Abstract
The embryonic development of neural crest cells and subsequent tissue differentiation are intricately regulated by specific transcription factors. Among these, SOX10, a member of the SOX gene family, stands out. Located on chromosome 22q13, the SOX10 gene encodes a transcription factor crucial for the differentiation, migration, and maintenance of tissues derived from neural crest cells. It plays a pivotal role in developing various tissues, including the central and peripheral nervous systems, melanocytes, chondrocytes, and odontoblasts. Mutations in SOX10 have been associated with congenital disorders such as Waardenburg–Shah Syndrome, PCWH syndrome, and Kallman syndrome, underscoring its clinical significance. Furthermore, SOX10 is implicated in neural and neuroectodermal tumors, such as melanoma, malignant peripheral nerve sheath tumors (MPNSTs), and schwannomas, influencing processes like proliferation, migration, and differentiation. In mesenchymal tumors, SOX10 expression serves as a valuable marker for distinguishing between different tumor types. Additionally, SOX10 has been identified in various epithelial neoplasms, including breast, ovarian, salivary gland, nasopharyngeal, and bladder cancers, presenting itself as a potential diagnostic and prognostic marker. However, despite these associations, further research is imperative to elucidate its precise role in these malignancies.
1. Introduction
During embryonic stages, the formation of the primitive neural crest gives rise to diverse neural structures. These neural crest cells undergo differentiation into various tissues, a process regulated by specific transcription factors with varying expression levels. The SRY-related HMG box, also known as the SOX gene, plays a multifaceted role in differentiating embryological and biological processes among neural crest cells. The SOX gene family comprises eight subfamilies.
Within the SoxE subfamily, SOX10 emerges as a distinctive transcription factor that significantly contributes to the enhancement of differentiation, migration, and maintenance of tissues derived from neural crest cells. Initially expressed in the dorsal neural tube, SOX10 guides the differentiation of tissues within the peripheral nervous system [1,2]. This gene’s pivotal role in embryonic development facilitates neural crest cell differentiation, giving rise to several sublineages, including the arachnoid and pia mater, melanocytes, odontoblasts, tracheal cartilage, laryngeal cartilage, and Schwann cells (Figure 1).
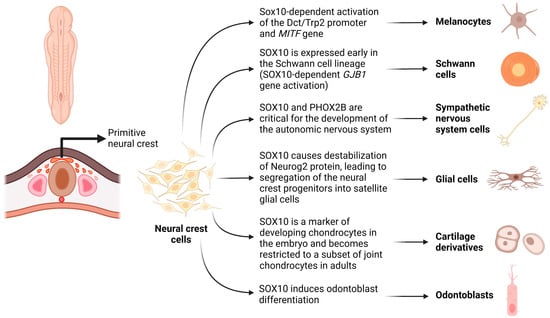
Figure 1.
Schematic demonstrating the role of SOX10 in embryonic development, facilitating neural crest cell differentiation, and giving rise to several sublineages, including melanocytes, Schwann cells, sympathetic nervous system cells, glial cells, odontoblasts, and cartilage derivatives (chondrocytes). Created with BioRender.com (2023).
The SOX10 gene is located on chromosome 22q13, encoding the SOX10 protein with an open reading frame consisting of 466 amino acids and a weight of 51 kDa [3]. The protein possesses a highly conserved dimerization domain at its N-terminus within the SoxE subfamily. Comprising 40 amino acids, this region facilitates the protein’s dimerization ability for binding target genes. Adjacent to the N-terminus is the high mobility group (HMG) domain, spanning 79 amino acids and maintaining a consistent structure across all SOX family members. This domain, characterized by three alpha helices forming an L-shape, is designed for binding DNA sequences within the minor groove, specifically containing the nucleotide sequence of C[A/T]TTG[A/T][A/T]. This binding modulates DNA molecules, creating a compatible structure for active transcriptional complexes [3].
Within the domain, an intron and K2 domain are present, along with a nuclear localization and export signal [3]. The K2 domain functions as a promoter-specific transactivation domain, TAM (transactivation domain in the middle of the protein), crucial for SOX10 expression in the peripheral nervous system [4]. On the opposite end of the protein, in the C-terminal region, 66 amino acids are located, marked by a high expression of serine, prolines, and glutamine sequences [5]. This C-terminus is essential for SOX10′s interaction with specific binding targets during tissue differentiation, facilitated by a transactivation domain (TA or TAC) [3].
The distinctive composition of SOX10 enables it to exist as a monomer or dimer, exerting influence on various DNA binding targets with differing affinities. Beyond this dual functionality, SOX10 also serves as a nucleocytoplasmic shuttle protein for transcriptional activation, potentially binding to cis elements on target genes to regulate their expression [6,7]. These specific functions are intricately regulated through the modification and expression of SOX10, involving various signal transduction pathways such as Wnt, BMPs, and FGFs pathways [2,3,8].
Wnt signaling, in particular, plays a crucial role in neural crest formation. Decreased levels of Wnt signaling inhibit neural crest formation, underscoring its necessity in this developmental process. A study demonstrated that blocking Wnt using a second messenger resulted in the suppression of SOX10 expression [2]. Moreover, over a dozen transcription factors bind to the N-terminus of the SOX10 HMG domain, regulating its transcriptional activity [9]. SOX9 and Slug are implicated in the regulation of SOX10, showing their necessity in neural crest cell development. Manipulating Slug and Sox9 expression, whether wild type or mutant, resulted in high or absent SOX10 expression, suggesting a mutual relationship between Slug and SOX10 [2].
Various modifications, including phosphorylation, acetylation, SUMOylation, and methylation, have been identified in different amino acid residues of SOX10. SUMOylation at three lysine residues (K55, K246, and K357) represses the transcriptional activation of target genes crucial for cell development and maintenance, such as MITF in melanocytes and GJB1 in Schwann cells [10]. Additionally, phosphorylation of Ser24 and Thr240, two highly conserved sites within the SoxE family, has been associated with melanoma [11].
SUMOylation of SoxE proteins is integral to the development of the inner ear. A yeast two-hybrid screen identified UCB9 and SUMO-1 in SoxE proteins, including SOX10 and SOX9, crucial for inner ear regulation. Both SOX10 and SOX9 feature two conserved SUMOylation sites—one at the N-terminal of an E1 domain and the other at the C-terminal of the activation domain. Specifically, SOX10 undergoes SUMOylation at K44 and K333, at the N-terminus and activation domain, respectively, in addition to other conserved sites [12]. SUMOylation may also occur at K55 and K357 sites within the SOX10 due to their involvement in the interaction of UBC9 and SOX10 [13]. Consequently, the absence of a SUMOylated site may indicate the non-expression of a lysine residue in a SOX10 variant.
The expression of SOX10 varies in response to SUMOylation or the absence of necessary residues in SOX9 [12]. This evolved ability of SOX10 to undergo SUMOylation plays a pivotal role in regulating the protein, enabling it to modulate distant proteins, up- and downregulate various cellular functions, and modify protein complex interactions.
Given the highly conserved expression of SOX10 within neural crest cells and their derivatives, the presence of mutated variants can result in a spectrum of severe to lethal diseases. Over half of the variations within the SOX10 family result from truncations. The remaining variants include non-truncating, missense, in-frame insertions or deletions, and partial copy number variants. Missense mutations typically cluster in the HMG domain [3]. These mutations can lead to conditions such as deafness, dysregulation of the peripheral and central nervous systems, embryonic lethality, colonic issues, and various neoplasms.
In cases of sensorineural hearing loss, various SOX10 mutations may lead to the agenesis or hypoplasia of semicircular canals and enlarged vestibules. Imaging modalities, including computed tomography (CT) and magnetic resonance imaging (MRI), have revealed a connection between SOX10 mutations and the absence or hypoplasia of these structures [3]. These malformations associated with SOX10 mutations have also been linked to dysregulation of WNT1 (regulating cell fate), KCNQ4 (potassium voltage-gated channel), STRC (stereocilin, associated with the hair bundle of the ear), and PAX6 (paired box 6) [3].
Considering the crucial role of SOX10 in myelin-containing glial cells, various mutations have been identified. Two frameshift mutations within the carboxy-terminal, resulting in truncations (SOX10Dom and SOX10-59), have been associated with dominant megacolon and Waardenburg–Hirschsprung disease [14]. A group of disorders collectively labeled as PCWH (peripheral demyelinating neuropathy, central demyelinating leukodystrophy, Waardenburg syndrome, and Hirschsprung disease) result from variants within the nervous system. Clinical presentations may include delayed motor and cognitive development, cerebral palsy, ataxia, spasticity, congenital nystagmus, hyporeflexia, distal sensory impairments, and distal muscle wasting [3]. Signs of Kallmann syndrome (KS) have also been observed in Waardenburg syndrome, suggesting that KS may result from SOX10 mutations. KS manifests with hypogonadotropic hypogonadism and anosmia. Many patients with KS may also present with hearing deficits and harbor SOX10 mutations [4]. The physiological basis of this disorder in relation to SOX10 is believed to involve the dysregulation of GnRH (gonadotropin-releasing hormone) as it travels through the neurons of the peripheral olfactory nerve, up to and through the olfactory bulb [3].
SOX10 plays a crucial role in the embryogenesis of neural crest cells, and deviations from its normal function can give rise to various congenital disorders. However, the impact of SOX10 variants extends beyond developmental disorders, contributing to the initiation and progression of different cancers due to its involvement in numerous tissues.
SOX10 expression has been identified in various cancer types, including breast tumors, glioma, glioblastoma, salivary adenoid cystic tumors, melanoma, and hepatocellular carcinoma. Intriguingly, SOX10 exhibits dual roles in these tumors, acting as a tumor suppressor and promoter. For instance, it functions as an oncogene in hepatocellular carcinoma and nasopharyngeal carcinoma while exerting tumor-suppressive effects in gastrointestinal neoplasms. Urothelial carcinoma shows an overexpression of SOX10, indicating its role as a tumor promoter [15]. The significance of SOX10 expression becomes evident when comparing its levels in different bladder cancers to normal bladder tissue [16].
These varied expressions underscore the need to study SOX10’s role and levels in both normal and pathological tissues. This comprehensive understanding is crucial for unraveling its precise role in cell biology and appreciating its clinical significance (Figure 2).
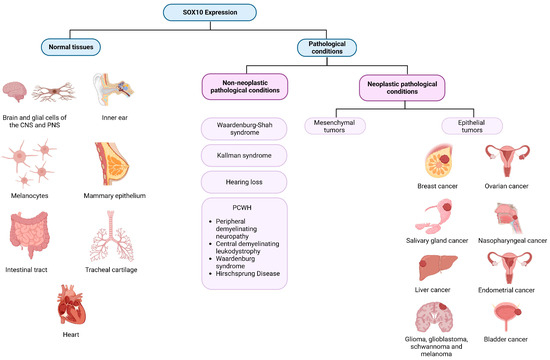
Figure 2.
SOX10 expression in normal tissues and across different pathological conditions. Created with BioRender.com (2023).
2. SOX10 Expression in Normal Tissues
The differentiation of various tissues from neural progenitor crest cells involves distinct processes. SOX10 expression remains elevated in tissues such as the brain, inner ear, intestinal tract, tracheal cartilage, and heart. In the early embryonic development of the inner ear, SOX10 shows high expression, gradually declining as hair cells mature. At this stage, SOX10 becomes specific to supporting cells, and an inability to express either SOX10 or SOX9 may result in the development of an enlarged or cystic otocyst [12,17,18].
Conversely, lower levels of SOX10 expression are observed in the prostate, testis, bladder, pancreas, and stomach [19]. During peripheral nervous system development, some neurons lose SOX10 expression while all mature glial cells maintain its expression. In the central nervous system, oligodendrocytes exhibit a high level of SOX10 expression. Similarly, melanocytes heavily rely on SOX10 for their specialization, maturation, and maintenance [2].
2.1. SOX10 Expression within the Peripheral Nervous System
Within the peripheral nervous system, SOX10 plays a pivotal role in facilitating the differentiation of both Schwann cells and glial cells, employing distinct biochemical processes in each cell type. In Schwann cell development, SOX10 directly targets the protein zero (P0) gene coding region, a myelin gene exclusively expressed in Schwann cells, tightly regulated by SOX10 [14].
Analysis of mouse embryos with mutated binding sequences on P0 for SOX10, compared to those with normal binding sequences, revealed robust SOX10 expression in mature Schwann cells with high P0 expression. This expression was further intensified when a SOX10 induction signal was introduced into these sequences, resulting in a ten-fold increase in P0 expression [14].
Neurogulin-1 has been identified as a key player in controlling the differentiation of neural crest cells into glia via the activation of ErbB receptors [20]. The absence of interaction between Neurogulins binding to the EGF receptor tyrosine kinase, ErbB3, has been associated with developmental defects in neural crest cells and their derivatives. The relationship between SOX10 and ErbB3 was investigated using the tet-on system, inducing SOX10 expression, leading to a significant increase in ErbB3 expression. However, whether this effect was direct or indirect remained unclear. Supporting this relationship, SOX10 mutant variations were found in ErbB3 mutant mice [20].
It is important to note that in certain cells, there was a high expression of SOX10 coupled with a low expression of P0, particularly in non-myelinating cells. This suggests that SOX10 typically does not function independently but, instead, interacts with different protein complexes. In unmyelinated Schwann cells, the downregulation of myelination may be attributed to SOX10’s regulation of various transcription factors, including SOX5, SOX6, NOTCH1, HMGA2, HES1, MYCN, ID4, and ID2. These regulators were found to oppose the process of myelination within Schwann cells [21]. Furthermore, in an experimental study on SOX10 expression within mammary glands, mouse embryos were manipulated to be homozygous dominant knockout for SOX10. In these specific mice, death was quickly encountered, in addition to the complete absence of Schwann cell production [22].
