Resistance of Human Liver Mesenchymal Stem Cells to FAS-Induced Cell Death
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation and Cultivation of Human Liver MSCs
2.2. Flow Cytometry Analysis of the MSC Markers and CD95
2.3. Cell Death Analysis
2.4. ATP Level in the L-MSCs
2.5. Assessment of the Mitochondrial Membrane Potential
2.6. Statistical Analysis
3. Results
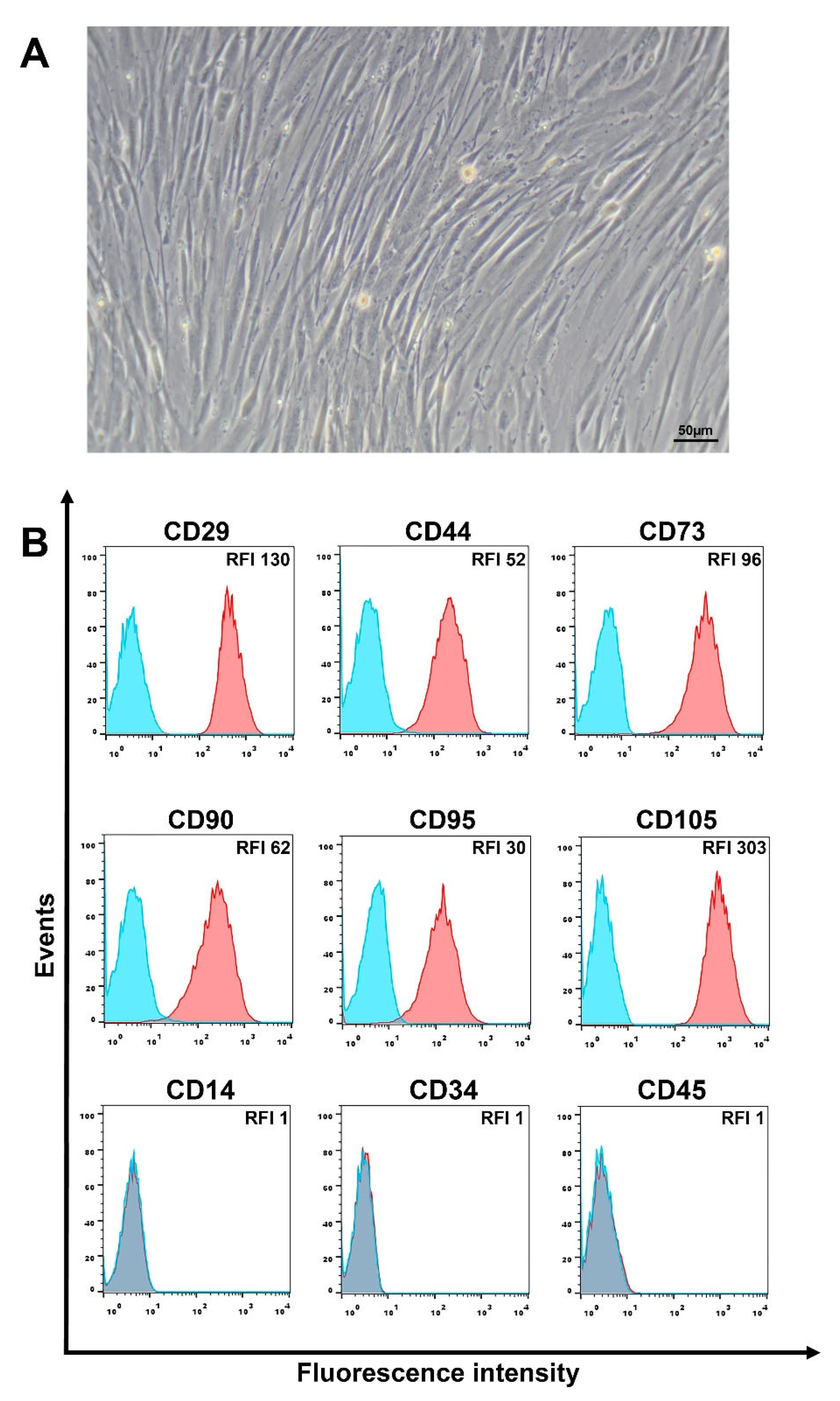
3.1. Phenotypic Characteristics of L-MSCs
3.2. Liver MSCs Are Resistant to Fas-Induced Cell Death
3.3. High Doses of Anti-Fas mAb or FasL Induce Changes in Mitochondrial Membrane Potential in Liver MSCs
3.4. Low Concentrations of Anti-Fas mAbs or FasL Induce an ATP Level Decline in L-MSCs
3.5. Pro-Inflammatory Cytokine TNF-α Does Not Increase the Sensitivity of L-MSCs to Fas-Induced Cell Death
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Song, N.; Scholtemeijer, M.; Shah, K. Mesenchymal Stem Cell Immunomodulation: Mechanisms and Therapeutic Potential. Trends Pharmacol. Sci. 2020, 41, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Krampera, M.; Le Blanc, K. Mesenchymal stromal cells: Putative microenvironmental modulators become cell therapy. Cell Stem Cell 2021, 28, 1708–1725. [Google Scholar] [CrossRef] [PubMed]
- Yarygin, K.N.; Namestnikova, D.D.; Sukhinich, K.K.; Gubskiy, I.L.; Majouga, A.G.; Kholodenko, I.V. Cell Therapy of Stroke: Do the Intra-Arterially Transplanted Mesenchymal Stem Cells Cross the Blood-Brain Barrier? Cells 2021, 10, 2997. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, W.Z.; Lin, Y.H.; Su, L.J.; Wu, M.S.; Jeng, H.Y.; Chang, H.C.; Huang, Y.H.; Ling, T.Y. Mesenchymal stem/stromal cell-based therapy: Mechanism, systemic safety and biodistribution for precision clinical applications. J. Biomed. Sci. 2021, 28, 28. [Google Scholar] [CrossRef] [PubMed]
- Poltavtseva, R.A.; Poltavtsev, A.V.; Lutsenko, G.V.; Svirshchevskaya, E.V. Myths, reality and future of mesenchymal stem cell therapy. Cell Tissue Res. 2019, 375, 563–574. [Google Scholar] [CrossRef]
- Preda, M.B.; Neculachi, C.A.; Fenyo, I.M.; Vacaru, A.M.; Publik, M.A.; Simionescu, M.; Burlacu, A. Short lifespan of syngeneic transplanted MSC is a consequence of in vivo apoptosis and immune cell recruitment in mice. Cell Death Dis. 2021, 12, 566. [Google Scholar] [CrossRef]
- Pang, S.H.M.; D’Rozario, J.; Mendonca, S.; Bhuvan, T.; Payne, N.L.; Zheng, D.; Hisana, A.; Wallis, G.; Barugahare, A.; Powell, D.; et al. Mesenchymal stromal cell apoptosis is required for their therapeutic function. Nat. Commun. 2021, 12, 6495. [Google Scholar] [CrossRef]
- Leibacher, J.; Dauber, K.; Ehser, S.; Brixner, V.; Kollar, K.; Vogel, A.; Spohn, G.; Schäfer, R.; Seifried, E.; Henschler, R. Human mesenchymal stromal cells undergo apoptosis and fragmentation after intravenous application in immune-competent mice. Cytotherapy 2017, 19, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.B.; Lin, Q.; Liu, Z.W. A study on the role of apoptotic human umbilical cord mesenchymal stem cells in bleomycin-induced acute lung injury in rat models. Eur. Rev. Med. Pharm. Sci. 2016, 20, 969–982. [Google Scholar]
