Exploring Various Transfection Approaches and Their Applications in Studying the Regenerative Potential of Dental Pulp Stem Cells
Abstract
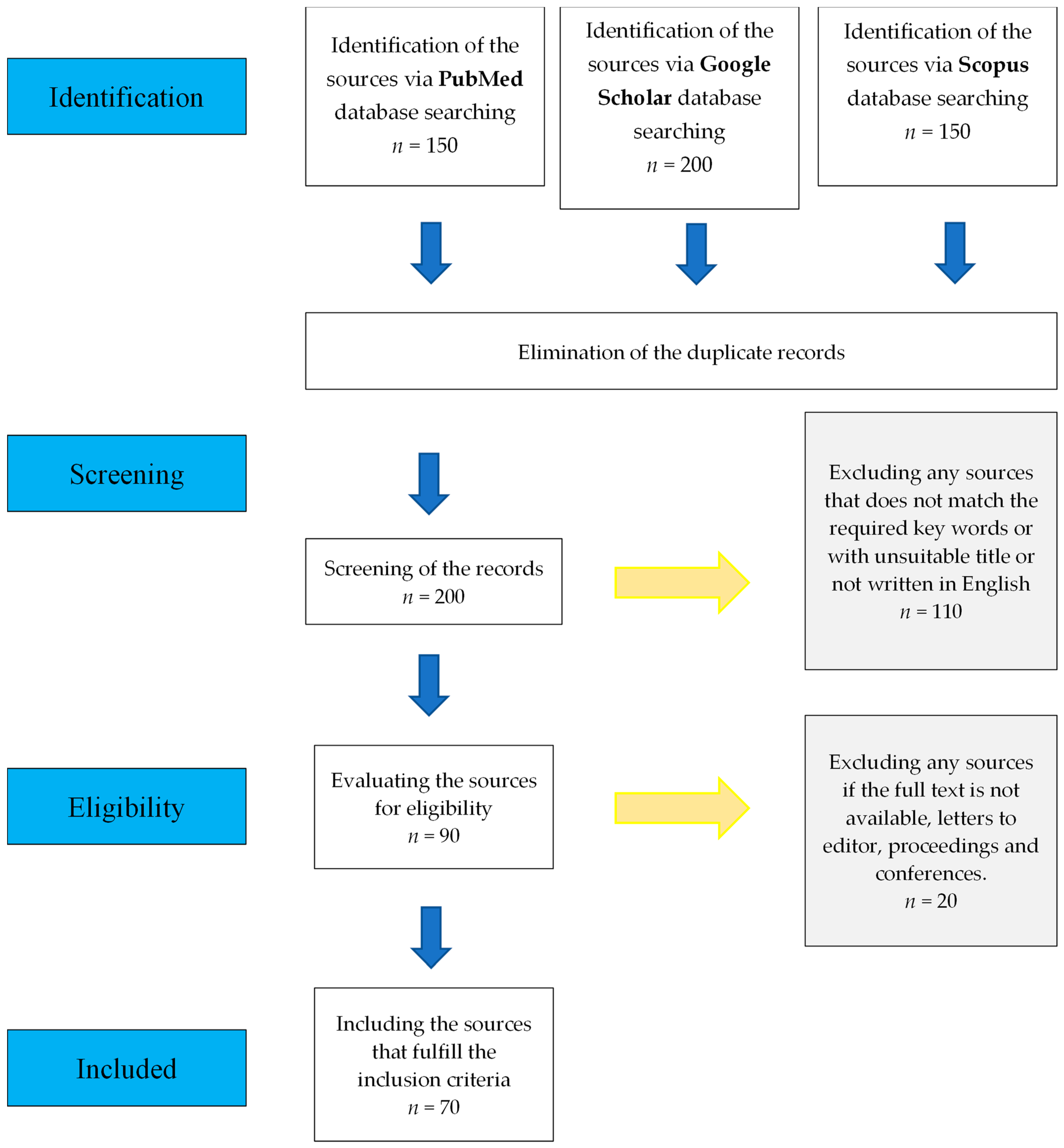
:1. Introduction
2. Methodology
3. Transfection Approaches
3.1. Viral Approach
3.2. Non-Viral Approach
3.2.1. Naked DNA Approach
3.2.2. Physical Approach
3.2.3. Chemical Approach
4. Transfection Outcomes
4.1. Stable Transfection
4.2. Transient Transfection
5. Uses of Transfection in Studying and Enhancing the Regenerative Capacity of DPSCs
5.1. Studying the Odontogenic Potential of DPSCs
5.2. Studying Osteogenic and Chondrogenic Differentiation Potential of DPSCs
5.3. Other Applications
6. Discussion
7. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Janebodin, K.; Chavanachat, R.; Hays, A.; Reyes Gil, M. Silencing VEGFR-2 hampers odontoblastic differentiation of dental pulp stem cells. Front. Cell Dev. Biol. 2021, 9, 665886. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Xiao, Q.; Sheng, R.; Jiang, S.; Yuan, Q.; Liu, W. Endogenous GDF11 regulates odontogenic differentiation of dental pulp stem cells. J. Cell. Mol. Med. 2020, 24, 11457–11464. [Google Scholar] [CrossRef] [PubMed]
- Blaese, R.M.; Culver, K.W.; Miller, A.D.; Carter, C.S.; Fleisher, T.; Clerici, M.; Shearer, G.; Chang, L.; Chiang, Y.; Tolstoshev, P. T lymphocyte-directed gene therapy for ADA− SCID: Initial trial results after 4 years. Science 1995, 270, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Nayerossadat, N.; Maedeh, T.; Ali, P.A. Viral and nonviral delivery systems for gene delivery. Adv. Biomed. Res. 2012, 1, 27. [Google Scholar] [CrossRef] [PubMed]
- Cotter, M.J.; Muruve, D.A. The induction of inflammation by adenovirus vectors used for gene therapy. Front. Biosci.-Landmark 2005, 10, 1098–1105. [Google Scholar] [CrossRef] [PubMed]
- Chow, Y.T.; Chen, S.; Wang, R.; Liu, C.; Kong, C.-w.; Li, R.A.; Cheng, S.H.; Sun, D. Single cell transfection through precise microinjection with quantitatively controlled injection volumes. Sci. Rep. 2016, 6, 24127. [Google Scholar] [CrossRef]
- Bushman, F.D. Retroviral integration and human gene therapy. J. Clin. Investig. 2007, 117, 2083–2086. [Google Scholar] [CrossRef]
- Liu, W.; Liu, Z.; Liu, L.; Xiao, Z.; Cao, X.; Cao, Z.; Xue, L.; Miao, L.; He, X.; Li, W. A novel human foamy virus mediated gene transfer of GAD67 reduces neuropathic pain following spinal cord injury. Neurosci. Lett. 2008, 432, 13–18. [Google Scholar] [CrossRef]
- Naso, M.F.; Tomkowicz, B.; Perry III, W.L.; Strohl, W.R. Adeno-associated virus (AAV) as a vector for gene therapy. BioDrugs 2017, 31, 317–334. [Google Scholar] [CrossRef]
- Ishida, M.; Selaru, F.M. miRNA-based therapeutic strategies. Curr. Pathobiol. Rep. 2013, 1, 63–70. [Google Scholar] [CrossRef]