2.2. SOX10 Expression within the Inner Ear
Moving beyond the peripheral nervous system to the inner ear, there is meticulous regulation of SOX10 and SOX9, which is crucial for normal development. During gastrulation and neural crest development, SOX10 is expressed in the otic vesicle, reaching its peak around stage 25 [2]. Both SoxE proteins, SOX10 and SOX9, undergo SUMOylation at different lysine residues and two conserved sequences [12]. The regulation of these modifications may have subsequent consequences, leading to progressive hearing loss.
SOX10 exhibits high expression in the otic vesicle from E9.5 onward until it becomes exclusively expressed in the supporting cells later in development. This sustained expression of SOX10 facilitates the maintenance of cochlear progenitors during the development of the organ of Corti and the otocyst [3].
2.3. SOX10 Expression in Melanocytes
The precise expression of SOX10 in melanocytes is indispensable for gene regulation within these cells. Before melanocyte development, SOX10 is highly expressed in the neural crest region, initially across all axial areas and later progressing to the expression only in the truncal region. Overexpression of SOX10 at this stage results in high expression in the Slug domain, both playing a role in the development of pigmented melanocytes [3].
Through a complex network, SOX10 collaborates with PAX3 to activate MITF, enhancing its expression. Increased MITF, in turn, works with SOX10 to promote DCT/TRP2 expression. Dominant SOX10 mutant mice exhibit a decrease in melanocyte markers Dct/Trp2, underscoring the pivotal role of SOX10 in pigment production [10]. Consistent with these cell markers, it has been shown that mutant SOX10 or low expressions lead to a proportional decrease in markers Trp2, c-kit, and Mitf [3]. The varying levels of these markers depend on the stage of melanocyte development within the embryo, starting with nonpigmented melanoblasts and eventually transitioning to melanocytes. It has been demonstrated that SOX10 could produce pigment at injected sites, while Slug alone could not [3].
2.4. SOX10 Expression in the Mammary Epithelium
An exception to the typical expression of SOX10 in neural crest cell derivatives is observed in the mammary epithelium, which originates from the ectoderm. The mammary gland houses epithelium that bifurcates into the ductal epithelial tree during puberty. The mammary epithelium undergoes dynamic changes in growth due to hormonal stimulation during puberty, pregnancy, lactation, and menopause. SOX10 expression in these cells initiates prenatally during the development of stem cells.
Within these stem cells, SOX10 responds to FGF signaling, facilitating their progression to mesenchymal tissue. A study manipulating mice embryos analyzed the effects of homozygous, heterozygous SOX10 knockouts, and wild type. Both heterozygous and homozygous knockout mice exhibited decreased mammary branching growth development. Furthermore, postnatal mammary development revealed that these adult mice were unable to lactate after pregnancy.
Continuing through the female reproductive process, mice were further analyzed during involution. Compared to wild-type mice, knockout mice started with substantially fewer epithelial cells in the mammary glands. However, during involution, the epithelial cell count decreased significantly more in wild-type mice. These findings suggest the involvement of SOX10 throughout the entire process, including the involution of expanded mammary epithelia. Although SOX10 may play a crucial role in this process, the presence of mammary growth indicates that SOX9 and SOX10 may work synergistically, with SOX9 contributing to the absence of SOX10 [22].
3. SOX10 Expression in Non-Neoplastic Pathological Conditions
3.1. SOX10 in Waardenburg–Shah Syndrome
SOX10 mutations have been implicated in disrupting neural crest development, leading to a diverse range of clinical phenotypes. The association of the SOX10 gene with congenital disorders was initially recognized in the context of Waardenburg–Shah syndrome, a subtype of Waardenburg syndrome (WS), also known as Waardenburg–Hirschsprung syndrome and WS type 4 [8]. WS4 is characterized by sensorineural hearing loss, depigmentation of hair, skin, and eyes, and Hirschsprung’s disease. The SOX10 gene was first identified as the mutant gene responsible for megacolon and depigmentation in Dom mutant mice (SOX10Dom) [1,23]. Specifically, a frameshift mutation in SOX10 causing haploinsufficiency was found to be the cause, with a homozygous mutation in mice proving lethal [24]. Based on this discovery, SOX10 mutations were screened for in human patients with Waardenburg–Hirschsprung disease, in whom a causative mutation had not yet been identified. Several cases were found to have a SOX10 mutation, confirming its involvement in the Waardenburg–Hirschsprung disease [8].
Waardenburg syndrome has been classified into four main presentations. Type I (WS1) presents with pigmentary abnormalities of the hair, heterochromia irides, sensorineural hearing loss, and the characteristic dystopia canthorum. Type 2 (WS2) has similar features with the absence of dystopia canthorum. Type 3 (WS3) is distinguished by abnormalities of the upper limb. While Waardenburg syndrome was initially classified by phenotypic presentation, detected mutations in patients with WS have been integrated into further subclassifications. For instance, WS4 has been split into WS4A, WS4B, and WS4C, with mutations in EDNRB, EDN3, and SOX10, respectively [25]. Another subtype, WS2E, is also caused by a SOX10 mutation [26].
3.2. PCWH
PWCH (Peripheral demyelinating neuropathy, central demyelinating leukodystrophy, Waardenburg syndrome, and Hirschsprung disease) represents a neurological variant of the previously discussed WS4, where a SOX10 mutation is also implicated. Patients with PWCH exhibit a similar presentation, including heterochromia irides, sensorineural hearing loss, and Hirschsprung’s disease, as observed in Waardenburg–Shah syndrome. Additionally, they experience neurological symptoms such as peripheral neuropathy, ataxia, and intellectual disability [27,28]. The syndrome was first described shortly after the discovery of SOX10 mutations in WS4. Due to the shared features, mutations in the SOX10 gene were investigated in patients with what is now termed PCWH. A de novo deletion mutation was identified in the coding region of SOX10, leading to an extension of the peptide and a toxic gain of function [29]. The discovery of a SOX10 mutation as a perpetrator (and the exclusion of other known mutations such as PMP22) in PCWH, a demyelinating disease, further supported the role of SOX10 in Schwann cell differentiation.
3.3. Kallman Syndrome
Due to the presence of hypogonadism and anosmia in subtypes of Waardenburg syndrome (e.g., WS2E), SOX10 was investigated as a potential candidate gene for Kallman syndrome (KS), which falls under the umbrella of congenital hypogonadotropic hypogonadism (HH). KS is characterized by anosmia, distinguishing it from idiopathic hypogonadotropic hypogonadism, which lacks anosmia. Both are considered manifestations of the same syndrome, and instances of each may coexist within the same family [30]. Although nine genes have been implicated in HH, demonstrating extensive genetic heterogeneity, they only account for 30% of KS cases. Therefore, SOX10 appeared to be a likely candidate mutation to explain the presence of anosmia within the disease spectrum.
In a mouse model study, SOX10 deficient mice exhibited an almost complete absence of olfactory ensheathing cells (OECs), misrouting of nerve fibers, impaired migration of GnRH cells, and disorganization of the olfactory nerve layer in the olfactory bulbs [31]. In the same study, a cohort of KS patients without known mutations were screened for SOX10 mutations. Six patients had novel SOX10 mutations, and five out of the six also had deafness.
The diversity and overlap of clinical features in patients with Waardenburg–Shah syndrome, PCWH, and Kallman syndrome underscore the role of SOX10 as a common factor for pathogenesis. However, the phenotypic variability among patients with the same mutations or in the same families emphasizes the need for further study of intermediate and downstream factors [3].
3.4. Hearing Loss
SOX10 mutations in the inner ear explain abnormalities in hearing, such as hypoplasia of semicircular canals, enlarged vestibular canals, vestibulocochlear nerve agenesis, and cochlear deformities [32,33]. Although the presence of sensorineural hearing loss among patients with Waardenburg–Shah syndrome varied among genotypes, Song et al. found that the prevalence of hearing loss in patients with a SOX10 mutation was 100% [33]. In mouse models, the expression of SOX10 in vestibulocochlear development has been studied, revealing an increase in SOX10 expression in the maturing cochleovestibular ganglion. In SOX10-deficient mice, there was a lack of glial cell development in this area [34]. Hearing loss is such a penetrant phenotype in patients with SOX10 mutations that it can manifest without any other features of WS or KS, resembling isolated hearing loss [35].
As of yet, there is no clear role for SOX10 in genetic screening or counseling for the discussed conditions. More effort is necessary to consolidate the range of phenotypes into one disease spectrum rather than individual syndromes.
4. SOX10 Expression in Neural and Neuroectodermal Tumors
SOX10’s role is of interest in the development of certain malignancies and as a potential differentiating marker with diagnostic use.
4.1. SOX10 Expression in Melanoma
The expression of SOX10 in melanoma has been conducted due to its significance in both diagnostic and therapeutic applications (Table 1). Bakos et al. investigated the expression of SOX10 through immunohistochemistry (IHC) in primary and metastatic melanoma cells and its association with neistin coexpression [36]. Nestin is an intermediate filament present in neural progenitor cells, melanomas, and melanocytic nevi. This study disclosed a significant co-expression of SOX10, SOX9, and neistin in early primary melanoma. However, no statistically significant co-expression was observed in the metastatic melanoma [36]. These results align with their in vitro findings, suggesting that SOX10 plays a crucial role in neistin activation during early melanoma development but is not associated with its expression in the more advanced stages of the disease [36]. These findings suggest that SOX10 may serve as a potential marker for determining melanoma stage.
In a separate study by Zhongyuan et al., the role of SOX10 in melanoma development was similarly investigated. According to that study, SOX10 plays an important role in regulating various factors involved in melanocyte proliferation and survival, including melanocyte inhibitory activity (MIA), MITF, p21/WAF1, and E2F1 [37]. A reduction in SOX10 expression resulted in reduced melanoma formation, and the knockout of the SOX10 gene led to the elimination of new tumor formation [37]. These findings provide additional evidence supporting the role of SOX10 in melanocyte proliferation. That study also aimed to establish the downstream pathway through which SOX10 affects melanocyte proliferation by observing its effects on the expression of the minichromosomal maintenance complex component (MCM5). The results demonstrated that the overexpression of MCM5 in SOX10-negative cells partially rescued the proliferation defect observed when SOX10 was absent [37]. Overall, these findings indicate that SOX10 is involved in multiple melanocyte proliferation pathways, with the SOX10-MCM5 axis playing a critical, though not exclusive, role in the proliferation [37].
Further evidence on the role of SOX10 on melanoma cell proliferation was reported in a study by Cronin et al., which revealed that the loss of SOX10 in melanoma cells resulted in cell arrest in the G1 phase [38]. Molecular studies of melanoma cells with absent SOX10 showed reduced expression of MITF, elevated expression of p21/WAF1 and p27KIP2, hypophosphorylated RB, and reduced levels of E2F1 [38]. These results suggest that the removal of SOX10 leads to cell arrest in the G1 phase [38]. Another study by Rosenbaum et al. examined the role of SOX10 in the regulation of the melanoma cell cycle, finding that knocking out SOX10 in immune-competent models led to a reduced expression of immune checkpoint proteins HVEM and CEACAM1 [39]. The loss of these immune checkpoint proteins promotes the proliferation of malignant melanoma cells by preventing cellular senesce and apoptosis [39].
Studies on SOX10 have extended beyond its role in proliferation with investigations into its involvement in the migration of melanoma cells. Seong et al. explored this aspect by studying the migration of B16F10 melanoma cell lines following the introduction of siRNA specific for SOX10. This was compared to a control group of the same cell line. That study demonstrated a significant reduction in migration in the experimental cell line with downregulated SOX10, as confirmed through a TUNEL assay. Additionally, microarray screening revealed a three-fold decrease in SOX10 and one of its downstream targets, MITF [40]. These findings highlight the significant role of SOX10 expression and its effect on MITF in B16F10 melanoma cells, suggesting a crucial role in cell migration and, consequently, metastasis [40]. Attempts to replicate these results using different melanoma cell lines (Cloudman S9 and Melan-A melanoma cell lines) yielded no statistically significant effects on cell migration, emphasizing the variability of SOX10 effects depending on the specific cell line being studied [40].
In light of the diverse yet persistent role of SOX10 in melanoma cells, its potential as a diagnostic histopathological marker has been explored. Clevenger et al. conducted a comparative study using a pan-melanoma cocktail, a SOX10 stain, and an MITF stain to identify melanoma cells of epithelioid origin, those with a predominantly spindle appearance. That study revealed a 100% SOX10 positive staining pattern in both epithelioid and spindle-shaped cells, demonstrating nuclear staining with a strong and diffused pattern. In contrast, the pan melanoma cocktail and MITF stain showed positive staining in 86% and 93% of cases, respectively, for epithelioid cells, and 86% for spindle-shaped melanoma cells [41]. The high rate of detection using SOX10 staining suggests its utility in detecting metastasis in locations where a small number of cells would be expected, such as the cerebrospinal fluid (CSF). However, caution is advised, and a more sensitive stain for melanoma should be considered due to the non-exclusive expression of SOX10 [41].
MITF, downstream of the SOX10 gene, plays a crucial role in the transcription control of melanocytes and retinal pigment cells and is strongly associated with malignancies [42]. Studies have shown that the absence or reduced activity of SOX10 consistently leads to cell death in melanocytic descent, particularly at the G1 stage of the cell cycle. The impact on lineage is associated with the type of knockout, whether it involves a complete knockout or an interruption in the product’s structure, or a reduction in the half-life [39,43].