- Laing, A.G.; Riffo-Vasquez, Y.; Sharif-Paghaleh, E.; Lombardi, G.; Sharpe, P.T. Immune modulation by apoptotic dental pulp stem cells in vivo. Immunotherapy 2018, 10, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Weiss, A.R.R.; Dahlke, M.H. Immunomodulation by Mesenchymal Stem Cells (MSCs): Mechanisms of Action of Living, Apoptotic, and Dead MSCs. Front. Immunol. 2019, 10, 1191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, X.; Hong, W.; Yang, J.; Lei, H.; Lu, T.; He, C.; Bi, Z.; Pan, X.; Liu, Y.; Dai, L.; et al. Spontaneous apoptosis of cells in therapeutic stem cell preparation exert immunomodulatory effects through release of phosphatidylserine. Signal Transduct. Target. Ther. 2021, 6, 270, Erratum in: Signal Transduct. Target. Ther. 2022, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, S.; Qiu, X.; Yang, X.; Bao, L.; Pu, F.; Liu, X.; Li, C.; Xuan, K.; Zhou, J.; et al. Donor MSCs release apoptotic bodies to improve myocardial infarction via autophagy regulation in recipient cells. Autophagy 2020, 16, 2140–2155. [Google Scholar] [CrossRef] [PubMed]
- Ham, O.; Lee, S.Y.; Song, B.W.; Cha, M.J.; Lee, C.Y.; Park, J.H.; Kim, I.K.; Lee, J.; Seo, H.H.; Seung, M.J.; et al. Modulation of Fas-Fas Ligand Interaction Rehabilitates Hypoxia-Induced Apoptosis of Mesenchymal Stem Cells in Ischemic Myocardium Niche. Cell Transpl. 2015, 24, 1329–1341. [Google Scholar] [CrossRef]
- Götherström, C.; Lundqvist, A.; Duprez, I.R.; Childs, R.; Berg, L.; le Blanc, K. Fetal and adult multipotent mesenchymal stromal cells are killed by different pathways. Cytotherapy 2011, 13, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Peter, M.E.; Barnhart, B.C.; Algeciras-Schimnich, A. CD95L/FasL and its receptor CD95 (APO-1/Fas). In Cytokine Hand Book, 4th ed.; Thomson, A., Lotze, M., Eds.; Academic Press: New York, NY, USA, 2003; Volume 2, pp. 885–911. [Google Scholar]
- Itoh, N.; Yonehara, S.; Ishii, A.; Yonehara, M.; Mizushima, S.; Sameshima, M.; Hase, A.; Seto, Y.; Nagata, S. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell 1991, 66, 233–243. [Google Scholar] [CrossRef]
- Barnhart, B.C.; Legembre, P.; Pietras, E.; Bubici, C.; Franzoso, G.; Peter, M.E. CD95 ligand induces motility and invasiveness of apoptosis-resistant tumor cells. EMBO J. 2004, 23, 3175–3185. [Google Scholar] [CrossRef] [Green Version]
- Kleber, S.; Sancho-Martinez, I.; Wiestler, B.; Beisel, A.; Gieffers, C.; Hill, O.; Thiemann, M.; Mueller, W.; Sykora, J.; Kuhn, A.; et al. Yes and PI3K bind CD95 to signal invasion of glioblastoma. Cancer Cell 2008, 13, 235–248. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Park, S.M.; Tumanov, A.V.; Hau, A.; Sawada, K.; Feig, C.; Turner, J.R.; Fu, Y.X.; Romero, I.L.; Lengyel, E.; et al. CD95 promotes tumour growth. Nature 2010, 465, 492–496. [Google Scholar] [CrossRef]
- Tamm, C.; Robertson, J.D.; Sleeper, E.; Enoksson, M.; Emgård, M.; Orrenius, S.; Ceccatelli, S. Differential regulation of the mitochondrial and death receptor pathways in neural stem cells. Eur. J. Neurosci. 2004, 19, 2613–2621. [Google Scholar] [CrossRef]
