Flow Cytometry Detection of Anthracycline-Treated Breast Cancer Cells: An Optimized Protocol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Cultures
2.2. Flow Cytometry Analysis of Apoptosis
2.3. Flow Cytometry Measurements of Anthracycline Autofluorescence
2.4. Statistics
3. Results
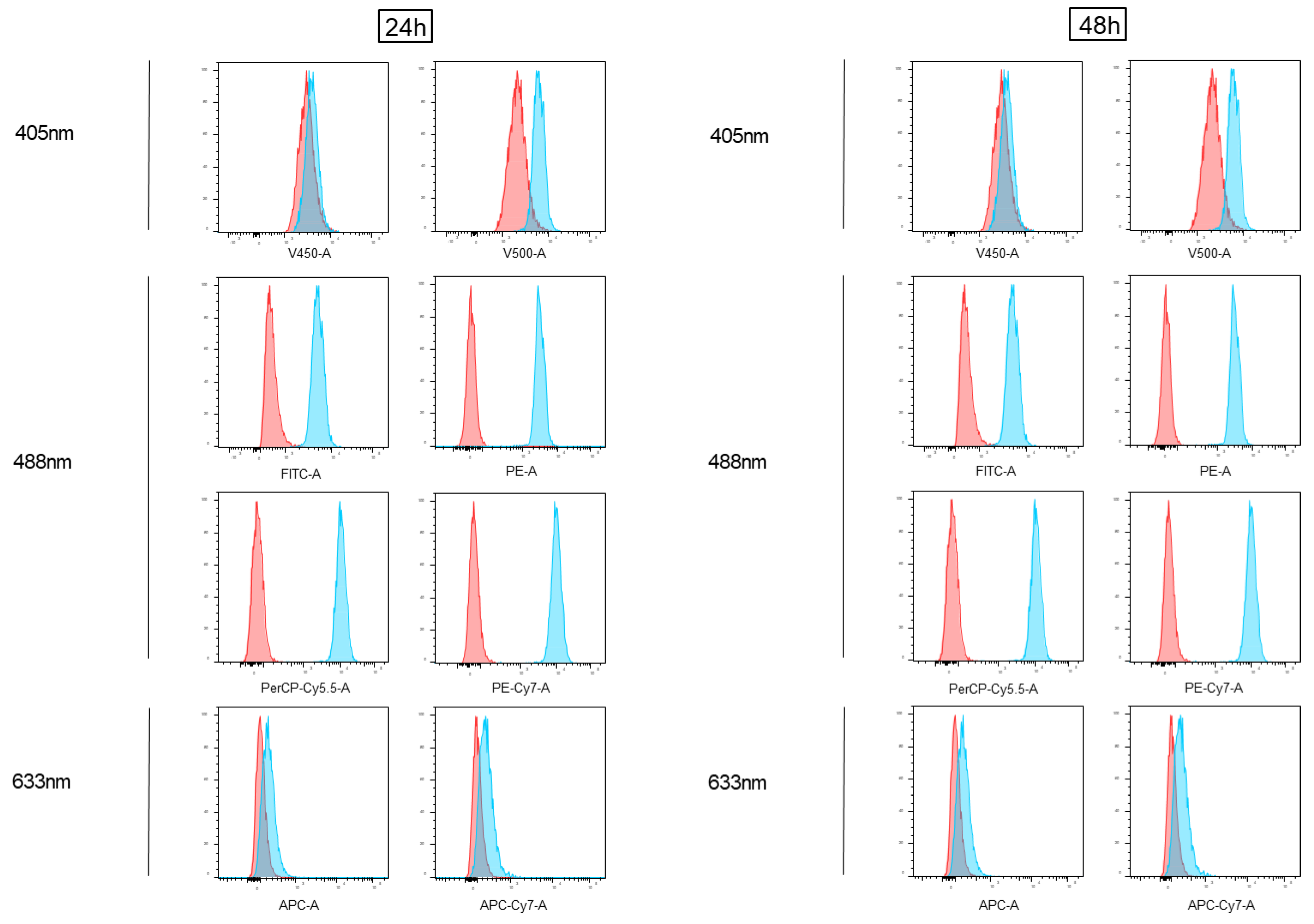
3.1. Impact of Doxorubicin Autofluorescence in Flow Cytometry Analyses
3.2. Impact of Epirubicin Autofluorescence in Flow Cytometry Analyses
3.3. Flow Cytometry Anthracycline Autofluorescence Is Dose-Dependent
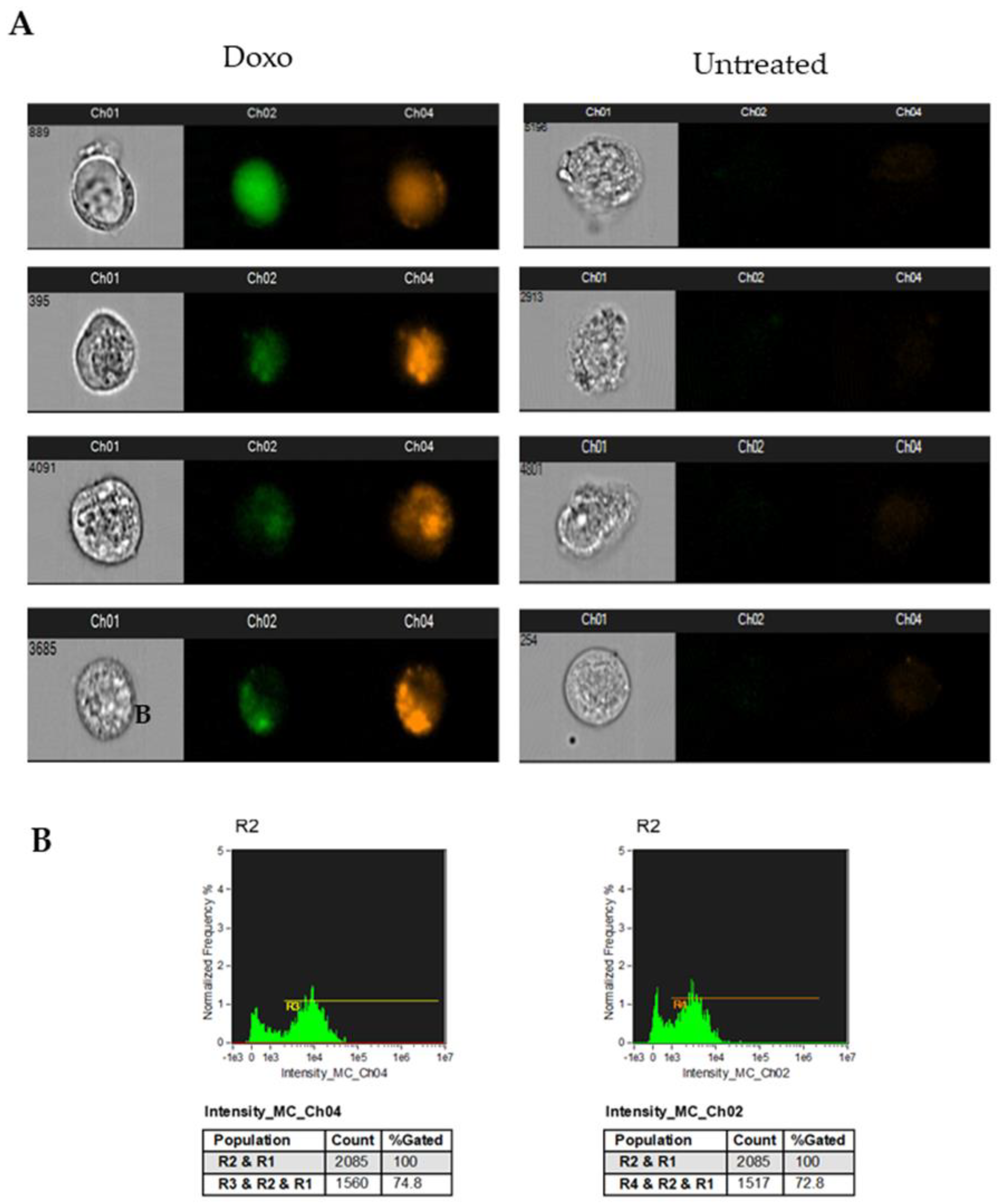
3.4. Analysis of Doxorubicin-Induced Apoptosis in Human Breast Cancer Models
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zoli, W.; Ricotti, L.; Tesei, A.; Barzanti, F.; Amadori, D. In Vitro Preclinical Models for a Rational Design of Chemotherapy Combinations in Human Tumors. Crit. Rev. Oncol. Hematol. 2001, 37, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Di Tomo, P.; Alessio, N.; Falone, S.; Pietrangelo, L.; Lanuti, P.; Cordone, V.; Santini, S.J.; Di Pietrantonio, N.; Marchisio, M.; Protasi, F.; et al. Endothelial Cells from Umbilical Cord of Women Affected by Gestational Diabetes: A Suitable in Vitro Model to Study Mechanisms of Early Vascular Senescence in Diabetes. FASEB J. 2021, 35, e21662. [Google Scholar] [CrossRef] [PubMed]
- Lanuti, P.; Bertagnolo, V.; Pierdomenico, L.; Bascelli, A.; Santavenere, E.; Alinari, L.; Capitani, S.; Miscia, S.; Marchisio, M. Enhancement of TRAIL Cytotoxicity by AG-490 in Human ALL Cells Is Characterized by Downregulation of CIAP-1 and CIAP-2 through Inhibition of Jak2/Stat3. Cell Res. 2009, 19, 1079–1089. [Google Scholar] [CrossRef] [Green Version]
- Lanuti, P.; Bertagnolo, V.; Gaspari, A.R.; Ciccocioppo, F.; Pierdomenico, L.; Bascelli, A.; Sabatino, G.; Miscia, S.; Marchisio, M. Parallel Regulation of PKC-Alpha and PKC-Delta Characterizes the Occurrence of Erythroid Differentiation from Human Primary Hematopoietic Progenitors. Exp. Hematol. 2006, 34, 1624–1634. [Google Scholar] [CrossRef] [PubMed]
