Expression of Human L-Dopa Decarboxylase (DDC) under Conditions of Oxidative Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Establishment of a Huh7.5-Based Cell Line with Stable Silencing of DDC
2.3. Assessment of Reactive Oxygen Species Levels
2.4. Detection of Cytotoxicity and Apoptosis Levels
2.5. RNA Quantification by Reverse Transcription-Quantitative PCR (RT-qPCR)
2.6. Determination of Protein Concentration
2.7. Gel Electrophoresis and Western Blot Analysis
2.8. Statistical Analysis
3. Results
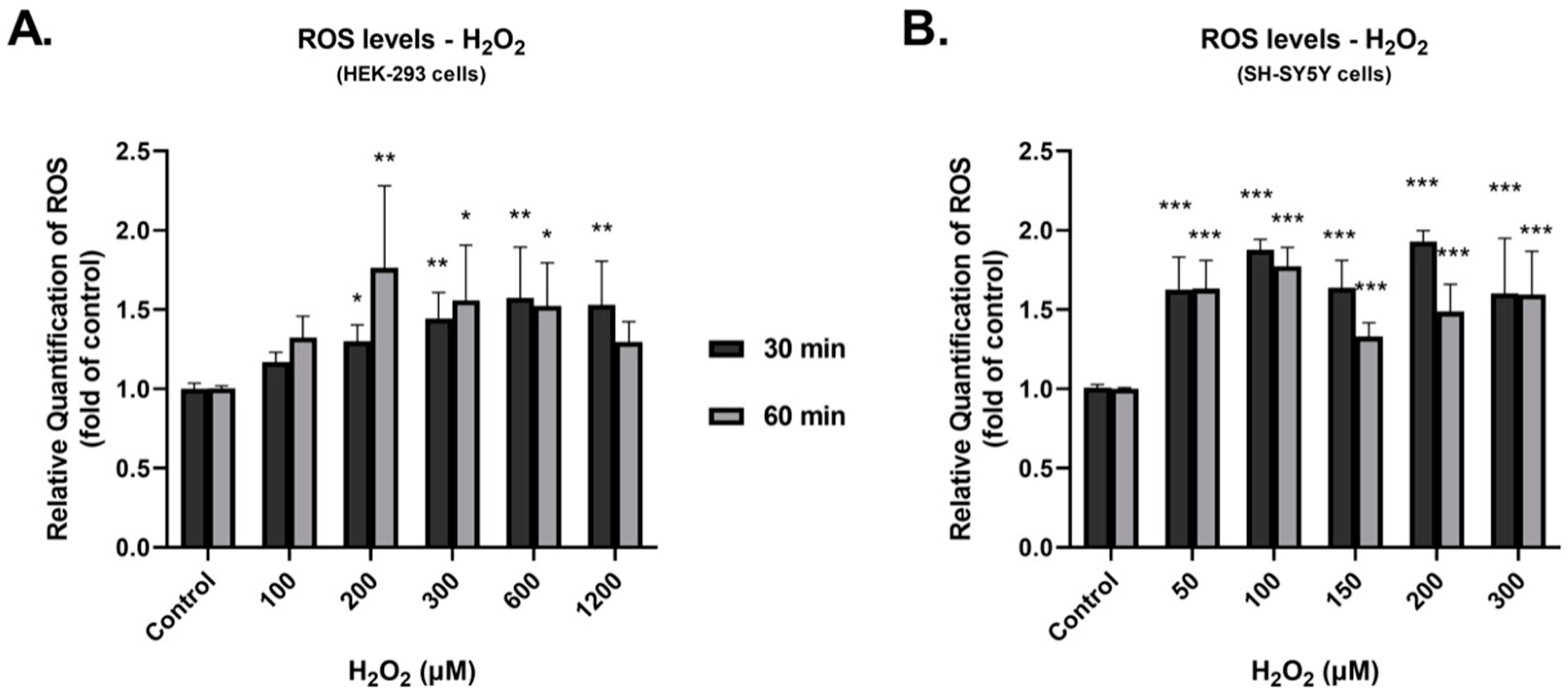
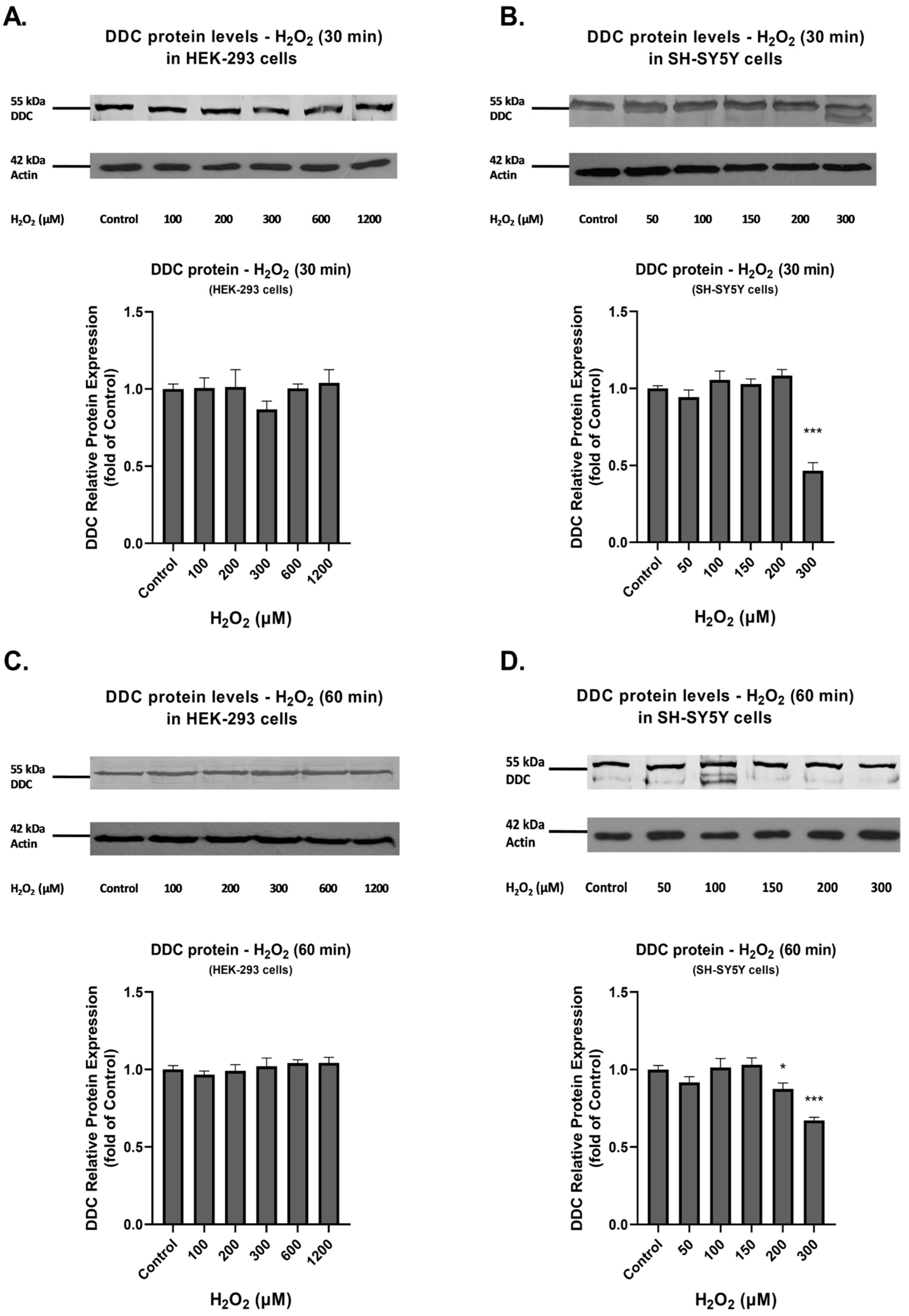
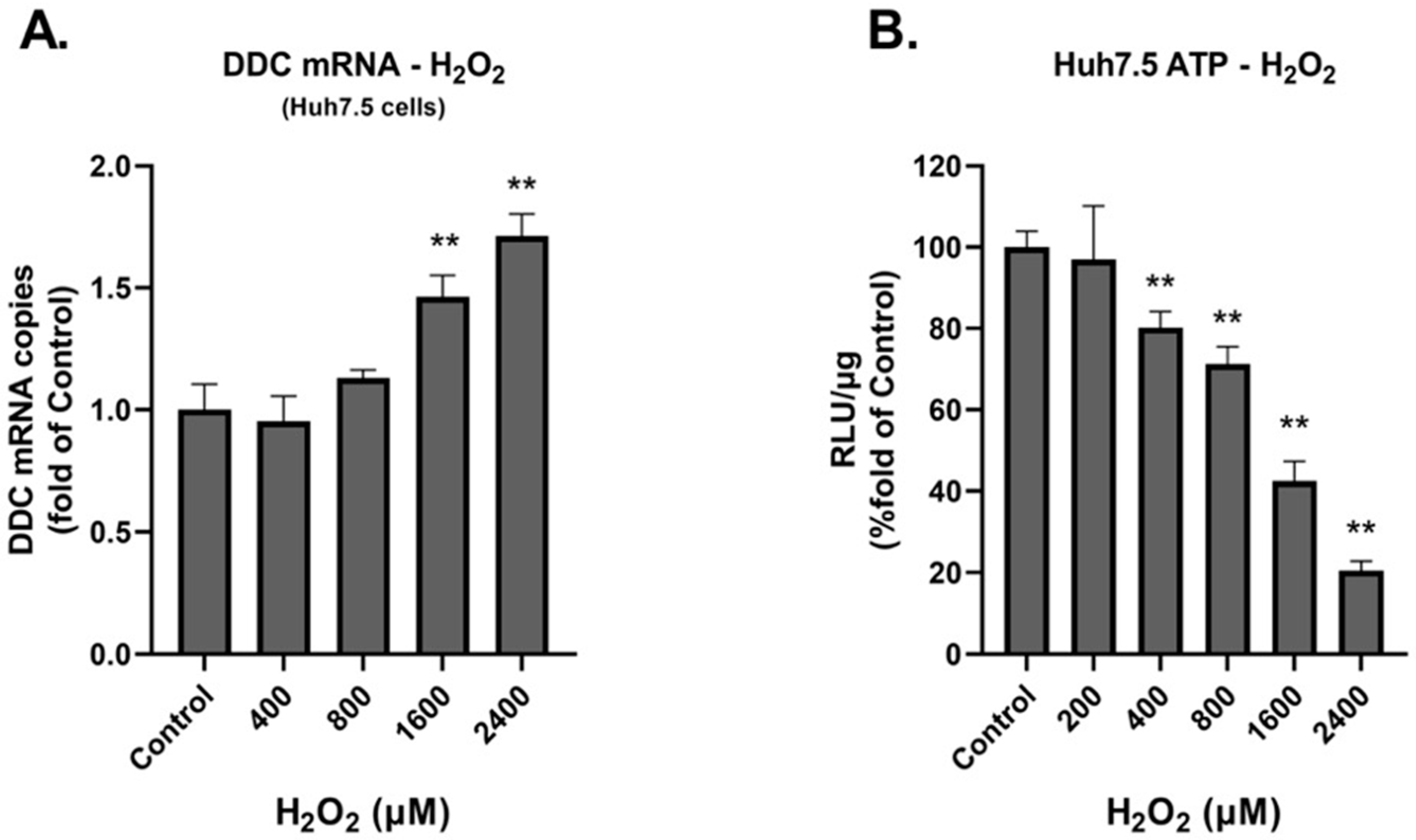
3.1. Investigation of the Effect of H2O2 on the Production of ROS and the Expression of DDC Protein
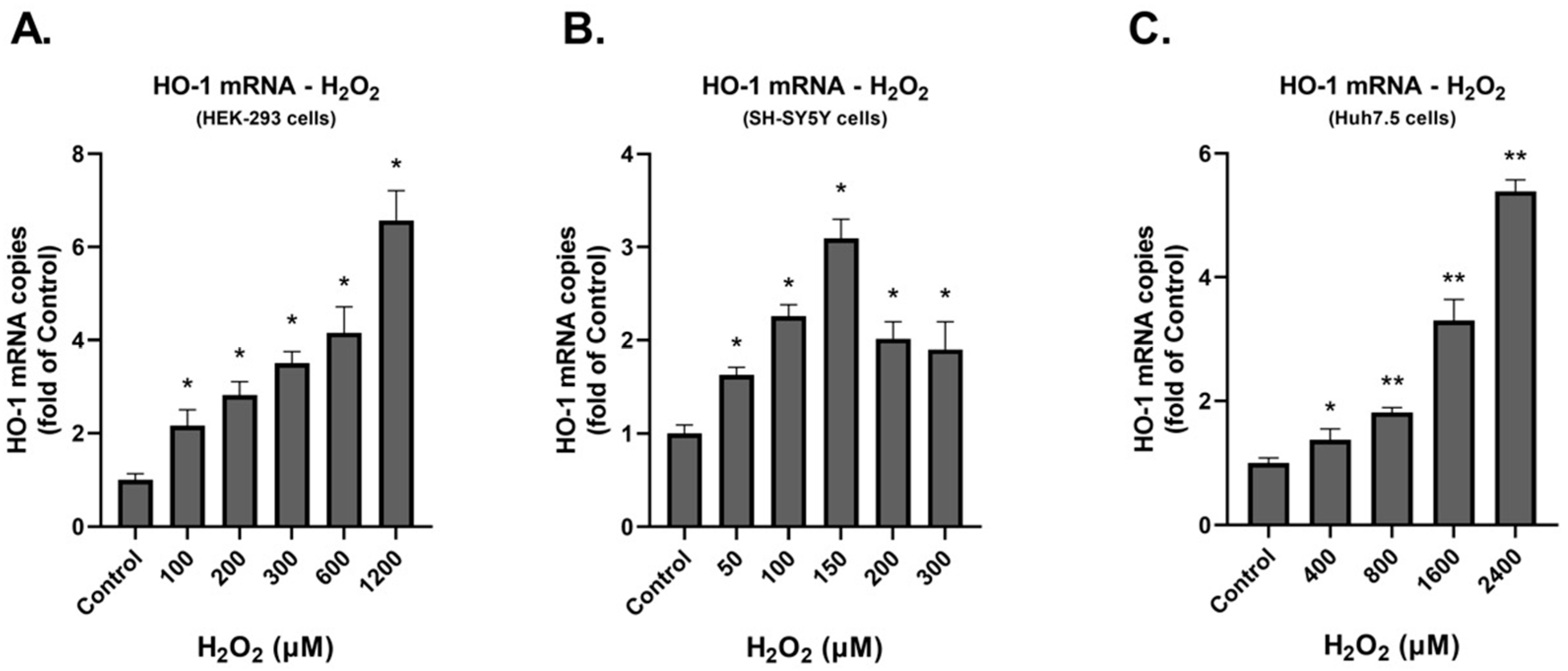
3.2. DDC mRNA and Protein Expression during Prolonged Exposure to Oxidative Conditions
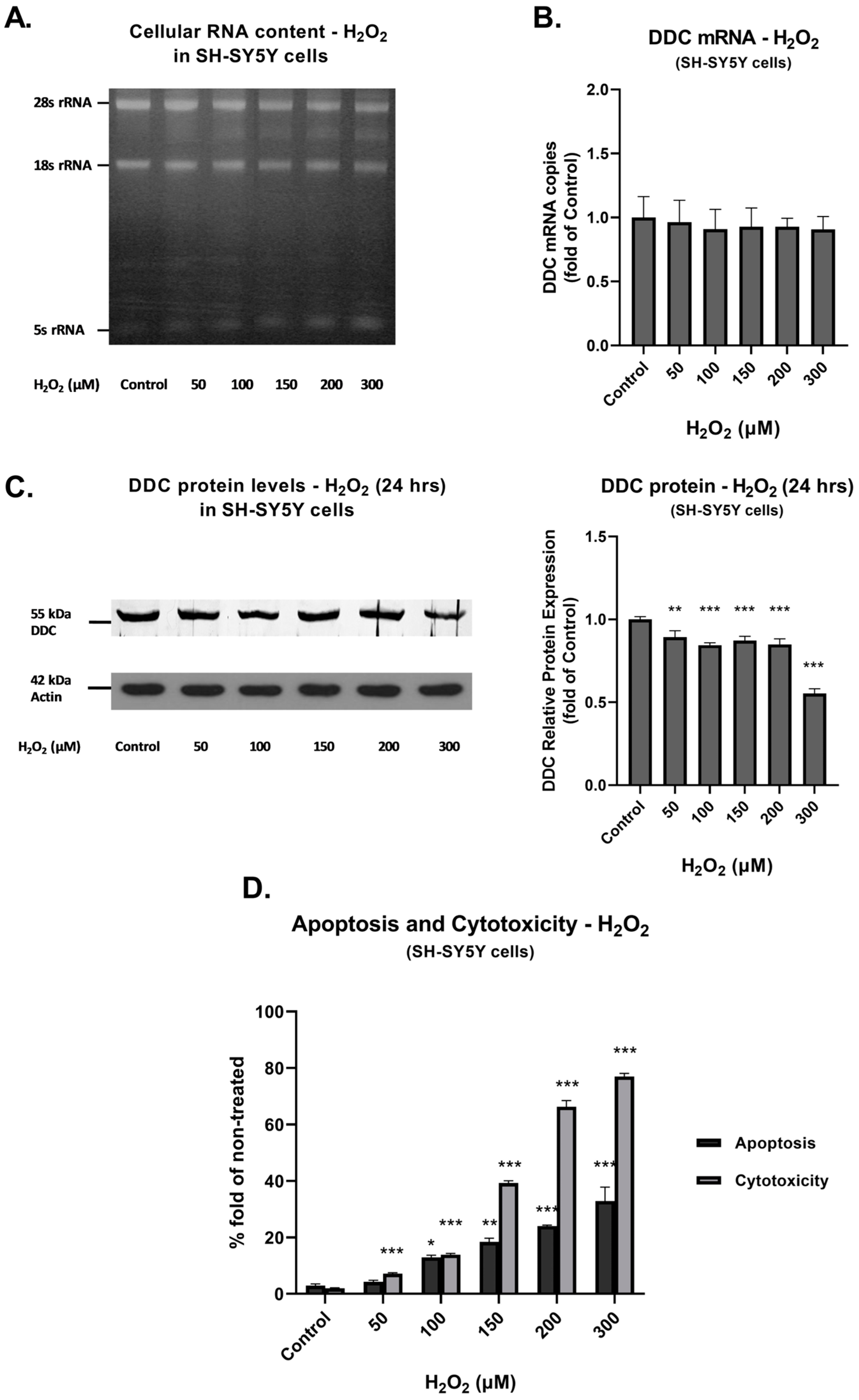
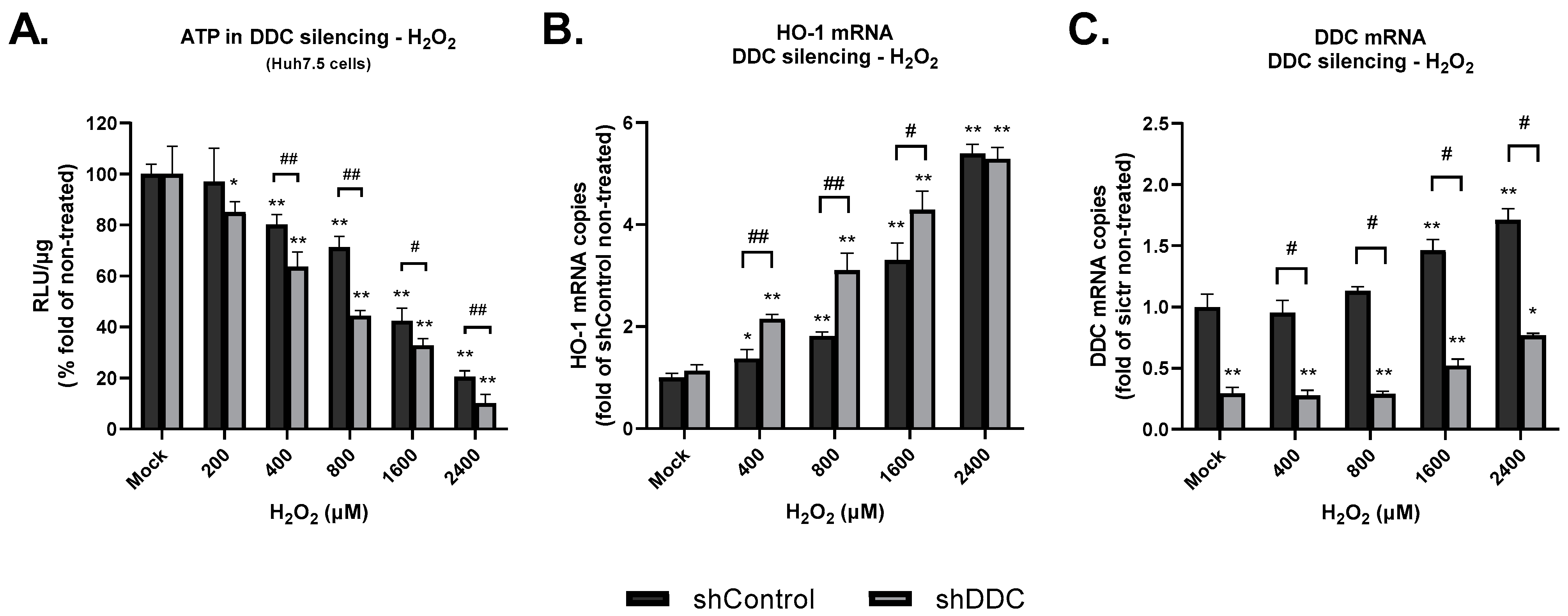
3.3. In DDC-Silenced Cells, the Effects of Prolonged Oxidative Stress Are more Pronounced
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Z.; Wu, J.; Deleo, C.J. RNA damage and surveillance under oxidative stress. IUBMB Life 2006, 58, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Rahal, A.; Kumar, A.; Singh, V.; Yadav, B.; Tiwari, R.; Chakraborty, S.; Dhama, K. Oxidative stress, prooxidants, and antioxidants: The interplay. BioMed Res. Int. 2014, 2014, 761274. [Google Scholar] [CrossRef] [PubMed]