- Corsini, N.S.; Sancho-Martinez, I.; Laudenklos, S.; Glagow, D.; Kumar, S.; Letellier, E.; Koch, P.; Teodorczyk, M.; Kleber, S.; Klussmann, S.; et al. The death receptor CD95 activates adult neural stem cells for working memory formation and brain repair. Cell Stem Cell 2009, 5, 178–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alderson, M.R.; Armitage, R.J.; Maraskovsky, E.; Tough, T.W.; Roux, E.; Schooley, K.; Ramsdell, F.; Lynch, D.H. Fas transduces activation signals in normal human T lymphocytes. J. Exp. Med. 1993, 178, 2231–2235. [Google Scholar] [CrossRef] [Green Version]
- Aggarwal, B.B.; Singh, S.; LaPushin, R.; Totpal, K. Fas antigen signals proliferation of normal human diploid fibroblast and its mechanism is different from tumor necrosis factor receptor. FEBS Lett. 1995, 364, 5–8. [Google Scholar] [CrossRef] [Green Version]
- Kennea, N.L.; Stratou, C.; Naparus, A.; Fisk, N.M.; Mehmet, H. Functional intrinsic and extrinsic apoptotic pathways in human fetal mesenchymal stem cells. Cell Death Differ. 2005, 12, 1439–1441. [Google Scholar] [CrossRef] [Green Version]
- Solodeev, I.; Meilik, B.; Volovitz, I.; Sela, M.; Manheim, S.; Yarkoni, S.; Zipori, D.; Gur, E.; Shani, N. Fas-L promotes the stem cell potency of adipose-derived mesenchymal cells. Cell Death Dis. 2018, 9, 695. [Google Scholar] [CrossRef]
- Rippo, M.R.; Babini, L.; Prattichizzo, F.; Graciotti, L.; Fulgenzi, G.; Tomassoni Ardori, F.; Olivieri, F.; Borghetti, G.; Cinti, S.; Poloni, A.; et al. Low FasL levels promote proliferation of human bone marrow-derived mesenchymal stem cells, higher levels inhibit their differentiation into adipocytes. Cell Death Dis. 2013, 4, e594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kholodenko, I.V.; Kurbatov, L.K.; Kholodenko, R.V.; Manukyan, G.V.; Yarygin, K.N. Mesenchymal Stem Cells in the Adult Human Liver: Hype or Hope? Cells 2019, 8, 1127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.; Choi, J.; Kang, S.; Kim, J.; Lee, R.; So, S.; Yoon, Y.I.; Kirchner, V.A.; Song, G.W.; Hwang, S.; et al. Hepatogenic Potential and Liver Regeneration Effect of Human Liver-derived Mesenchymal-Like Stem Cells. Cells 2020, 9, 1521. [Google Scholar] [CrossRef]
- Yigitbilek, F.; Conley, S.M.; Tang, H.; Saadiq, I.M.; Jordan, K.L.; Lerman, L.O.; Taner, T. Comparable in vitro Function of Human Liver-Derived and Adipose Tissue-Derived Mesenchymal Stromal Cells: Implications for Cell-Based Therapy. Front. Cell Dev. Biol. 2021, 9, 641792. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, S.; Park, H.J.; Kim, Y.A.; Lee, S.K. Human Liver Stem Cell Transplantation Alleviates Liver Fibrosis in a Rat Model of CCl4-Induced Liver Fibrosis. Int. J. Stem Cells 2021, 14, 475–484. [Google Scholar] [CrossRef]
- Herrera Sanchez, M.B.; Bruno, S.; Grange, C.; Tapparo, M.; Cantaluppi, V.; Tetta, C.; Camussi, G. Human liver stem cells and derived extracellular vesicles improve recovery in a murine model of acute kidney injury. Stem Cell Res. Ther. 2014, 5, 124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarro-Tableros, V.; Gai, C.; Gomez, Y.; Giunti, S.; Pasquino, C.; Deregibus, M.C.; Tapparo, M.; Pitino, A.; Tetta, C.; Brizzi, M.F.; et al. Islet-Like Structures Generated In Vitro from Adult Human Liver Stem Cells Revert Hyperglycemia in Diabetic SCID Mice. Stem Cell Rev. Rep. 2019, 15, 93–111. [Google Scholar] [CrossRef] [Green Version]
