Optimization of Plant Growth Regulators for In Vitro Mass Propagation of a Disease-Free ‘Shine Muscat’ Grapevine Cultivar
Abstract
:1. Introduction
2. Materials and Methods
2.1. Culture Establishment and Media Composition
2.2. Shoot Multiplication and Shoot-Related Data Collection
2.3. Root Induction, Root-Related Data Collection, and Acclimatization
2.4. Genetic Stability Analysis Using ISSR Markers
2.5. Statistical Analysis
3. Results and Discussion
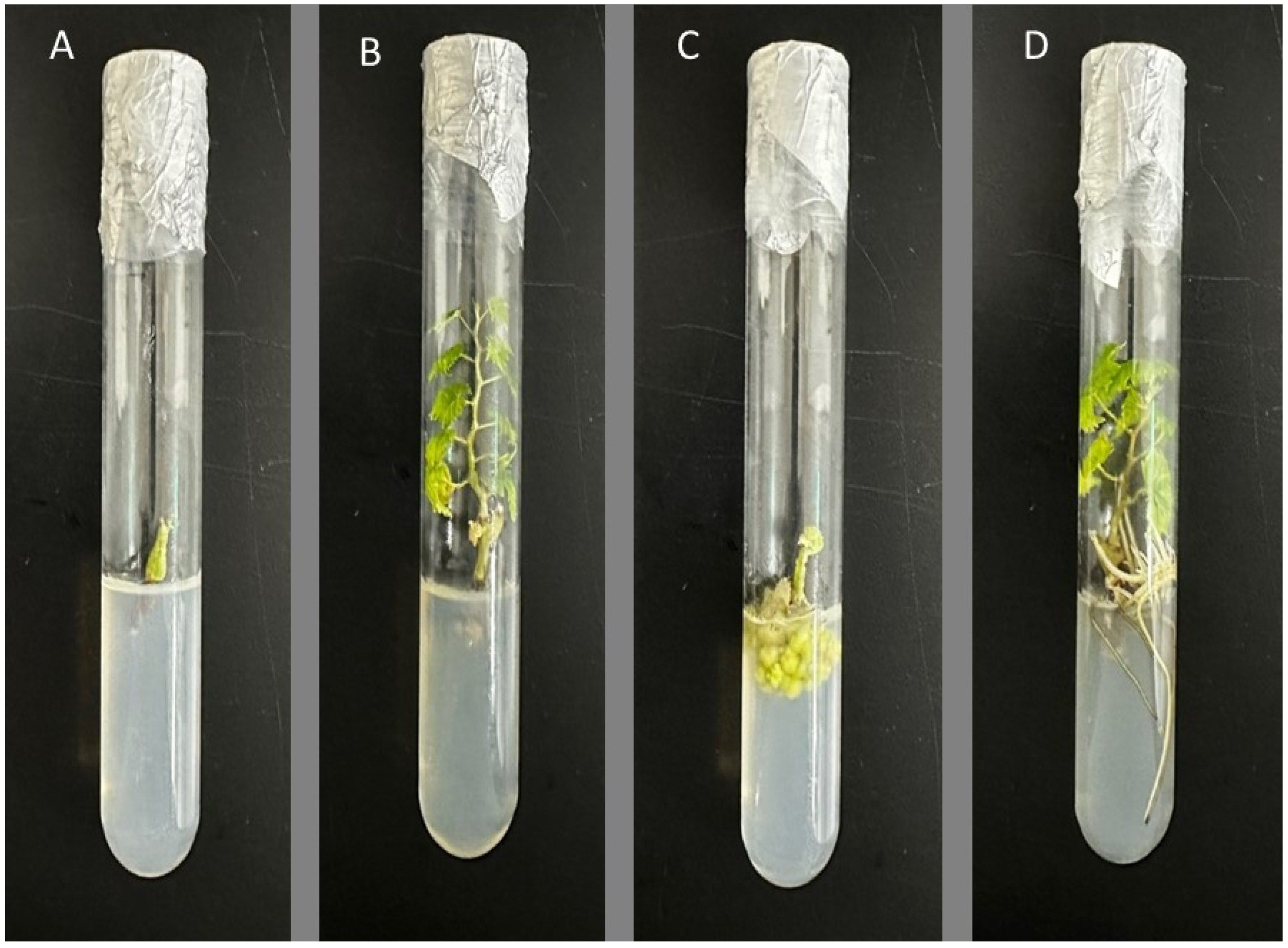
3.1. Assessment of the Impact of Hormones on the Initiation of Culture
3.2. Assessment of the Impact of Hormones on the Growth and Multiplication of Shoots
3.3. Investigation of the Influence of Hormones on the Initiation and Development of Roots
3.4. Evaluation of Genetic Stability of ‘Shine Muscat’ Using ISSR Markers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fortes, A.M.; Pais, M.S. Grape (Vitis Species). In Nutritional Composition of Fruit Cultivars; Elsevier: Amsterdam, The Netherlands, 2016; pp. 257–286. [Google Scholar]
- Doshi, P.; Adsule, P.; Banerjee, K.; Oulkar, D. Phenolic Compounds, Antioxidant Activity and Insulinotropic Effect of Extracts Prepared from Grape (Vitis vinifera L.) Byproducts. J. Food Sci. Technol. 2015, 52, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Jiménez, Y.; Palma, M.; Guillén-Sánchez, D.A.; García-Moreno, M.V. Study of the Cluster Thinning Grape as a Source of Phenolic Compounds and Evaluation of Its Antioxidant Potential. Biomolecules 2021, 11, 227. [Google Scholar] [CrossRef] [PubMed]
- Mannini, F.; Digiaro, M. The Effects of Viruses and Viral Diseases on Grapes and Wine. In Grapevine Viruses: Molecular Biology, Diagnostics and Management; Springer: Cham, Switzerland, 2017; pp. 453–482. [Google Scholar]
- Shirasawa, K.; Hirakawa, H.; Azuma, A.; Taniguchi, F.; Yamamoto, T.; Sato, A.; Ghelfi, A.; Isobe, S.N. De Novo Whole-Genome Assembly in an Interspecific Hybrid Table Grape,‘Shine Muscat’. DNA Res. 2022, 29, dsac040. [Google Scholar] [CrossRef]
- Lim, Y.-S.; Hassan, O.; Chang, T. First Report of Anthracnose of Shine Muscat Caused by Colletotrichum Fructicola in Korea. Mycobiology 2020, 48, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.-K.; Kang, K.-J.; Park, H.; Lee, U.-S.; Seo, J.-H. Comparison of Fruit Quality According to Harvest Time of Shine Muscat Grape (Vitis labruscana Bailey× V. vinifera L.). In Proceedings of the XXXI International Horticultural Congress (IHC2022): International Symposium on Integrative Approaches to Product Quality in 1353, Angers, France, 14–20 August 2022; pp. 317–322. [Google Scholar]