Notably, the knockout of SOX10, when simultaneously treating advanced melanoma, can confer resistive mechanisms against chemotherapeutic medications. Using Vemurafenib to treat advanced melanoma with an observed BRAFV600E mutation, cells acquiring a somatic SOX10 mutation that hinders proper gene product formation allow the tumor to grow unchallenged by therapeutic treatments that would otherwise be effective [42,44]. This underscores the intricacies and complex integration of SOX10, which primarily directs proliferation and steers cells toward differentiated paths. Acting as an oversight system for downstream transcription factors, such as MITF [45], the gene gains unregulated function to promote transcriptive and translative efforts within the cell, allowing malignancies to establish their proliferative roots [39,44]. However, the knockout of SOX10 in existing cancers can lead to acquired resistance against chemotherapeutic efforts. In other iterations of malignancies, knocking out the gene has been found to suspend cell proliferation, restrain cell growth, and reduce overall tumor size [39,42,43]. As melanomas approach their proliferative limits or the threshold for potential invasion, SOX10 has been observed to become downregulated within the tumor cells. This change induces a phenotypic shift from melanocytic cell lineages to undifferentiated mesenchymal cell lines, characterized by their invasive nature and ability to resist targeted therapeutic regiments against malignancy [42].
Not all mutations of the SOX10 locus are somatic. In studies focusing on childhood melanoma, almost all congenital melanomas were found to be SOX10 positive. The significance of this positivity, whether it represents an unhindered function or a gain-of-function mutation, is yet to be determined. Regardless, its presence signifies its key integration in the early stages of skin neoplasms [46]. Studies supporting SOX10 as a more sensitive marker for melanoma, compared to MITF, the previous standard marker for neoplastic testing within this sector, further highlight its diagnostic potential [3,45].
In research by Shakova et al. concerning the significance of SOX10 in melanoma and congenital giant melanocytic nevus, a pre-cancerous lesion heavily associated with melanoma formation, it was confirmed in mouse subjects and later human cell lines that the knockout of this transcription factor showed effective results in blocking tumorigenesis. Furthermore, the knockout or inactivation of the SOX10 gene established its role as a prerequisite for the formation and maintenance of pre-melanoma lesions [46]. In observed human cell lines, the absence of SOX10 activity resulted in an estimated nine-fold increase in apoptotic cells due to the disrupted regulation of apoptotic control factors. Examples of this dysregulation were noted from the increases in these control factors, such as caspases and proteins related to the tumor necrosis factor (TNF) pathway [46].
A study by Capparelli et al. demonstrated that SOX10 plays a crucial role in mediating phenotypic switching in cutaneous melanoma. The loss of SOX10 led to the development of an invasive, slow-cycling state in melanoma cells, promoting tolerance to BRAF and/or MEK inhibitors, which are commonly used in melanoma treatment. That study also identified a vulnerability in SOX10-deficient melanoma cells, specifically an up-regulation of cellular inhibitors of apoptosis-2 (cIAP2). The use of cIAP1/2 inhibitors selectively induced cell death in SOX10-deficient cells, providing a potential therapeutic strategy to target and eliminate these cells. Additionally, combining cIAP1/2 inhibitors with BRAF/MEK inhibitors delayed the onset of acquired resistance in melanomas in vivo [47].
4.2. SOX10 Expression in Malignant Peripheral Nerve Sheath Tumor and Schwannomas
While much of the existing data on the role of SOX10 in neoplasms primarily focuses on melanoma, this gene is also implicated in other neural and neuroectodermal tumors. Malignant peripheral nerve sheath tumor (MPNST) is one such malignancy where the role of SOX10 has been investigated. A study by Kang et al. aimed to assess SOX10 as a marker for distinguishing MPNST from synovial sarcoma, given the histopathological similarities that can make differentiation challenging [48]. SOX10 staining revealed a 67% positivity rate in MPNST cells compared to only 7% in synovial sarcomas. The overall results demonstrated a 67% sensitivity rate and a high specificity rate of 93% for SOX10 staining in MPNST, with a positive predictive value of 82% and a negative predictive value of 89% [48]. These findings suggest that SOX10 staining is moderately sensitive but highly specific, serving as a valuable marker for differentiating MPNST from synovial sarcomas in cases where there is a diagnostic discrepancy [48].
Another study by Pekmezci et al. investigated the use of SOX10 as a differentiating marker between MPNST and schwannomas, revealing a positive diffuse SOX10 expression pattern seen only in cellular schwannomas [49]. The results imply that SOX10 expression is significantly more prevalent in cellular schwannomas, and its loss of expression is indicative of MPNST when compared to cellular schwannomas [49]. Doddrell et al. explored SOX10 expression in merlin-null schwannomas, finding reduced expression of SOX10 and two proteins crucial for the myelinating function of Schwann cells: KROX20 and OCT6 [50]. Reintroducing the SOX10 gene in schwannoma cells showed a small increase in KROX20 expression, which significantly increased with the introduction of cAMP [50]. Overall, the results suggest that the loss of SOX10 in Schwann cells leads to cellular abnormalities resembling schwannomas [50]. Collectively, these studies indicate that SOX10 expression is a relatively effective marker for differentiating between specific malignancies that may pose diagnostic challenges. Moreover, a recurring pattern in the reported results suggests that SOX10 expression tends to decrease as cells undergo a transition from normal to malignant states in tumors.

Table 1.
Studies demonstrating SOX10 expression in neural and neuroectodermal tumors.
Table 1.
Studies demonstrating SOX10 expression in neural and neuroectodermal tumors.
| Tumor | References | Findings |
|---|---|---|
| Melanoma | [51] | Role of SOX10 in Melanoma:
|
| [45] |
| |
| [39] |
| |
| [40] | SOX10 in Melanoma Cell Migration and Metastasis:
| |
| [41] | SOX10 as a Diagnostic Marker for Melanoma:
| |
| [36] | SOX10 and Nestin in Melanoma Development:
| |
| Malignant peripheral nerve sheath tumor | [48,49] | SOX10 in Differentiating MPNSTs and Synovial Sarcomas:
|
| Merlin-null schwannoma | [50] | SOX10 in Schwannomas and Normal Schwann Cell Function:
|
5. SOX10 Expression in Mesenchymal Tumors
In a study conducted by Miettinen et al., the expression of SOX10 was analyzed in 1645 non-neurogenic mesenchymal tumors. Among non-nerve sheath tumors, positive SOX10 tumor cells were identified only in alveolar rhabdomyosarcoma (2/27) and ossifying fibromyxoid tumors (2/47). Thirty-three other types of mesenchymal tissues analyzed (1571 samples), including fibroblastic-myofibroblastic tumors, benign fibrous histiocytoma and subtypes, solitary fibrous tumor/hemangiopericytoma of the peripheral soft tissues and intracranial space, and undifferentiated pleomorphic sarcomas, were negative for SOX10. Synovial sarcomas, desmoid fibromatosis, and glomus tumors showed fewer than 5% of SOX10-positive nuclei, possibly representing entangled neural components [52].
Research by Karamchandani et al. aimed to validate the use of SOX10 and S100 protein as reliable markers in soft tissue neoplasms of both neural crest and non-neural crest origin. SOX10 and S100 mRNA levels were evaluated in 122 cases of peripheral nerve sheath tumors and synovial sarcomas, and IHC was used for SOX10 and S100 protein expression in 1012 tissue specimens [53]. Synovial sarcomas expressed significantly higher levels of S100 than SOX10, and no significant SOX10 mRNA expression was identified in synovial sarcoma [53]. The majority of schwannomas and neurofibromas showed increased expression of both SOX10 and S100 mRNA [53]. MPNSTs revealed highly correlated, variable levels of SOX10 and S100 mRNA expression. Of the non-neural, nonmelanocytic sarcomas, only one rhabdomyosarcoma sample was positive for SOX10. In summary, SOX10 was positive in only 5 of 668 cases with a 99% specificity for non-schwannian, nonmelanocytic tumors [53].
Kang et al. evaluated the diagnostic utility of SOX10 IHC in differentiating between synovial sarcoma and MPNST due to similar histomorphology and immunophenotype [48]. Forty-eight cases of MPNST and 97 cases of synovial sarcoma, including four intraneural synovial sarcomas, were stained for SOX10. Sixty-seven percent of MPNST (32/48) and only 7% (7/97) of synovial sarcomas were positive for SOX10. Nevertheless, there is uncertainty as to whether SOX10-positive cells in intraneural synovial sarcoma represent entangled Schwann cells, synovial sarcoma cells, or both [48].
In an attempt to demonstrate the clinical and morphological heterogeneity between gastrointestinal mesenchymal tumors with neurotrophic tyrosine receptor kinase (NTRK) gene rearrangements and gastrointestinal stromal tumors, Atiq et al. reported consistently absent SOX10 expression in eight mesenchymal tumors in the gastrointestinal tract with NTRK1 or NTRK3 rearrangements [54].
Research by Chiang et al. focused on classifying a newly discovered category of high-grade uterine sarcomas. Four NTRK fusion-positive uterine sarcomas were identified and distinguished from both undifferentiated uterine sarcomas and more commonly aggressive leiomyosarcomas. All four mesenchymal tumors lacked SOX10 expression [55].
6. SOX10 Expression in Epithelial Neoplasms
When examining the impact of SOX10 on epithelial neoplasms, its influence is extensive and continues to unfold with further investigations. This transcription factor plays a crucial role in regulating the proliferation and specialization processes of melanocyte and Schwannian lineages, exhibiting high expression levels in melanoma malignancies and those affecting the central nervous system [3]. While many of the studied mutations indicate somatic changes, there are instances of inherited cases [46]. In the observed cases, SOX10 expression is more prevalent in malignancies during proliferative stages compared to those found in invasive or metastatic stages [44] (Table 2).
Beyond tumors involving melanocytic lineages, research has provided substantial evidence of SOX10 expression in the salivary gland, breast, and ovarian neoplasms affecting epithelial cells. Although this evidence has accumulated in recent years, sensitivity for diagnostic differentiation, particularly in salivary gland tumors, remains less reliable [56]. Conversely, concerning ovarian cancers, distinguishing between SOX10 expression within the nucleus and cytoplasm has shown promise in estimating grade and prognosis.
In the context of salivary gland neoplasms, SOX10 expression has been identified in tumors arising from acinar and intercalated ductal cells [57,58,59]. Notably, tumors lacking SOX10 have been associated with the appearance of excretory ducts or striated ducts [59]. SOX10-expressing neoplasms in the salivary glands include acinic cell carcinoma, epithelial-myoepithelial carcinoma, adenoid cystic carcinoma, and polymorphous adenocarcinoma [59,60]. Adenoid cystic carcinoma and polymorphous adenocarcinoma have consistently demonstrated SOX10 expression in virtually all cases studied [57,58]. Distinctively, acinic cell carcinoma can be differentiated from metastatic renal cell carcinoma in the parotid gland, as the latter does not express SOX10 on staining [58].
However, certain salivary gland neoplasms either show no SOX10 representation or exhibit focal expression in staining. These include salivary duct carcinoma, mucoepidermoid carcinoma (MEC), squamous cell carcinoma (SCC), and oncocytic carcinoma, which originate from excretory and serous ductal cells within the salivary glands. While MEC tumors were initially considered SOX10-negative, further investigation revealed a subgroup of SOX10-positive MEC with distinct morphology and colloid-like secretion [57,59]. Additionally, SOX10 has been found to be positive in other tumors, such as basal cell carcinomas (BCCs) and low-grade salivary duct carcinomas [57]. In the case of SCC secondary to HPV infection, SOX10 is not a reliable diagnostic marker due to similar staining distributions with HPV-related multiphenotypic sinonasal carcinoma [61]. While SOX10 staining can aid in categorizing tumors based on cell origins, negative staining does not necessarily imply the absence of SOX10 mutation, as inactivating or truncating mutations can result in reduced or absent SOX10 expression [59,61,62]. Despite this, SOX10 is considered a valuable protein expression marker for the diagnostic identification of salivary gland neoplasms, contributing to increased diagnostic accuracy [58].
SOX10 protein expression has also been observed in breast carcinomas, particularly in approximately 66–74% of triple-negative breast carcinomas [63]. Triple-negative breast carcinoma has shown SOX10 expression in a substantial number of cases, ranging from 38 to 67% in the literature [62]. SOX10 has been associated with CD117 and vimentin expression in triple-negative breast carcinomas, though its prognostic value remains inconclusive and is mainly considered a marker for aiding in differential diagnoses [62]. While evidence suggests a potential prognostic value due to associations with malignant characteristics of triple-negative breast carcinomas, further research is needed to establish its definitive prognostic significance [64]. In cases where homozygous deletions and point mutations eliminate SOX10 staining presence, GATA3, a common marker in breast carcinoma, has been used in conjunction with SOX10 to address this limitation. Approximately 60% of triple-negative breast carcinomas have been identified using this dual-staining method, making SOX10 a useful marker in identifying epithelial neoplasms of the breast [62,65].
In the context of ovarian epithelial tumors, SOX10 has shown value in differentiating cell origin and estimating prognosis. Contrary to previous claims that suggested no application for SOX10 in the study and diagnosis of ovarian epithelial tumors, Kwon et al. demonstrated its utility. Ovarian epithelial neoplasms, including serous, mucinous, and endometrioid subtypes, can be differentiated based on the localization of staining. Serous neoplasms show nuclear localization, while mucinous and endometrioid neoplasms exhibit cytoplasmic localization [66]. Staining in both regions is possible, but the diffuse characteristic of SOX10 staining helps distinguish between subtypes. The intensity of the stain within the nucleus correlates with the prognosis of the patient, emphasizing its potential as a prognostic marker [66]. While SOX10’s involvement in ovarian carcinomas was assessed, other common expression markers studied for ovarian cancer include SOX8 and, notably, SOX9, which has been implicated in various signaling pathways in ovarian cancer development [67,68,69,70,71].
In nasopharyngeal carcinomas, SOX10 is markedly overexpressed, and this overexpression is associated with a poorer prognosis, particularly in T classification and lymph node metastasis. The correlation with poor prognosis is linked to SOX10’s involvement in tumor development and metastatic-seeding ability in breast cancer cells. The overexpression of SOX10 in nasopharyngeal tumors highlights its potential importance as a diagnostic and prognostic marker for patients with nasopharyngeal carcinoma [72].
