Presynaptic Purinergic Modulation of the Rat Neuro-Muscular Transmission
Abstract
1. Introduction
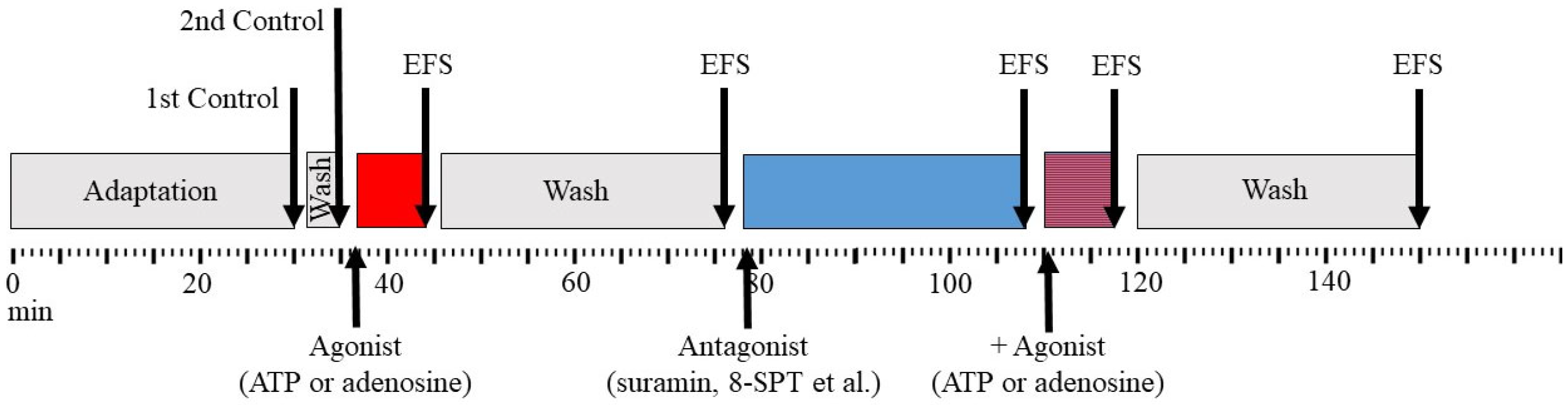
2. Materials and Methods
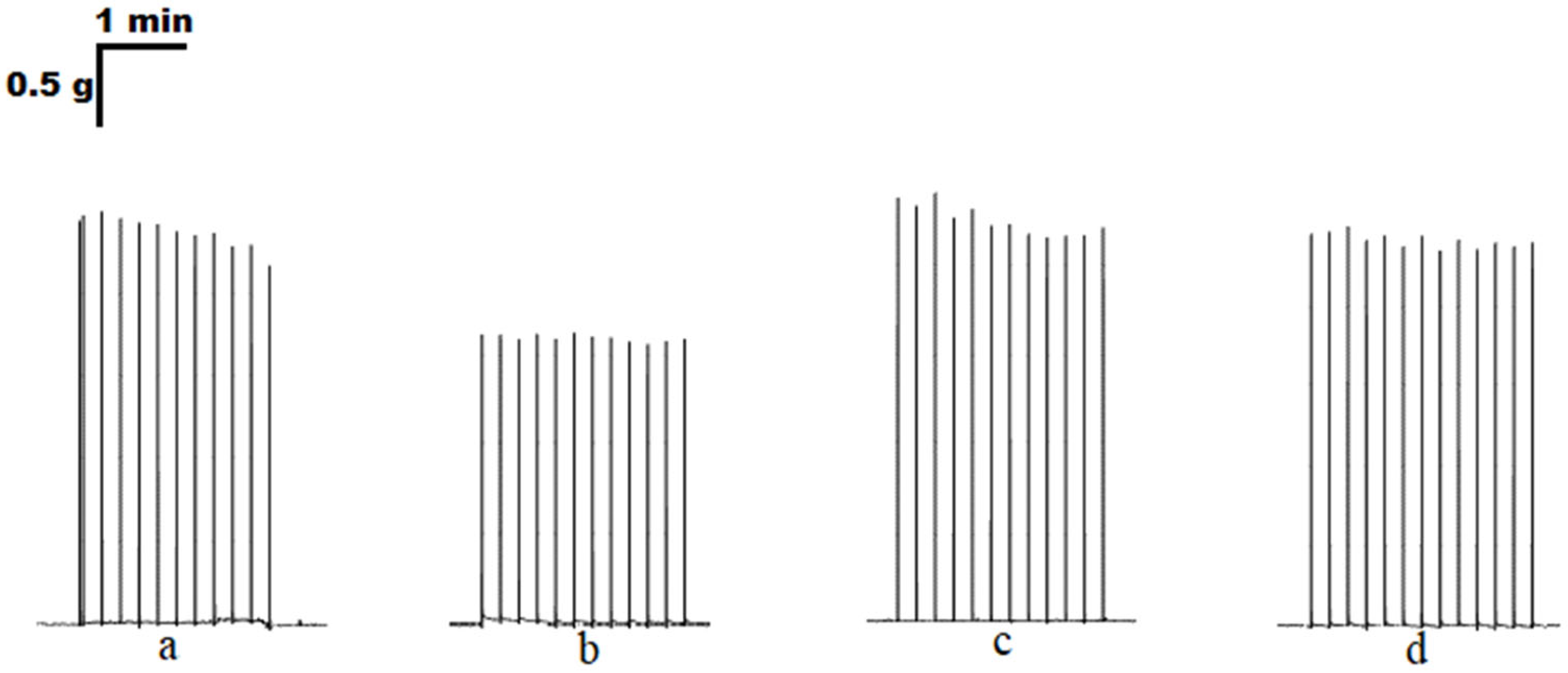
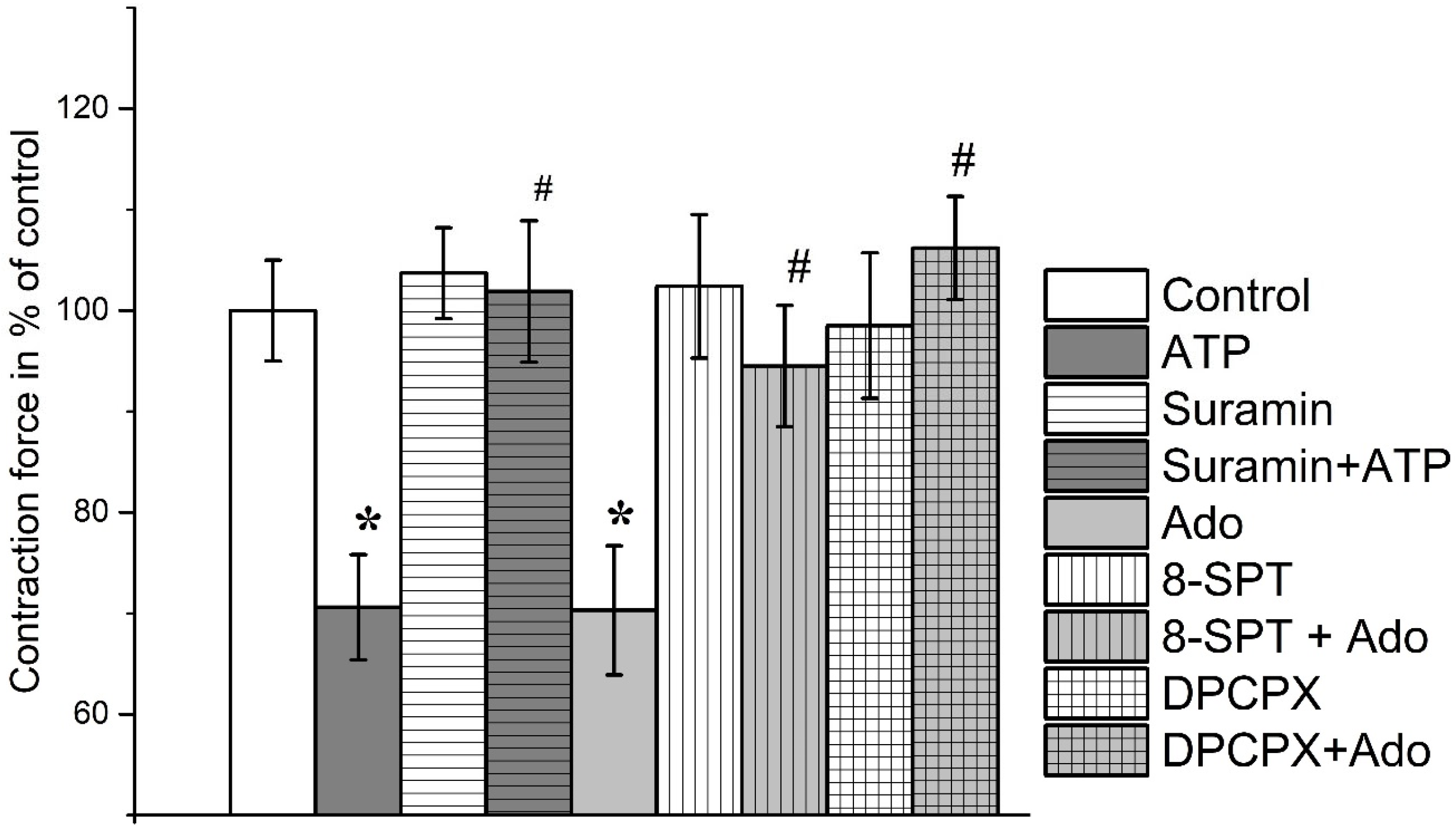
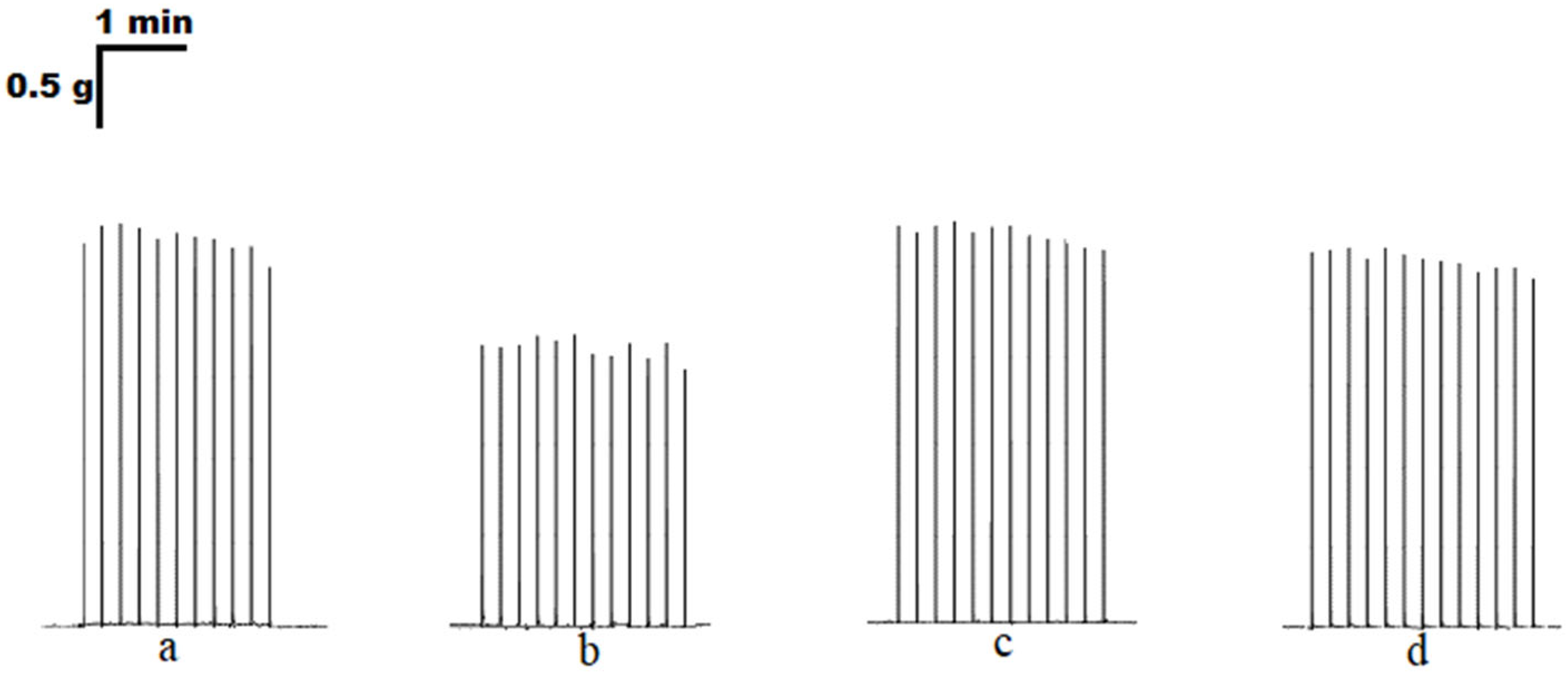
3. Results
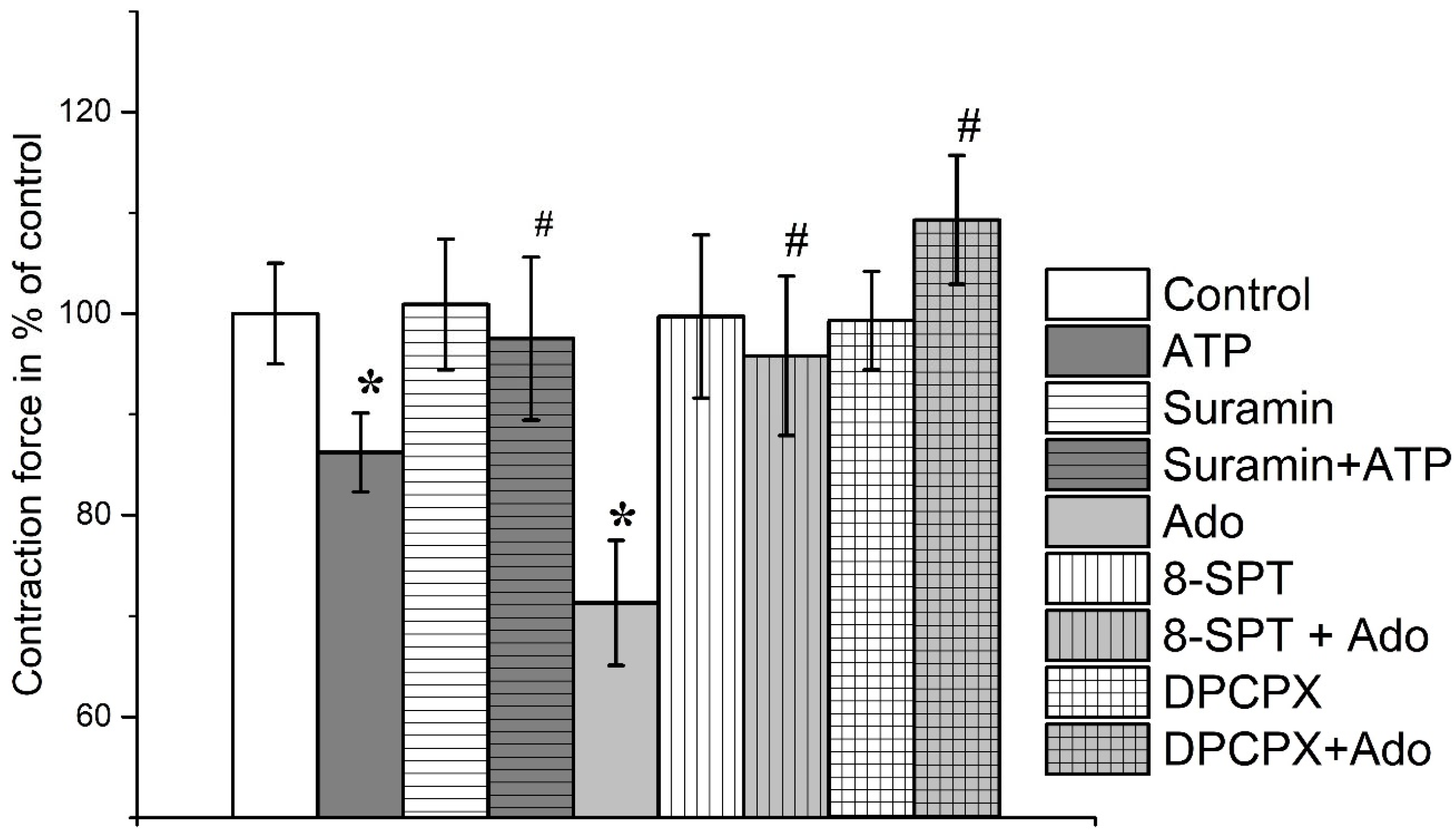
3.1. The Effect of Purines on the Contraction Parameters of Rat m. soleus and m. EDL
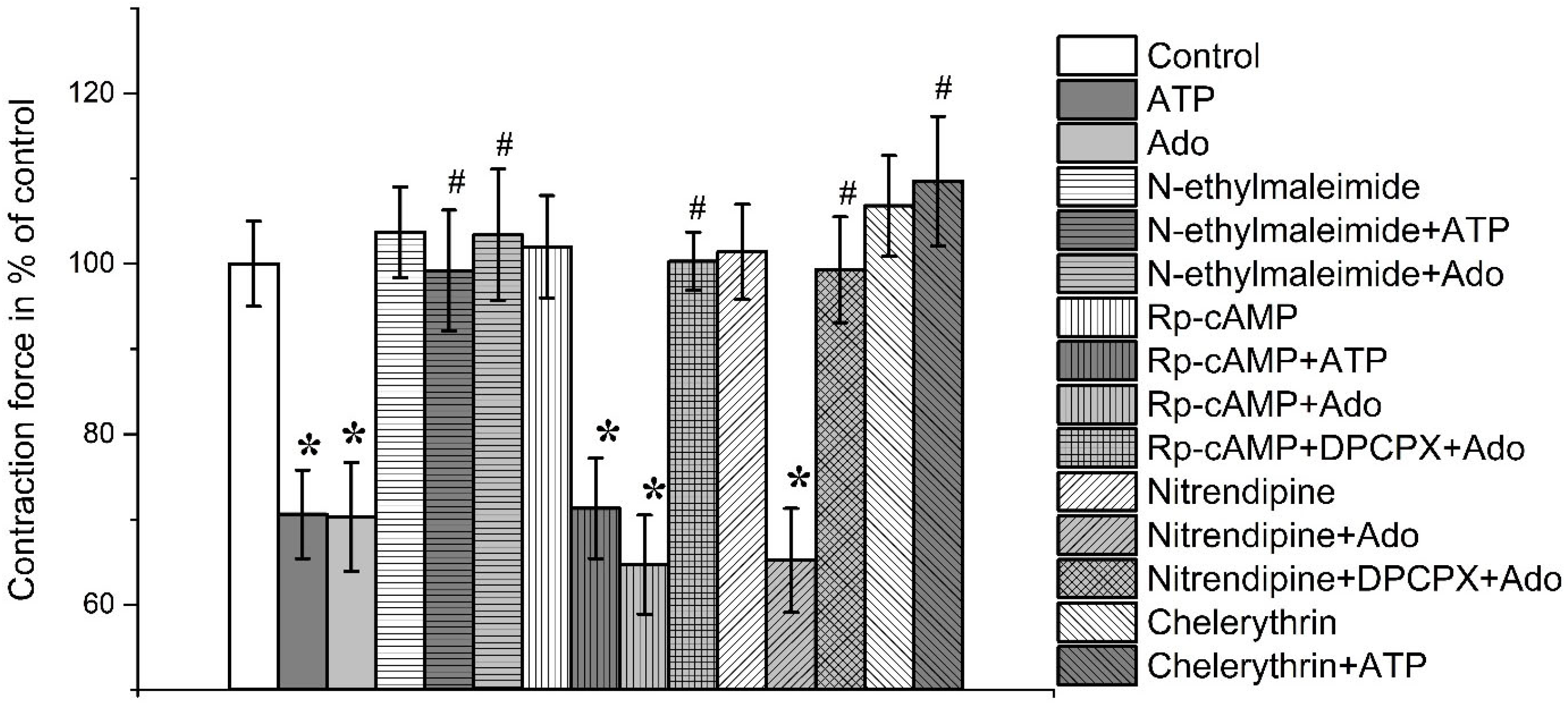
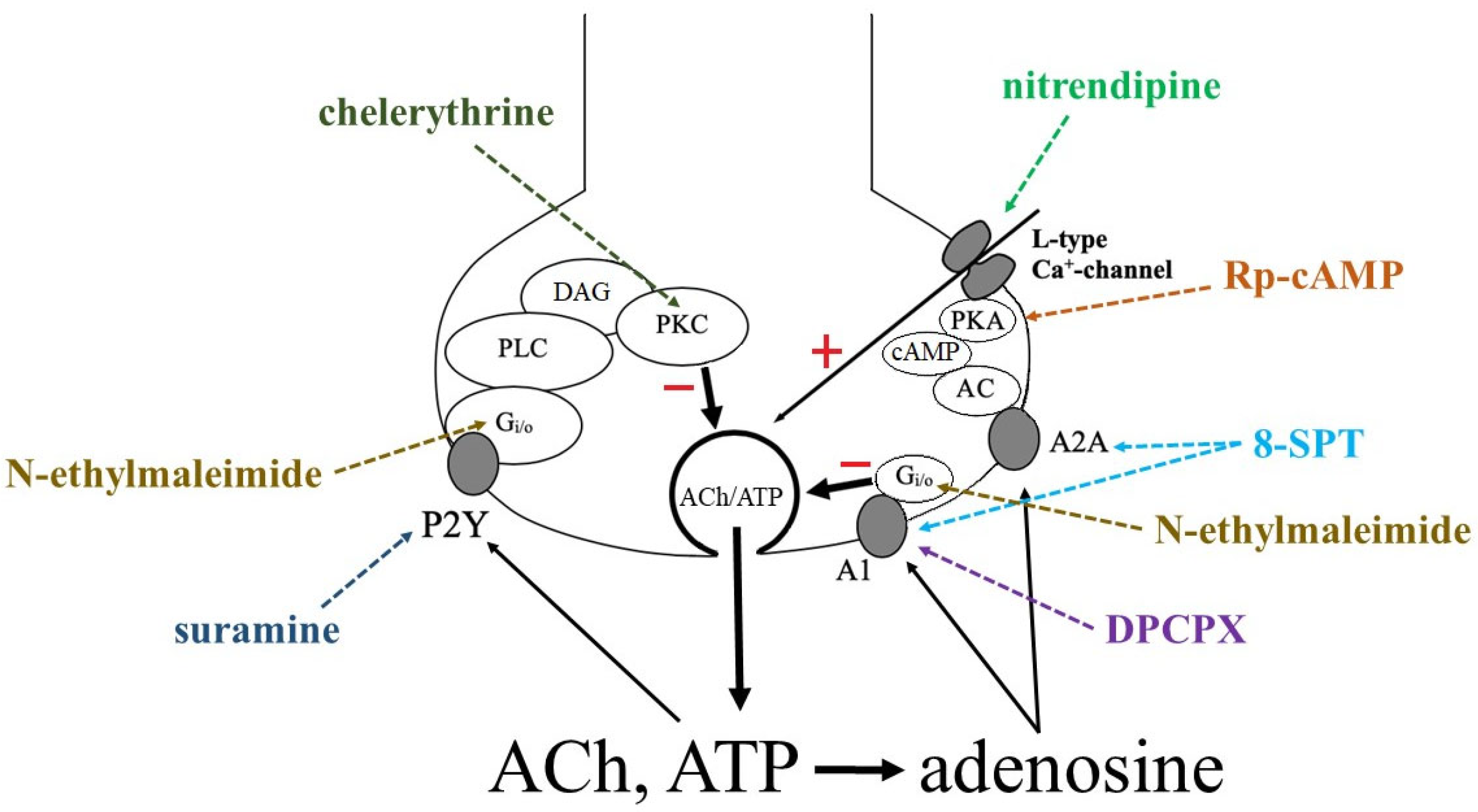
3.2. Identification of Intracellular Mechanisms Involved in the Effects of Adenosine and ATP
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 8-SPT | 8-(p-sulfophenyl)-theophylline |
| AC | adenylate cyclase. |
| ACH | acetylcholine |
| ATP | adenosine-5′-triphosphate |
| cAMP | 3′-5′-cyclic adenosine monophosphate, |