- Zhong, T.; Gao, Y.; Qiao, H.; Zhou, H.; Liu, Y. Elevated osteogenic potential of stem cells from inflammatory dental pulp tissues by Wnt4 overexpression for treating bone defect in rats. Ann. Palliat. Med 2020, 9, 2962–2969. [Google Scholar] [CrossRef] [PubMed]
- Chong, Z.X.; Yeap, S.K.; Ho, W.Y. Transfection types, methods and strategies: A technical review. PeerJ 2021, 9, e11165. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.-L.; Zhang, Y.-J.; Wang, J.-W.; Tian, F.; Wang, C.-F. Studies on microRNA regulation of multidirectional differentiation of dental pulp stem cells: A narrative review. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 1816–1824. [Google Scholar] [PubMed]
- Costela-Ruiz, V.J.; Melguizo-Rodríguez, L.; Bellotti, C.; Illescas-Montes, R.; Stanco, D.; Arciola, C.R.; Lucarelli, E. Different sources of mesenchymal stem cells for tissue regeneration: A guide to identifying the most favorable one in orthopedics and dentistry applications. Int. J. Mol. Sci. 2022, 23, 6356. [Google Scholar] [CrossRef] [PubMed]
- Tatullo, M.; Marrelli, M.; Shakesheff, K.M.; White, L.J. Dental pulp stem cells: Function, isolation and applications in regenerative medicine. J. Tissue Eng. Regen. Med. 2015, 9, 1205–1216. [Google Scholar] [CrossRef] [PubMed]
- Smojver, I.; Katalinić, I.; Bjelica, R.; Gabrić, D.; Matišić, V.; Molnar, V.; Primorac, D. Mesenchymal Stem Cells Based Treatment in Dental Medicine: A Narrative Review. Int. J. Mol. Sci. 2022, 23, 1662. [Google Scholar] [CrossRef] [PubMed]
- Smeda, M.; Galler, K.M.; Woelflick, M.; Rosendahl, A.; Moehle, C.; Lenhardt, B.; Buchalla, W.; Widbiller, M. Molecular biological comparison of dental pulp-and apical papilla-derived stem cells. Int. J. Mol. Sci. 2022, 23, 2615. [Google Scholar] [CrossRef]
- Costa, L.A.; Eiro, N.; Vaca, A.; Vizoso, F.J. Towards a New Concept of Regenerative Endodontics Based on Mesenchymal Stem Cell-Derived Secretomes Products. Bioengineering 2022, 10, 4. [Google Scholar] [CrossRef]
- Genova, T.; Cavagnetto, D.; Tasinato, F.; Petrillo, S.; Ruffinatti, F.A.; Mela, L.; Carossa, M.; Munaron, L.; Roato, I.; Mussano, F. Isolation and characterization of buccal fat pad and dental pulp MSCS from the same donor. Biomedicines 2021, 9, 265. [Google Scholar] [CrossRef]
- Huang, G.-J.; Gronthos, S.; Shi, S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: Their biology and role in regenerative medicine. J. Dent. Res. 2009, 88, 792–806. [Google Scholar] [CrossRef]
- Chamila Prageeth Pandula, P.; Samaranayake, L.; Jin, L.; Zhang, C. Periodontal ligament stem cells: An update and perspectives. J. Investig. Clin. Dent. 2014, 5, 81–90. [Google Scholar] [CrossRef]
- Zhang, Y.; Xing, Y.; Jia, L.; Ji, Y.; Zhao, B.; Wen, Y.; Xu, X. An in vitro comparative study of multisource derived human mesenchymal stem cells for bone tissue engineering. Stem Cells Dev. 2018, 27, 1634–1645. [Google Scholar] [CrossRef] [PubMed]