- Lanuti, P.; Marchisio, M.; Cantilena, S.; Paludi, M.; Bascelli, A.; Gaspari, A.R.; Grifone, G.; Centurione, M.A.; Papa, S.; Di Pietro, R.; et al. A Flow Cytometry Procedure for Simultaneous Characterization of Cell DNA Content and Expression of Intracellular Protein Kinase C-Zeta. J. Immunol. Methods 2006, 315, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Bologna, G.; Lanuti, P.; D’Ambrosio, P.; Tonucci, L.; Pierdomenico, L.; D’Emilio, C.; Celli, N.; Marchisio, M.; D’Alessandro, N.; Santavenere, E.; et al. Water-Soluble Platinum Phthalocyanines as Potential Antitumor Agents. Biometals 2014, 27, 575–589. [Google Scholar] [CrossRef]
- Di Tomo, P.; Pipino, C.; Lanuti, P.; Morabito, C.; Pierdomenico, L.; Sirolli, V.; Bonomini, M.; Miscia, S.; Mariggiò, M.A.; Marchisio, M.; et al. Calcium Sensing Receptor Expression in Ovine Amniotic Fluid Mesenchymal Stem Cells and the Potential Role of R-568 during Osteogenic Differentiation. PLoS ONE 2013, 8, e73816. [Google Scholar] [CrossRef]
- D’Alimonte, I.; Nargi, E.; Zuccarini, M.; Lanuti, P.; Di Iorio, P.; Giuliani, P.; Ricci-Vitiani, L.; Pallini, R.; Caciagli, F.; Ciccarelli, R. Potentiation of Temozolomide Antitumor Effect by Purine Receptor Ligands Able to Restrain the in Vitro Growth of Human Glioblastoma Stem Cells. Purinergic Signal 2015, 11, 331–346. [Google Scholar] [CrossRef] [Green Version]
- Veschi, S.; De Lellis, L.; Florio, R.; Lanuti, P.; Massucci, A.; Tinari, N.; De Tursi, M.; di Sebastiano, P.; Marchisio, M.; Natoli, C.; et al. Effects of Repurposed Drug Candidates Nitroxoline and Nelfinavir as Single Agents or in Combination with Erlotinib in Pancreatic Cancer Cells. J. Exp. Clin. Cancer Res. 2018, 37, 236. [Google Scholar] [CrossRef]
- Di Tomo, P.; Lanuti, P.; Di Pietro, N.; Baldassarre, M.P.A.; Marchisio, M.; Pandolfi, A.; Consoli, A.; Formoso, G. Liraglutide Mitigates TNF-α Induced pro-Atherogenic Changes and Microvesicle Release in HUVEC from Diabetic Women. Diabetes Metab. Res. Rev. 2017, 33, e2925. [Google Scholar] [CrossRef]