| DAG | diacylglycerol, |
| DPCPX | 1,3-dipropyl-8-cyclopentylxanthine |
| EFS | electrical field stimulation |
| m. EDL | extensor digitorum longus; |
| PKA | protein kinase A, |
| PKC | protein kinase C, |
| PLC | phospholipase C, |
| Rp-cAMPS | 6-(6-aminopurin-9-yl)-2-hydroxy-2-sulfanylidene-4a,6,7,7a-tetrahydro-4H-furo[3,2-d][1,3,2]dioxaphosphinin-7-ol;N,N-diethylethanamine |
References
- Silinsky, E.M.; Redman, R.S. Synchronous release of ATP and neurotransmitter within milliseconds of a motor nerve impulse in the frog. J. Physiol. 1996, 492, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Giniatullin, R.A.; Sokolova, E.M. Modulating role of ATP in the neuromuscular junction. Russ. J. Physiol. 1998, 84, 1132–1138. [Google Scholar]
- Ziganshin, A.U.; Kamaliev, R.R.; Grishin, S.N.; Ziganshina, L.E.; Zefirov, A.L.; Burnstock, G. The influence of hypothermia on P2 receptor-mediated responses of frog skeletal muscle. Eur. J. Pharmacol. 2005, 509, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Giniatullin, R.A.; Sokolova, E.M. ATP and adenosine inhibit transmitter release at the frog neuromuscular junction through distinct presynaptic receptors. Br. J. Pharmacol. 1998, 124, 839–844. [Google Scholar] [CrossRef]
- Galkin, A.V.; Giniatullin, R.A.; Mukhtarov, M.R.; Svandova, I.; Grishin, S.N.; Vyskocil, F. ATP but not adenosine inhibits nonquantal acetylcholine release at the mouse neuromuscular junction. Eur. J. Neurosci. 2001, 13, 2047–2053. [Google Scholar] [CrossRef]
- Alexander, S.P.H.; Christopoulos, A.; Davenport, A.P.; Kelly, E.; Mathie, A.; Peters, J.A.; Veale, E.L.; Armstrong, J.F.; Faccenda, E.; Harding, S.D.; et al. The concise guide to pharmacology 2019/20: G protein-coupled receptors. Br. J. Pharmacol. 