SOX10 has also been associated with metaplastic bladder cancers, where it exhibits elevated expression in bladder cancer tissues compared to healthy tissue. Knockdown experiments targeting SOX10 confirmed its prognostic value by significantly impacting the growth and metastatic ability of bladder cancer. The suspected mechanism involves SOX10 influencing the expression of other components such as B-catenin and Met. Targeting SOX10 as a marker for diagnosis, prognosis, and treatment may prove useful in the context of bladder cancer [16].
In summary, while further research is needed to fully understand the extent of SOX10 expression in various epithelial neoplasms, it remains a promising marker for the diagnosis and prognosis development of several carcinomas. Additionally, it shows potential as a treatment target in certain cancers.

Table 2.
SOX10 expression in epithelial neoplasms.
Table 2.
SOX10 expression in epithelial neoplasms.
| Epithelial Neoplasm | SOX10 Expression | Implications |
|---|---|---|
| Ovarian serous, mucinous, and endometrioid carcinoma | Overexpressed |
|
| Triple-negative breast cancer | Overexpressed |
|
| Nasopharyngeal carcinomas | Overexpressed |
|
| Bladder carcinomas | Overexpressed |
|
| Salivary gland neoplasms | Overexpressed |
|
| Gastrointestinal Mesenchymal Tumors | Lost |
|
| Uterine Sarcomas | Lost |
|
7. Expression of Other Members of the “SRY-Related HMG Box” in Cancers
7.1. The HMG Box Family
The HMG box is a versatile protein domain consisting of about 75 amino acids that plays a crucial role in DNA binding and various transcription and translation processes. The name “High Mobility” originates from the initial discovery of these proteins in the acid extracts of mammalian chromatin, where they exhibited significant electrophoretic mobility [73].
HMG box domains can be broadly categorized into two types based on their DNA binding specificity: non-sequence specific; and sequence specific [74]. Both types of HMG box domains exhibit a high affinity for non-B-type DNA structures, which include bent, kinked, and unwound DNA. Additionally, these domains are involved in diverse protein-protein interactions, such as DNA bending, looping, and unwinding [74,75].
7.1.1. Non-Sequence Specific HMG Box Domains
- Proteins in this category, such as HMGB1-4, typically possess two HMG boxes or four to six HMG boxes in the presence of transcription factor UBF [75];
- Mammals have four HMGB proteins (HMGB1-4), and they function as DNA chaperones, contributing to processes like transcription and DNA repair. However, each of these proteins has distinct characteristics [75].
7.1.2. Sequence Specific HMG Box Domains
- Proteins classified as sequence-specific usually have a single HMG box and lack acidic C-tails, which are common in non-sequence-specific HMG box proteins [74];
- Examples of proteins in this category include TCF, SRY, and SOX [75];
- Despite recognizing specific DNA sequences, these proteins form few base-specific hydrogen bonds, resulting in less sequence specificity [75].
7.2. SRY-Related HMG Box
The SOX genes, a subset of HMG box-type proteins, are encoded by 20 different genes in both humans and mice. These genes, located within the SRY gene on the Y chromosome, play pivotal roles in various cellular processes, including stemness maintenance, cell lineage determination, differentiation, proliferation, and even cell death. Unlike typical DNA modification mechanisms, SOX genes achieve their functions by binding specifically to the minor groove of pre-existing DNA, thereby influencing its shape and facilitating higher affinity binding of DNA to various transcription factors [76]. Key features of the SOX genes include the below.
7.2.1. Genetic Organization
- SOX genes are organized into eight groups (A–H), with group B further divided into subgroups B1 and B2 [76];
- Within the same group, SOX proteins share a high degree of structural and identity similarity, ranging from 70% to 95%, both in the HMG box domain and in external characteristics;
- Groups outside the same group have partial similarities in identities (>46%) in the HMG box domain and none in the external domains [76];
7.2.2. Functions and Mechanisms
- SOX genes play crucial roles in DNA replication and mutations, contributing to diverse cellular processes [76];
7.2.3. Individual SOX Genes
- The specific locus and schematic of the different SOX genes are detailed in Table 3
The following sections will provide insights into the implications of individual SOX gene groups in the genesis and progression of common cancers. The diversity within the SOX gene family allows for a wide range of functions and regulatory roles in cellular processes, making them essential players in normal development as well as potential contributors to cancer development [76].

Table 3.
Specific locus and schematic of the different SOX genes. The blue oval represents the HMG box domain. The text within the blue oval indicates which SOX gene the schematic correlates to. The hexagon with “TA” indicates a transactivation domain. The hexagon with “TR” indicates a trans-repression domain. The gray diamond with “D” indicates a dimerization domain. Schematics were created with BioRender.com (2023).
Table 3.
Specific locus and schematic of the different SOX genes. The blue oval represents the HMG box domain. The text within the blue oval indicates which SOX gene the schematic correlates to. The hexagon with “TA” indicates a transactivation domain. The hexagon with “TR” indicates a trans-repression domain. The gray diamond with “D” indicates a dimerization domain. Schematics were created with BioRender.com (2023).
| Group | Gene | Locus | Schematic |
|---|---|---|---|
| A | SRY | YC3 |  |
| B1 | SOX1 | 8 A1–A2 | 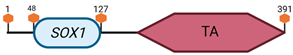 |
| SOX2 | 3 A2–B | 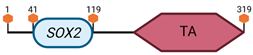 | |
| SOX3 | X A7.3–B | 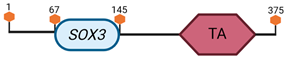 | |
| B2 | SOX14 | 9 E3.3 | 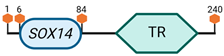 |
| SOX21 | 14 E4 | 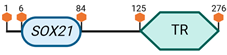 | |
| C | SOX4 | 13 A3–A5 |  |
| SOX11 | 12 A3 |  | |
| SOX12 | 2 G3 |  | |
| D | SOX5 | 6 G3 | 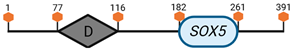 |
| L-SOX5 | 6 G3 | 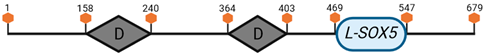 | |
| SOX6 | 7 F1 |  | |
| SOX13 | 1 E4 |  | |
| E | SOX8 | 17 A3 |  |
| SOX9 | 11 E2 | 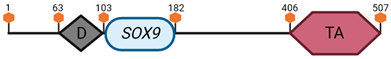 | |
| SOX10 | 15 E1 | 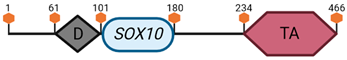 | |
| F | SOX7 | 14 C3 | 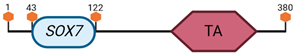 |
| SOX17 | 1 A1 |  | |
| SOX18 | 2 H4 |  | |
| G | SOX15 | 11 B3 |  |
| H | SOX30 | 11 B1.1 |  |
Group A
Group A of the SOX gene family consists of a single member, SRY (Sex-determining Region Y), and its primary function is to determine sex in mammals [77,78]. In the context of cancer, particularly in prostate cancer, the role of SRY is not well understood, and it is unclear whether SRY acts as a tumor suppressor or has other implications in cancer development [79]. Downregulation of SRY has been observed in prostate cancer, but it often occurs concurrently with the downregulation of other Y chromosome-specific genes [79]. Therefore, it would be premature to attribute the development and proliferation of prostate cancer solely to the downregulation of SRY. Further research is needed to elucidate the specific role of SRY and its potential contributions to prostate cancer and other cancers.
Group B (B1 + B2)
Group B of the SOX gene family consists of SOX1, SOX2, SOX3, SOX14, and SOX21. SOX1, SOX2, and SOX3 belong to subgroup B1, while SOX14 and SOX21 fall into subgroup B2 [77,80,81,82].
SOX1
- Function: SOX1 plays a crucial role in maintaining stem cell lineage, particularly in embryogenesis, differentiation, and mammalian brain development. It is essential for the survival and function of dopaminergic neurons [80];
- Oncogenic properties: SOX1 has been implicated in the development of small cell lung, central nervous system, breast, and ovarian cancers. In small-cell lung cancer, SOX1 collaborates with NKX2.1 to maintain its identity and function. In central nervous system tumors like glioblastomas, SOX1 extends the survivability of cancer cells [83]. In breast and ovarian cancer, SOX1 acts as a tumor suppressor by inhibiting the Wnt/B-Catenin and STAT3 signaling pathways [84,85];
SOX2
- Function: SOX2 is a transcription factor that prolongs stemness in both embryonic and adult stem cells [86];
- Oncogenic properties: Dysregulation of SOX2 expression is associated with increased proliferation and metastasis in the central nervous system and lung carcinomas [86];
SOX3
- Function: SOX3 is upregulated in esophageal SCC, ovarian carcinoma, and osteosarcoma, promoting proliferation and migration [87]. It induces apoptosis in human breast cancer cell lines [87];
SOX14
- Function: SOX14 is involved in the development of cervical cancer, inducing P53 activation, which leads to apoptosis in cervical carcinoma cell lines [88]. It also promotes proliferation and invasion through the Wnt/B-catenin pathway [88,89];
SOX21
- Function: SOX21 has a tumor suppressor-like function in central nervous system cancers [90], inhibiting the carcinogenic properties of SOX2 [91]. Forced expression of SOX21 induces cellular apoptosis in glioma cells and enables differentiation, preventing glioma formation [90].
These SOX genes in Group B exhibit diverse functions and play critical roles in various cancers, either promoting or inhibiting oncogenic processes. Their involvement underscores the complexity of SOX gene functions in different cellular contexts and cancer types;
Group C
The SOX genes that have been classified into group C include SOX4, SOX11, and SOX12;
SOX4
- Function: SOX4 is implicated in embryogenesis and tissue development [92,93,94,95,96,97];
- Cancer associations: Elevated SOX4 expression is observed in leukemia, colorectal, lung, breast, and hepatocellular cancers [92,93,94,95,96,97]. In hepatocellular carcinoma, increased SOX4 expression inhibits P53-directed apoptosis by restricting BAX expression [96];
SOX11
- Function: SOX11 serves as both a causative and protective agent in various tumors;
- Cancer associations: Upregulation of SOX11 is seen in medulloblastoma, mantle cell lymphoma, endometrial and breast cancer, Burkitt’s lymphoma, colorectal cancer, lung adenocarcinoma, lung SCC, and ovarian cancer [98,99,100,101,102,103,104]. SOX11 expression is a unique feature in certain cancers and helps distinguish them from other malignancies [105,106];
- Prognostic factors: High SOX11 expression in gastric and ovarian cancers is linked to higher survival rates, while in breast cancers, the opposite is observed [102,107];
SOX12
- Function: Hepatocellular carcinomas positive for SOX12 exhibit increased proliferation, malignant potential, and higher resistance to cisplatin, a common chemotherapy agent [108];
- Cancer associations: SOX12 is involved in gastric, lung, hepatocellular, colorectal, renal carcinomas, and thyroid cancers (elevated levels) [51,108,109,110]. Increased SOX12 expression in thyroid cancer cells is associated with promoting carcinogenic properties [51].
The SOX genes in Group C play diverse roles in embryonic development and tissue maintenance and are implicated in various cancers. They contribute to the complexity of cancer biology by either promoting or inhibiting tumorigenesis depending on the specific context and cancer type;
Group D
The SOX genes that have been classified into group D include SOX5, SOX6, and SOX13;
SOX5
- Function: SOX5 plays a role in the development and differentiation of embryonic germ cell lines [111];
- Cancer associations: Similar to other SRY-related HMG box genes, SOX5 elevates the ability of cancer to grow, metastasize, and invade through angiogenesis. It is implicated in hepatocellular, breast, and gastric cancer [112,113,114];
- Unique properties: SOX5 can mediate the epithelial-to-mesenchymal transition (EMT), a fundamental process in metastasis, by regulating the expression of E-cadherin and vimentin [112,115,116];
SOX6
- Function: SOX6 exhibits both tumor suppressor and oncogenic properties depending on the cancer type;
- Cancer associations: SOX6 is downregulated in osteosarcoma [117], esophageal SCC [118], hepatocellular carcinoma [119], and pancreatic β-cell cancers [120]. It shows oncogenic properties in gliomas [121] and endometrial cancers [122];
- Unique properties: SOX6 induces autophagy in cervical cancer cell lines, leading to increased resistance to cisplatin chemotherapy and enhanced survivability [123];
SOX13
- Cancer associations: SOX13 is highly expressed in oligodendrogliomas, gliomas, gastric carcinomas, and hepatocellular carcinomas [123,124,125,126]. SOX13 overexpression in hepatocellular carcinoma activates TWIST1, a major transcription factor in embryonic development, promoting cancer metastasis [126]. SOX13 supports stem-like properties in hepatocellular carcinoma, contributing to increased self-renewal, resistance to chemotherapy, and tumorgenicity [127].
These SOX genes, namely SOX5, SOX6, and SOX13, demonstrate diverse roles in embryonic development and cancer biology. Their involvement in processes like EMT, autophagy induction, and support for stem-like properties highlights their significance in the complex landscape of cancer progression and metastasis;
Group E
The SOX genes that have been classified into group E include SOX8, SOX9, and SOX10;
SOX8
- Function: SOX8 has some minor effects on the specification and differentiation of glial cells;
- Cancer associations: SOX8 expression is greatest during central nervous system development in immature cells. Elevated levels of SOX8 indicate an undifferentiated state in the gliomas [124];
SOX9
- Function: SOX9 is involved in multiple cancers in a variety of ways;
- Cancer associations: In some breast cancer subtypes, SOX9 is involved in a positive feedback loop through Wnt/β-catenin activation [128]. Prostate cancer tends to be correlated with elevated levels of SOX9 [129]. SOX9 contributes to cell proliferation and invasion in renal cell carcinoma. MiRNA-138-induced SOX9 suppression prevents renal cell carcinoma progression [130]. Through the WNT/β-catenin pathway, SOX9 is involved in cancer cell proliferation and invasion in papillary thyroid cancer [131]. SOX9 increases LGR5 expression, imparting the ability of glioblastoma cells to undergo tumorigenesis [77]. Elevated levels of SOX9 expression in colorectal cancers are associated with lower 5-year survival rates [132]. SOX9 levels are increased in non-small lung cancer [77] due to tumor-associated macrophages, which release TGF-β [133]. In skin cancers, SOX9 levels are elevated too [77]. Increased SOX9 levels cause melanoma cells to metastasize [134]. SOX9-involved keratinocyte proliferation also occurs in cutaneous BCC and cutaneous SCC [135].
