Verbascoside Inhibits/Repairs the Damage of LPS-Induced Inflammation by Regulating Apoptosis, Oxidative Stress, and Bone Remodeling
Abstract
:1. Introduction
2. Material and Methods
2.1. Cell Culture and Viability
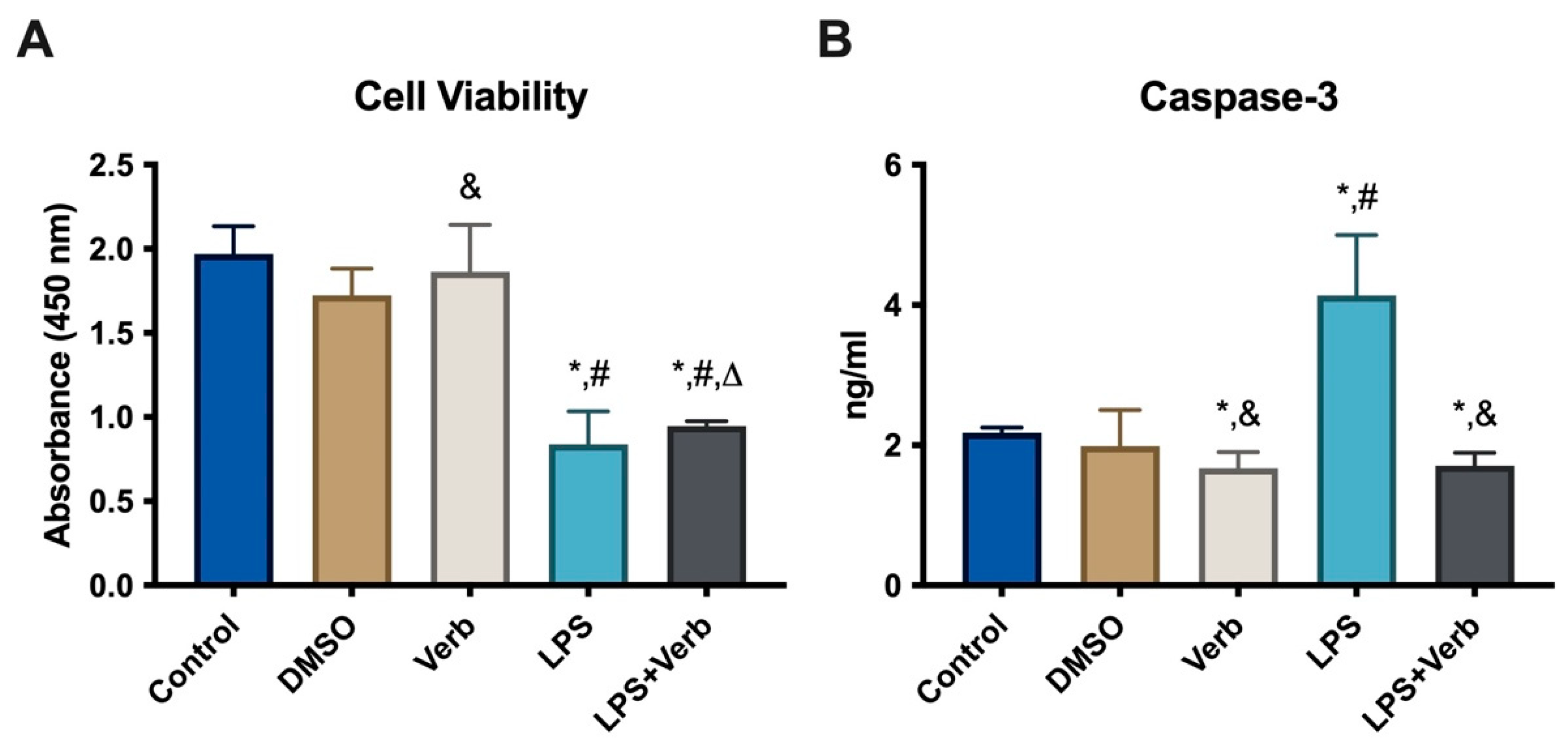
2.2. CAT, SOD, GSH, NOX and Caspase-3 Measurements
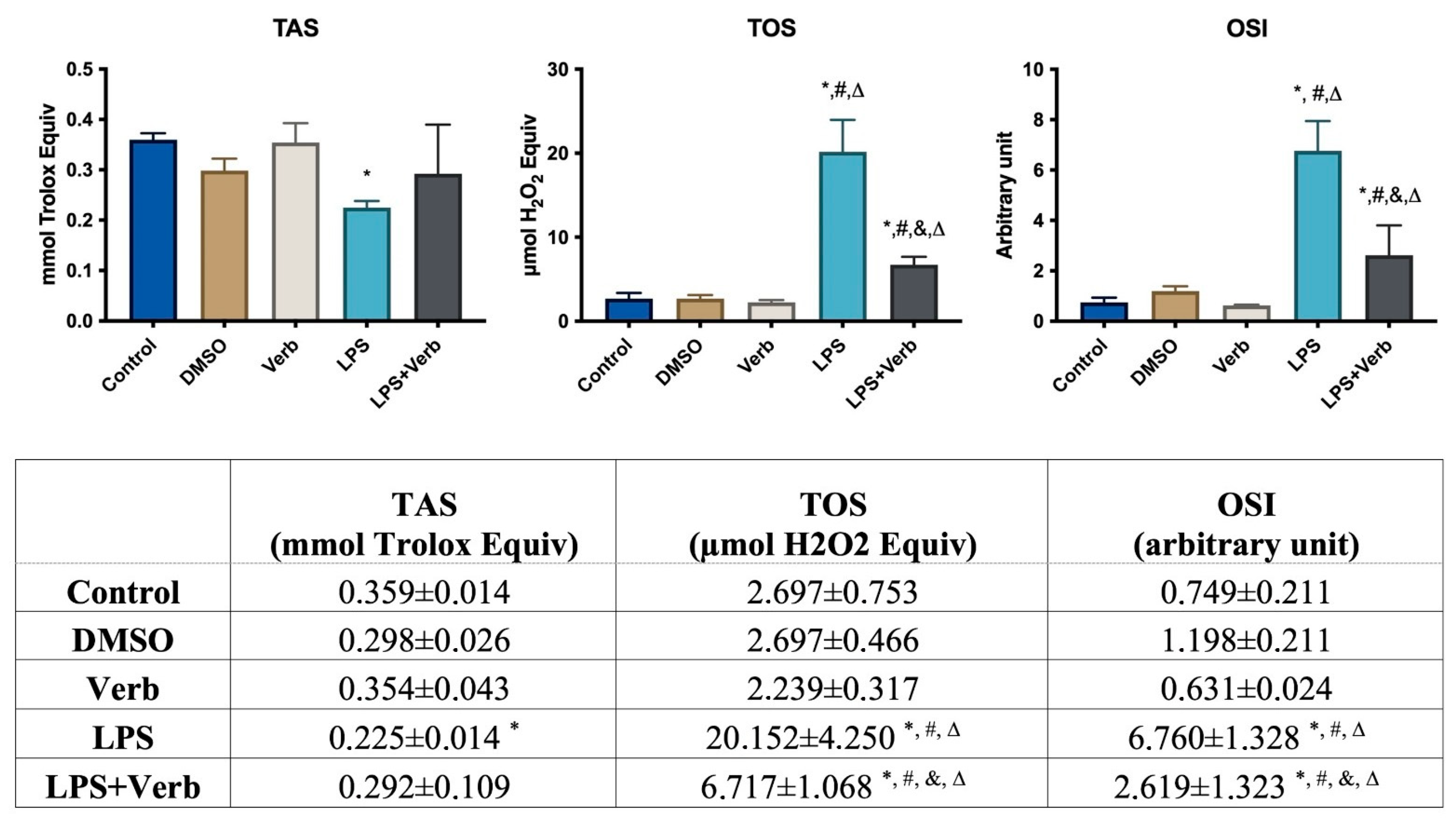
2.3. Determination of the Total Antioxidant Status, Total Oxidant Status, and Oxidative Stress Index
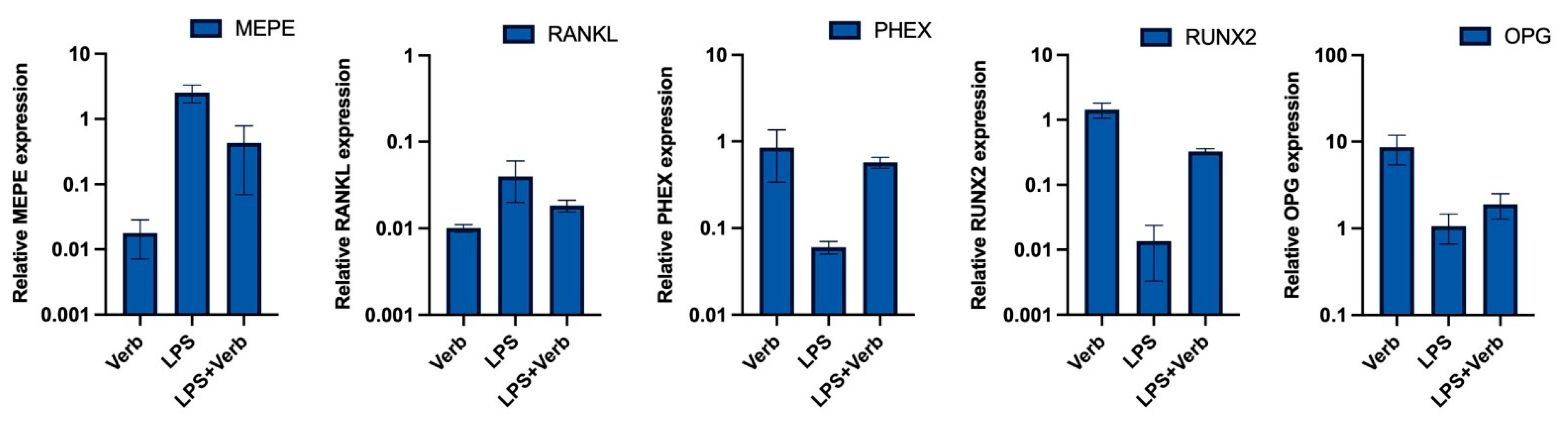
2.4. RNA Isolation, cDNA Synthesis, and Quantitative Real Time Polymerase Chain Reaction (qRT-PCR)
2.5. Statistical Analysis
3. Results
3.1. The Effects of Verbascoside Cell Viability in MLO-Y4 Cells
3.2. Verbascoside Regulated the Activity of Oxidant and Antioxidant
3.3. Verbascoside Regulates the Balance of Mineralization and Demineralization via PHEX, RUNX2, OPG, RANKL, MEPE Genes
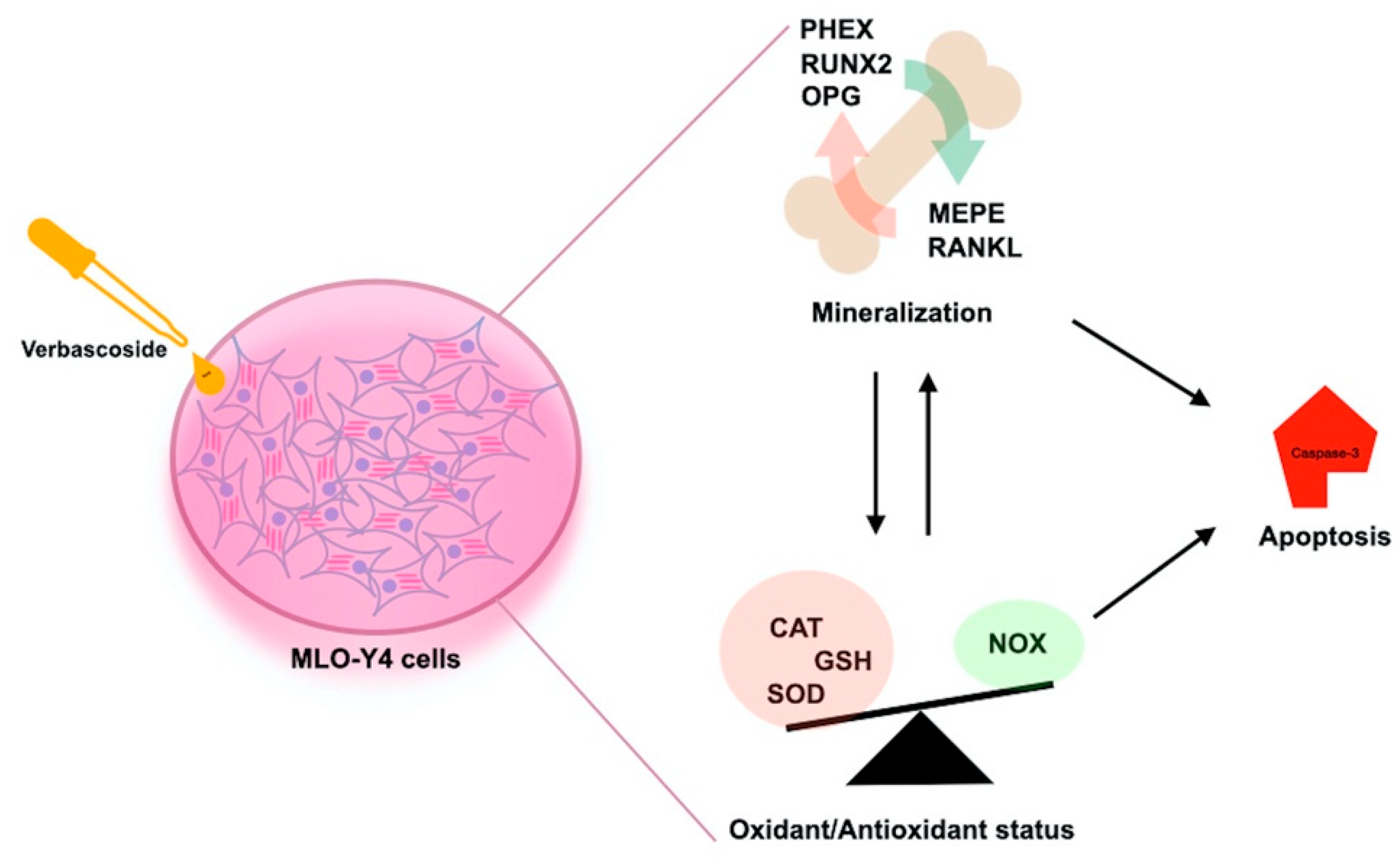
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Compton, J.T.; Lee, F.Y. A review of osteocyte function and the emerging importance of sclerostin. J. Bone Joint. Surg Am. 2014, 96, 1659–1668. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, C.; Ferretti, M. The Osteocyte: From “Prisoner” to “Orchestrator”. J. Funct. Morphol. Kinesiol. 2021, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Bonewald, L.F. The amazing osteocyte. J. Bone Miner. Res. 2011, 26, 229–238. [Google Scholar] [CrossRef]
- Florencio-Silva, R.; da Silva Sasso, G.R.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. BioMed Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef]
- Metzger, C.E.; Narayanan, S.A. The Role of Osteocytes in Inflammatory Bone Loss. Front. Endocrinol. 2019, 10, 285. [Google Scholar] [CrossRef] [PubMed]