- Bienert, G.P.; Schjoerring, J.K.; Jahn, T.P. Membrane transport of hydrogen peroxide. Biochim. Biophys. Acta 2006, 1758, 994–1003. [Google Scholar] [CrossRef] [PubMed]
- Stone, J.R.; Yang, S. Hydrogen peroxide: A signaling messenger. Antioxid. Redox Signal. 2006, 8, 243–270. [Google Scholar] [CrossRef] [PubMed]
- Dasuri, K.; Zhang, L.; Keller, J.N. Oxidative stress, neurodegeneration, and the balance of protein degradation and protein synthesis. Free Radic. Biol. Med. 2013, 62, 170–185. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef] [PubMed]
- Bertoldi, M. Mammalian Dopa decarboxylase: Structure, catalytic activity and inhibition. Arch. Biochem. Biophys. 2014, 546, 1–7. [Google Scholar] [CrossRef]
- Lovenberg, W.; Weissbach, H.; Udenfriend, S. Aromatic L-amino acid decarboxylase. J. Biol. Chem. 1962, 237, 89–93. [Google Scholar] [CrossRef]
- Rahman, M.K.; Nagatsu, T.; Kato, T. Determination of aromatic L-amino acid decarboxylase in serum of various animals by high-performance liquid chromatography with electrochemical detection. Life Sci. 1981, 28, 485–492. [Google Scholar] [CrossRef]
- Lindström, P.; Sehlin, J. Mechanisms underlying the effects of 5-hydroxytryptamine and 5-hydroxytryptophan in pancreatic islets. A proposed role for L-aromatic amino acid decarboxylase. Endocrinology 1983, 112, 1524–1529. [Google Scholar] [CrossRef]