2019, 176, 21–141. [Google Scholar] [CrossRef]
- Alexander, S.P.H.; Mathie, A.; Peters, J.A.; Veale, E.L.; Striessnig, J.; Kelly, E.; Armstrong, J.F.; Faccenda, E.; Harding, S.D.; Pawson, A.J.; et al. The concise guide to pharmacology 2019/20: Ion Channels. Br. J. Pharmacol. 2019, 176, 142–228. [Google Scholar] [CrossRef]
- Ribeiro, J.A.; Sebastião, A.M. On the role, inactivation and origin of endogenous adenosine at the frog neuromuscular junction. J. Physiol. 1987, 384, 571–585. [Google Scholar] [CrossRef]
- Sokolova, E.; Grishin, S.; Shakirzyanova, A.; Talantova, M.; Giniatullin, R. Distinct receptors and different transduction mechanisms for ATP and adenosine at the frog motor nerve endings. Eur. J. Neurosci. 2003, 18, 1254–1264. [Google Scholar] [CrossRef]
- Fu, W.M. Regulatory role of ATP at developing neuromuscular junctions. Prog. Neurobiol. 1995, 47, 31–44. [Google Scholar] [CrossRef]
- Salgado, A.I.; Cunha, R.A.; Ribeiro, J.A. Facilitation by P(2) receptor activation of acetylcholine release from rat motor nerve terminals: Interaction with presynaptic nicotinic receptors. Brain Res. 2000, 877, 245–250. [Google Scholar] [CrossRef]
- González Sanabria, J.; Hurtado Paso, M.; Frontera, T.; Losavio, A. Effect of endogenous purines on electrically evoked ACh release at the mouse neuromuscular junction. J. Neurosci. Res. 2022, 100, 1933–1950. [Google Scholar] [CrossRef] [PubMed]
- Ziganshin, A.U.; Khairullin, A.E.; Teplov, A.Y.; Gabdrakhmanov, A.I.; Ziganshina, L.E.; Hoyle, C.H.V.; Ziganshin, B.A.; Grishin, S.N. The effects of ATP on the contractions of rat and mouse fast skeletal muscle. Muscle Nerve. 2019, 59, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, M.S.; Wollmuth, L.P.; Hille, B. Modulation of Ca2+ channels by PTX-sensitive G-proteins is blocked by N-ethylmaleimide in rat sympathetic neurons. J. Neurosci. 1994, 14, 7109–7116. [Google Scholar] [CrossRef]
- Sousa-Soares, C.; Noronha-Matos, J.B.; Correia-de-Sá, P. Purinergic Tuning of the Tripartite Neuromuscular Synapse. Mol. Neurobiol. 2023, 60, 4084–4104. [Google Scholar] [CrossRef] [PubMed]
- Jimsheleishvili, S.; Marwaha, K.; Sherman, A.I. Physiology, Neuromuscular Transmission. StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2019; Available online: https://pubmed.ncbi.nlm.nih.gov/31082177/ (accessed on 28 August 2023).
