Treatment following Triple-AAV Delivery in Mature Murine Model of Human CDH23-Associated Hearing Loss
Abstract
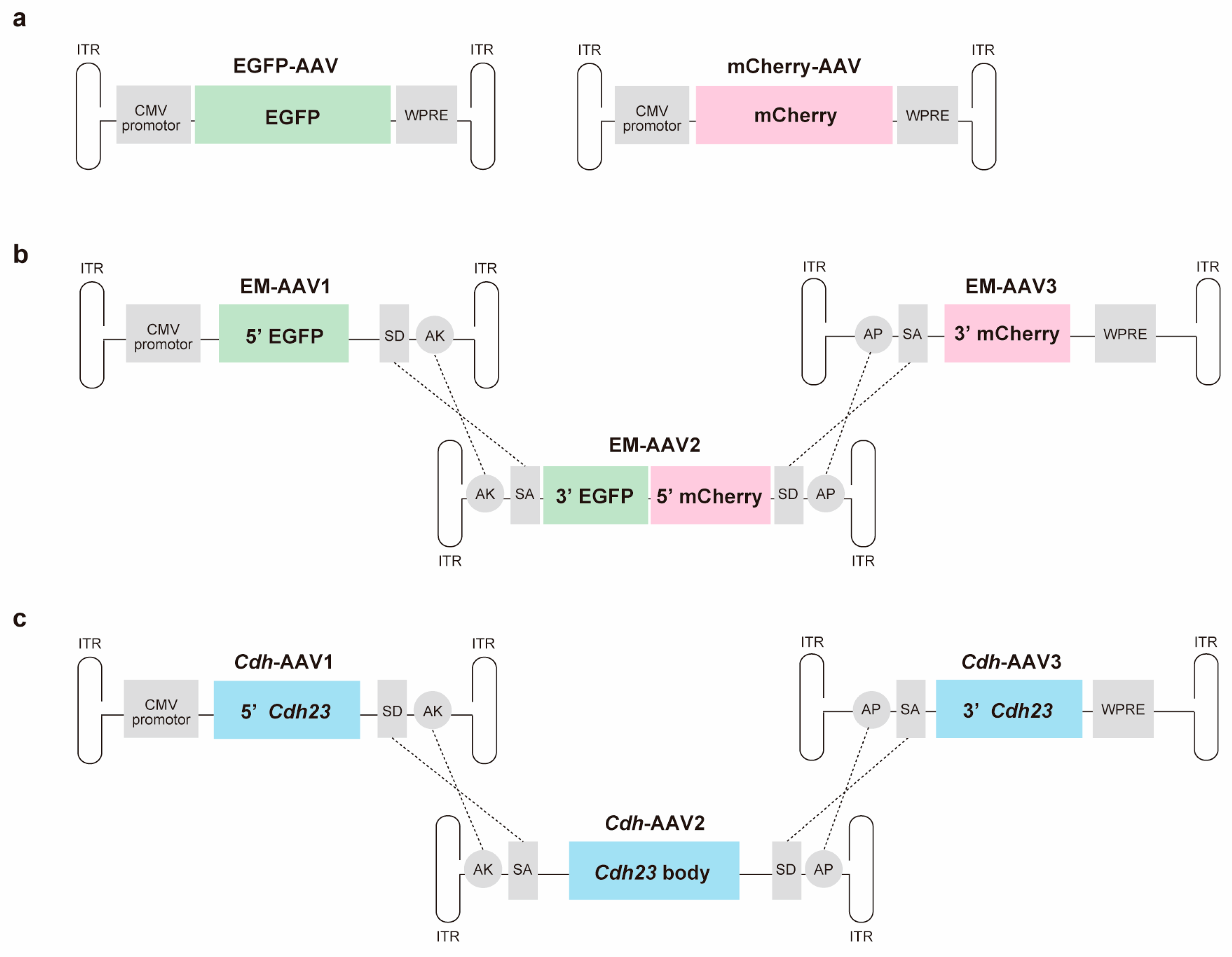
:1. Introduction
2. Materials and Methods
2.1. Virus Production
2.2. Animal Models
2.3. Animal Surgery
2.4. Auditory Testing
2.5. Immunohistochemistry, Cell Counts, and Transduction Efficiency Analysis
2.6. Statistical Analysis
3. Results
3.1. Injection of a Single AAV Vector Demonstrates Robust Transgene Expression in IHCs
3.2. Injection of Triple AAV Vector Demonstrates Limited IHC Transduction
3.3. Delivery of Triple Cdh23-AAV Vectors Does Not Alter Hearing Deterioration in Cdh23ahl/ahl Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Morton, C.C.; Nance, W.E. Newborn hearing screening—A silent revolution. N. Engl. J. Med. 2006, 354, 2151–2164. [Google Scholar] [CrossRef]
- Yoshimura, H.; Nishio, S.Y.; Usami, S.I. Milestones toward cochlear gene therapy for patients with hereditary hearing loss. Laryngoscope Investig. Otolaryngol. 2021, 6, 958–967. [Google Scholar] [CrossRef] [PubMed]
- Akil, O.; Dyka, F.; Calvet, C.; Emptoz, A.; Lahlou, G.; Nouaille, S.; Boutet de Monvel, J.; Hardelin, J.P.; Hauswirth, W.W.; Avan, P.; et al. Dual AAV-mediated gene therapy restores hearing in a DFNB9 mouse model. Proc. Natl. Acad. Sci. USA 2019, 116, 4496–4501. [Google Scholar] [CrossRef] [PubMed]
- Akil, O.; Seal, R.P.; Burke, K.; Wang, C.; Alemi, A.; During, M.; Edwards, R.H.; Lustig, L.R. Restoration of hearing in the VGLUT3 knockout mouse using virally mediated gene therapy. Neuron 2012, 75, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Al-Moyed, H.; Cepeda, A.P.; Jung, S.; Moser, T.; Kugler, S.; Reisinger, E. A dual-AAV approach restores fast exocytosis and partially rescues auditory function in deaf otoferlin knock-out mice. EMBO Mol. Med. 2019, 11, e9396. [Google Scholar] [CrossRef]
- Askew, C.; Rochat, C.; Pan, B.; Asai, Y.; Ahmed, H.; Child, E.; Schneider, B.L.; Aebischer, P.; Holt, J.R. Tmc gene therapy restores auditory function in deaf mice. Sci. Transl. Med. 2015, 7, 295ra108. [Google Scholar] [CrossRef]
- Ivanchenko, M.V.; Hathaway, D.M.; Klein, A.J.; Pan, B.; Strelkova, O.; De-la-Torre, P.; Wu, X.; Peters, C.W.; Mulhall, E.M.; Booth, K.T.; et al. Mini-PCDH15 gene therapy rescues hearing in a mouse model of Usher syndrome type 1F. Nat. Commun. 2023, 14, 2400. [Google Scholar] [CrossRef]