- Stanko, P.; Altanerova, U.; Jakubechova, J.; Repiska, V.; Altaner, C. Dental mesenchymal stem/stromal cells and their exosomes. Stem Cells Int. 2018, 2018, 8973613. [Google Scholar] [CrossRef]
- Angelopoulos, I.; Brizuela, C.; Khoury, M. Gingival mesenchymal stem cells outperform haploidentical dental pulp-derived mesenchymal stem cells in proliferation rate, migration ability, and angiogenic potential. Cell Transplant. 2018, 27, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Stanko, P.; Kaiserova, K.; Altanerova, V.; Altaner, C. Comparison of human mesenchymal stem cells derived from dental pulp, bone marrow, adipose tissue, and umbilical cord tissue by gene expression. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc. Czech. Repub. 2014, 158, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Winning, L.; El Karim, I.A.; Lundy, F.T. A comparative analysis of the osteogenic potential of dental mesenchymal stem cells. Stem Cells Dev. 2019, 28, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Yamato, E.; Miyazaki, J.-I. Intravenous delivery of naked plasmid DNA for in vivo cytokine expression. Biochem. Biophys. Res. Commun. 2001, 289, 1088–1092. [Google Scholar] [CrossRef]
- Maruyama, H.; Higuchi, N.; Kameda, S.; Miyazaki, J.-I.; Gejyo, F. Rat liver-targeted naked plasmid DNA transfer by tail vein injection. Mol. Biotechnol. 2004, 26, 165–172. [Google Scholar] [CrossRef]
- Ni, R.; Zhou, J.; Hossain, N.; Chau, Y. Virus-inspired nucleic acid delivery system: Linking virus and viral mimicry. Adv. Drug Deliv. Rev. 2016, 106, 3–26. [Google Scholar] [CrossRef]
- Salkın, H.; Gönen, Z.B.; Özcan, S.; Bahar, D.; Lekesizcan, A.; Taheri, S.; Kütük, N.; Alkan, A. Effects of combination TGF-B1 transfection and platelet rich plasma (PRP) on three-dimension chondrogenic differentiation of rabbit dental pulp-derived mesenchymal stem cells. Connect. Tissue Res. 2021, 62, 226–237. [Google Scholar] [CrossRef]
- Yamano, S.; Dai, J.; Moursi, A.M. Comparison of transfection efficiency of nonviral gene transfer reagents. Mol. Biotechnol. 2010, 46, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Wolf, G.D.; Schmidt-Wolf, I.G. Non-viral and hybrid vectors in human gene therapy: An update. Trends Mol. Med. 2003, 9, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, P.; Giri, J.; Rieken, F.; Koch, C.; Mykhaylyk, O.; Döblinger, M.; Banerjee, R.; Bahadur, D.; Plank, C. Targeted temperature sensitive magnetic liposomes for thermo-chemotherapy. J. Control. Release 2010, 142, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Varga, C.M.; Hong, K.; Lauffenburger, D.A. Quantitative analysis of synthetic gene delivery vector design properties. Mol. Ther. 2001, 4, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.; Xue, M.; Wang, Y.; Wang, Y.; Li, D.; Zhao, X.; Li, X. An improved method for increasing the efficiency of gene transfection and transduction. Int. J. Physiol. Pathophysiol. Pharmacol. 2018, 10, 95. [Google Scholar]