- Kholodenko, I.V.; Kholodenko, R.V.; Manukyan, G.V.; Burunova, V.V.; Yarygin, K.N. Mesenchymal-epithelial transition in culture of stromal progenitor cells isolated from the liver of a patient with alcoholic cirrhosis. Bull. Exp. Biol. Med. 2016, 162, 115–119. [Google Scholar] [CrossRef]
- Kholodenko, R.; Kholodenko, I.; Sorokin, V.; Tolmazova, A.; Sazonova, O.; Buzdin, A. Anti-apoptotic effect of retinoic acid on retinal progenitor cells mediated by a protein kinase A-dependent mechanism. Cell Res. 2007, 17, 151–162. [Google Scholar] [CrossRef]
- Doronin, I.I.; Vishnyakova, P.A.; Kholodenko, I.V.; Ponomarev, E.D.; Ryazantsev, D.Y.; Molotkovskaya, I.M.; Kholodenko, R.V. Ganglioside GD2 in reception and transduction of cell death signal in tumor cells. BMC Cancer 2014, 14, 295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reers, M.; Smith, T.W.; Chen, L.B. J-aggregate formation of a carbocyanine as a quantitative fluorescent indicator of membrane potential. Biochemistry 1991, 30, 4480–4486. [Google Scholar] [CrossRef]
- Tsujimoto, Y. Apoptosis and necrosis: Intracellular ATP level as a determinant for cell death modes. Cell Death Differ. 1997, 4, 429–434. [Google Scholar] [CrossRef] [Green Version]
- Gleiss, B.; Gogvadze, V.; Orrenius, S.; Fadeel, B. Fas-triggered phosphatidylserine exposure is modulated by intracellular ATP. FEBS Lett. 2002, 519, 153–158. [Google Scholar] [CrossRef] [Green Version]
- Imamura, H.; Sakamoto, S.; Yoshida, T.; Matsui, Y.; Penuela, S.; Laird, D.W.; Mizukami, S.; Kikuchi, K.; Kakizuka, A. Single-cell dynamics of pannexin-1-facilitated programmed ATP loss during apoptosis. Elife 2020, 9, e61960. [Google Scholar] [CrossRef] [PubMed]
- Sagoo, P.; Chan, G.; Larkin, D.F.; George, A.J. Inflammatory cytokines induce apoptosis of corneal endothelium through nitric oxide. Investig. Ophthalmol. Vis. Sci. 2004, 45, 3964–3973. [Google Scholar] [CrossRef] [PubMed]
- Dettmer, R.; Niwolik, I.; Cirksena, K.; Yoshimoto, T.; Tang, Y.; Mehmeti, I.; Gurgul-Convey, E.; Naujok, O. Proinflammatory cytokines induce rapid, NO-independent apoptosis, expression of chemotactic mediators and interleukin-32 secretion in human pluripotent stem cell-derived beta cells. Diabetologia 2022, 65, 829–843. [Google Scholar] [CrossRef] [PubMed]
- Faletti, L.; Peintner, L.; Neumann, S.; Sandler, S.; Grabinger, T.; Mac Nelly, S.; Merfort, I.; Huang, C.H.; Tschaharganeh, D.; Kang, T.W.; et al. TNFα sensitizes hepatocytes to FasL-induced apoptosis by NFκB-mediated Fas upregulation. Cell Death Dis. 2018, 9, 909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rex, J.; Lutz, A.; Faletti, L.E.; Albrecht, U.; Thomas, M.; Bode, J.G.; Borner, C.; Sawodny, O.; Merfort, I. IL-1β and TNFα Differentially Influence NF-κB Activity and FasL-Induced Apoptosis in Primary Murine Hepatocytes During LPS-Induced Inflammation. Front. Physiol. 2019, 10, 117. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.M.; Seki, E. TNFα in liver fibrosis. Curr. Pathobiol. Rep. 2015, 3, 253–261. [Google Scholar] [CrossRef] [Green Version]