- Pathak, J.L.; Bakker, A.D.; Luyten, F.P.; Verschueren, P.; Lems, W.F.; Klein-Nulend, J.; Bravenboer, N. Systemic Inflammation Affects Human Osteocyte-Specific Protein and Cytokine Expression. Calcif. Tissue Int. 2016, 98, 596–608. [Google Scholar] [CrossRef]
- Badran, Z.; Struillou, X.; Verner, C.; Clee, T.; Rakic, M.; Martinez, M.C.; Soueidan, A. Periodontitis as a risk factor for systemic disease: Are microparticles the missing link? Med. Hypotheses 2015, 84, 555–556. [Google Scholar] [CrossRef]
- Denis, G.V.; Sebastiani, P.; Bertrand, K.A.; Strissel, K.J.; Tran, A.H.; Slama, J.; Medina, N.D.; Andrieu, G.; Palmer, J.R. Inflammatory signatures distinguish metabolic health in African American women with obesity. PLoS ONE 2018, 13, e0196755. [Google Scholar] [CrossRef]
- Tanaka, K.-I.; Yamaguchi, T.; Kanazawa, I.; Sugimoto, T. Effects of high glucose and advanced glycation end products on the expressions of sclerostin and RANKL as well as apoptosis in osteocyte-like MLO-Y4-A2 cells. Biochem. Biophys. Res. Commun. 2015, 461, 193–199. [Google Scholar] [CrossRef] [PubMed]
- van Staa, T.P.; Geusens, P.; Bijlsma, J.W.J.; Leufkens, H.G.M.; Cooper, C. Clinical assessment of the long-term risk of fracture in patients with rheumatoid arthritis. Arthritis Rheum. 2006, 54, 3104–3112. [Google Scholar] [CrossRef] [PubMed]
- Ogdie, A.; Harter, L.; Shin, D.; Baker, J.; Takeshita, J.; Choi, H.K.; Love, T.J.; Gelfand, J.M. The risk of fracture among patients with psoriatic arthritis and psoriasis: A population-based study. Ann. Rheum. Dis. 2017, 76, 882–885. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Windle, J.J.; Koop, B.A.; Mundy, G.R.; Bonewald, L.F. Establishment of an osteocyte-like cell line, MLO-Y4. J. Bone Miner. Res. 1997, 12, 2014–2023. [Google Scholar] [CrossRef]