- Sork, H.; Nordin, J.Z.; Turunen, J.J.; Wiklander, O.P.; Bestas, B.; Zaghloul, E.M.; Margus, H.; Padari, K.; Duru, A.D.; Corso, G. Lipid-based transfection reagents exhibit cryo-induced increase in transfection efficiency. Mol. Ther.-Nucleic Acids 2016, 5, e290. [Google Scholar] [CrossRef]
- Chen, J.; Guo, Z.; Tian, H.; Chen, X. Production and clinical development of nanoparticles for gene delivery. Mol. Ther.-Methods Clin. Dev. 2016, 3, 16023. [Google Scholar] [CrossRef]
- Borawski, J.; Lindeman, A.; Buxton, F.; Labow, M.; Gaither, L.A. Optimization procedure for small interfering RNA transfection in a 384-well format. J. Biomol. Screen. 2007, 12, 546–559. [Google Scholar] [CrossRef]
- Nakashima, M.; Tachibana, K.; Iohara, K.; Ito, M.; Ishikawa, M.; Akamine, A. Induction of reparative dentin formation by ultrasound-mediated gene delivery of growth/differentiation factor 11. Hum. Gene Ther. 2003, 14, 591–597. [Google Scholar] [CrossRef]
- Arnold, A.S.; Laporte, V.; Dumont, S.; Appert-Collin, A.; Erbacher, P.; Coupin, G.; Levy, R.; Poindron, P.; Gies, J.P. Comparing reagents for efficient transfection of human primary myoblasts: FuGENE 6, Effectene and ExGen 500. Fundam. Clin. Pharmacol. 2006, 20, 81–89. [Google Scholar] [CrossRef]
- Van Camp, J.; Beckers, S.; Zegers, D.; Van Hul, W. Wnt signaling and the control of human stem cell fate. Stem Cell Rev. Rep. 2014, 10, 207–229. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Dissanayaka, W.L.; Zhang, C. Dental pulp stem cells overexpressing stromal-derived factor-1α and vascular endothelial growth factor in dental pulp regeneration. Clin. Oral Investig. 2019, 23, 2497–2509. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.K.; Eberwine, J.H. Mammalian cell transfection: The present and the future. Anal. Bioanal. Chem. 2010, 397, 3173–3178. [Google Scholar] [CrossRef] [PubMed]
- Yau, J.; Zuo, P.; Rabie, A.; Wong, R. BMP7 transfection induces in-vitro osteogenic differentiation of dental pulp mesenchymal stem cells. APOS Trends Orthod. J. Asian Pac. Orthod. Soc. 2013, 3, 9–14. [Google Scholar]
- Audouy, S.A.; de Leij, L.F.; Hoekstra, D.; Molema, G. In vivo characteristics of cationic liposomes as delivery vectors for gene therapy. Pharm. Res. 2002, 19, 1599–1605. [Google Scholar] [CrossRef] [PubMed]
- Xin, T.; Zhang, T.; Li, Q.; Yu, T.; Zhu, Y.; Yang, R.; Zhou, Y. A novel mutation of MSX1 in oligodontia inhibits odontogenesis of dental pulp stem cells via the ERK pathway. Stem Cell Res. Ther. 2018, 9, 221. [Google Scholar] [CrossRef] [PubMed]
- Scherer, F.; Anton, M.; Schillinger, U.; Henke, J.; Bergemann, C.; Krüger, A.; Gänsbacher, B.; Plank, C. Magnetofection: Enhancing and targeting gene delivery by magnetic force in vitro and in vivo. Gene Ther. 2002, 9, 102–109. [Google Scholar] [CrossRef]