SOX8, SOX9, and SOX10 play diverse roles in different cancers, influencing processes such as differentiation, proliferation, invasion, and tumorigenesis. Understanding their specific functions in various cancer types is crucial for developing targeted therapeutic approaches;
Group F
The SOX genes that have been classified into group F include SOX7, SOX17, and SOX18;
SOX7
- Cancer associations: SOX7 is implicated in several cancers. In breast cancer, SOX7 functions as a tumor suppressor [136]. Hypermethylation-mediated silencing of the SOX7 promoter is associated with greater carcinogenesis in breast cancer [136]. SOX7 can be used as a marker for prognosis in prostate cancer. Its downregulation may be involved in the castration-resistant progression of prostate cancer [129]. SOX7 also exhibits tumor-suppressive effects in gastric cancer through potential involvement in abnormalities with the SOX7-associated WNT/β-catenin pathway [137]. SOX7’s tumor suppressor effects have also been delineated in non-small-cell lung cancer, targeted by microRNA-9 [138];
SOX17
- Cancer associations: SOX17 is associated with several cancers. Hypermethylation-dependent silencing of the SOX17 promoter may induce inappropriate activation of the Wnt pathway, giving rise to breast cancer, thyroid cancer, gliomas, and gastrointestinal tumors [139,140,141,142]. Melanoma pathogenesis is also associated with decreased SOX17 expression; however, the mechanism is unclear [143];
SOX18
- Function: SOX18 takes part in the development of blood and lymphatic vessels, as well as hair follicles [144]. Wound healing also involves SOX18 [145].
- Cancer associations: SOX18 is associated with breast, lung, and skin cancers. In breast cancer, there is a positive correlation between SOX18 and vascular endothelial growth factor D (VEGF-D), suggesting that SOX18 positively influences angiogenesis [144]. In non-small-cell lung cancer, SOX18 expression is noted in cells and vessels, and its expression may be used as a prognostic marker [145]. In skin cancers, elevated SOX18 expression is involved in the formation of BCC and SCC [146].
Understanding the roles of SOX7, SOX17, and SOX18 in various cancers provides insights into their potential as diagnostic markers and therapeutic targets in cancer treatment;
Group G
The sole member of this group is SOX15. Compared to the other members of the SOX family, it has been relatively understudied. Overexpression of SOX15 is linked to lower proliferation of testicular embryonic cancer cell lines [147]. SOX15 serves as a potential tumor suppressor gene and is negatively associated with the development of pancreatic ductal adenocarcinoma through the Wnt/B-catenin pathway [148]. Additionally, SOX15 is repeatedly underexpressed among cancer cell lines, including colon, prostate, stomach, and uterine cancers, and overexpressed in some subsets of lung carcinomas [149].
SOX15, despite being relatively understudied compared to other SOX family members, demonstrates potential significance in regulating proliferation and acting as a tumor suppressor in specific cancer types, such as testicular embryonic cancer and pancreatic ductal adenocarcinoma. Its differential expression across various cancers suggests a context-dependent role, and further research may unveil its precise mechanisms and therapeutic implications;
Group H
SOX30 is the sole member of Group H [77]. It acts as a tumor suppressor by activating P53 transcription, leading to apoptosis. SOX30 inhibits T-cell factor (TCF) either by binding to β-catenin or inhibiting β-catenin transcription [77,150,151]. Regarding lung adenocarcinoma specifically, the latter can be associated with hypermethylation of the SOX30 gene. SOX30’s inhibition of TCF can contribute to the development of lung cancer. It also functions as a tumor suppressor by activating desmosomal genes, impeding cancer growth and spread [77,152].
8. Conclusions and Future Directions
In conclusion, SOX10 emerges as a pivotal transcription factor with a multifaceted role extending from embryonic development to the pathogenesis of diverse pathological conditions. Its critical significance is exemplified by its association with congenital disorders such as Waardenburg–Shah Syndrome, PCWH syndrome, and Kallman syndrome, where mutations disrupt neural crest development. Within neural and neuroectodermal tumors, SOX10 serves as a key player influencing proliferation and differentiation, making it a promising diagnostic and therapeutic marker.
The spotlight on SOX10 intensifies in melanoma, where its impact on crucial factors like MITF and cell migration shapes tumor progression and treatment responses. In mesenchymal tumors, SOX10 expression becomes a valuable tool for distinguishing between different tumor types, thereby facilitating accurate diagnoses and informed treatment decisions.
Epithelial neoplasms further underscore SOX10’s clinical relevance. Its expression or absence provides crucial insights into tumor cell origins, prognosis, and treatment responses. Particularly in ovarian cancer, SOX10’s involvement in chemoresistance highlights its significance in clinical settings.
The multifunctionality of SOX10 positions it as a promising candidate for extensive research and clinical applications across various pathological conditions. As we delve deeper into its intricacies, there is potential for improved diagnostic accuracy and the development of more effective therapeutic strategies. SOX10 stands at the intersection of basic research and clinical utility, holding promise for advancements that could reshape our approach to a spectrum of diseases.
Author Contributions
Conceptualization, H.F.B.; methodology, H.F.B., A.T., K.S., J.G., V.A., S.O., A.R.A., R.A., A.H.A. and S.H.; investigation, H.F.B., A.T.; K.S., J.G., V.A., S.O., A.R.A., R.A. and A.H.A.; resources, H.F.B., A.T., K.S., J.G., V.A., S.O., A.R.A., R.A., A.H.A. and S.H.; data curation, H.F.B., A.T., K.S., J.G., V.A., S.O., A.R.A., R.A. and A.H.A.; writing—original draft preparation, H.F.B., A.T., K.S., J.G., V.A., S.O., A.R.A., R.A., A.H.A. and S.H.; writing—review and editing, R.P.; visualization, R.P.; supervision, R.P.; project administration, H.F.B.; funding acquisition, H.F.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
We would like to thank all members of the Department of Pathology and Laboratory Medicine, Mount Sinai Medical Center of Florida (Miami Beach, FL, USA) for their help with this work. Figures were created with BioRender.com (accessed on 7 October 2023). All rights and ownership of BioRender content are reserved by BioRender (Agreement numbers JK25Y0GP09, JF25Y0I2WN, CJ25Y0KPLG, and YD25Y0KPPH). BioRender content included in the completed graphic is not licensed for any commercial uses beyond publication in a journal.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Herbarth, B.; Pingault, V.; Bondurand, N.; Kuhlbrodt, K.; Hermans-Borgmeyer, I.; Puliti, A.; Lemort, N.; Goossens, M.; Wegner, M. Mutation of the Sry-related Sox10 gene in Dominant megacolon, a mouse model for human Hirschsprung disease. Proc. Natl. Acad. Sci. USA 1998, 95, 5161–5165. [Google Scholar] [CrossRef]
- Aoki, Y.; Saint-Germain, N.; Gyda, M.; Magner-Fink, E.; Lee, Y.H.; Credidio, C.; Saint-Jeannet, J.P. Sox10 regulates the development of neural crest-derived melanocytes in Xenopus. Dev. Biol. 2003, 259, 19–33. [Google Scholar] [CrossRef]
- Pingault, V.; Zerad, L.; Bertani-Torres, W.; Bondurand, N. SOX10: 20 years of phenotypic plurality and current understanding of its developmental function. J. Med. Genet. 2022, 59, 105–114. [Google Scholar] [CrossRef]
- Schreiner, S.; Cossais, F.; Fischer, K.; Scholz, S.; Bösl, M.R.; Holtmann, B.; Sendtner, M.; Wegner, M. Hypomorphic Sox10 alleles reveal novel protein functions and unravel developmental differences in glial lineages. Development 2007, 134, 3271–3281. [Google Scholar] [CrossRef] [PubMed]
- Pusch, C.; Hustert, E.; Pfeifer, D.; Südbeck, P.; Kist, R.; Roe, B.; Wang, Z.; Balling, R.; Blin, N.; Scherer, G. The SOX10/Sox10 gene from human and mouse: Sequence, expression, and transactivation by the encoded HMG domain transcription factor. Hum. Genet. 1998, 103, 115–123. [Google Scholar] [CrossRef]
- Sommer, L. Generation of melanocytes from neural crest cells. Pigment. Cell Melanoma Res. 2011, 24, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Yalan, L.; Hua, Z.; Yong, F. Progress in the study of syndromic hearing loss resulted from neural crest abnormalities. Yi Chuan 2014, 36, 1131–1144. [Google Scholar] [PubMed]
- Pingault, V.; Bondurand, N.; Kuhlbrodt, K.; Goerich, D.E.; Préhu, M.O.; Puliti, A.; Herbarth, B.; Hermans-Borgmeyer, I.; Legius, E.; Matthijs, G.; et al. SOX10 mutations in patients with Waardenburg-Hirschsprung disease. Nat. Genet. 1998, 18, 171–173. [Google Scholar] [CrossRef]
- Wissmüller, S.; Kosian, T.; Wolf, M.; Finzsch, M.; Wegner, M. The high-mobility-group domain of Sox proteins interacts with DNA-binding domains of many transcription factors. Nucleic Acids Res. 2006, 34, 1735–1744. [Google Scholar] [CrossRef] [PubMed]
- Schock, E.N.; LaBonne, C. Sorting Sox: Diverse Roles for Sox Transcription Factors During Neural Crest and Craniofacial Development. Front. Physiol. 2020, 11, 606889. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.A.C.; Soufi, A.; Pollard, S.M. Post-translational modification of SOX family proteins: Key biochemical targets in cancer? Semin. Cancer Biol. 2020, 67, 30–38. [Google Scholar] [CrossRef]
- Taylor, K.M.; Labonne, C. SoxE factors function equivalently during neural crest and inner ear development and their activity is regulated by SUMOylation. Dev. Cell 2005, 9, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Girard, M.; Goossens, M. Sumoylation of the SOX10 transcription factor regulates its transcriptional activity. FEBS Lett. 2006, 580, 1635–1641. [Google Scholar] [CrossRef] [PubMed]
- Peirano, R.I.; Goerich, D.E.; Riethmacher, D.; Wegner, M. Protein zero gene expression is regulated by the glial transcription factor Sox10. Mol. Cell Biol. 2000, 20, 3198–3209. [Google Scholar] [CrossRef] [PubMed]
- Amer, S.; Ibrahim, H.; Elkordy, M. The Immunohistochemical Expression of SOX-10 in Urothelial Carcinoma and the Non Neoplastic Urothelium; and a Correlation with the Tumor Features. Asian Pac. J. Cancer Prev. 2022, 23, 1425–1432. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Qin, C.; Zhao, Y.; Du, Y.; Sheng, Z.; Wang, Q.; Song, Q.; Chen, L.; Liu, C.; Xu, T. SOX10 is over-expressed in bladder cancer and contributes to the malignant bladder cancer cell behaviors. Clin. Transl. Oncol. 2017, 19, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Takeda, K.; Katori, Y.; Ikeda, K.; Oshima, T.; Yasumoto, K.; Saito, H.; Takasaka, T.; Shibahara, S. Expression of the Sox10 gene during mouse inner ear development. Brain Res. Mol. Brain Res. 2000, 84, 141–145. [Google Scholar] [CrossRef]
- Locher, H.; Frijns, J.H.; van Iperen, L.; de Groot, J.C.; Huisman, M.A.; Chuva de Sousa Lopes, S.M. Neurosensory development and cell fate determination in the human cochlea. Neural Dev. 2013, 8, 20. [Google Scholar] [CrossRef]
- Qi, J.; Ma, L.; Guo, W. Recent advances in the regulation mechanism of SOX10. J. Otol. 2022, 17, 247–252. [Google Scholar] [CrossRef]