- Zhou, M.; Li, S.; Pathak, J.L. Pro-inflammatory Cytokines and Osteocytes. Curr. Osteoporos. Rep. 2019, 17, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Sindhu, S.; Akhter, N.; Wilson, A.; Thomas, R.; Arefanian, H.; Al Madhoun, A.; Al-Mulla, F.; Ahmad, R. MIP-1α Expression Induced by Co-Stimulation of Human Monocytic Cells with Palmitate and TNF-α Involves the TLR4-IRF3 Pathway and Is Amplified by Oxidative Stress. Cells 2020, 9, 1799. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, R.N.; Bakker, A.D.; Everts, V.; Klein-Nulend, J. Inhibition of osteoclastogenesis by mechanically loaded osteocytes: Involvement of MEPE. Calcif. Tissue Int. 2010, 87, 461–468. [Google Scholar] [CrossRef]
- Zhang, C.; Bakker, A.D.; Klein-Nulend, J.; Bravenboer, N. Studies on Osteocytes in Their 3D Native Matrix Versus 2D In Vitro Models. Curr. Osteoporos. Rep. 2019, 17, 207–216. [Google Scholar] [CrossRef]
- Robling, A.G.; Bonewald, L.F. The Osteocyte: New Insights. Annu. Rev. Physiol. 2020, 82, 485–506. [Google Scholar] [CrossRef]
- Guo, D.; Keightley, A.; Guthrie, J.; Veno, P.A.; Harris, S.E.; Bonewald, L.F. Identification of osteocyte-selective proteins. Proteomics 2010, 10, 3688–3698. [Google Scholar] [CrossRef]
- Patalong-Wójcik, M.; Golara, A.; Zając, K.; Sokołowska, A.; Kozłowski, M.; Tołoczko-Grabarek, A.; Krzyścin, M.; Brodowska, A.; Janiec, A.; Myszka, A.; et al. Influence of Muscle Mass and Strength on Bone Mineralisation with Consideration of Sclerostin Concentration. Biomedicines 2023, 11, 1574. [Google Scholar] [CrossRef]