- Shibata, S.B.; Ranum, P.T.; Moteki, H.; Pan, B.; Goodwin, A.T.; Goodman, S.S.; Abbas, P.J.; Holt, J.R.; Smith, R.J.H. RNA Interference Prevents Autosomal-Dominant Hearing Loss. Am. J. Hum. Genet. 2016, 98, 1101–1113. [Google Scholar] [CrossRef]
- Yoshimura, H.; Shibata, S.B.; Ranum, P.T.; Moteki, H.; Smith, R.J.H. Targeted Allele Suppression Prevents Progressive Hearing Loss in the Mature Murine Model of Human TMC1 Deafness. Mol. Ther. 2019, 27, 681–690. [Google Scholar] [CrossRef]
- Akil, O. Dual and triple AAV delivery of large therapeutic gene sequences into the inner ear. Hear. Res. 2020, 394, 107912. [Google Scholar] [CrossRef]
- Omichi, R.; Shibata, S.B.; Morton, C.C.; Smith, R.J.H. Gene therapy for hearing loss. Hum. Mol. Genet. 2019, 28, R65–R79. [Google Scholar] [CrossRef] [PubMed]
- Omichi, R.; Yoshimura, H.; Shibata, S.B.; Vandenberghe, L.H.; Smith, R.J.H. Hair Cell Transduction Efficiency of Single- and Dual-AAV Serotypes in Adult Murine Cochleae. Mol. Ther. Methods Clin. Dev. 2020, 17, 1167–1177. [Google Scholar] [CrossRef] [PubMed]
- Maddalena, A.; Tornabene, P.; Tiberi, P.; Minopoli, R.; Manfredi, A.; Mutarelli, M.; Rossi, S.; Simonelli, F.; Naggert, J.K.; Cacchiarelli, D.; et al. Triple Vectors Expand AAV Transfer Capacity in the Retina. Mol. Ther. 2018, 26, 524–541. [Google Scholar] [CrossRef] [PubMed]
- Sloan-Heggen, C.M.; Bierer, A.O.; Shearer, A.E.; Kolbe, D.L.; Nishimura, C.J.; Frees, K.L.; Ephraim, S.S.; Shibata, S.B.; Booth, K.T.; Campbell, C.A.; et al. Comprehensive genetic testing in the clinical evaluation of 1119 patients with hearing loss. Hum. Genet. 2016, 135, 441–450. [Google Scholar] [CrossRef]
- Usami, S.I.; Nishio, S.Y. The genetic etiology of hearing loss in Japan revealed by the social health insurance-based genetic testing of 10K patients. Hum. Genet. 2021, 141, 665–681. [Google Scholar] [CrossRef]
- Miyagawa, M.; Nishio, S.Y.; Usami, S. Prevalence and clinical features of hearing loss patients with CDH23 mutations: A large cohort study. PLoS ONE 2012, 7, e40366. [Google Scholar] [CrossRef]