- Codagnone, M.; Recchiuti, A.; Lanuti, P.; Pierdomenico, A.M.; Cianci, E.; Patruno, S.; Mari, V.C.; Simiele, F.; Di Tomo, P.; Pandolfi, A.; et al. Lipoxin A4 Stimulates Endothelial MiR-126-5p Expression and Its Transfer via Microvesicles. FASEB J. 2017, 31, 1856–1866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minotti, G.; Menna, P.; Salvatorelli, E.; Cairo, G.; Gianni, L. Anthracyclines: Molecular Advances and Pharmacologic Developments in Antitumor Activity and Cardiotoxicity. Pharmacol. Rev. 2004, 56, 185–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGowan, J.V.; Chung, R.; Maulik, A.; Piotrowska, I.; Walker, J.M.; Yellon, D.M. Anthracycline Chemotherapy and Cardiotoxicity. Cardiovasc. Drugs Ther. 2017, 31, 63–75. [Google Scholar] [CrossRef] [Green Version]
- Megías-Vericat, J.E.; Martínez-Cuadrón, D.; Sanz, M.Á.; Poveda, J.L.; Montesinos, P. Daunorubicin and Cytarabine for Certain Types of Poor-Prognosis Acute Myeloid Leukemia: A Systematic Literature Review. Expert Rev. Clin. Pharmacol. 2019, 12, 197–218. [Google Scholar] [CrossRef]
- Antolín, S.; Acea, B.; Albaina, L.; Concha, Á.; Santiago, P.; García-Caballero, T.; Mosquera, J.J.; Varela, J.R.; Soler, R.; Calvo, L. Primary Systemic Therapy in HER2-Positive Operable Breast Cancer Using Trastuzumab and Chemotherapy: Efficacy Data, Cardiotoxicity and Long-Term Follow-up in 142 Patients Diagnosed from 2005 to 2016 at a Single Institution. Breast Cancer Dove Med. Press 2019, 11, 29–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, M.; Seetharam, M. First-Line Therapy for Metastatic Soft Tissue Sarcoma. Curr. Treat. Options Oncol. 2019, 20, 6. [Google Scholar] [CrossRef]
- Singal, P.K.; Iliskovic, N. Doxorubicin-Induced Cardiomyopathy. N. Engl. J. Med. 1998, 339, 900–905. [Google Scholar] [CrossRef]
- Salvatorelli, E.; Guarnieri, S.; Menna, P.; Liberi, G.; Calafiore, A.M.; Mariggiò, M.A.; Mordente, A.; Gianni, L.; Minotti, G. Defective One- or Two-Electron Reduction of the Anticancer Anthracycline Epirubicin in Human Heart. Relative Importance of Vesicular Sequestration and Impaired Efficiency of Electron Addition. J. Biol. Chem. 2006, 281, 10990–11001. [Google Scholar] [CrossRef] [Green Version]