- Yamada, M.; Yamane, H.; Sato, A.; Hirakawa, N.; Iwanami, H.; Yoshinaga, K.; Ozawa, T.; Mitani, N.; Shiraishi, M.; Yoshioka, M. New Grape Cultivar ‘Shine Muscat’. Bull. Natl. Inst. Fruit Tree Sci. 2008, 7, 21–38. [Google Scholar]
- Si-Hong, K.I.M.; Jeong, S.-H.; Jae-Yun, H.E.O. Incidence of 14 Grapevine Viruses in Korean Vineyards. Not. Bot. Horti Agrobot. Cluj-Napoca 2021, 49, 1–7. [Google Scholar]
- Hamdeni, I.; Louhaichi, M.; Slim, S.; Boulila, A.; Bettaieb, T. Incorporation of Organic Growth Additives to Enhance in Vitro Tissue Culture for Producing Genetically Stable Plants. Plants 2022, 11, 3087. [Google Scholar] [CrossRef] [PubMed]
- Poudel, P.R.; Kataoka, I.; Mochioka, R. Effect of Plant Growth Regulators on in Vitro Propagation of Vitis ficifolia Var. Ganebu and Its Interspecific Hybrid Grape. Asian J. Plant Sci. 2005, 4, 466–471. [Google Scholar] [CrossRef]
- Yerbolova, L.S.; Ryabushkina, N.A.; Oleichenko, S.N.; Kampitova, G.A.; Galiakparov, N.N. The Effect of Growth Regulators on in Vitro Culture of Some Vitis vinifera L. Cultivars. World Appl. Sci. J. 2013, 23, 76–80. [Google Scholar]
- Weremczuk-Jeżyna, I.; Kuźma, Ł.; Kiss, A.K.; Grzegorczyk-Karolak, I. Effect of Cytokinins on Shoots Proliferation and Rosmarinic and Salvianolic Acid B Production in Shoot Culture of Dracocephalum forrestii WW Smith. Acta Physiol. Plant. 2018, 40, 189. [Google Scholar] [CrossRef]
- Nowakowska, K.; Pińkowska, A.; Siedlecka, E.; Pacholczak, A. The Effect of Cytokinins on Shoot Proliferation, Biochemical Changes and Genetic Stability of Rhododendron ‘Kazimierz Odnowiciel’ in the in Vitro Cultures. Plant Cell Tissue Organ Cult. 2022, 149, 675–684. [Google Scholar] [CrossRef]
- Chapman, E.J.; Estelle, M. Cytokinin and Auxin Intersection in Root Meristems. Genome Biol. 2009, 10, 210. [Google Scholar] [CrossRef]
- Qiu, Y.; Guan, S.C.; Wen, C.; Li, P.; Gao, Z.; Chen, X. Auxin and Cytokinin Coordinate the Dormancy and Outgrowth of Axillary Bud in Strawberry Runner. BMC Plant Biol. 2019, 19, 528. [Google Scholar] [CrossRef] [PubMed]
- Edelmann, H.G. Plant Root Development: Is the Classical Theory for Auxin-Regulated Root Growth False? Protoplasma 2022, 259, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Cleland, R.E. Auxin and Cell Elongation. In Plant Hormones and Their Role in Plant Growth and Development; Springer: Dordrecht, The Netherlands, 1987; pp. 132–148. [Google Scholar]