- Noben-Trauth, K.; Zheng, Q.Y.; Johnson, K.R. Association of cadherin 23 with polygenic inheritance and genetic modification of sensorineural hearing loss. Nat. Genet. 2003, 35, 21–23. [Google Scholar] [CrossRef]
- Kane, K.L.; Longo-Guess, C.M.; Gagnon, L.H.; Ding, D.; Salvi, R.J.; Johnson, K.R. Genetic background effects on age-related hearing loss associated with Cdh23 variants in mice. Hear. Res. 2012, 283, 80–88. [Google Scholar] [CrossRef]
- Chien, W.W. A CRISPR Way to Restore Hearing. N. Engl. J. Med. 2018, 378, 1255–1256. [Google Scholar] [CrossRef]
- von Ilberg, C.; Kiefer, J.; Tillein, J.; Pfenningdorff, T.; Hartmann, R.; Sturzebecher, E.; Klinke, R. Electric-acoustic stimulation of the auditory system. New technology for severe hearing loss. ORL J. Otorhinolaryngol. Relat. Spec. 1999, 61, 334–340. [Google Scholar] [CrossRef]
- von Ilberg, C.A.; Baumann, U.; Kiefer, J.; Tillein, J.; Adunka, O.F. Electric-acoustic stimulation of the auditory system: A review of the first decade. Audiol. Neurootol. 2011, 16 (Suppl. 2), 1–30. [Google Scholar] [CrossRef] [PubMed]
- Welch, C.; Dillon, M.T.; Pillsbury, H.C. Electric and Acoustic Stimulation in Cochlear Implant Recipients with Hearing Preservation. Semin. Hear. 2018, 39, 414–427. [Google Scholar] [CrossRef] [PubMed]
- Tarabichi, O.; Correa, T.; Kul, E.; Phillips, S.; Darkazanly, B.; Young, S.M., Jr.; Hansen, M.R. Development and evaluation of helper dependent adenoviral vectors for inner ear gene delivery. Hear. Res. 2023, 435, 108819. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshimura, H.; Yokota, S.; Takumi, Y. Treatment following Triple-AAV Delivery in Mature Murine Model of Human CDH23-Associated Hearing Loss. Curr. Issues Mol. Biol. 2023, 45, 9413-9421. https://doi.org/10.3390/cimb45120590
Yoshimura H, Yokota S, Takumi Y. Treatment following Triple-AAV Delivery in Mature Murine Model of Human CDH23-Associated Hearing Loss. Current Issues in Molecular Biology. 2023; 45(12):9413-9421. https://doi.org/10.3390/cimb45120590
Chicago/Turabian StyleYoshimura, Hidekane, Shu Yokota, and Yutaka Takumi. 2023. "Treatment following Triple-AAV Delivery in Mature Murine Model of Human CDH23-Associated Hearing Loss" Current Issues in Molecular Biology 45, no. 12: 9413-9421. https://doi.org/10.3390/cimb45120590