- Gewirtz, D.A. A Critical Evaluation of the Mechanisms of Action Proposed for the Antitumor Effects of the Anthracycline Antibiotics Adriamycin and Daunorubicin. Biochem. Pharmacol. 1999, 57, 727–741. [Google Scholar] [CrossRef]
- Venkatesh, P.; Kasi, A. Anthracyclines; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Christidi, E.; Brunham, L.R. Regulated Cell Death Pathways in Doxorubicin-Induced Cardiotoxicity. Cell Death Discov. 2021, 12, 339. [Google Scholar] [CrossRef]
- Wlodkowic, D.; Skommer, J.; Darzynkiewicz, Z. Flow Cytometry-Based Apoptosis Detection. Methods Mol. Biol. 2009, 559, 19–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, J.C.; Sims, J.R.; Gupta, A.; Hagoel, T.J.; Gao, L.; Lynch, M.L.; Woloszynska, A.; Melendy, T.; Kane, J.F.; Kuechle, J.; et al. CDC7 Kinase (DDK) Inhibition Disrupts DNA Replication Leading to Mitotic Catastrophe in Ewing Sarcoma. Cell Death Discov. 2022, 8, 85. [Google Scholar] [CrossRef] [PubMed]
- Huigsloot, M.; Tijdens, I.B.; Mulder, G.J.; van de Water, B. Differential Regulation of Doxorubicin-Induced Mitochondrial Dysfunction and Apoptosis by Bcl-2 in Mammary Adenocarcinoma (MTLn3) Cells. J. Biol. Chem. 2002, 277, 35869–35879. [Google Scholar] [CrossRef] [Green Version]
- Wilson and Gisvold’s Textbook of Organic Medicinal and Pharmaceutical Chemistry, 11th ed.; Wilson, C.O.; Gisvold, O.; Block, J.H.; Beale, J.M.; Wilson, C.O. (Eds.) Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2004; ISBN 978-0-7817-3481-3. [Google Scholar]
- Kauffman, M.K.; Kauffman, M.E.; Zhu, H.; Jia, Z.; Li, Y.R. Fluorescence-Based Assays for Measuring Doxorubicin in Biological Systems. React Oxyg Species (Apex NC) 2016, 2, 432–439. [Google Scholar] [CrossRef] [Green Version]
- Dine, T.; Brunet, C.; Luyckx, M.; Cazin, M.; Gosselin, P.; Cazin, J.L. Rapid Quantitative Determination of Epirubicin and Its Metabolites in Plasma Using High Performance Liquid Chromatography and Fluorescence Detection. Biomed. Chromatogr. 1990, 4, 20–23. [Google Scholar] [CrossRef]