- Ya, R.; Li, J.; Zhang, N.; Yu, Q.; Xu, W. Phenotypically Abnormal Cotyledonary Vitis vinifera Embryos Differ in Anatomy, Endogenous Hormone Levels and Transcriptome Profiles. Tree Physiol. 2023, 43, 467–485. [Google Scholar] [CrossRef]
- Ganguly, A.; Lee, S.H.; Cho, M.; Lee, O.R.; Yoo, H.; Cho, H.-T. Differential Auxin-Transporting Activities of PIN-FORMED Proteins in Arabidopsis Root Hair Cells. Plant Physiol. 2010, 153, 1046–1061. [Google Scholar] [CrossRef]
- Bennett, T.; Hines, G.; van Rongen, M.; Waldie, T.; Sawchuk, M.G.; Scarpella, E.; Ljung, K.; Leyser, O. Connective Auxin Transport in the Shoot Facilitates Communication between Shoot Apices. PLoS Biol. 2016, 14, e1002446. [Google Scholar] [CrossRef]
- Ribeiro, Y.R. de S.; Aragão, V.P.M.; Sousa, K.R. de; Macedo, A.F.; Floh, E.I.S.; Silveira, V.; Santa-Catarina, C. Involvement of Differentially Accumulated Proteins and Endogenous Auxin in Adventitious Root Formation in Micropropagated Shoot Cuttings of Cedrela fissilis Vellozo (Meliaceae). Plant Cell Tissue Organ. Cult. 2022, 148, 119–135. [Google Scholar]
- Jing, H.; Strader, L.C. Interplay of Auxin and Cytokinin in Lateral Root Development. Int. J. Mol. Sci. 2019, 20, 486. [Google Scholar] [CrossRef]
- Kurepa, J.; Smalle, J.A. Auxin/Cytokinin Antagonistic Control of the Shoot/Root Growth Ratio and Its Relevance for Adaptation to Drought and Nutrient Deficiency Stresses. Int. J. Mol. Sci. 2022, 23, 1933. [Google Scholar] [CrossRef]
- Sato, M.; Hosokawa, M.; Doi, M. Somaclonal Variation Is Induced de Novo via the Tissue Culture Process: A Study Quantifying Mutated Cells in Saintpaulia. PLoS ONE 2011, 6, e23541. [Google Scholar] [CrossRef] [PubMed]
- Samarfard, S.; Kadir, M.A.; Kadzimin, S.B.; Saud, H.M.; Ravanfar, S.A.; Danaee, M. In Vitro Propagation and Detection of Somaclonal Variation in Phalaenopsis Gigantea as Affected by Chitosan and Thidiazuron Combinations. HortScience 2014, 49, 82–88. [Google Scholar] [CrossRef]
- Krishna, H.; Alizadeh, M.; Singh, D.; Singh, U.; Chauhan, N.; Eftekhari, M.; Sadh, R.K. Somaclonal Variations and Their Applications in Horticultural Crops Improvement. 3 Biotech 2016, 6, 54. [Google Scholar] [CrossRef]
- Aljuaid, B.S.; Ismail, I.A.; Attia, A.O.; El Dessoky, S.D. Genetic Stability of in Vitro Propagated Grapevine (Vitis vinifera L.) Cv. Al-Bayadi. J. Agric. Crop. 2022, 8, 12–19. [Google Scholar] [CrossRef]
- Gemmill, C.E.C.; Grierson, E.R.P. Inter-Simple Sequence Repeats (ISSR), Microsatellite-Primed Genomic Profiling Using Universal Primers. Mol. Plant Taxon. Methods Protoc. 2021, 2222, 249–262. [Google Scholar]