- Craig, S.P.; Thai, A.L.; Weber, M.; Craig, I.W. Localisation of the gene for human aromatic L-amino acid decarboxylase (DDC) to chromosome 7p13-->p11 by in situ hybridization. Cytogenet. Cell Genet. 1992, 61, 114–116. [Google Scholar] [CrossRef] [PubMed]
- Ichinose, H.; Sumi-Ichinose, C.; Ohye, T.; Hagino, Y.; Fujita, K.; Nagatsu, T. Tissue-specific alternative splicing of the first exon generates two types of mRNAs in human aromatic L-amino acid decarboxylase. Biochemistry 1992, 31, 11546–11550. [Google Scholar] [CrossRef] [PubMed]
- Albert, V.R.; Lee, M.R.; Bolden, A.H.; Wurzburger, R.J.; Aguanno, A. Distinct promoters direct neuronal and nonneuronal expression of rat aromatic L-amino acid decarboxylase. Proc. Natl. Acad. Sci. USA 1992, 89, 12053–12057. [Google Scholar] [CrossRef] [PubMed]
- Sumi-Ichinose, C.; Hasegawa, S.; Ichinose, H.; Sawada, H.; Kobayashi, K.; Sakai, M.; Fujii, T.; Nomura, H.; Nomura, T.; Nagatsu, I.; et al. Analysis of the alternative promoters that regulate tissue-specific expression of human aromatic L-amino acid decarboxylase. J. Neurochem. 1995, 64, 514–524. [Google Scholar] [CrossRef] [PubMed]