- Chin, K.-Y.; Ng, B.N.; Rostam, M.K.I.; Fadzil, N.F.D.M.; Raman, V.; Yunus, F.M.; Mark-Lee, W.F.; Chong, Y.Y.; Qian, J.; Zhang, Y.; et al. Effects of E’Jiao on Skeletal Mineralisation, Osteocyte and WNT Signalling Inhibitors in Ovariectomised Rats. Life 2023, 13, 570. [Google Scholar] [CrossRef]
- Fisher, L.W.; Fedarko, N.S. Six genes expressed in bones and teeth encode the current members of the SIBLING family of proteins. Connect. Tissue Res. 2003, 44 (Suppl. S1), 33–40. [Google Scholar] [CrossRef] [PubMed]
- Kao, R.S.; Abbott, M.J.; Louie, A.; O’Carroll, D.; Lu, W.; Nissenson, R. Constitutive protein kinase A activity in osteocytes and late osteoblasts produces an anabolic effect on bone. Bone 2013, 55, 277–287. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, B.; Liu, J.; Dong, Y.; Li, Y.; Li, N.; Zhao, X.; Snooks, H.; Hu, C.; Ma, X. Protective Effect of Acteoside on Ovariectomy-Induced Bone Loss in Mice. Int. J. Mol. Sci. 2019, 20, 2974. [Google Scholar] [CrossRef] [PubMed]
- Chiou, W.F.; Lin, L.C.; Chen, C.F. Acteoside protects endothelial cells against free radical-induced oxidative stress. J. Pharm. Pharmacol. 2004, 56, 743–748. [Google Scholar] [CrossRef]
- Pastore, S.; Potapovich, A.; Kostyuk, V.; Mariani, V.; Lulli, D.; De Luca, C.; Korkina, L. Plant polyphenols effectively protect HaCaT cells from ultraviolet C–triggered necrosis and suppress inflammatory chemokine expression. Ann. N. Y. Acad. Sci. 2009, 1171, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.-C.; Fan, Y.-G.; Liu, J.-R.; Zeng, Y.-R.; Yi, C.-Z.; Yan, L. Experimental study of directional differentiation of bone mesenchymal stem cells (BMSCs) to osteoblasts guided by serum containing cistanche deserticola. Zhongguo Gu Shang 2010, 23, 606–608. [Google Scholar] [PubMed]