- Fabregat, I.; Moreno-Càceres, J.; Sánchez, A.; Dooley, S.; Dewidar, B.; Giannelli, G.; Ten Dijke, P. IT-LIVER Consortium. TGF-β signalling and liver disease. FEBS J. 2016, 283, 2219–2232. [Google Scholar] [CrossRef] [Green Version]
- Weng, H.L.; Wang, B.E.; Jia, J.D.; Wu, W.F.; Xian, J.Z.; Mertens, P.R.; Cai, W.M.; Dooley, S. Effect of interferon-gamma on hepatic fibrosis in chronic hepatitis B virus infection: A randomized controlled study. Clin. Gastroenterol. Hepatol. 2005, 3, 819–828. [Google Scholar] [CrossRef]
- Naseem, S.; Hussain, T.; Manzoor, S. Interleukin-6: A promising cytokine to support liver regeneration and adaptive immunity in liver pathologies. Cytokine Growth Factor Rev. 2018, 39, 36–45. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Kikuiri, T.; Akiyama, K.; Chen, C.; Xu, X.; Yang, R.; Chen, W.; Wang, S.; Shi, S. Mesenchymal stem cell-based tissue regeneration is governed by recipient T lymphocytes via IFN-γ and TNF-α. Nat. Med. 2011, 17, 1594–1601. [Google Scholar] [CrossRef]
- Dang, S.; Xu, H.; Xu, C.; Cai, W.; Li, Q.; Cheng, Y.; Jin, M.; Wang, R.X.; Peng, Y.; Zhang, Y.; et al. Autophagy regulates the therapeutic potential of mesenchymal stem cells in experimental autoimmune encephalomyelitis. Autophagy 2014, 10, 1301–1315. [Google Scholar] [CrossRef] [Green Version]
- Herrera, M.B.; Bruno, S.; Buttiglieri, S.; Tetta, C.; Gatti, S.; Deregibus, M.C.; Bussolati, B.; Camussi, G. Isolation and characterization of a stem cell population from adult human liver. Stem Cells 2006, 24, 2840–2850. [Google Scholar] [CrossRef]
- Sokal, E.M.; Stéphenne, X.; Ottolenghi, C.; Jazouli, N.; Clapuyt, P.; Lacaille, F.; Najimi, M.; de Lonlay, P.; Smets, F. Liver engraftment and repopulation by in vitro expanded adult derived human liver stem cells in a child with ornithine carbamoyltransferase deficiency. JIMD Rep. 2014, 13, 65–72. [Google Scholar] [PubMed] [Green Version]
- Smets, F.; Dobbelaere, D.; McKiernan, P.; Dionisi-Vici, C.; Broué, P.; Jacquemin, E.; Lopes, A.I.; Gonçalves, I.; Mandel, H.; Pawlowska, J.; et al. Phase I/II Trial of Liver Derived Mesenchymal Stem Cells in Pediatric Liver Based Metabolic Disorders: A Prospective, Open Label, Multicenter, Partially Randomized, Safety Study of One Cycle of Heterologous Human Adult Liver-Derived Progenitor Cells (HepaStem®) in Urea Cycle Disorders and Crigler-Najjar Syndrome patients. Transplantation 2019, 103, 1903–1915. [Google Scholar] [PubMed]
- Yang, Y.H.K.; Ogando, C.R.; Wang See, C.; Chang, T.Y.; Barabino, G.A. Changes in phenotype and differentiation potential of human mesenchymal stem cells aging in vitro. Stem Cell Res. Ther. 2018, 9, 131. [Google Scholar] [CrossRef] [Green Version]
- Walter, S.G.; Randau, T.M.; Hilgers, C.; Haddouti, E.M.; Masson, W.; Gravius, S.; Burger, C.; Wirtz, D.C.; Schildberg, F.A. Molecular and Functional Phenotypes of Human Bone Marrow-Derived Mesenchymal Stromal Cells Depend on Harvesting Techniques. Int. J. Mol. Sci. 2020, 21, 4382. [Google Scholar] [CrossRef] [PubMed]