- Jahng, J.W.; Wessel, T.C.; Houpt, T.A.; Son, J.H.; Joh, T.H. Alternate promoters in the rat aromatic L-amino acid decarboxylase gene for neuronal and nonneuronal expression: An in situ hybridization study. J. Neurochem. 1996, 66, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Siaterli, M.Z.; Vassilacopoulou, D.; Fragoulis, E.G. Cloning and expression of human placental L-Dopa decarboxylase. Neurochem. Res. 2003, 28, 797–803. [Google Scholar] [CrossRef]
- Kokkinou, I.; Nikolouzou, E.; Hatzimanolis, A.; Fragoulis, E.G.; Vassilacopoulou, D. Expression of enzymatically active L-DOPA decarboxylase in human peripheral leukocytes. Blood Cells Mol. Dis. 2009, 42, 92–98. [Google Scholar] [CrossRef]
- Chalatsa, I.; Nikolouzou, E.; Fragoulis, E.G.; Vassilacopoulou, D. L-Dopa decarboxylase expression profile in human cancer cells. Mol. Biol. Rep. 2011, 38, 1005–1011. [Google Scholar] [CrossRef]
- Vassiliou, A.G.; Siaterli, M.-Z.; Frakolaki, E.; Gkogkosi, P.; Paspaltsis, I.; Sklaviadis, T.; Vassilacopoulou, D.; Vassilaki, N. L-Dopa decarboxylase interaction with the major signaling regulator ΡΙ3Κ in tissues and cells of neural and peripheral origin. Biochimie 2019, 160, 76–87. [Google Scholar] [CrossRef]
- O’Malley, K.L.; Harmon, S.; Moffat, M.; Wong, S.; Uhland-Smith, A. The human aromatic L-amino acid decarboxylase gene can be alternatively spliced to generate unique protein isoforms. J. Neurochem. 1995, 65, 2409–2416. [Google Scholar] [CrossRef]