- Guo, B.; Tam, A.; Santi, S.A.; Parissenti, A.M. Role of Autophagy and Lysosomal Drug Sequestration in Acquired Resistance to Doxorubicin in MCF-7 Cells. BMC Cancer 2016, 16, 762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lovitt, C.J.; Shelper, T.B.; Avery, V.M. Doxorubicin Resistance in Breast Cancer Cells Is Mediated by Extracellular Matrix Proteins. BMC Cancer 2018, 18, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chattopadhyay, P.K.; Hogerkorp, C.-M.; Roederer, M. A Chromatic Explosion: The Development and Future of Multiparameter Flow Cytometry. Immunology 2008, 125, 441–449. [Google Scholar] [CrossRef]
- Lanuti, P.; Ciccocioppo, F.; Bonanni, L.; Marchisio, M.; Lachmann, R.; Tabet, N.; Pierdomenico, L.; Santavenere, E.; Catinella, V.; Iacone, A.; et al. Amyloid-Specific T-Cells Differentiate Alzheimer’s Disease from Lewy Body Dementia. Neurobiol. Aging 2012, 33, 2599–2611. [Google Scholar] [CrossRef]
- Dumas, M.-P.; Xia, S.; Bear, C.E.; Ratjen, F. Perspectives on the Translation of In-Vitro Studies to Precision Medicine in Cystic Fibrosis. EBioMedicine 2021, 73, 103660. [Google Scholar] [CrossRef]
- Pauli, C.; Hopkins, B.D.; Prandi, D.; Shaw, R.; Fedrizzi, T.; Sboner, A.; Sailer, V.; Augello, M.; Puca, L.; Rosati, R.; et al. Personalized In Vitro and In Vivo Cancer Models to Guide Precision Medicine. Cancer Discov. 2017, 7, 462–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schilsky, R.L. Personalized Medicine in Oncology: The Future Is Now. Nat. Rev. Drug Discov. 2010, 9, 363–366. [Google Scholar] [CrossRef] [PubMed]
- Krishan, A.; Ganapathi, R. Laser Flow Cytometric Studies on the Intracellular Fluorescence of Anthracyclines. Cancer Res. 1980, 40, 3895–3900. [Google Scholar] [PubMed]
- Motlagh, N.S.H.; Parvin, P.; Ghasemi, F.; Atyabi, F. Fluorescence Properties of Several Chemotherapy Drugs: Doxorubicin, Paclitaxel and Bleomycin. Biomed. Opt. Express 2016, 7, 2400–2406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karukstis, K.K.; Thompson, E.H.; Whiles, J.A.; Rosenfeld, R.J. Deciphering the Fluorescence Signature of Daunomycin and Doxorubicin. Biophys. Chem. 1998, 73, 249–263. [Google Scholar] [CrossRef]