- Li, T.M.; Huang, H.C.; Su, C.M.; Ho, T.Y.; Wu, C.M.; Chen, W.C.; Fong, Y.C.; Tang, C.H. Cistanche deserticola extract increases bone formation in osteoblasts. J. Pharm. Pharmacol. 2012, 64, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, Z.; Wang, W.; Yao, H.; Ma, X. Therapeutic Effect of Cistanoside A on Bone Metabolism of Ovariectomized Mice. Molecules 2017, 22, 197. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, K.S.; Yi, S.H.; Kook, S.H.; Lee, J.C. Acteoside suppresses RANKL-mediated osteoclastogenesis by inhibiting c-Fos induction and NF-kappaB pathway and attenuating ROS production. PLoS ONE 2013, 8, e80873. [Google Scholar]
- Bott, K.N.; Yumol, J.L.; Comelli, E.M.; Klentrou, P.; Peters, S.J.; Ward, W.E. Trabecular and cortical bone are unaltered in response to chronic lipopolysaccharide exposure via osmotic pumps in male and female CD-1 mice. PLoS ONE 2021, 16, e0243933. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, S.; Leng, S.X. Chronic Low-grade Inflammatory Phenotype (CLIP) and Senescent Immune Dysregulation. Clin. Ther. 2019, 41, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Droke, E.A.; Hager, K.A.; Lerner, M.R.; Lightfoot, S.A.; Stoecker, B.J.; Brackett, D.J.; Smith, B.J. Soy isoflavones avert chronic inflammation-induced bone loss and vascular disease. J. Inflamm. 2007, 4, 17. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.J.; Chongwatpol, P.; Rendina-Ruedy, E.; Stoecker, B.J.; Clarke, S.L.; Lucas, E. Implications of compromised zinc status on bone loss associated with chronic inflammation in C57BL/6 mice. J. Inflamm. Res. 2015, 8, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.J.; Gregoire, B.R.; Shen, C.