- Vassilacopoulou, D.; Sideris, D.C.; Vassiliou, A.G.; Fragoulis, E.G. Identification and characterization of a novel form of the human L-dopaL-Dopa decarboxylase mRNA. Neurochem. Res. 2014, 29, 1817–1823. [Google Scholar] [CrossRef] [PubMed]
- Adamopoulous, P.G.; Tsiakanikas, P.; Kontos, C.K.; Panagiotou, A.; Vassilacopoulou, D.; Scorilas, A. Identification of novel alternative splice variants of the human L-DOPA decarboxylase (DDC) gene in human cancer cells, using high-throughput sequencing approaches. Gene 2019, 719, 144075. [Google Scholar] [CrossRef]
- Paterson, I.A.; Juorio, A.V.; Boulton, A.A. 2-Phenylethylamine: A modulator of catecholamine transmission in the mammalian central nervous system? J. Neurochem. 1990, 55, 1827–1837. [Google Scholar] [CrossRef] [PubMed]
- Kow, R.L.; Sikkema, C.; Wheeler, J.M.; Wilkinson, C.W.; Kraemer, B.C. DOPA Decarboxylase Modulates Tau Toxicity. Biol. Psychiatry 2018, 83, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Devos, D.; Lejeune, S.; Cormier-Dequaire, F.; Tahiri, K.; Charbonnier-Beaupel, F.; Rouaix, N.; Duhamel, A.; Sablonnière, B.; Bonnet, A.-M.; Bonnet, C.; et al. Dopa-decarboxylase gene polymorphisms affect the motor response to L-dopa in Parkinson’s disease. Park. Relat. Disord. 2014, 20, 170–175. [Google Scholar] [CrossRef]
- Li, L.; Lin, H.; Hua, P.; Yan, L.; Dong, H.; Li, T.; Liu, W. Polymorphism of the Dopa-Decarboxylase Gene Modifies the Motor Response to Levodopa in Chinese Patients with Parkinson’s Disease. Front. Neurol. 2020, 11, 520934. [Google Scholar] [CrossRef]