- Mukund, K.; Subramaniam, S. Skeletal muscle: A review of molecular structure and function, in health and disease. Wiley Interdiscip. Rev. Syst. Biol. Med. 2020, 12, 1462. [Google Scholar] [CrossRef] [PubMed]
- Pasnoor, M.; Dimachkie, M.M. Approach to Muscle and Neuromuscular Junction Disorders. Continuum 2019, 25, 1536–1563. [Google Scholar] [CrossRef]
- Ribeiro, J.A.; Cunha, R.A.; Correia-de-Sá, P.; Sebastião, A.M. Purinergic regulation of acetylcholine release. Prog. Brain. Res. 1996, 109, 231–241. [Google Scholar] [CrossRef]
- Vizi, E.S.; Nitahara, K.; Sato, K.; Sperlágh, B. Stimulation-dependent release, breakdown, and action of endogenous ATP in mouse hemidiaphragm preparation: The possible role of ATP in neuromuscular transmission. J. Auton. Nerv. Syst. 2000, 81, 278–284. [Google Scholar] [CrossRef]
- Fryer, M.W.; Stephenson, D.G. Total and sarcoplasmic reticulum calcium contents of skinned fibres from rat skeletal muscle. J. Physiol. 1996, 493, 357–370. [Google Scholar] [CrossRef]
- Macdonald, W.A.; Stephenson, D.G. Effect of ADP on slow-twitch muscle fibres of the rat: Implications for muscle fatigue. J. Physiol. 2006, 573, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Ziganshin, A.U.; Khairullin, A.E.; Hoyle, C.H.V.; Grishin, S.N. Modulatory Roles of ATP and Adenosine in Cholinergic Neuromuscular Transmission. Int. J. Mol. Sci. 2020, 21, 6423. [Google Scholar] [CrossRef] [PubMed]
- Fredholm, B.B.; Ijzerman, A.P.; Jacobson, K.A.; Linden, J.; Müller, C.E. International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and classifcation of adenosine receptors—An update. Pharmacol. Rev. 2011, 63, 1–34. [Google Scholar] [CrossRef]
- Sebastião, A.M.; Cristóvão-Ferreira, S.; Ribeiro, J.A. Downstream Pathways of Adenosine. In Adenosine; Masino, S., Boison, D., Eds.; Springer: New York, NY, USA, 2013. [Google Scholar] [CrossRef]
- Jacobson, K.A.; Gao, Z.G. Adenosine receptors as therapeutic targets. Nat. Rev. Drug. Discov. 2006, 5, 247–264. [Google Scholar] [CrossRef] [PubMed]
- Correia-de-Sá, P.; Sebastião, A.M.; Ribeiro, J.A. Inhibitory and excitatory effects of adenosine receptor agonists on evoked transmitter release from phrenic nerve ending of the rat. Br. J. Pharmacol. 1991, 103, 1614–1620. [Google Scholar] [CrossRef] [PubMed]
- Barros-Barbosa, A.R.; Ferreirinha, F.; Oliveira, Â.; Mendes, M.; Lobo, M.G.; Santos, A.; Rangel, R.; Pelletier, J.; Sévigny, J.; Cordeiro, J.M.; et al. Adenosine A2A receptor and ecto-5’-nucleotidase/CD73 are upregulated in hippocampal astrocytes of human patients with mesial temporal lobe epilepsy (MTLE). Purinergic Signal. 2016, 12, 719–734. [Google Scholar] [CrossRef]
- Vieira, C.; Magalhães-Cardoso, M.T.; Ferreirinha, F.; Silva, I.; Dias, A.S.; Pelletier, J.; Sévigny, J.; Correia-de-Sá, P. Feed-forward inhibition of CD73 and upregulation of adenosine deaminase contribute to the loss of adenosine neuromodulation in postinfammatory ileitis. Mediat. Infamm. 2014, 2014, 254640. [Google Scholar] [CrossRef]
- Augusto, E.; Matos, M.; Sévigny, J.; El-Tayeb, A.; Bynoe, M.S.; Müller, C.E.; Cunha, R.A.; Chen, J.F. Ecto-5’-nucleotidase (CD73)- mediated formation of adenosine is critical for the striatal adenosine A2A receptor functions. J. Neurosci. 2013, 33, 11390–11399. [Google Scholar] [CrossRef]