- Britsch, S.; Goerich, D.E.; Riethmacher, D.; Peirano, R.I.; Rossner, M.; Nave, K.A.; Birchmeier, C.; Wegner, M. The transcription factor Sox10 is a key regulator of peripheral glial development. Genes. Dev. 2001, 15, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, C.; Law, W.D.; Rodríguez-Molina, J.F.; Prasad, A.B.; Song, L.; Crawford, G.E.; Mullikin, J.C.; Svaren, J.; Antonellis, A. Stringent comparative sequence analysis reveals SOX10 as a putative inhibitor of glial cell differentiation. BMC Genom. 2016, 17, 887. [Google Scholar] [CrossRef]
- Mertelmeyer, S.; Weider, M.; Baroti, T.; Reiprich, S.; Fröb, F.; Stolt, C.C.; Wagner, K.U.; Wegner, M. The transcription factor Sox10 is an essential determinant of branching morphogenesis and involution in the mouse mammary gland. Sci. Rep. 2020, 10, 17807. [Google Scholar] [CrossRef]
- Southard-Smith, E.M.; Kos, L.; Pavan, W.J. Sox10 mutation disrupts neural crest development in Dom Hirschsprung mouse model. Nat. Genet. 1998, 18, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Lane, P.W.; Liu, H.M. Association of megacolon with a new dominant spotting gene (Dom) in the mouse. J. Hered. 1984, 75, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Veronique, P.; Dorothée, E.; Florence Dastot-Le, M.; Michel, G.; Sandrine, M.; Nadege, B. Review and update of mutations causing Waardenburg syndrome. Hum. Mutat. 2010, 31, 391–406. [Google Scholar] [CrossRef]
- Bondurand, N.; Dastot-Le Moal, F.; Stanchina, L.; Collot, N.; Baral, V.; Marlin, S.; Attie-Bitach, T.; Giurgea, I.; Skopinski, L.; Reardon, W.; et al. Deletions at the SOX10 gene locus cause Waardenburg syndrome types 2 and 4. Am. J. Hum. Genet. 2007, 81, 1169–1185. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Shilo, K.; Boerkoel, C.F.; Crowe, C.; Sawady, J.; Lupski, J.R.; Agamanolis, D.P. Congenital hypomyelinating neuropathy, central dysmyelination, and Waardenburg-Hirschsprung disease: Phenotypes linked by SOX10 mutation. Ann. Neurol. 2002, 52, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Touraine, R.L.; Attié-Bitach, T.; Manceau, E.; Korsch, E.; Sarda, P.; Pingault, V.; Encha-Razavi, F.; Pelet, A.; Augé, J.; Nivelon-Chevallier, A.; et al. Neurological phenotype in Waardenburg syndrome type 4 correlates with novel SOX10 truncating mutations and expression in developing brain. Am. J. Hum. Genet. 2000, 66, 1496–1503. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Tanabe, Y.; Lupski, J.R. Myelin deficiencies in both the central and the peripheral nervous systems associated with a SOX10 mutation. Ann. Neurol. 1999, 46, 313–318. [Google Scholar] [CrossRef]
- Lieblich, J.M.; Rogol, A.D.; White, B.J.; Rosen, S.W. Syndrome of anosmia with hypogonadotropic hypogonadism (Kallmann syndrome): Clinical and laboratory studies in 23 cases. Am. J. Med. 1982, 73, 506–519. [Google Scholar] [CrossRef]
- Veronique, P.; Virginie, B.; Viviane, B.; Séverine, M.; Yuli, W.; Asma, C.; Corinne, F.; Chrystel, L.; Verier-Mine, O.; Christine, F.; et al. Loss-of-Function Mutations in SOX10 Cause Kallmann Syndrome with Deafness. Am. J. Hum. Genet. 2013, 92, 707–724. [Google Scholar] [CrossRef]
- Elmaleh-Bergès, M.; Baumann, C.; Noël-Pétroff, N.; Sekkal, A.; Couloigner, V.; Devriendt, K.; Wilson, M.; Marlin, S.; Sebag, G.; Pingault, V. Spectrum of Temporal Bone Abnormalities in Patients with Waardenburg Syndrome and SOX10 Mutations. Am. J. Neuroradiol. 2013, 34, 1257–1263. [Google Scholar] [CrossRef] [PubMed]
- Jian, S.; Yong, F.; Frederic, R.A.; Frederic, A.; Paul, C.; Kris, V.; Ingeborg, D. Hearing loss in Waardenburg syndrome: A systematic review. Clin. Genet. 2016, 89, 416–425. [Google Scholar] [CrossRef]
- Breuskin, I.; Bodson, M.; Thelen, N.; Thiry, M.; Borgs, L.; Nguyen, L.; Stolt, C.; Wegner, M.; Lefebvre, P.P.; Malgrange, B. Glial but not neuronal development in the cochleo-vestibular ganglion requires Sox10. J. Neurochem. 2010, 114, 1827–1839. [Google Scholar] [CrossRef] [PubMed]
- Veronique, P.; Emmanuelle, F.; Viviane, B.; Souad, G.; Natalie, L.; Vincent, C.; Françoise, D.; Noël-Pétroff, N.; Pointe, H.D.L.; Monique, E.; et al. SOX10 mutations mimic isolated hearing loss. Clin. Genet. 2015, 88, 352–359. [Google Scholar] [CrossRef]
- Bakos, R.M.; Maier, T.; Besch, R.; Mestel, D.S.; Ruzicka, T.; Sturm, R.A.; Berking, C. Nestin and SOX9 and SOX10 transcription factors are coexpressed in melanoma. Exp. Dermatol. 2010, 19, e89–e94. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Zheng, X.; Zhang, X.; Wang, Y.; Zhu, S.; Lu, F.; Qu, J.; Hou, L. Sox10 regulates skin melanocyte proliferation by activating the DNA replication licensing factor MCM5. J. Dermatol. Sci. 2017, 85, 216–225. [Google Scholar] [CrossRef]
- Cronin, J.C.; Watkins-Chow, D.E.; Incao, A.; Hasskamp, J.H.; Schönewolf, N.; Aoude, L.G.; Hayward, N.K.; Bastian, B.C.; Dummer, R.; Loftus, S.K.; et al. SOX10 Ablation Arrests Cell Cycle, Induces Senescence, and Suppresses Melanomagenesis. Cancer Res. 2013, 73, 5709–5718. [Google Scholar] [CrossRef]
- Rosenbaum, S.R.; Tiago, M.; Caksa, S.; Capparelli, C.; Purwin, T.J.; Kumar, G.; Glasheen, M.; Pomante, D.; Kotas, D.; Chervoneva, I.; et al. SOX10 requirement for melanoma tumor growth is due, in part, to immune-mediated effects. Cell Rep. 2021, 37, 110085. [Google Scholar] [CrossRef]
- Seong, I.; Min, H.J.; Lee, J.H.; Yeo, C.Y.; Kang, D.M.; Oh, E.S.; Hwang, E.S.; Kim, J. Sox10 controls migration of B16F10 melanoma cells through multiple regulatory target genes. PLoS ONE 2012, 7, e31477. [Google Scholar] [CrossRef]
- Clevenger, J.; Joseph, C.; Dawlett, M.; Guo, M.; Gong, Y. Reliability of immunostaining using pan-melanoma cocktail, SOX10, and microphthalmia transcription factor in confirming a diagnosis of melanoma on fine-needle aspiration smears. Cancer Cytopathol. 2014, 122, 779–785. [Google Scholar] [CrossRef]
- Cronin, J.C.; Loftus, S.K.; Baxter, L.L.; Swatkoski, S.; Gucek, M.; Pavan, W.J. Identification and functional analysis of SOX10 phosphorylation sites in melanoma. PLoS ONE 2018, 13, e0190834. [Google Scholar] [CrossRef]
- Wouters, J.; Kalender-Atak, Z.; Minnoye, L.; Spanier, K.I.; De Waegeneer, M.; Bravo González-Blas, C.; Mauduit, D.; Davie, K.; Hulselmans, G.; Najem, A.; et al. Robust gene expression programs underlie recurrent cell states and phenotype switching in melanoma. Nat. Cell Biol. 2020, 22, 986–998. [Google Scholar] [CrossRef]
- Verfaillie, A.; Imrichova, H.; Atak, Z.K.; Dewaele, M.; Rambow, F.; Hulselmans, G.; Christiaens, V.; Svetlichnyy, D.; Luciani, F.; Van den Mooter, L.; et al. Decoding the regulatory landscape of melanoma reveals TEADS as regulators of the invasive cell state. Nat. Commun. 2015, 6, 6683. [Google Scholar] [CrossRef] [PubMed]
- Cronin, J.C.; Wunderlich, J.; Loftus, S.K.; Prickett, T.D.; Wei, X.; Ridd, K.; Vemula, S.; Burrell, A.S.; Agrawal, N.S.; Lin, J.C.; et al. Frequent mutations in the MITF pathway in melanoma. Pigment. Cell Melanoma Res. 2009, 22, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Shakhova, O.; Zingg, D.; Schaefer, S.M.; Hari, L.; Civenni, G.; Blunschi, J.; Claudinot, S.; Okoniewski, M.; Beermann, F.; Mihic-Probst, D.; et al. Sox10 promotes the formation and maintenance of giant congenital naevi and melanoma. Nat. Cell Biol. 2012, 14, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Capparelli, C.; Purwin, T.J.; Glasheen, M.; Caksa, S.; Tiago, M.; Wilski, N.; Pomante, D.; Rosenbaum, S.; Nguyen, M.Q.; Cai, W.; et al. Targeting SOX10-deficient cells to reduce the dormant-invasive phenotype state in melanoma. Nat. Commun. 2022, 13, 1381. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Pekmezci, M.; Folpe, A.L.; Ersen, A.; Horvai, A.E. Diagnostic utility of SOX10 to distinguish malignant peripheral nerve sheath tumor from synovial sarcoma, including intraneural synovial sarcoma. Mod. Pathol. 2014, 27, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Pekmezci, M.; Reuss, D.E.; Hirbe, A.C.; Dahiya, S.; Gutmann, D.H.; von Deimling, A.; Horvai, A.E.; Perry, A. Morphologic and immunohistochemical features of malignant peripheral nerve sheath tumors and cellular schwannomas. Mod. Pathol. 2015, 28, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Doddrell, R.D.; Dun, X.P.; Shivane, A.; Feltri, M.L.; Wrabetz, L.; Wegner, M.; Sock, E.; Hanemann, C.O.; Parkinson, D.B. Loss of SOX10 function contributes to the phenotype of human Merlin-null schwannoma cells. Brain 2013, 136, 549–563. [Google Scholar] [CrossRef][Green Version]
- Su, Z.; Bao, W.; Yang, G.; Liu, J.; Zhao, B. SOX12 promotes thyroid cancer cell proliferation and invasion by regulating the expression of POU2F1 and POU3F1. Yonsei Med. J. 2022, 63, 591. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, M.; McCue, P.A.; Sarlomo-Rikala, M.; Biernat, W.; Czapiewski, P.; Kopczynski, J.; Thompson, L.D.; Lasota, J.; Wang, Z.; Fetsch, J.F. Sox10—A marker for not only schwannian and melanocytic neoplasms but also myoepithelial cell tumors of soft tissue: A systematic analysis of 5134 tumors. Am. J. Surg. Pathol. 2015, 39, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Karamchandani, J.R.; Nielsen, T.O.; van de Rijn, M.; West, R.B. Sox10 and S100 in the diagnosis of soft-tissue neoplasms. Appl. Immunohistochem. Mol. Morphol. 2012, 20, 445–450. [Google Scholar] [CrossRef]
- Atiq, M.A.; Davis, J.L.; Hornick, J.L.; Dickson, B.C.; Fletcher, C.D.M.; Fletcher, J.A.; Folpe, A.L.; Mariño-Enríquez, A. Mesenchymal tumors of the gastrointestinal tract with NTRK rearrangements: A clinicopathological, immunophenotypic, and molecular study of eight cases, emphasizing their distinction from gastrointestinal stromal tumor (GIST). Mod. Pathol. 2021, 34, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Chiang, S.; Cotzia, P.; Hyman, D.M.; Drilon, A.; Tap, W.D.; Zhang, L.; Hechtman, J.F.; Frosina, D.; Jungbluth, A.A.; Murali, R.; et al. NTRK Fusions Define a Novel Uterine Sarcoma Subtype With Features of Fibrosarcoma. Am. J. Surg. Pathol. 2018, 42, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kang, H.J.; Yoo, C.W.; Park, W.S.; Ryu, J.S.; Jung, Y.S.; Choi, S.W.; Park, J.Y.; Han, N. PLAG1, SOX10, and Myb Expression in Benign and Malignant Salivary Gland Neoplasms. J. Pathol. Transl. Med. 2019, 53, 23–30. [Google Scholar] [CrossRef]
- Hsieh, M.S.; Lee, Y.H.; Chang, Y.L. SOX10-positive salivary gland tumors: A growing list, including mammary analogue secretory carcinoma of the salivary gland, sialoblastoma, low-grade salivary duct carcinoma, basal cell adenoma/adenocarcinoma, and a subgroup of mucoepidermoid carcinoma. Hum. Pathol. 2016, 56, 134–142. [Google Scholar] [CrossRef]
- Schmitt, A.C.; Cohen, C.; Siddiqui, M.T. Expression of SOX10 in Salivary Gland Oncocytic Neoplasms: A Review and a Comparative Analysis with Other Immunohistochemical Markers. Acta Cytol. 2015, 59, 384–390. [Google Scholar] [CrossRef]
- Zhu, S.; Schuerch, C.; Hunt, J. Review and updates of immunohistochemistry in selected salivary gland and head and neck tumors. Arch. Pathol. Lab. Med. 2015, 139, 55–66. [Google Scholar] [CrossRef]
- Ohtomo, R.; Mori, T.; Shibata, S.; Tsuta, K.; Maeshima, A.M.; Akazawa, C.; Watabe, Y.; Honda, K.; Yamada, T.; Yoshimoto, S.; et al. SOX10 is a novel marker of acinus and intercalated duct differentiation in salivary gland tumors: A clue to the histogenesis for tumor diagnosis. Mod. Pathol. 2013, 26, 1041–1050. [Google Scholar] [CrossRef]