- Frakolaki, E.; Kalliampakou, K.I.; Kaimou, P.; Moraiti, M.; Kolaitis, N.; Boleti, H.; Koskinas, J.; Vassilacopoulou, D.; Vassilaki, N. Emerging Role of l-Dopa Decarboxylase in Flaviviridae Virus Infections. Cells 2019, 8, 837. [Google Scholar] [CrossRef]
- Chalatsa, I.; Arvanitis, N.; Arvanitis, D.; Tsakou, A.C.; Kalantzis, E.D.; Vassiliou, A.G.; Sideris, D.C.; Frakolaki, E.; Vassilaki, N.; Vassilacopoulou, D. Human L-DopaL-Dopa decarboxylase interaction with annexin V and expression during apoptosis. Biochimie 2020, 177, 78–86. [Google Scholar] [CrossRef]
- Blight, K.J.; McKeating, J.A.; Rice, C.M. Highly Permissive Cell Lines for Subgenomic and Genomic Hepatitis C Virus RNA Replication. J. Virol. 2002, 76, 13001–13014. [Google Scholar] [CrossRef]
- Mpekoulis, G.; Tsopela, V.; Chalari, A.; Kalliampakou, K.I.; Panos, G.; Frakolaki, E.; Milona, R.S.; Sideris, D.C.; Vassilacopoulou, D.; Vassilaki, N. Dengue Virus Replication Is Associated with Catecholamine Biosynthesis and Metabolism in Hepatocytes. Viruses 2022, 14, 564. [Google Scholar] [CrossRef]