- Duarte-Araújo, M.; Nascimento, C.; Timóteo, M.A.; Magalhães-Cardoso, M.T.; Correia-de-Sá, P. Dual efects of adenosine on acetylcholine release from myenteric motoneurons are mediated by junctional facilitatory A2A and extrajunctional inhibitory A1 receptors. Br. J. Pharmacol. 2004, 141, 925–934. [Google Scholar] [CrossRef][Green Version]
- Correia-de-Sá, P.; Timóteo, M.A.; Ribeiro, J.A. A(2A) adenosine receptor facilitation of neuromuscular transmission: Infuence of stimulus paradigm on calcium mobilization. J. Neurochem. 2000, 74, 2462–2469. [Google Scholar] [CrossRef]
- Noronha-Matos, J.B.; Morais, T.; Trigo, D.; Timóteo, M.A.; Magalhães Cardoso, M.T.; Oliveira, L.; Correia-de-Sá, P. Tetanic failure due to decreased endogenous adenosine A2A tonus operating neuronal Cav1 (L-type) infux in myasthenia gravis. J. Neurochem. 2011, 117, 797–811. [Google Scholar] [CrossRef]
- Oliveira, L.; Timóteo, M.A.; Correia-de-Sá, P. Tetanic depression is overcome by tonic adenosine A2A receptor facilitation of L-type Ca2+ infux into rat motor nerve terminals. J. Physiol. 2004, 560, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Correia-de-Sá, P.; Ribeiro, J.A. Evidence that the presynaptic A2A-adenosine receptor of the rat motor nerve endings is positively coupled to adenylate cyclase. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1994, 350, 514–522. [Google Scholar] [CrossRef]
- Correia-de-Sá, P.; Ribeiro, J.A. Facilitation of [3H]-ACh release by forskolin depends on A2-adenosine receptor activation. Neurosci. Lett. 1993, 151, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.; Correia-de-Sá, P. Protein kinase A and Cav1 (L-type) channels are common targets to facilitatory adenosine A2A and muscarinic M1 receptors on rat motoneurons. Neurosignals 2005, 14, 262–272. [Google Scholar] [CrossRef]
- Tarasova, E.O.; Miteva, A.S.; Gaidukov, A.E.; Balezina, O.P. The role of adenosine receptors and L-type calcium channels in the regulation of the mediator secretion in mouse motor synapses. Biochem. Suppl. Ser. A Membr. Cell. Biol. 2015, 9, 318–328. [Google Scholar] [CrossRef]
- Oliveira, L.; Timóteo, M.A.; Correia-de-Sá, P. Modulation by adenosine of both muscarinic M1-facilitation and M2-inhibition of [3H]-acetylcholine release from the rat motor nerve terminals. Eur. J. Neurosci. 2002, 15, 1728–1736. [Google Scholar] [CrossRef] [PubMed]
- Correia-de-Sá, P.; Timóteo, M.A.; Ribeiro, J.A. Presynaptic A1 inhibitory/A2A facilitatory adenosine receptor activation balance depends on motor nerve stimulation paradigm at the rat hemidiaphragm. J. Neurophysiol. 1996, 76, 3910–3919. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khairullin, A.E.; Grishin, S.N.; Ziganshin, A.U. Presynaptic Purinergic Modulation of the Rat Neuro-Muscular Transmission. Curr. Issues Mol. Biol. 2023, 45, 8492-8501. https://doi.org/10.3390/cimb45100535
Khairullin AE, Grishin SN, Ziganshin AU. Presynaptic Purinergic Modulation of the Rat Neuro-Muscular Transmission. Current Issues in Molecular Biology. 2023; 45(10):8492-8501. https://doi.org/10.3390/cimb45100535
Chicago/Turabian StyleKhairullin, Adel E., Sergey N. Grishin, and Ayrat U. Ziganshin. 2023. "Presynaptic Purinergic Modulation of the Rat Neuro-Muscular Transmission" Current Issues in Molecular Biology 45, no. 10: 8492-8501. https://doi.org/10.3390/cimb45100535
APA StyleKhairullin, A. E., Grishin, S. N., & Ziganshin, A. U. (2023). Presynaptic Purinergic Modulation of the Rat Neuro-Muscular Transmission. Current Issues in Molecular Biology, 45(10), 8492-8501. https://doi.org/10.3390/cimb45100535