- Rojewski, M.T.; Weber, B.M.; Schrezenmeier, H. Phenotypic Characterization of Mesenchymal Stem Cells from Various Tissues. Transfus. Med. Hemotherapy 2008, 35, 168–184. [Google Scholar] [CrossRef] [Green Version]
- Qiao, C.; Xu, W.; Zhu, W.; Hu, J.; Qian, H.; Yin, Q.; Jiang, R.; Yan, Y.; Mao, F.; Yang, H.; et al. Human mesenchymal stem cells isolated from the umbilical cord. Cell Biol. Int. 2008, 32, 8–15. [Google Scholar] [CrossRef]
- Moravcikova, E.; Meyer, E.M.; Corselli, M.; Donnenberg, V.S.; Donnenberg, A.D. Proteomic Profiling of Native Unpassaged and Culture-Expanded Mesenchymal Stromal Cells (MSC). Cytom. A 2018, 93, 894–904. [Google Scholar] [CrossRef] [Green Version]
- Mazar, J.; Thomas, M.; Bezrukov, L.; Chanturia, A.; Pekkurnaz, G.; Yin, S.; Kuznetsov, S.A.; Robey, P.G.; Zimmerberg, J. Cytotoxicity mediated by the Fas ligand (FasL)-activated apoptotic pathway in stem cells. J. Biol. Chem. 2009, 284, 22022–22028. [Google Scholar] [CrossRef] [Green Version]
- Akiyama, K.; Chen, C.; Wang, D.; Xu, X.; Qu, C.; Yamaza, T.; Cai, T.; Chen, W.; Sun, L.; Shi, S. Mesenchymal-stem-cell-induced immunoregulation involves FAS-ligand-/FAS-mediated T cell apoptosis. Cell Stem Cell 2012, 10, 544–555. [Google Scholar] [CrossRef] [Green Version]
- Vacaru, A.M.; Dumitrescu, M.; Vacaru, A.M.; Fenyo, I.M.; Ionita, R.; Gafencu, A.V.; Simionescu, M. Enhanced Suppression of Immune Cells In Vitro by MSC Overexpressing FasL. Int. J. Mol Sci. 2020, 22, 348. [Google Scholar] [CrossRef]
- Vacaru, A.M.; Mazilu, A.M.; Dumitrescu, M.; Fenyo, I.M.; Gafencu, A.V.; Vacaru, A.M. Treatment with Mesenchymal Stromal Cells Overexpressing Fas-Ligand Ameliorates Acute Graft-versus-Host Disease in Mice. Int. J. Mol. Sci. 2022, 23, 534. [Google Scholar] [CrossRef]
- Rodrigues, M.; Turner, O.; Stolz, D.; Griffith, L.; Wells, A. Production of reactive oxygen species by multipotent stromal cells/mesenchymal stem cells upon exposure to Fas Ligand. Cell Transpl. 2012, 21, 2171–2187. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Gao, L.; Wang, P.; Xie, Z.; Cen, S.; Li, Y.; Wu, X.; Wang, L.; Su, H.; Deng, W.; et al. TNF-α Induced the Enhanced Apoptosis of Mesenchymal Stem Cells in Ankylosing Spondylitis by Overexpressing TRAIL-R2. Stem Cells Int. 2017, 2017, 4521324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliver, L.; Hue, E.; Rossignol, J.; Bougras, G.; Hulin, P.; Naveilhan, P.; Heymann, D.; Lescaudron, L.; Vallette, F.M. Distinct roles of Bcl-2 and Bcl-Xl in the apoptosis of human bone marrow mesenchymal stem cells during differentiation. PLoS ONE 2011, 6, e19820. [Google Scholar] [CrossRef] [PubMed]
- Flusberg, D.A.; Sorger, P.K. Surviving apoptosis: Life-death signaling in single cells. Trends Cell Biol. 2015, 25, 446–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Micheau, O.; Thome, M.; Schneider, P.; Holler, N.; Tschopp, J.; Nicholson, D.W.; Briand, C.; Grütter, M.G. The long form of FLIP is an activator of caspase-8 at the Fas death-inducing signaling complex. J. Biol. Chem. 2002, 277, 45162–45171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jost, P.J.; Vucic, D. Regulation of Cell Death and Immunity by XIAP. Cold Spring Harb. Perspect. Biol. 2020, 12, a036426. [Google Scholar] [CrossRef]