- Dai, X.; Yue, Z.; Eccleston, M.E.; Swartling, J.; Slater, N.K.H.; Kaminski, C.F. Fluorescence Intensity and Lifetime Imaging of Free and Micellar-Encapsulated Doxorubicin in Living Cells. Nanomedicine 2008, 4, 49–56. [Google Scholar] [CrossRef] [Green Version]
- Trevisan, M.G.; Poppi, R.J. Determination of Doxorubicin in Human Plasma by Excitation–Emission Matrix Fluorescence and Multi-Way Analysis. Anal. Chim. Acta 2003, 493, 69–81. [Google Scholar] [CrossRef]
- Prakash, J.; Mishra, A.K. Quantification of Doxorubicin in Biofluids Using White Light Excitation Fluorescence. J. Biophotonics 2014, 7, 607–616. [Google Scholar] [CrossRef]
- Martínez Ferreras, F.; Wolfbeis, O.S.; Gorris, H.H. Dual Lifetime Referenced Fluorometry for the Determination of Doxorubicin in Urine. Anal. Chim. Acta 2012, 729, 62–66. [Google Scholar] [CrossRef]
- Hulspas, R.; O’Gorman, M.R.G.; Wood, B.L.; Gratama, J.W.; Sutherland, D.R. Considerations for the Control of Background Fluorescence in Clinical Flow Cytometry. Cytom. B Clin. Cytom. 2009, 76, 355–364. [Google Scholar] [CrossRef]
- Sebestyén, Z.; Nagy, P.; Horváth, G.; Vámosi, G.; Debets, R.; Gratama, J.W.; Alexander, D.R.; Szöllosi, J. Long Wavelength Fluorophores and Cell-by-Cell Correction for Autofluorescence Significantly Improves the Accuracy of Flow Cytometric Energy Transfer Measurements on a Dual-Laser Benchtop Flow Cytometer. Cytometry 2002, 48, 124–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanders, C.K.; Mourant, J.R. Advantages of Full Spectrum Flow Cytometry. J. Biomed. Opt. 2013, 18, 037004. [Google Scholar] [CrossRef] [PubMed]


| Channel | Filters | SNR Doxorubicin | SNR Epirubicin | |
|---|---|---|---|---|
| FACS CantoII | FITC | 502 LP 530/30 | 8.45 | 12.92 |
| PE | 556 LP 585/42 | 94.80 | 164.06 | |
| PerCP-Cy5-5 | 655 LP 670 LP | 171.29 | 412.03 | |
| PE-Cy7 | 735 LP 780/60 | 148.60 | 391.92 | |