- Rooper, L.M.; McCuiston, A.M.; Westra, W.H.; Bishop, J.A. SOX10 Immunoexpression in Basaloid Squamous Cell Carcinomas: A Diagnostic Pitfall for Ruling out Salivary Differentiation. Head. Neck Pathol. 2019, 13, 543–547. [Google Scholar] [CrossRef]
- Kriegsmann, K.; Flechtenmacher, C.; Heil, J.; Kriegsmann, J.; Mechtersheimer, G.; Aulmann, S.; Weichert, W.; Sinn, H.P.; Kriegsmann, M. Immunohistological Expression of SOX-10 in Triple-Negative Breast Cancer: A Descriptive Analysis of 113 Samples. Int. J. Mol. Sci. 2020, 21, 6407. [Google Scholar] [CrossRef]
- Cimino-Mathews, A. Novel uses of immunohistochemistry in breast pathology: Interpretation and pitfalls. Mod. Pathol. 2021, 34, 62–77. [Google Scholar] [CrossRef]
- Liu, J.L.; Chen, D.S.; Cheng, Z.Q.; Hu, J.T. Expression of SOX10 and GATA3 in breast cancer and their significance. Zhonghua Bing Li Xue Za Zhi 2022, 51, 536–541. [Google Scholar] [PubMed]
- Adkins, B.D.; Geromes, A.; Zhang, L.Y.; Chernock, R.; Kimmelshue, K.; Lewis, J., Jr.; Ely, K. SOX10 and GATA3 in Adenoid Cystic Carcinoma and Polymorphous Adenocarcinoma. Head. Neck Pathol. 2020, 14, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Kwon, A.Y.; Heo, I.; Lee, H.J.; Kim, G.; Kang, H.; Heo, J.H.; Kim, T.H.; An, H.J. Sox10 expression in ovarian epithelial tumors is associated with poor overall survival. Virchows Arch. 2016, 468, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Raspaglio, G.; Petrillo, M.; Martinelli, E.; Li Puma, D.D.; Mariani, M.; De Donato, M.; Filippetti, F.; Mozzetti, S.; Prislei, S.; Zannoni, G.F.; et al. Sox9 and Hif-2α regulate TUBB3 gene expression and affect ovarian cancer aggressiveness. Gene 2014, 542, 173–181. [Google Scholar] [CrossRef]
- Siu, M.K.Y.; Jiang, Y.X.; Wang, J.J.; Leung, T.H.Y.; Han, C.Y.; Tsang, B.K.; Cheung, A.N.Y.; Ngan, H.Y.S.; Chan, K.K.L. Hexokinase 2 Regulates Ovarian Cancer Cell Migration, Invasion and Stemness via FAK/ERK1/2/MMP9/NANOG/SOX9 Signaling Cascades. Cancers 2019, 11, 813. [Google Scholar] [CrossRef]
- Lu, R.; Tang, P.; Zhang, D.; Lin, S.; Li, H.; Feng, X.; Sun, M.; Zhang, H. SOX9/NFIA promotes human ovarian cancer metastasis through the Wnt/β-catenin signaling pathway. Pathol. Res. Pract. 2023, 248, 154602. [Google Scholar] [CrossRef]
- Hou, R.; Jiang, L. LINC00115 promotes stemness and inhibits apoptosis of ovarian cancer stem cells by upregulating SOX9 and inhibiting the Wnt/β-catenin pathway through competitively binding to microRNA-30a. Cancer Cell Int. 2021, 21, 360. [Google Scholar] [CrossRef]
- Malki, S.; Bibeau, F.; Notarnicola, C.; Roques, S.; Berta, P.; Poulat, F.; Boizet-Bonhoure, B. Expression and biological role of the prostaglandin D synthase/SOX9 pathway in human ovarian cancer cells. Cancer Lett. 2007, 255, 182–193. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Z.G.; Tang, J.; Zou, R.F.; Chen, X.Y.; Jiang, G.M.; Qiu, Y.F.; Wang, H. High expression of Sox10 correlates with tumor aggressiveness and poor prognosis in human nasopharyngeal carcinoma. Onco Targets Ther. 2016, 9, 1671–1677. [Google Scholar] [CrossRef]
- Goodwin, G.H.; Sanders, C.; Johns, E.W. A new group of chromatin-associated proteins with a high content of acidic and basic amino acids. Eur. J. Biochem. 1973, 38, 14–19. [Google Scholar] [CrossRef]
- Štros, M.; Launholt, D.; Grasser, K.D. The HMG-box: A versatile protein domain occurring in a wide variety of DNA-binding proteins. Cell. Mol. Life Sci. 2007, 64, 2590–2606. [Google Scholar] [CrossRef]
- Štros, M. HMGB proteins: Interactions with DNA and chromatin. Biochim. Biophys. Acta (BBA)-Gene Regul. Mech. 2010, 1799, 101–113. [Google Scholar] [CrossRef]
- Lefebvre, V.; Dumitriu, B.; Penzo-Méndez, A.; Han, Y.; Pallavi, B. Control of cell fate and differentiation by Sry-related high-mobility-group box (Sox) transcription factors. Int. J. Biochem. Cell Biol. 2007, 39, 2195–2214. [Google Scholar] [CrossRef] [PubMed]
- Grimm, D.; Bauer, J.; Wise, P.; Krüger, M.; Simonsen, U.; Wehland, M.; Infanger, M.; Corydon, T.J. The role of SOX family members in solid tumours and metastasis. Semin. Cancer Biol. 2020, 67, 122–153. [Google Scholar] [CrossRef] [PubMed]
- Kashimada, K.; Koopman, P. Sry: The master switch in mammalian sex determination. Development 2010, 137, 3921–3930. [Google Scholar] [CrossRef] [PubMed]
- Lau, Y.F.C.; Zhang, J. Expression analysis of thirty one Y chromosome genes in human prostate cancer. Mol. Carcinog. Publ. Coop. Univ. Tex. MD Anderson Cancer Cent. 2000, 27, 308–321. [Google Scholar] [CrossRef]
- Kanwore, K.; Guo, X.-X.; Abdulrahman, A.A.; Kambey, P.A.; Nadeem, I.; Gao, D. SOX1 is a backup gene for brain neurons and glioma stem cell protection and proliferation. Mol. Neurobiol. 2021, 58, 2634–2642. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zou, Y.; Lu, Q.; Tang, N.; Heng, A.; Islam, I.; Tong, H.J.; Dawe, G.S.; Cao, T. Efficient derivation of dopaminergic neurons from SOX1—Floor plate cells under defined culture conditions. J. Biomed. Sci. 2016, 23, 34. [Google Scholar] [CrossRef] [PubMed]
- Nitta, K.R.; Takahashi, S.; Haramoto, Y.; Fukuda, M.; Onuma, Y.; Asashima, M. Expression of Sox1 during Xenopus early embryogenesis. Biochem. Biophys. Res. Commun. 2006, 351, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Garcia, I.; Aldaregia, J.; Marjanovic Vicentic, J.; Aldaz, P.; Moreno-Cugnon, L.; Torres-Bayona, S.; Carrasco-Garcia, E.; Garros-Regulez, L.; Egaña, L.; Rubio, A. Oncogenic activity of SOX1 in glioblastoma. Sci. Rep. 2017, 7, 46575. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Liu, T.; Li, C.; Yan, G.; Li, C.; Zhang, J.; Wang, L. The MRTF-A/miR-155/SOX1 pathway mediates gastric cancer migration and invasion. Cancer Cell Int. 2020, 20, 1–13. [Google Scholar] [CrossRef]
- Guan, Z.; Zhang, J.; Wang, J.; Wang, H.; Zheng, F.; Peng, J.; Xu, Y.; Yan, M.; Liu, B.; Cui, B. SOX1 down-regulates β-catenin and reverses malignant phenotype in nasopharyngeal carcinoma. Mol. Cancer 2014, 13, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Novak, D.; Hüser, L.; Elton, J.J.; Umansky, V.; Altevogt, P.; Utikal, J. SOX2 in development and cancer biology. Semin. Cancer Biol. 2020, 67, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.H.d.S.; Underwood, A.; Almeida, C.P.; Ribeiro, T.S.; Souza-Fagundes, E.M.; Martins, A.S.; Eliezeck, M.; Guatimosim, S.; Andrade, L.O.; Rezende, L. Transcription factor SOX3 upregulated pro-apoptotic genes expression in human breast cancer. Med. Oncol. 2022, 39, 212. [Google Scholar] [CrossRef]
- Zhao, J.; Cao, H.; Zhang, W.; Fan, Y.; Shi, S.; Wang, R. SOX14 hypermethylation as a tumour biomarker in cervical cancer. BMC Cancer 2021, 21, 675. [Google Scholar] [CrossRef]
- Li, F.; Wang, T.; Tang, S. SOX14 promotes proliferation and invasion of cervical cancer cells through Wnt/β-catenin pathway. Int. J. Clin. Exp. Pathol. 2015, 8, 1698. [Google Scholar]
- Caglayan, D.; Lundin, E.; Kastemar, M.; Westermark, B.; Ferletta, M. SOX21 inhibits glioma progression in vivo by forming complexes with SOX2 and stimulating aberrant differentiation. Int. J. Cancer 2013, 133, 1345–1356. [Google Scholar] [CrossRef]
- Ferletta, M.; Caglayan, D.; Mokvist, L.; Jiang, Y.; Kastemar, M.; Uhrbom, L.; Westermark, B. Forced expression of SOX21 inhibits SOX2 and induces apoptosis in human glioma cells. Int. J. Cancer 2011, 129, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Vervoort, S.J.; van Boxtel, R.; Coffer, P.J. The role of SRY-related HMG box transcription factor 4 (SOX4) in tumorigenesis and metastasis: Friend or foe? Oncogene 2013, 32, 3397–3409. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liang, Q.; Lei, Y.; Yao, M.; Li, L.; Gao, X.; Feng, J.; Zhang, Y.; Gao, H.; Liu, D.-X. SOX4 induces epithelial–mesenchymal transition and contributes to breast cancer progression. Cancer Res. 2012, 72, 4597–4608. [Google Scholar] [CrossRef]
- Hanieh, H.; Ahmed, E.A.; Vishnubalaji, R.; Alajez, N.M. SOX4: Epigenetic regulation and role in tumorigenesis. Semin. Cancer Biol. 2020, 67, 91–104. [Google Scholar] [CrossRef]
- Medina, P.P.; Castillo, S.D.; Blanco, S.; Sanz-Garcia, M.; Largo, C.; Alvarez, S.; Yokota, J.; Gonzalez-Neira, A.; Benitez, J.; Clevers, H.C. The SRY-HMG box gene, SOX4, is a target of gene amplification at chromosome 6p in lung cancer. Hum. Mol. Genet. 2009, 18, 1343–1352. [Google Scholar] [CrossRef] [PubMed]
- Hur, W.; Rhim, H.; Jung, C.K.; Kim, J.D.; Bae, S.H.; Jang, J.W.; Yang, J.M.; Oh, S.-T.; Kim, D.G.; Wang, H.J. SOX4 overexpression regulates the p53-mediated apoptosis in hepatocellular carcinoma: Clinical implication and functional analysis in vitro. Carcinogenesis 2010, 31, 1298–1307. [Google Scholar] [CrossRef]
- Jafarnejad, S.M.; Ardekani, G.S.; Ghaffari, M.; Li, G. Pleiotropic function of SRY-related HMG box transcription factor 4 in regulation of tumorigenesis. Cell. Mol. Life Sci. 2013, 70, 2677–2696. [Google Scholar] [CrossRef]
- Weigle, B.; Ebner, R.; Temme, A.; Schwind, S.; Schmitz, M.; Kiessling, A.; Rieger, M.A.; Schackert, G.; Schackert, H.K.; Rieber, E.P. Highly specific overexpression of the transcription factor SOX11 in human malignant gliomas. Oncol. Rep. 2005, 13, 139–144. [Google Scholar] [CrossRef]
- de Bont, J.M.; Kros, J.M.; Passier, M.M.; Reddingius, R.E.; Smitt, P.A.S.; Luider, T.M.; Boer, M.L.d.; Pieters, R. Differential expression and prognostic significance of SOX genes in pediatric medulloblastoma and ependymoma identified by microarray analysis. Neuro-Oncol. 2008, 10, 648–660. [Google Scholar] [CrossRef]
- Ek, S.; Dictor, M.; Jerkeman, M.; Jirström, K.; Borrebaeck, C.A. Nuclear expression of the non-B-cell lineage SOX11 transcription factor identifies mantle cell lymphoma. Blood J. Am. Soc. Hematol. 2008, 111, 800–805. [Google Scholar] [CrossRef]
- Dictor, M.; Ek, S.; Sundberg, M.; Warenholt, J.; György, C.; Sernbo, S.; Gustavsson, E.; Abu-Alsoud, W.; Wadström, T.; Borrebaeck, C. Strong lymphoid nuclear expression of SOX11 transcription factor defines lymphoblastic neoplasms, mantle cell lymphoma and Burkitt’s lymphoma. Haematologica 2009, 94, 1563. [Google Scholar] [CrossRef] [PubMed]
- Brennan, D.J.; Ek, S.; Doyle, E.; Drew, T.; Foley, M.; Flannelly, G.; O’Connor, D.P.; Gallagher, W.M.; Kilpinen, S.; Kallioniemi, O.-P. The transcription factor Sox11 is a prognostic factor for improved recurrence-free survival in epithelial ovarian cancer. Eur. J. Cancer 2009, 45, 1510–1517. [Google Scholar] [CrossRef] [PubMed]
- Zvelebil, M.; Oliemuller, E.; Gao, Q.; Wansbury, O.; Mackay, A.; Kendrick, H.; Smalley, M.J.; Reis-Filho, J.S.; Howard, B.A. Embryonic mammary signature subsets are activated in Brca1-/-and basal-like breast cancers. Breast Cancer Res. 2013, 15, R25. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Yuan, Z.; Shi, H.; Bian, Y.; Guo, R. miR-145 targets the SOX11 3’UTR to suppress endometrial cancer growth. Am. J. Cancer Res. 2017, 7, 2305. [Google Scholar] [PubMed]
- Prat, A.; Adamo, B.; Fan, C.; Peg, V.; Vidal, M.; Galván, P.; Vivancos, A.; Nuciforo, P.; Palmer, H.G.; Dawood, S. Genomic analyses across six cancer types identify basal-like breast cancer as a unique molecular entity. Sci. Rep. 2013, 3, 3544. [Google Scholar] [CrossRef] [PubMed]
- Tsang, S.M.; Oliemuller, E.; Howard, B.A. Regulatory roles for SOX11 in development, stem cells and cancer. Semin. Cancer Biol. 2020, 67, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Zhou, C.; Zhang, J.; Cai, Q.; Li, J.; Du, T.; Zhu, Z.; Cui, X.; Liu, B. The metastasis suppressor SOX11 is an independent prognostic factor for improved survival in gastric cancer. Int. J. Oncol. 2014, 44, 1512–1520. [Google Scholar] [CrossRef]