- Tsujimoto, Y. Role of Bcl-2 family proteins in apoptosis: Apoptosomes or mitochondria? Genes Cells 1998, 3, 697–707. [Google Scholar] [CrossRef]
- Santiago, B.; Galindo, M.; Palao, G.; Pablos, J.L. Intracellular regulation of Fas-induced apoptosis in human fibroblasts by extracellular factors and cycloheximide. J. Immunol. 2004, 172, 560–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vorobjev, I.; Barteneva, N.S. Temporal Heterogeneity Metrics in Apoptosis Induced by Anticancer Drugs. J. Histochem. Cytochem. 2015, 63, 494–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tait, S.W.; Parsons, M.J.; Llambi, F.; Bouchier-Hayes, L.; Connell, S.; Muñoz-Pinedo, C.; Green, D.R. Resistance to caspase-independent cell death requires persistence of intact mitochondria. Dev. Cell. 2010, 18, 802–813. [Google Scholar] [CrossRef] [Green Version]
- Bock, F.J.; Tait, S.W.G. Mitochondria as multifaceted regulators of cell death. Nat. Rev. Mol. Cell Biol. 2020, 21, 85–100. [Google Scholar] [CrossRef]
- Colell, A.; Ricci, J.E.; Tait, S.; Milasta, S.; Maurer, U.; Bouchier-Hayes, L.; Fitzgerald, P.; Guio-Carrion, A.; Waterhouse, N.J.; Li, C.W.; et al. GAPDH and autophagy preserve survival after apoptotic cytochrome c release in the absence of caspase activation. Cell 2007, 129, 983–997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, P.; Yu, X.Y.; Xie, X.H.; Chen, C.H.; Zhang, P.; Yang, C.; Peng, X.; Wang, Y.T. Mitophagy is a protective response against oxidative damage in bone marrow mesenchymal stem cells. Life Sci. 2019, 229, 36–45. [Google Scholar] [CrossRef]
- Sato, K.; Chitose, S.I.; Sato, K.; Sato, F.; Ono, T.; Umeno, H. Glycolytic activity of the tissue stem cells in the macula flava of the human vocal fold. Laryngoscope Investig. Otolaryngol. 2021, 6, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Suda, T. Metabolic requirements for the maintenance of self-renewing stem cells. Nat. Rev. Mol. Cell Biol. 2014, 15, 243–256. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.T.; Shih, Y.R.; Kuo, T.K.; Lee, O.K.; Wei, Y.H. Coordinated changes of mitochondrial biogenesis and antioxidant enzymes during osteogenic differentiation of human mesenchymal stem cells. Stem Cells 2008, 26, 960–968. [Google Scholar] [CrossRef]
- Matsuura, K.; Canfield, K.; Feng, W.; Kurokawa, M. Metabolic Regulation of Apoptosis in Cancer. Int. Rev. Cell Mol. Biol. 2016, 327, 43–87. [Google Scholar] [PubMed] [Green Version]
- Pradelli, L.A.; Bénéteau, M.; Chauvin, C.; Jacquin, M.A.; Marchetti, S.; Muñoz-Pinedo, C.; Auberger, P.; Pende, M.; Ricci, J.E. Glycolysis inhibition sensitizes tumor cells to death receptors-induced apoptosis by AMP kinase activation leading to Mcl-1 block in translation. Oncogene 2010, 29, 1641–1652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaudet, S.; Spencer, S.L.; Chen, W.W.; Sorger, P.K. Exploring the contextual sensitivity of factors that determine cell-to-cell variability in receptor-mediated apoptosis. PLoS Comput. Biol. 2012, 8, e1002482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomzikova, M.O.; James, V.; Rizvanov, A.A. Mitochondria Donation by Mesenchymal Stem Cells: Current Understanding and Mitochondria Transplantation Strategies. Front. Cell Dev. Biol. 2021, 9, 653322. [Google Scholar] [CrossRef] [PubMed]