- Kalajzic, I.; Stover, M.; Liu, P.; Kalajzic, Z.; Rowe, D.; Lichtler, A. Use of VSV-G pseudotyped retroviral vectors to target murine osteoprogenitor cells. Virology 2001, 284, 37–45. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dull, T.; Zufferey, R.; Kelly, M.; Mandel, R.J.; Nguyen, M.; Trono, D.; Naldini, L. A third-generation lentivirus vector with a conditional packaging system. J. Virol. 1998, 72, 8463–8471. [Google Scholar] [CrossRef] [PubMed]
- Eruslanov, E.; Kusmartsev, S. Identification of ROS using oxidized DCFDA and flow-cytometry. Methods Mol. Biol. 2010, 594, 57–72. [Google Scholar] [PubMed]
- Louis, K.S.; Siegel, A.C. Cell viability analysis using trypan blue: Manual and automated methods. Methods Mol. Biol. 2011, 740, 7–12. [Google Scholar] [PubMed]
- Gibb, R.K.; Gercel-Taylor, C. Use of diphenylamine in the detection of apoptosis. Methods Mol. Med. 2001, 39, 679–680. [Google Scholar] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Towbin, H.; Staehelin, T.; Gordon, J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc. Natl. Acad. Sci. USA 1979, 76, 4350–4354. [Google Scholar] [CrossRef]
- Batteiger, B.; Newhall, W.J.; Jones, R.B. The use of Tween 20 as a blocking agent in the immunological detection of proteins transferred to nitrocellulose membranes. J. Immunol. Methods 1982, 55, 297–307. [Google Scholar] [CrossRef]