- Johnson, L.J.; Brookfield, J.F.Y. A Test of the Master Gene Hypothesis for Interspersed Repetitive DNA Sequences. Mol. Biol. Evol. 2006, 23, 235–239. [Google Scholar] [CrossRef]
- Babu, K.N.; Sheeja, T.E.; Minoo, D.; Rajesh, M.K.; Samsudeen, K.; Suraby, E.J.; Kumar, I.P.V. Random Amplified Polymorphic DNA (RAPD) and Derived Techniques. Mol. Plant Taxon. Methods Protoc. 2021, 1115, 219–247. [Google Scholar]
- Sheeja, T.E.; Kumar, I.P.V.; Giridhari, A.; Minoo, D.; Rajesh, M.K.; Babu, K.N. Amplified Fragment Length Polymorphism: Applications and Recent Developments. Mol. Plant Taxon. Methods Protoc. 2021, 2222, 187–218. [Google Scholar]
- Motha, K.; Singh, S.K.; Singh, R.; Ram, C.; Srivastav, M.; Verma, M.K.; Alizadeh, M.; Bhardwaj, C.; Dev, R. Comparative in Vitro Propagation of Stress Tolerant Grape (Vitis spp.) Rootstocks and Assessment of Clonal Fidelity of Plantlets. Indian J. Hortic. 2017, 74, 317–325. [Google Scholar] [CrossRef]
- Benke, A.P.; Krishna, R.; Samarth, R.R.; Dhumal, S.S.; Ansari, W.A.; Shelke, P.V.; Dukare, S.S.; Singh, M. Development of an Embryo Germination Protocol for Shy-Seeded Grape (Vitis vinifera L.). Plant Genet. Resour. 2021, 19, 252–260. [Google Scholar] [CrossRef]
- Classic Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bioassays with Tobacco Tissue Cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Marconi, M.; Wabnik, K. Shaping the Organ: A Biologist Guide to Quantitative Models of Plant Morphogenesis. Front. Plant Sci. 2021, 12, 2171. [Google Scholar] [CrossRef]
- Jamwal, M.; Singh, B.; Sharma, N.; Kumar, R.; Sharma, A.; Sharma, R.M.; Parmar, A.M. In Vitro Regeneration of Grape (Vitis vinifera L.) Cv. Perlette. World J. Agric. Sci. 2013, 9, 161–166. [Google Scholar]
- Joon-Ho, K.; Young-Sik, P.; Si-Hong, K.I.M.; Jae-Yun, H.E.O. Evaluation of Genetic Stability and Effects of Plant Growth Regulators for in Vitro Propagation of Underutilized Vitis amurensis ‘Cheongsan’. Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 987–994. [Google Scholar]
- San José, M.C.; Cernadas, M.J.; Janeiro, L.V. Optimization of Micropropagation Protocols in Some Woody Plants Using Meta-Topolin. In Meta-Topolin: A Growth Regulator for Plant Biotechnology and Agriculture; Springer: Singapore, 2021; pp. 221–240. [Google Scholar]
- Murvanidze, N.; Nisler, J.; Leroux, O.; Werbrouck, S.P.O. Cytokinin Oxidase/Dehydrogenase Inhibitors Stimulate 2iP to Induce Direct Somatic Embryogenesis in Coffea Arabica. Plant Growth Regul. 2021, 94, 195–200. [Google Scholar] [CrossRef]