- Vorburger, S.A.; Hunt, K.K. Adenoviral gene therapy. Oncology 2002, 7, 46–59. [Google Scholar] [CrossRef]
- Roser, A.E.; Caldi Gomes, L.; Schünemann, J.; Maass, F.; Lingor, P. Circulating miRNAs as diagnostic biomarkers for Parkinson’s disease. Front. Neurosci. 2018, 12, 625. [Google Scholar] [CrossRef]
- Fus-Kujawa, A.; Prus, P.; Bajdak-Rusinek, K.; Teper, P.; Gawron, K.; Kowalczuk, A.; Sieron, A.L. An overview of methods and tools for transfection of eukaryotic cells in vitro. Front. Bioeng. Biotechnol. 2021, 9, 701031. [Google Scholar] [CrossRef]
- Chang, K.; Chen, R.-S.; Chang, F.-H.; Chen, M.-H. Promoting dentinogenesis of DPSCs through inhibiting microRNA-218 by using magnetic nanocarrier delivery. J. Formos. Med. Assoc. 2019, 118, 1005–1013. [Google Scholar] [CrossRef]
- Herweijer, H.; Wolff, J. Progress and prospects: Naked DNA gene transfer and therapy. Gene Ther. 2003, 10, 453–458. [Google Scholar] [CrossRef]
- Rizk, A.; Rabie, B.M. Electroporation for transfection and differentiation of dental pulp stem cells. BioResearch Open Access 2013, 2, 155–162. [Google Scholar] [CrossRef]
- Tomizawa, M.; Shinozaki, F.; Motoyoshi, Y.; Sugiyama, T.; Yamamoto, S.; Sueishi, M. Sonoporation: Gene transfer using ultrasound. World J. Methodol. 2013, 3, 39. [Google Scholar] [CrossRef]
- Son, K.K.; Tkach, D.; Hall, K.J. Efficient in vivo gene delivery by the negatively charged complexes of cationic liposomes and plasmid DNA. Biochim. Et Biophys. Acta (BBA)-Biomembr. 2000, 1468, 6–10. [Google Scholar] [CrossRef]
- Nakashima, M.; Mizunuma, K.; Murakami, T.; Akamine, A. Induction of dental pulp stem cell differentiation into odontoblasts by electroporation-mediated gene delivery of growth/differentiation factor 11 (Gdf11). Gene Ther. 2002, 9, 814–818. [Google Scholar] [CrossRef]
- Salkın, H.; Gönen, Z.B.; Ergen, E.; Bahar, D.; Çetin, M. Effects of TGF-β1 overexpression on biological characteristics of human dental pulp-derived mesenchymal stromal cells. Int. J. Stem. Cells 2019, 12, 170–182. [Google Scholar] [CrossRef]
- Soda, M.; Saitoh, I.; Murakami, T.; Inada, E.; Iwase, Y.; Noguchi, H.; Shibasaki, S.; Kurosawa, M.; Sawami, T.; Terunuma, M. Repeated human deciduous tooth-derived dental pulp cell reprogramming factor transfection yields multipotent intermediate cells with enhanced iPS cell formation capability. Sci. Rep. 2019, 9, 1490. [Google Scholar] [CrossRef]
- Zeng, K.; Kang, Q.; Li, Y.; Li, W.; Cheng, Q.; Xia, W. EVL Promotes Osteo-/Odontogenic Differentiation of Dental Pulp Stem Cells via Activating JNK Signaling Pathway. Stem Cells Int. 2023, 2023, 7585111. [Google Scholar] [CrossRef]
