Procoagulant Activity in Amniotic Fluid Is Associated with Fetal-Derived Extracellular Vesicles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Amniotic Fluid Preparation
2.2. Preparation of Plasma Samples
2.3. Fibrin Generation Assay
2.4. Flow Cytometry
2.5. Statistical Analysis
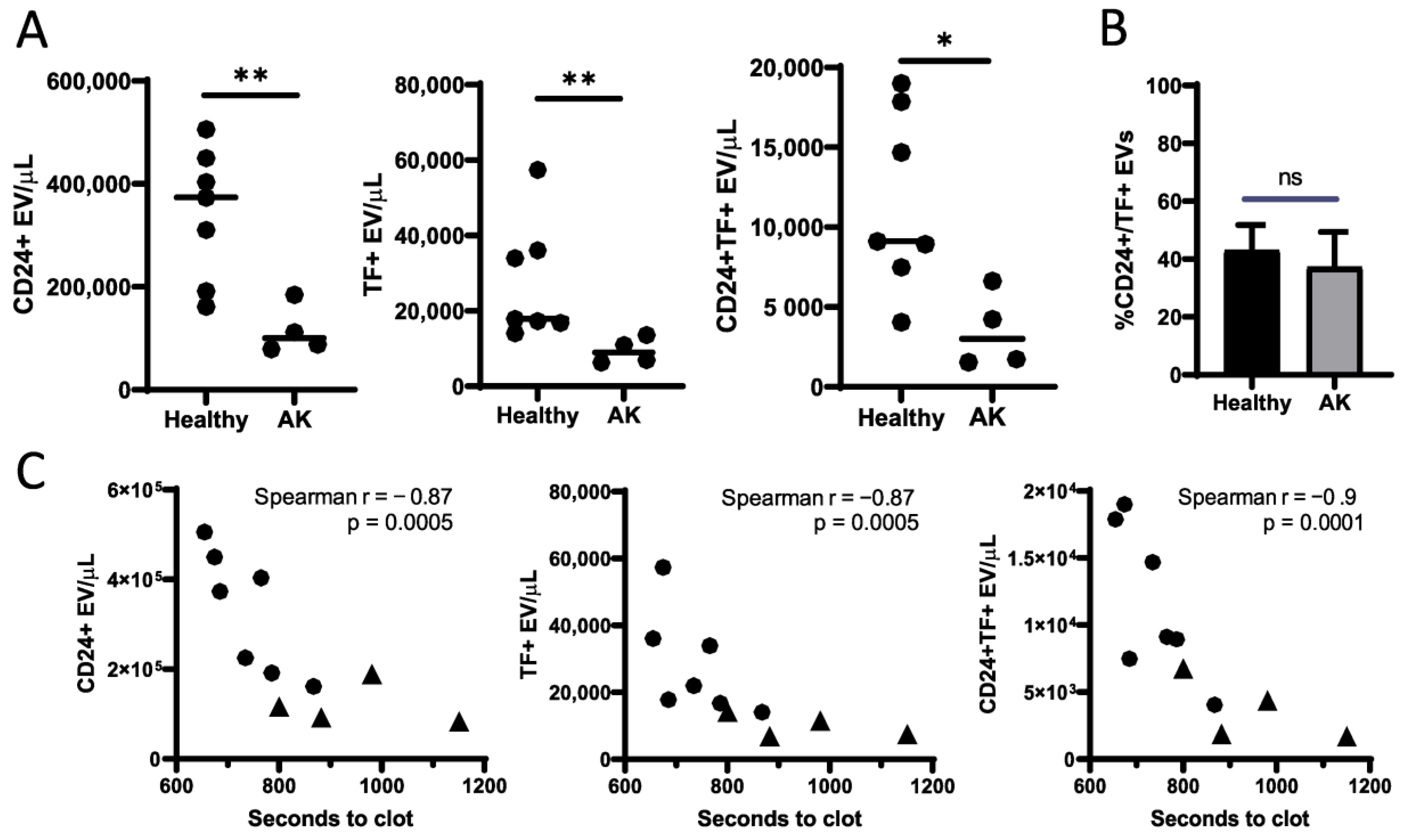
3. Results and Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clark, S.L. New Concepts of Amniotic Fluid Embolism. Obstet. Gynecol. Surv. 1990, 45, 360–368. Available online: http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00006254-199006000-00003 (accessed on 15 June 2020). [CrossRef] [PubMed]
- Clark, S.L. Amniotic Fluid Embolism. Obstet. Gynecol. Clin. Obstet. Gynecol. 2014, 53, 322–328. Available online: http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00006250-201402000-00016 (accessed on 1 June 2020).