- Kaviani, B.; Barandan, A.; Tymoszuk, A.; Kulus, D. Optimization of in Vitro Propagation of Pear (Pyrus communis L.) ‘Pyrodwarf®(S)’ Rootstock. Agronomy 2023, 13, 268. [Google Scholar] [CrossRef]
- Ouyang, Y.; Chen, Y.; Lü, J.; Teixeira da Silva, J.A.; Zhang, X.; Ma, G. Somatic embryogenesis and enhanced shoot organogenesis in Metabriggsia ovalifolia WT Wang. Sci. Rep. 2016, 6, 24662. [Google Scholar] [CrossRef]
- Nautiyal, A.; Rashid, A.; Agnihotri, A. Induction of Multiple Shoots in Oryza Sativa: Roles of Thidiazuron, 6-Benzylaminopurine, Decapitation, Flooding, and Ethrel® Treatments. Vitro Cell. Dev. Biol. 2022, 58, 1126–1137. [Google Scholar] [CrossRef]
- Yucesan, B.; Turker, A.U.; Gurel, E. TDZ-Induced High Frequency Plant Regeneration through Multiple Shoot Formation in Witloof Chicory (Cichorium intybus L.). Plant Cell. Tissue Organ Cult. 2007, 91, 243–250. [Google Scholar] [CrossRef]
- Vinoth, A.; Ravindhran, R. In Vitro Morphogenesis of Woody Plants Using Thidiazuron. In Thidiazuron: From Urea Derivative to Plant Growth Regulator; Springer: Singapore, 2018; pp. 211–229. [Google Scholar]
- Novikova, T.I.; Zaytseva, Y.G. TDZ-Induced Morphogenesis Pathways in Woody Plant Culture BT-Thidiazuron: From Urea Derivative to Plant Growth Regulator; Springer: Singapore, 2018. [Google Scholar]
- Ikeuchi, M.; Favero, D.S.; Sakamoto, Y.; Iwase, A.; Coleman, D.; Rymen, B.; Sugimoto, K. Molecular Mechanisms of Plant Regeneration. Annu. Rev. Plant Biol. 2019, 70, 377–406. [Google Scholar] [CrossRef]
- Smart, D.R.; Kocsis, L.; Andrew Walker, M.; Stockert, C. Dormant Buds and Adventitious Root Formation by Vitis and Other Woody Plants. J. Plant Growth Regul. 2002, 21, 296–314. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, Y.; Jiang, C.; Lu, M.-Z.; Zhang, J. Exogenous Hormones Supplementation Improve Adventitious Root Formation in Woody Plants. Front. Bioeng. Biotechnol. 2022, 10, 1009531. [Google Scholar] [CrossRef] [PubMed]
- Frick, E.M.; Strader, L.C. Roles for IBA-Derived Auxin in Plant Development. J. Exp. Bot. 2018, 69, 169–177. [Google Scholar] [CrossRef]
- Matsuoka, H.; Hinata, K. NAA-Induced Organogenesis and Embryogenesis in Hypocotyl Callus of Solarium melongena L. J. Exp. Bot. 1979, 30, 363–370. [Google Scholar] [CrossRef]
- Villalobos-Olivera, A.; Ferreira, C.F.; Yanes-Paz, E.; Lorente, G.Y.; Souza, F.V.; Engelmann, F.; Martínez-Montero, M.E.; Lorenzo, J.C. Inter Simple Sequence Repeat (ISSR) Markers Reveal DNA Stability in Pineapple Plantlets after Shoot Tip Cryopreservation. Vegetos 2022, 35, 360–366. [Google Scholar] [CrossRef]