-L. A High-Fat Diet Decreases Bone Mass in Growing Mice with Systemic Chronic Inflammation Induced by Low-Dose, Slow-Release Lipopolysaccharide Pellets. J. Nutr. 2017, 147, 1909–1916. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.; Lerner, M.; Bu, S.; Lucas, E.; Hanas, J.; Lightfoot, S.; Postier, R.; Bronze, M.; Brackett, D. Systemic bone loss and induction of coronary vessel disease in a rat model of chronic inflammation. Bone 2006, 38, 378–386. [Google Scholar] [CrossRef]
- Shen, C.-L.; Yeh, J.K.; Cao, J.J.; Tatum, O.L.; Dagda, R.Y.; Wang, J.-S. Green tea polyphenols mitigate bone loss of female rats in a chronic inflammation-induced bone loss model. J. Nutr. Biochem. 2010, 21, 968–974. [Google Scholar] [CrossRef]
- Shen, C.-L.; Yeh, J.K.; Samathanam, C.; Cao, J.J.; Stoecker, B.J.; Dagda, R.Y.; Chyu, M.-C.; Dunn, D.M.; Wang, J.-S. Green tea polyphenols attenuate deterioration of bone microarchitecture in female rats with systemic chronic inflammation. Osteoporos. Int. 2011, 22, 327–337. [Google Scholar] [CrossRef]
- Erel, O. A new automated colorimetric method for measuring total oxidant status. Clin. Biochem. 2005, 38, 1103–1111. [Google Scholar] [CrossRef]
- Alipieva, K.; Korkina, L.; Orhan, I.E.; Georgiev, M.I. Verbascoside—A review of its occurrence, (bio)synthesis and pharmacological significance. Biotechnol. Adv. 2014, 32, 1065–1076. [Google Scholar] [CrossRef]
- Cardinali, A.; Pati, S.; Minervini, F.; D’antuono, I.; Linsalata, V.; Lattanzio, V. Verbascoside, Isoverbascoside, and Their Derivatives Recovered from Olive Mill Wastewater as Possible Food Antioxidants. J. Agric. Food Chem. 2012, 60, 1822–1829. [Google Scholar] [CrossRef]