- Pacheco, L.D.; Clark, S.L.; Klassen, M.; Hankins, G.D. Amniotic fluid embolism: Principles of early clinical management. Am. J. Obstet. Gynecol. 2019, 222, 48–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conde-Agudelo, A.; Romero, R. Amniotic fluid embolism: An evidence-based review. Am. J. Obstet. Gynecol. 2009, 201, 445.e1–445.e13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hell, L.; Wisgrill, L.; Ay, C.; Spittler, A.; Schwameis, M.; Jilma, B.; Pabinger, I.; Altevogt, P.; Thaler, J. Procoagulant extracellular vesicles in amniotic fluid. Transl. Res. 2017, 184, 12–20. [Google Scholar] [CrossRef]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Tripisciano, C.; Weiss, R.; Eichhorn, T.; Spittler, A.; Heuser, T.; Fischer, M.B.; Weber, V. Different Potential of Extracellular Vesicles to Support Thrombin Generation: Contributions of Phosphatidylserine, Tissue Factor, and Cellular Origin. Sci. Rep. 2017, 7, 6522. [Google Scholar] [CrossRef]
- Mackman, N.; Grover, S.P.; Antoniak, S. Tissue factor expression, extracellular vesicles, and thrombosis after infection with the respiratory viruses influenza A virus and coronavirus. J. Thromb. Haemost. 2021, 19, 2652–2658. [Google Scholar] [CrossRef]
- Rosell, A.; Havervall, S.; Von Meijenfeldt, F.; Hisada, Y.; Aguilera, K.; Grover, S.P.; Lisman, T.; Mackman, N.; Thålin, C. Patients With COVID-19 Have Elevated Levels of Circulating Extracellular Vesicle Tissue Factor Activity That Is Associated With Severity and Mortality—Brief Report. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 878–882. [Google Scholar] [CrossRef]
- Hisada, Y.; Alexander, W.; Kasthuri, R.; Voorhees, P.; Mobarrez, F.; Taylor, A.; McNamara, C.; Wallen, H.; Witkowski, M.; Key, N.S.; et al. Measurement of microparticle tissue factor activity in clinical samples: A summary of two tissue factor-dependent FXa generation assays. Thromb. Res. 2016, 139, 90–97. [Google Scholar] [CrossRef]
- Beall, M.; Wijngaard, J.V.D.; van Gemert, M.; Ross, M. Amniotic Fluid Water Dynamics. Placenta 2007, 28, 816–823. [Google Scholar] [CrossRef]
- Mann, S.E.; Nijland, M.J.; Ross, M.G. Mathematic modeling of human amniotic fluid dynamics. Am. J. Obstet. Gynecol. 1996, 175, 937–944. [Google Scholar] [CrossRef]
- Sherer, D.M. A Review of Amniotic Fluid Dynamics and the Enigma of Isolated Oligohydramnios. Am. J. Perinatol. 2002, 19, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Keller, S.; Rupp, C.; Stoeck, A.; Runz, S.; Fogel, M.; Lugert, S.; Hager, H.-D.; Abdel-Bakky, M.; Gutwein, P.; Altevogt, P. CD24 is a marker of exosomes secreted into urine and amniotic fluid. Kidney Int. 2007, 72, 1095–1102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wendt, T.; Zhang, Y.M.; Bierhaus, A.; Kriegsmann, J.; Deng, Y.; Waldherr, R.; Teske, T.; Luther, T.; Fünfstück, R.; Nawroth, P.P.; et al. Tissue factor expression in an animal model of hydronephrosis. Nephrol. Dial. Transplant. 1995, 10, 1820–1828. [Google Scholar] [PubMed]