| ISSR Primer | Annealing Temperature (°C) | Nucleotide Sequence (5′-3′) | No. of Distinct Band Classes | Total Number of Bands Amplified | % Similarity |
|---|---|---|---|---|---|
| UBC 808 | 50 | (AG)8C | 8 | 80 | 100 |
| UBC 812 | 50 | (GA)8C | 8 | 80 | 100 |
| UBC 815 | 55 | (CT)8G | 4 | 40 | 100 |
| UBC 823 | 50 | (TC)8C | 4 | 40 | 100 |
| UBC 825 | 55 | (AC)8T | 9 | 90 | 100 |
| UBC 836 | 50 | (AG)7ACYA | 8 | 80 | 100 |
| UBC 840 | 50 | (GA)8Y | 8 | 80 | 100 |
| UBC 873 | 50 | (GACA)4 | 13 | 130 | 100 |
| UBC 878 | 55 | (GGAT)4 | 8 | 80 | 100 |
| UBC 881 | 53 | GGGT(GGGGT)2G | 8 | 80 | 100 |
| Plant Growth Regulators/Concentrations | Bud Induction Rate/Nodal Segment (%) | No. of Shoots/ Nodal segment | No. of Nodes/ Nodal Segment | Length of Main Shoot (cm) |
|---|---|---|---|---|
| Control (MS medium) | 54.3 b | 0.72 d | 2.31 d | 1.31 c |
| BA 1.0 μM | 90.4 a | 1.05 bc | 3.79 abc | 2.07 ab |
| BA 2.0 μM | 97.8 a | 1.21 abc | 4.29 a | 2.37 a |
| BA 4.0 μM | 92.3 a | 1.31 ab | 4.32 a | 2.21 ab |
| BA 8.0 μM | 93.7 a | 1.38 a | 4.43 a | 1.93 ab |
| BA 16.0 μM | 94.3 a | 1.29 ab | 4.57 a | 1.62 bc |
| KIN 1.0 μM | 53.7 b | 0.67 d | 2.27 d | 1.69 bc |
| KIN 2.0 μM | 60.0 b | 0.65 d | 2.84 bcd | 1.81 bc |
| KIN 4.0 μM | 43.7 b | 0.57 d | 2.47 d | 2.24 ab |
| KIN 8.0 μM | 37.5 b | 0.51 d | 2.34 d | 1.91 ab |
| KIN 16.0 μM | 30.4 b | 0.43 d | 1.97 b | 1.57 bc |
| TDZ 1.0 μM | 89.4 a | 0.97 c | 3.64 abc | 1.97 ab |
| TDZ 2.0 μM | 92.3 a | 1.04 bc | 4.21 a | 2.35 a |
| TDZ 4.0 μM | 94.5 a | 1.20 abc | 4.32 a | 2.32 a |
| TDZ 8.0 μM | 92.4 a | 1.09 bc | 4.01 a | 2.26 ab |
| TDZ 16.0 μM | 86.4 a | 0.94 c | 3.81 abc | 2.14 ab |
| Plant Growth Regulators/Concentrations | Bud Induction Rate/Nodal Segment (%) | No. of Shoots/ Nodal Segment | No. of Nodes/ Nodal Segment | Length of Main Shoot (cm) |
|---|---|---|---|---|
| Control (MS medium) | 42.1 b | 0.41 d | 1.38 c | 0.51 b |
| BA 1.0 μM | 93.2 a | 1.88 abc | 5.24 ab | 1.53 ab |
| BA 2.0 μM | 92.1 a | 2.14 a | 6.51 a | 1.69 a |
| BA 4.0 μM | 84.3 a | 1.89 abc | 5.87 ab | 1.45 ab |
| BA 8.0 μM | 82.9 a | 1.54 bc | 5.54 ab | 1.33 ab |
| BA 16.0 μM | 81.5 a | 1.31 bcd | 5.42 ab | 1.21 ab |
| TDZ 1.0 μM | 89.7 a | 1.04 c | 4.32 b | 1.48 ab |
| TDZ 2.0 μM | 85.4 a | 1.24 bc | 4.47 ab | 1.51 ab |
| TDZ 4.0 μM | 83.3 a | 1.31 bcd | 4.87 ab | 1.55 ab |
| TDZ 8.0 μM | 81.9 a | 1.58 bc | 5.27 ab | 1.58 ab |
| TDZ 16.0 μM | 81.3 a | 1.39 bcd | 5.09 ab | 1.49 ab |
