the Administration of Pentadecapeptide BPC 157 Therapy" Current Issues in Molecular Biology 44, no. 5: 1901-1927. https://doi.org/10.3390/cimb44050130
APA StylePerovic, D., Milavic, M., Dokuzovic, S., Krezic, I., Gojkovic, S., Vranes, H., Bebek, I., Bilic, V., Somun, N., Brizic, I., Skorak, I., Hriberski, K., Sikiric, S., Lovric, E., Strbe, S., Kubat, M., Boban Blagaic, A., Skrtic, A., Seiwerth, S., & Sikiric, P. (2022). Novel Therapeutic Effects in Rat Spinal Cord Injuries: Recovery of the Definitive and Early Spinal Cord Injury by the Administration of Pentadecapeptide BPC 157 Therapy. Current Issues in Molecular Biology, 44(5), 1901-1927. https://doi.org/10.3390/cimb44050130