- Erlich, J.H.; Apostolopoulos, J.; Wun, T.C.; Kretzmer, K.K.; Holdsworth, S.R.; Tipping, P.G. Renal expression of tissue factor pathway inhibitor and evidence for a role in crescentic glomerulonephritis in rabbits. J. Clin. Investig. 1996, 98, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Iyer, A.; Humphries, T.L.R.; Owens, E.P.; Zhao, K.-N.; Masci, P.P.; Johnson, D.W.; Nikolic-Paterson, D.; Gobe, G.C.; Fairlie, D.P.; Vesey, D.A. PAR2 Activation on Human Kidney Tubular Epithelial Cells Induces Tissue Factor Synthesis, That Enhances Blood Clotting. Front. Physiol. 2021, 12, 249. [Google Scholar] [CrossRef]
- Bahado-Singh, R.O.; Lynch, L.; Deren, O.; Morroti, R.; Copel, J.A.; Mahoney, M.J.; Williams, J. First-trimester growth restriction and fetal aneuploidy: The effect of type of aneuploidy and gestational age. Am. J. Obstet. Gynecol. 1997, 176, 976–980. [Google Scholar] [CrossRef]
- Ariel, I.; Wells, T.R.; Landing, B.H.; Singer, D.B. The urinary system in Down syndrome: A study of 124 autopsy cases. Pediatr. Pathol. 1991, 11, 879–888. [Google Scholar] [CrossRef]
- Fitzsimmons, J.; Droste, S.; Shepard, T.H.; Pascoe-Mason, J.; Fantel, A. Growth failure in second-trimester fetuses with trisomy. Teratology 1990, 42, 337–345. [Google Scholar] [CrossRef]
- Toka, H.R.; Toka, O.; Hariri, A.; Nguyen, H.T. Congenital Anomalies of Kidney and Urinary Tract. Semin. Nephrol. 2010, 30, 374–386. [Google Scholar] [CrossRef]
- Kupferman, J.C.; Druschel, C.M.; Kupchik, G.S. Increased Prevalence of Renal and Urinary Tract Anomalies in Children with Down Syndrome. Pediatrics 2009, 124, e615–e621. [Google Scholar] [CrossRef]
- Postolache, L.; Parsa, A.; Simoni, P.; Boitsios, G.; Ismaili, K.; Schurmans, T.; Monier, A.; Casimir, G.; Albert, A.; Parsa, C.F. Widespread kidney anomalies in children with Down syndrome. Pediatr. Nephrol. 2022, 17, 1032–1037. [Google Scholar] [CrossRef]
- Kaur, K.; Bhardwaj, M.; Kumar, P.; Singhal, S.; Singh, T.; Hooda, S. Amniotic fluid embolism. J. Anaesthesiol. Clin. Pharmacol. 2016, 32, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Zucker, M.; Seligsohn, U.; Salomon, O.; Wolberg, A.S. Abnormal plasma clot structure and stability distinguish bleeding risk in patients with severe factor XI deficiency. J. Thromb. Haemost. 2014, 12, 1121–1130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gidley, G.N.; Holle, L.A.; Burthem, J.; Bolton-Maggs, P.H.B.; Lin, F.-C.; Wolberg, A.S. Abnormal plasma clot formation and fibrinolysis reveal bleeding tendency in patients with partial factor XI deficiency. Blood Adv. 2018, 2, 1076–1088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, C.-K.J.; Shan, S.J.; Winsor, E.J.; Diamandis, E.P. Proteomics Analysis of Human Amniotic Fluid. Mol. Cell. Proteom. 2007, 6, 1406–1415. [Google Scholar] [CrossRef] [Green Version]