- Luchetti, F.; Carloni, S.; Nasoni, M.G.; Reiter, R.J.; Balduini, W. Tunneling nanotubes and mesenchymal stem cells: New insights into the role of melatonin in neuronal recovery. J. Pineal Res. 2022, 13, e12800. [Google Scholar] [CrossRef] [PubMed]
- Kondo, M.; Murakawa, Y.; Harashima, N.; Kobayashi, S.; Yamaguchi, S.; Harada, M. Roles of proinflammatory cytokines and the Fas/Fas ligand interaction in the pathogenesis of inflammatory myopathies. Immunology 2009, 128, e589–e599. [Google Scholar] [CrossRef] [PubMed]
- Aschkenazi, S.; Straszewski, S.; Verwer, K.M.; Foellmer, H.; Rutherford, T.; Mor, G. Differential regulation and function of the Fas/Fas ligand system in human trophoblast cells. Biol. Reprod. 2002, 66, 1853–1861. [Google Scholar] [CrossRef]
- Elzey, B.D.; Griffith, T.S.; Herndon, J.M.; Barreiro, R.; Tschopp, J.; Ferguson, T.A. Regulation of Fas ligand-induced apoptosis by TNF. J. Immunol. 2001, 167, 3049–3056. [Google Scholar] [CrossRef] [Green Version]

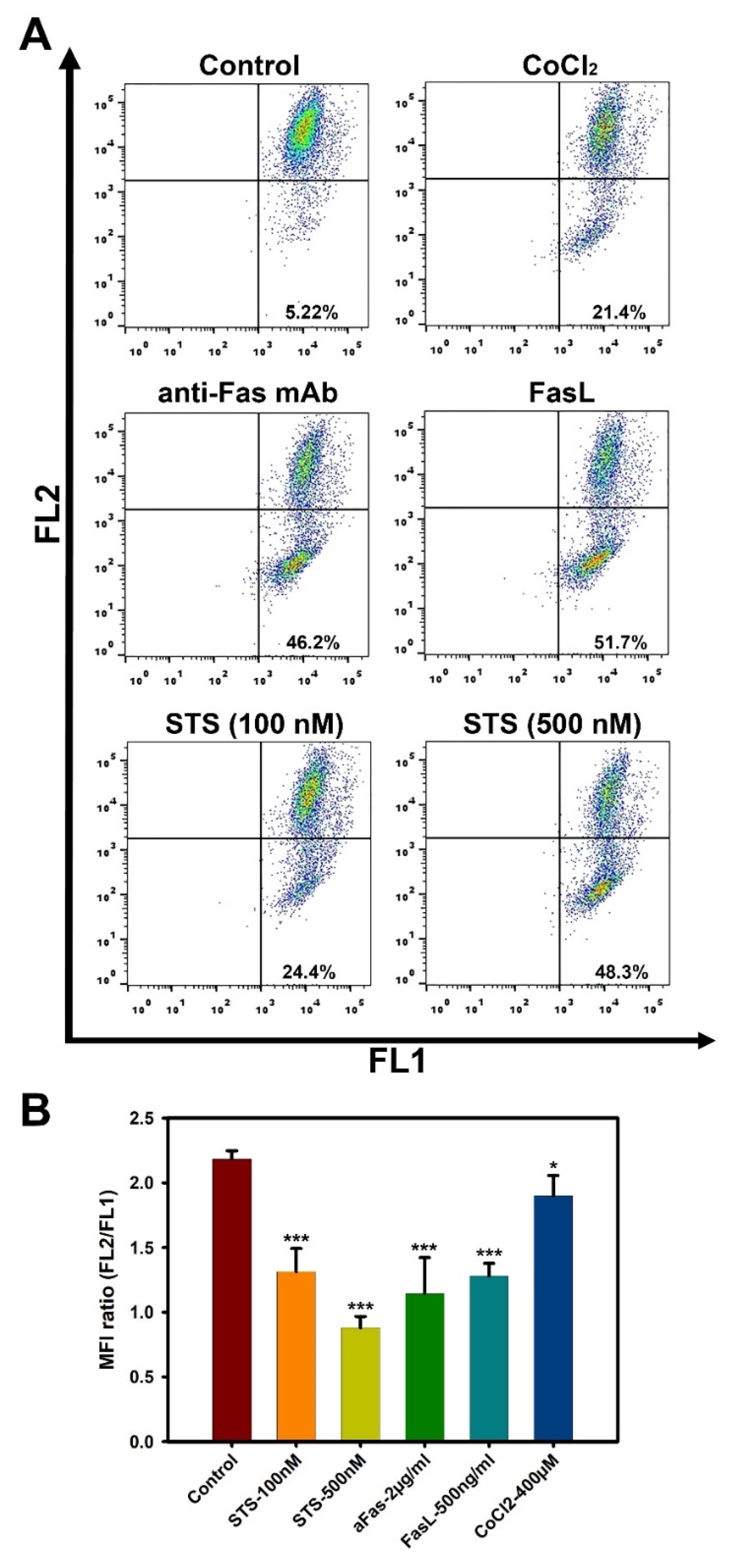


| Liver MSC#1 | Liver MSC#2 | Liver MSC#3 | |
|---|---|---|---|
| Control | 5.2 ± 0.9 | 4.41 ± 0.8 | 7.3 ± 2.8 |
| CoCl2, 400 µM | 21.4 ± 0.9 | 19.3 ± 0.9 | 22.5 ± 3.2 |
| Anti-Fas mAb, 2 µg/mL | 46.2 ± 4.8 | 40.4 ± 2.5 | 48.9 ± 6.0 |
| FasL, 500 ng/mL | 51.7 ± 4.2 | 56.2 ± 5.1 | 47.5 ± 2.5 |
| STS, 100 nM | 24.4 ± 3.2 | 20.2 ± 1.9 | 26.7 ± 5.9 |
| STS, 500 nM | 48.3 ± 4.0 | 43.1 ± 3.6 | 52.4 ± 5.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kholodenko, I.V.; Gisina, A.M.; Manukyan, G.V.; Majouga, A.G.; Svirshchevskaya, E.V.; Kholodenko, R.V.; Yarygin, K.N. Resistance of Human Liver Mesenchymal Stem Cells to FAS-Induced Cell Death. Curr. Issues Mol. Biol. 2022, 44, 3428-3443. https://doi.org/10.3390/cimb44080236
Kholodenko IV, Gisina AM, Manukyan GV, Majouga AG, Svirshchevskaya EV, Kholodenko RV, Yarygin KN. Resistance of Human Liver Mesenchymal Stem Cells to FAS-Induced Cell Death. Current Issues in Molecular Biology. 2022; 44(8):3428-3443. https://doi.org/10.3390/cimb44080236
Chicago/Turabian StyleKholodenko, Irina V., Alisa M. Gisina, Garik V. Manukyan, Alexander G. Majouga, Elena V. Svirshchevskaya, Roman V. Kholodenko, and Konstantin N. Yarygin. 2022. "Resistance of Human Liver Mesenchymal Stem Cells to FAS-Induced Cell Death" Current Issues in Molecular Biology 44, no. 8: 3428-3443. https://doi.org/10.3390/cimb44080236
APA StyleKholodenko, I. V., Gisina, A. M., Manukyan, G. V., Majouga, A. G., Svirshchevskaya, E. V., Kholodenko, R. V., & Yarygin, K. N. (2022). Resistance of Human Liver Mesenchymal Stem Cells to FAS-Induced Cell Death. Current Issues in Molecular Biology, 44(8), 3428-3443. https://doi.org/10.3390/cimb44080236