- Heo, S.C.; Keum, B.R.; Seo, E.J.; Yoon, J.; Jeong, S.; Tigyi, G.J.; Norman, D.; Jang, I.H.; Kim, H.J. Lysophosphatidic acid induces proliferation and osteogenic differentiation of human dental pulp stem cell through lysophosphatidic acid receptor 3/extracellular signal-regulated kinase signaling axis. J. Dent. Sci. 2023, 18, 1219–1226. [Google Scholar] [CrossRef]
- Elgundi, Z.; Sifniotis, V.; Reslan, M.; Cruz, E.; Kayser, V. Laboratory scale production and purification of a therapeutic antibody. JoVE J. Vis. Exp. 2017, 119, e55153. [Google Scholar]
- Zhu, S.; Ying, Y.; He, Y.; Zhong, X.; Ye, J.; Huang, Z.; Chen, M.; Wu, Q.; Zhang, Y.; Xiang, Z. Hypoxia response element-directed expression of bFGF in dental pulp stem cells improve the hypoxic environment by targeting pericytes in SCI rats. Bioact. Mater. 2021, 6, 2452–2466. [Google Scholar] [CrossRef] [PubMed]
- Mehri-Ghahfarrokhi, A.; Pourteymourfard-Tabrizi, Z.; Farrokhi, E.; Chaleshtori, M.H.; Jami, M.-S. Increased levels of miR-124 in human dental pulp stem cells alter the expression of neural markers. J. Otol. 2019, 14, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Liu, Y.; Dong, F.; Dou, Y.; Li, W.; Wang, J. Knockdown of microRNA-584 promotes dental pulp stem cells proliferation by targeting TAZ. Cell Cycle 2020, 19, 1048–1058. [Google Scholar] [CrossRef]
- Cucco, C.; Zhang, Z.; Botero, T.M.; Chiego, D.J.; Castilho, R.M.; Nör, J.E. SCF/c-Kit signaling induces self-renewal of dental pulp stem cells. J. Endod. 2020, 46, S56–S62. [Google Scholar] [CrossRef]
- Du, Z.; Shi, X.; Guan, A. lncRNA H19 facilitates the proliferation and differentiation of human dental pulp stem cells via EZH2-dependent LATS1 methylation. Mol. Ther.-Nucleic Acids 2021, 25, 116–126. [Google Scholar] [CrossRef]
- Baek, K.; Tu, C.; Zoldan, J.; Suggs, L.J. Gene transfection for stem cell therapy. Curr. Stem Cell Rep. 2016, 2, 52–61. [Google Scholar] [CrossRef]

| DPSC Properties | Key Findings |
|---|---|
| Proliferation |
|
| Migration capacity | The migration capacity of stem cells obtained from gingival tissue is higher compared to DPSCs. |
| Clonogenicity | Colony-forming ability is higher in stem cells obtained from gingival tissue compared to DPSCs. |
| Angiogenic capacity | Stem cells obtained from gingival tissue showed higher angiogenic capacity compared to cells from DPSCs. |
| Differentiation potential |
|
| Viral Vector | Descriptive Comparisons |
| Retroviruses |
|
| Adenovirus |
|
| Adeno-associated virus |
|
| Herpes virus |
|
| Transfection Techniques | Goal of the Transfection |
|---|---|
| Non-viral approach using siRNA to silence the growth/differentiation factor 11 (GDF11) | To study the effect of growth/differentiation factor 11 (GDF11) on the odontogenic differentiation potential of DPSCs |
| Viral approach using lentiviral transfection system to introduce the recombinant Wnt4 siRNA into the cells | To study the effect of Wnt4 on the odontogenic and osteogenic differentiation potential of DPSCs |