- Sendur, O.F.; Turan, Y.; Tastaban, E.; Serter, M. Antioxidant status in patients with osteoporosis: A controlled study. Joint Bone Spine 2009, 76, 514–518. [Google Scholar] [CrossRef]
- Ray, G.; Husain, S.A. Oxidants, antioxidants and carcinogenesis. Indian J. Exp. Biol. 2002, 40, 1213–1232. [Google Scholar]
- Fraser, J.; Helfrich, M.; Wallace, H.; Ralston, S. Hydrogen peroxide, but not superoxide, stimulates bone resorption in mouse calvariae. Bone 1996, 19, 223–226. [Google Scholar] [CrossRef]
- Bánhegyi, G.; Csala, M.; Szarka, A.; Varsányi, M.; Benedetti, A.; Mandl, J. Role of ascorbate in oxidative protein folding. BioFactors 2003, 17, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Schröder, K. NADPH oxidases in bone homeostasis and osteoporosis. Cell. Mol. Life Sci. 2014, 72, 25–38. [Google Scholar] [CrossRef]
- Gowen, L.C.; Petersen, D.N.; Mansolf, A.L.; Qi, H.; Stock, J.L.; Tkalcevic, G.T.; Simmons, H.A.; Crawford, D.T.; Chidsey-Frink, K.L.; Ke, H.Z.; et al. Targeted Disruption of the Osteoblast/Osteocyte Factor 45 Gene (OF45) Results in Increased Bone Formation and Bone Mass. J. Biol. Chem. 2003, 278, 1998–2007. [Google Scholar] [CrossRef]
- David, V.; Martin, A.; Hedge, A.-M.; Rowe, P.S.N. Matrix Extracellular Phosphoglycoprotein (MEPE) Is a New Bone Renal Hormone and Vascularization Modulator. Endocrinology 2009, 150, 4012–4023. [Google Scholar] [CrossRef]
- Donmez, B.O.; Karagur, E.R.; Donmez, A.C.; Choi, J.; Akkus, O. Calcium-dependent activation of PHEX, MEPE and DMP1 in osteocytes. Mol. Med. Rep. 2022, 26, 359. [Google Scholar] [CrossRef] [PubMed]
- Che, C.-T.; Wong, M.S.; Lam, C.W.K. Natural Products from Chinese Medicines with Potential Benefits to Bone Health. Molecules 2016, 21, 239. [Google Scholar] [CrossRef] [PubMed]

| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| RUNX2 | GGCGTCAAACAGCCTCTTCA | GCTCACGTCGCTCATCTTGC |
| OPG | CGAGTGATGAATGCGTGTACTG | CTTCGCACAGGGTGACATCTATT |
| RANKL | AGCGAAGACACAGAAGCACTAC | TTTATGGGAACCCGATGGGATG |
| PHEX | TACTGCCTGAAGCCAGAATG | CCACAGAAAGATTTACTTTGCTCA |
| MEPE | ACTGTTCCTCTTCAGTATGAC | TGATATTTCTGAGGAGGGTG |
| GAPDH | AGGTCGGTGTGAACGGATTTG | GGGGTCGTTGATGGCAACA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akyer, S.P.; Karagur, E.R.; Ata, M.T.; Toprak, E.K.; Donmez, A.C.; Donmez, B.O. Verbascoside Inhibits/Repairs the Damage of LPS-Induced Inflammation by Regulating Apoptosis, Oxidative Stress, and Bone Remodeling. Curr. Issues Mol. Biol. 2023, 45, 8755-8766. https://doi.org/10.3390/cimb45110550
Akyer SP, Karagur ER, Ata MT, Toprak EK, Donmez AC, Donmez BO. Verbascoside Inhibits/Repairs the Damage of LPS-Induced Inflammation by Regulating Apoptosis, Oxidative Stress, and Bone Remodeling. Current Issues in Molecular Biology. 2023; 45(11):8755-8766. https://doi.org/10.3390/cimb45110550
Chicago/Turabian StyleAkyer, Sahika Pinar, Ege Rıza Karagur, Melek Tunc Ata, Emine Kilic Toprak, Aysegul Cort Donmez, and Baris Ozgur Donmez. 2023. "Verbascoside Inhibits/Repairs the Damage of LPS-Induced Inflammation by Regulating Apoptosis, Oxidative Stress, and Bone Remodeling" Current Issues in Molecular Biology 45, no. 11: 8755-8766. https://doi.org/10.3390/cimb45110550
APA StyleAkyer, S. P., Karagur, E. R., Ata, M. T., Toprak, E. K., Donmez, A. C., & Donmez, B. O. (2023). Verbascoside Inhibits/Repairs the Damage of LPS-Induced Inflammation by Regulating Apoptosis, Oxidative Stress, and Bone Remodeling. Current Issues in Molecular Biology, 45(11), 8755-8766. https://doi.org/10.3390/cimb45110550