- Tsangaris, G.T.; Karamessinis, P.; Kolialexi, A.; Garbis, S.D.; Antsaklis, A.; Mavrou, A.; Fountoulakis, M. Proteomic analysis of amniotic fluid in pregnancies with Down syndrome. Proteomics 2006, 6, 4410–4419. [Google Scholar] [CrossRef]
- Kolialexi, A.; Tounta, G.; Mavrou, A.; Tsangaris, G.T. Proteomic analysis of amniotic fluid for the diagnosis of fetal aneuploidies. Expert Rev. Proteom. 2011, 8, 175–185. [Google Scholar] [CrossRef]
- Uszyński, W.; Żekanowska, E.; Uszyński, M.; Żyliński, A.; Kuczyński, J. New observations on procoagulant properties of amniotic fluid: Microparticles (MPs) and tissue factor-bearing MPs (MPs-TF), comparison with maternal blood plasma. Thromb. Res. 2013, 132, 757–760. [Google Scholar] [CrossRef]
- Uszynski, M.; Zekanowska, E.; Uszynski, W.; Kuczynski, J. Tissue factor (tf) and tissue factor pathway inhibitor (tfpi) in amniotic fluid and blood plasma: Implications for the mechanism of amniotic fluid embolism. Eur. J. Obstet. Gynecol. Reprod. Biol. 2001, 95, 163–166. [Google Scholar] [CrossRef]

| Patient No. | Pregnancy Week | Diagnosis |
|---|---|---|
| 1 | 18–19 | Normal Karyotype |
| 2 | 18–19 | Normal Karyotype |
| 3 | 17–18 | Normal Karyotype |
| 4 | 16–17 | Normal Karyotype |
| 5 | 18–19 | Normal Karyotype |
| 6 | 19–20 | Normal Karyotype |
| 7 | 18–19 | Normal Karyotype |
| 8 | 18–19 | Trisomy 21 |
| 9 | 18–19 | Trisomy 21, Trisomy X |
| 10 | 18–19 | Trisomy 21 |
| 11 | 18–19 | Trisomy 21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Butov, K.R.; Karetnikova, N.A.; Pershin, D.Y.; Trofimov, D.Y.; Panteleev, M.A. Procoagulant Activity in Amniotic Fluid Is Associated with Fetal-Derived Extracellular Vesicles. Curr. Issues Mol. Biol. 2022, 44, 2710-2716. https://doi.org/10.3390/cimb44060185
Butov KR, Karetnikova NA, Pershin DY, Trofimov DY, Panteleev MA. Procoagulant Activity in Amniotic Fluid Is Associated with Fetal-Derived Extracellular Vesicles. Current Issues in Molecular Biology. 2022; 44(6):2710-2716. https://doi.org/10.3390/cimb44060185
Chicago/Turabian StyleButov, Kirill R., Natalia A. Karetnikova, Dmitry Y. Pershin, Dmitry Y. Trofimov, and Mikhail A. Panteleev. 2022. "Procoagulant Activity in Amniotic Fluid Is Associated with Fetal-Derived Extracellular Vesicles" Current Issues in Molecular Biology 44, no. 6: 2710-2716. https://doi.org/10.3390/cimb44060185
APA StyleButov, K. R., Karetnikova, N. A., Pershin, D. Y., Trofimov, D. Y., & Panteleev, M. A. (2022). Procoagulant Activity in Amniotic Fluid Is Associated with Fetal-Derived Extracellular Vesicles. Current Issues in Molecular Biology, 44(6), 2710-2716. https://doi.org/10.3390/cimb44060185