- Zou, S.; Wang, C.; Liu, J.; Wang, Q.; Zhang, D.; Zhu, S.; Xu, S.; Kang, M.; He, S. Sox12 is a cancer stem-like cell marker in hepatocellular carcinoma. Mol. Cells 2017, 40, 847. [Google Scholar]
- Qu, M.; Zhu, Y.; Jin, M. MicroRNA-138 inhibits SOX12 expression and the proliferation, invasion and migration of ovarian cancer cells. Exp. Ther. Med. 2018, 16, 1629–1638. [Google Scholar] [CrossRef]
- Du, F.; Feng, W.; Chen, S.; Wu, S.; Cao, T.; Yuan, T.; Tian, D.; Nie, Y.; Wu, K.; Fan, D. Sex determining region Y-box 12 (SOX12) promotes gastric cancer metastasis by upregulating MMP7 and IGF1. Cancer Lett. 2019, 452, 103–118. [Google Scholar] [CrossRef]
- Yuan, W.-M.; Fan, Y.-G.; Cui, M.; Luo, T.; Wang, Y.-E.; Shu, Z.-J.; Zhao, J.; Zheng, J.; Zeng, Y. SOX5 regulates cell proliferation, apoptosis, migration and invasion in KSHV-infected cells. Virol. Sin. 2021, 36, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Han, S.; Wang, X.; Peng, R.; Li, X. SOX5 promotes epithelial–mesenchymal transition and cell invasion via regulation of Twist1 in hepatocellular carcinoma. Med. Oncol. 2015, 32, 461. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Ban, Y.; Wang, K.; Sun, Y.; Zhao, Z. SOX5 promotes breast cancer proliferation and invasion by transactivation of EZH2. Oncol. Lett. 2019, 17, 2754–2762. [Google Scholar] [CrossRef]
- You, J.; Zhao, Q.; Fan, X.; Wang, J. SOX5 promotes cell invasion and metastasis via activation of twist-mediated epithelial–mesenchymal transition in gastric cancer. OncoTargets Ther. 2019, 12, 2465. [Google Scholar] [CrossRef]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial–mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef]
- Niknami, Z.; Muhammadnejad, A.; Ebrahimi, A.; Harsani, Z.; Shirkoohi, R. Significance of E-cadherin and Vimentin as epithelial-mesenchymal transition markers in colorectal carcinoma prognosis. EXCLI J. 2020, 19, 917. [Google Scholar] [PubMed]
- Wang, Z.; Li, J.; Li, K.; Xu, J. SOX6 is downregulated in osteosarcoma and suppresses the migration, invasion and epithelial-mesenchymal transition via TWIST1 regulation. Mol. Med. Rep. 2018, 17, 6803–6811. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.-R.; Tang, H.; Xie, F.; Liu, H.; Zhu, Y.; Ai, J.; Chen, L.; Li, Y.; Kwong, D.L.; Fu, L. Characterization of tumor-suppressive function of SOX6 in human esophageal squamous cell carcinoma. Clin. Cancer Res. 2011, 17, 46–55. [Google Scholar] [CrossRef]
- Guo, X.; Yang, M.; Gu, H.; Zhao, J.; Zou, L. Decreased expression of SOX6 confers a poor prognosis in hepatocellular carcinoma. Cancer Epidemiol. 2013, 37, 732–736. [Google Scholar] [CrossRef]
- Iguchi, H.; Urashima, Y.; Inagaki, Y.; Ikeda, Y.; Okamura, M.; Tanaka, T.; Uchida, A.; Yamamoto, T.T.; Kodama, T.; Sakai, J. SOX6 suppresses cyclin D1 promoter activity by interacting with β-catenin and histone deacetylase 1, and its down-regulation induces pancreatic β-cell proliferation. J. Biol. Chem. 2007, 282, 19052–19061. [Google Scholar] [CrossRef]
- Ueda, R.; Ohkusu-Tsukada, K.; Fusaki, N.; Soeda, A.; Kawase, T.; Kawakami, Y.; Toda, M. Identification of HLA-A2-and A24-restricted T-cell epitopes derived from SOX6 expressed in glioma stem cells for immunotherapy. Int. J. Cancer 2010, 126, 919–929. [Google Scholar] [CrossRef]
- Lin, M.; Lei, T.; Zheng, J.; Chen, S.; Du, L.; Xie, H. UBE2S mediates tumor progression via SOX6/β-Catenin signaling in endometrial cancer. Int. J. Biochem. Cell Biol. 2019, 109, 17–22. [Google Scholar] [CrossRef]
- Huang, H.; Han, Q.; Zheng, H.; Liu, M.; Shi, S.; Zhang, T.; Yang, X.; Li, Z.; Xu, Q.; Guo, H. MAP4K4 mediates the SOX6-induced autophagy and reduces the chemosensitivity of cervical cancer. Cell Death Dis. 2021, 13, 13. [Google Scholar] [CrossRef]
- Schlierf, B.; Friedrich, R.; Roerig, P.; Felsberg, J.; Reifenberger, G.; Wegner, M. Expression of SOXE and SOXD genes in human gliomas. Neuropathol. Appl. Neurobiol. 2007, 33, 621–630. [Google Scholar] [CrossRef]
- Bie, L.-Y.; Li, D.; Wei, Y.; Li, N.; Chen, X.-B.; Luo, S.-X. SOX13 dependent PAX8 expression promotes the proliferation of gastric carcinoma cells. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3180–3187. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Fang, F.; Fang, T.; Jiao, H.; You, S.; Wang, X.; Zhao, W. SOX13 promotes hepatocellular carcinoma metastasis by transcriptionally activating Twist1. Lab. Investig. 2020, 100, 1400–1410. [Google Scholar] [CrossRef] [PubMed]
- Jiao, H.; Fang, F.; Fang, T.; You, Y.; Feng, M.; Wang, X.; Yin, Z.; Zhao, W. SOX13 regulates cancer stem-like properties and tumorigenicity in hepatocellular carcinoma cells. Am. J. Cancer Res. 2021, 11, 760. [Google Scholar] [PubMed]
- Wang, H.; He, L.; Ma, F.; Regan, M.M.; Balk, S.P.; Richardson, A.L.; Yuan, X. SOX9 regulates low density lipoprotein receptor-related protein 6 (LRP6) and T-cell factor 4 (TCF4) expression and Wnt/β-catenin activation in breast cancer. J. Biol. Chem. 2013, 288, 6478–6487. [Google Scholar] [CrossRef]
- Zhong, W.-d.; Qin, G.-q.; Dai, Q.-s.; Han, Z.-d.; Chen, S.-m.; Ling, X.-h.; Fu, X.; Cai, C.; Chen, J.-h.; Chen, X.-b. SOXs in human prostate cancer: Implication as progression and prognosis factors. BMC Cancer 2012, 12, 248. [Google Scholar] [CrossRef]
- Hu, B.; Wang, J.; Jin, X. MicroRNA-138 suppresses cell proliferation and invasion of renal cell carcinoma by directly targeting SOX9. Oncol. Lett. 2017, 14, 7583–7588. [Google Scholar] [CrossRef][Green Version]
- Huang, J.; Guo, L. Knockdown of SOX9 inhibits the proliferation, invasion, and EMT in thyroid cancer cells. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2017, 25, 167–176. [Google Scholar] [CrossRef]
- Lü, B.; Fang, Y.; Xu, J.; Wang, L.; Xu, F.; Xu, E.; Huang, Q.; Lai, M. Analysis of SOX9 expression in colorectal cancer. Am. J. Clin. Pathol. 2008, 130, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Che, D.; Yang, F.; Chi, C.; Meng, H.; Shen, J.; Qi, L.; Liu, F.; Lv, L.; Li, Y. Tumor-associated macrophages promote tumor metastasis via the TGF-β/SOX9 axis in non-small cell lung cancer. Oncotarget 2017, 8, 99801. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liang, R.; Liu, C.; Liu, J.A.; Cheung, M.P.L.; Liu, X.; Man, O.Y.; Guan, X.-Y.; Lung, H.L.; Cheung, M. SOX9 is a dose-dependent metastatic fate determinant in melanoma. J. Exp. Clin. Cancer Res. 2019, 38, 17. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Sohn, K.-C.; Li, Z.; Choi, D.-K.; Park, Y.M.; Kim, J.-H.; Fan, Y.-M.; Nam, Y.H.; Kim, S.; Im, M. Expression and functional role of SOX9 in human epidermal keratinocytes. PLoS ONE 2013, 8, e54355. [Google Scholar] [CrossRef] [PubMed]
- Stovall, D.B.; Wan, M.; Miller, L.D.; Cao, P.; Maglic, D.; Zhang, Q.; Stampfer, M.R.; Liu, W.; Xu, J.; Sui, G. The regulation of SOX7 and its tumor suppressive role in breast cancer. Am. J. Pathol. 2013, 183, 1645–1653. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Xi, H.; Cai, A.; Bian, S.; Wei, B.; Chen, L. Decreased expression of SOX7 correlates with the upregulation of the Wnt/β-catenin signaling pathway and the poor survival of gastric cancer patients. Int. J. Mol. Med. 2014, 34, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Wang, W.; Ding, W.; Zhang, L. MiR-9 is involved in TGF-β1-induced lung cancer cell invasion and adhesion by targeting SOX7. J. Cell. Mol. Med. 2017, 21, 2000–2008. [Google Scholar] [CrossRef]
- Fu, D.-Y.; Wang, Z.-M.; Wang, B.-L.; Shen, Z.-Z.; Huang, W.; Shao, Z.-M. SOX17, the canonical Wnt antagonist, is epigenetically inactivated by promoter methylation in human breast cancer. Breast Cancer Res. Treat. 2010, 119, 601–612. [Google Scholar] [CrossRef]
- Li, J.-Y.; Han, C.; Zheng, L.-L.; Guo, M.-Z. Epigenetic regulation of Wnt signaling pathway gene SRY-related HMG-box 17 in papillary thyroid carcinoma. Chin. Med. J. 2012, 125, 3526–3531. [Google Scholar]
- Majchrzak-Celińska, A.; Słocińska, M.; Barciszewska, A.-M.; Nowak, S.; Baer-Dubowska, W. Wnt pathway antagonists, SFRP1, SFRP2, SOX17, and PPP2R2B, are methylated in gliomas and SFRP1 methylation predicts shorter survival. J. Appl. Genet. 2016, 57, 189–197. [Google Scholar] [CrossRef]
- Du, Y.C.; Oshima, H.; Oguma, K.; Kitamura, T.; Itadani, H.; Fujimura, T.; Piao, Y.S.; Yoshimoto, T.; Minamoto, T.; Kotani, H. Induction and down-regulation of SOX17 and its possible roles during the course of gastrointestinal tumorigenesis. Gastroenterology 2009, 137, 1346–1357. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, G.; Cheng, Y.; Tang, Y.; Dong, Z.; McElwee, K.J.; Li, G. Reduced expression of SRY-box containing gene 17 correlates with an unfavorable melanoma patient survival. Oncol. Rep. 2014, 32, 2571–2579. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pula, B.; Olbromski, M.; Wojnar, A.; Gomulkiewicz, A.; Witkiewicz, W.; Ugorski, M.; Dziegiel, P.; Podhorska-Okolow, M. Impact of SOX18 expression in cancer cells and vessels on the outcome of invasive ductal breast carcinoma. Cell. Oncol. 2013, 36, 469–483. [Google Scholar] [CrossRef] [PubMed]
- Jethon, A.; Pula, B.; Olbromski, M.; Werynska, B.; Muszczynska-Bernhard, B.; Witkiewicz, W.; Dziegiel, P.; Podhorska-Okolow, M. Prognostic significance of SOX18 expression in non-small cell lung cancer. Int. J. Oncol. 2015, 46, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Ornat, M.; Kobierzycki, C.; Grzegrzolka, J.; Pula, B.; Zamirska, A.; Bieniek, A.; Szepietowski, J.C.; Dziegiel, P.; Okolow, M.P. SOX18 expression in non-melanoma skin cancer. Anticancer. Res. 2016, 36, 2379–2383. [Google Scholar] [PubMed]
- Yan, H.-T.; Shinka, T.; Sato, Y.; Yang, X.-J.; Chen, G.; Sakamoto, K.; Kinoshita, K.; Aburatani, H.; Nakahori, Y. Overexpression of SOX15 inhibits proliferation of NT2/D1 cells derived from a testicular embryonal cell carcinoma. Mol. Cells 2007, 24, 323–328. [Google Scholar] [PubMed]
- Thu, K.; Radulovich, N.; Becker-Santos, D.; Pikor, L.; Pusic, A.; Lockwood, W.; Lam, W.; Tsao, M. SOX15 is a candidate tumor suppressor in pancreatic cancer with a potential role in Wnt/β-catenin signaling. Oncogene 2014, 33, 279–288. [Google Scholar] [CrossRef]
- Thu, K.L.; Becker-Santos, D.D.; Radulovich, N.; Pikor, L.A.; Lam, W.L.; Tsao, M.-S. SOX15 and other SOX family members are important mediators of tumorigenesis in multiple cancer types. Oncoscience 2014, 1, 326. [Google Scholar] [CrossRef]
- Han, F.; Liu, W.; Jiang, X.; Shi, X.; Yin, L.; Ao, L.; Cui, Z.; Li, Y.; Huang, C.; Cao, J. SOX30, a novel epigenetic silenced tumor suppressor, promotes tumor cell apoptosis by transcriptional activating p53 in lung cancer. Oncogene 2015, 34, 4391–4402. [Google Scholar] [CrossRef]
- Han, F.; Liu, W.-B.; Shi, X.-Y.; Yang, J.-T.; Zhang, X.; Li, Z.-M.; Jiang, X.; Yin, L.; Li, J.-J.; Huang, C.-S. SOX30 inhibits tumor metastasis through attenuating Wnt-signaling via transcriptional and posttranslational regulation of β-catenin in lung cancer. EBioMedicine 2018, 31, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Han, F.; Ma, B.; Zhang, N.; Chen, H.; Jiang, X.; Yin, L.; Liu, W.; Ao, L.; Cao, J. SOX30 is a key regulator of desmosomal gene suppressing tumor growth and metastasis in lung adenocarcinoma. J. Exp. Clin. Cancer Res. 2018, 37, 111. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).