| The electroporation-mediated gene delivery approach used to overexpress the recombinant human GDF11 in the cells | To study the effect of GDF11 on the healing of injured pulp tissue |
| Sonoporation is used to transfer the GDF11 plasmid DNA into the cells | To study the effect of GDF11 on the odontogenic differentiation potential of DPSCs |
| Viral approach using lentiviral carriers to deliver VEGF and SDF-1α into the cells | To study the effect of vascular endothelial growth factor (VEGF) on dental pulp regeneration, proliferation, and endothelial cell migration |
| Viral approach using shRNA to silence the expression of the VEGFR-2 gene in the targeted cells | To study the effect of vascular endothelial growth factor-2 (VEGFR-2) on the dentinogenesis capacity of DPSCs |
| The electroporation-mediated gene delivery approach used to transfect cells with a transforming growth factor-beta 1 (TGF-B1) gene plasmid | To study the effect of TGF-B1 on the osteogenic and chondrogenic differentiation potential of DPSCs |
| Non-viral approach using siRNA to overexpress Ena/VASP-like protein (EVL) | To study the effect of EVL on the osteogenic differentiation potential of DPSCs |
| Non-viral approach using siRNA to silence lysophosphatidic acid (LPA) | To study the effect of LPA on the osteogenic differentiation potential of DPSCs |
| Viral approach using lentiviral carriers expressing shRNA to modulate the stem cell factor (SCF) signaling through its receptor tyrosine kinase | To study the influence of SCF signaling through its receptor tyrosine kinase on the self-renewal ability of DPSCs |
| Transfection Technique | Advantages | Disadvantages |
|---|---|---|
| siRNA |
|
|
| Electroporation |
|
|
| Sonoporation |
|
|
| Transfection with viral vector |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkharobi, H. Exploring Various Transfection Approaches and Their Applications in Studying the Regenerative Potential of Dental Pulp Stem Cells. Curr. Issues Mol. Biol. 2023, 45, 10026-10040. https://doi.org/10.3390/cimb45120626
Alkharobi H. Exploring Various Transfection Approaches and Their Applications in Studying the Regenerative Potential of Dental Pulp Stem Cells. Current Issues in Molecular Biology. 2023; 45(12):10026-10040. https://doi.org/10.3390/cimb45120626
Chicago/Turabian StyleAlkharobi, Hanaa. 2023. "Exploring Various Transfection Approaches and Their Applications in Studying the Regenerative Potential of Dental Pulp Stem Cells" Current Issues in Molecular Biology 45, no. 12: 10026-10040. https://doi.org/10.3390/cimb45120626
APA StyleAlkharobi, H. (2023). Exploring Various Transfection Approaches and Their Applications in Studying the Regenerative Potential of Dental Pulp Stem Cells. Current Issues in Molecular Biology, 45(12), 10026-10040. https://doi.org/10.3390/cimb45120626