| APC | 660/20 | 2.31 | 2.12 | |
| APC-Cy7 | 735 LP 780/60 | 2.52 | 2.58 | |
| Pacific Blue | 450/50 | 1.08 | 1.01 | |
| AmCyan | 502 LP 510/50 | 1.14 | 1.14 | |
| FACS Verse | FITC | 507 LP 527/32 | 10.15 | 12.89 |
| PE | 560 LP 586/42 | 116.96 | 171.54 | |
| PerCP-Cy5-5 | 665 LP 700/54 | 219.69 | 459.87 | |
| PE-Cy7 | 752 LP 783/56 | 190.82 | 411.52 | |
| APC | 610/610 660/10 | 2.17 | 2.22 | |
| APC-Cy7 | 752 LP 783/56 | 2.36 | 2.43 | |
| V450 | 448/45 448/45 | 1.25 | 1.16 | |
| V500 | 500 LP 528/45 | 2.71 | 3.08 | |
| CytoFLEX | FITC | 525/40 | 11.27 | 14.82 |
| PE | 585/42 | 105.67 | 156.59 | |
| ECD | 610/20 | 179.03 | 323.83 | |
| PC5.5 | 690/50 | 219.21 | 469.39 | |
| PC7 | 780/60 | 188.31 | 446.30 | |
| APC | 660/10 | 2.46 | 2.41 | |
| APC-A700 | 712/25 | 2.47 | 2.54 | |
| APC-A750 | 780/60 | 2.51 | 2.75 | |
| PB450 | 450/45 | 1.29 | 1.24 | |
| KO525 | 525/40 | 2.20 | 2.45 | |
| Violet 610 | 610/20 | 48.19 | 90.47 | |
| Violet 780 | 780/60 | 47.78 | 122.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catitti, G.; De Fabritiis, S.; Brocco, D.; Simeone, P.; De Bellis, D.; Vespa, S.; Veschi, S.; De Lellis, L.; Tinari, N.; Verginelli, F.; et al. Flow Cytometry Detection of Anthracycline-Treated Breast Cancer Cells: An Optimized Protocol. Curr. Issues Mol. Biol. 2023, 45, 164-174. https://doi.org/10.3390/cimb45010013
Catitti G, De Fabritiis S, Brocco D, Simeone P, De Bellis D, Vespa S, Veschi S, De Lellis L, Tinari N, Verginelli F, et al. Flow Cytometry Detection of Anthracycline-Treated Breast Cancer Cells: An Optimized Protocol. Current Issues in Molecular Biology. 2023; 45(1):164-174. https://doi.org/10.3390/cimb45010013
Chicago/Turabian StyleCatitti, Giulia, Simone De Fabritiis, Davide Brocco, Pasquale Simeone, Domenico De Bellis, Simone Vespa, Serena Veschi, Laura De Lellis, Nicola Tinari, Fabio Verginelli, and et al. 2023. "Flow Cytometry Detection of Anthracycline-Treated Breast Cancer Cells: An Optimized Protocol" Current Issues in Molecular Biology 45, no. 1: 164-174. https://doi.org/10.3390/cimb45010013