| Plant Growth Regulators/Concentrations | % Rooting | % Callusing | Root Number | Root Length (cm) | Shoot Length (cm) |
|---|---|---|---|---|---|
| Control (MS medium) | 37.4 b | 0 d | 0.39 d | 0.68 c | 0.57 c |
| IAA 0.25 μM | 69.7 ab | 0 d | 2.18 bcd | 1.37 a | 1.11 abcd |
| IAA 0.50 μM | 70.5 ab | 0 d | 2.49 abcd | 1.24 ab | 1.18 abcd |
| IAA 1.0 μM | 70.8 ab | 0 d | 2.59 abcd | 1.18 abc | 1.23 abcd |
| IAA 2.0 μM | 67.8 ab | 0 d | 2.71 abcd | 1.04 abc | 1.46 abc |
| IAA 4.0 μM | 65.3 ab | 0 d | 2.54 abcd | 0.87 bc | 1.39 abc |
| IBA 0.25 μM | 81.1 a | 0 d | 2.29 bcd | 1.12 abc | 1.53 ab |
| IBA 0.50 μM | 83.7 a | 0 d | 2.79 abcd | 1.24 ab | 1.47 abc |
| IBA 1.0 μM | 86.8 a | 0 d | 3.42 ab | 1.29 ab | 1.63 a |
| IBA 2.0 μM | 70.4 ab | 11.8 cd | 2.57 abcd | 0.91 bc | 1.22 abcd |
| IBA 4.0 μM | 69.7 ab | 24.8 c | 1.84 c | 0.89 bc | 1.09 bcd |
| NAA 0.25 μM | 66.1 ab | 47.5 b | 1.80 c | 0.84 bc | 1.08 bcd |
| NAA 0.50 μM | 68.2 ab | 63.7 ab | 2.61 abcd | 1.04 abc | 0.89 de |
| NAA 1.0 μM | 70.3 ab | 70.5 ab | 2.97 abcd | 1.12 abc | 0.00 f |
| NAA 2.0 μM | 71.8 ab | 73.8 ab | 3.71 a | 0.94 abc | 0.00 f |
| NAA 4.0 μM | 75.7 ab | 80.2 a | 3.34 abc | 0.73 c | 0.00 f |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-H.; Zebro, M.; Jang, D.-C.; Sim, J.-E.; Park, H.-K.; Kim, K.-Y.; Bae, H.-M.; Tilahun, S.; Park, S.-M. Optimization of Plant Growth Regulators for In Vitro Mass Propagation of a Disease-Free ‘Shine Muscat’ Grapevine Cultivar. Curr. Issues Mol. Biol. 2023, 45, 7721-7733. https://doi.org/10.3390/cimb45100487
Kim S-H, Zebro M, Jang D-C, Sim J-E, Park H-K, Kim K-Y, Bae H-M, Tilahun S, Park S-M. Optimization of Plant Growth Regulators for In Vitro Mass Propagation of a Disease-Free ‘Shine Muscat’ Grapevine Cultivar. Current Issues in Molecular Biology. 2023; 45(10):7721-7733. https://doi.org/10.3390/cimb45100487
Chicago/Turabian StyleKim, Si-Hong, Mewuleddeg Zebro, Dong-Cheol Jang, Jeong-Eun Sim, Han-Kyeol Park, Kyeong-Yeon Kim, Hyung-Min Bae, Shimeles Tilahun, and Sung-Min Park. 2023. "Optimization of Plant Growth Regulators for In Vitro Mass Propagation of a Disease-Free ‘Shine Muscat’ Grapevine Cultivar" Current Issues in Molecular Biology 45, no. 10: 7721-7733. https://doi.org/10.3390/cimb45100487
APA StyleKim, S.-H., Zebro, M., Jang, D.-C., Sim, J.-E., Park, H.-K., Kim, K.-Y., Bae, H.-M., Tilahun, S., & Park, S.-M. (2023). Optimization of Plant Growth Regulators for In Vitro Mass Propagation of a Disease-Free ‘Shine Muscat’ Grapevine Cultivar. Current Issues in Molecular Biology, 45(10), 7721-7733. https://doi.org/10.3390/cimb45100487