- Moreno-Loshuertos, R.; Acín-Pérez, R.; Fernández-Silva, P. Differences in reactive oxygen species production explain the phenotypes associated with common mouse mitochondrial DNA variants. Nat. Genet. 2006, 38, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Paravicini, T.M.; Touyz, R.M. Redox signaling in hypertension. Cardiovasc. Res. 2020, 71, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Lian, G. ROS and diseases: Role in metabolism and energy supply. Mol. Cell Biochem. 2020, 467, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Fahn, S.; Sulzer, D. Neurodegeneration and neuroprotection in Parkinson disease. NeuroRx 2014, 1, 139–154. [Google Scholar] [CrossRef] [PubMed]
- Mahul-Mellier, A.-L.; Burtscher, J.; Maharjan, N.; Weerens, L.; Croisier, M.; Kuttler, F.; Leleu, M.; Knott, G.W.; Lashuel, H.A. The process of Lewy body formation, rather than simply α-synuclein fibrillization, is one of the major drivers of neurodegeneration. Proc. Natl. Acad. Sci. USA 2020, 117, 4971–4982. [Google Scholar] [CrossRef] [PubMed]
- Conway, K.A.; Rochet, J.C.; Bieganski, R.M.; Lansbury, P.T. Kinetic stabilization of the alpha-synuclein protofibril by a dopamine-alpha-synuclein adduct. Science 2001, 294, 1346–1349. [Google Scholar] [CrossRef]
- Caudle, W.M.; Colebrooke, R.E.; Emson, P.C.; Miller, G.W. Altered vesicular dopamine storage in Parkinson’s disease: A premature demise. Trends Neurosci. 2008, 31, 303–308. [Google Scholar] [CrossRef]
- Westlund, K.N.; Denney, R.M.; Rose, R.M.; Abell, C.W. Localization of distinct monoamine oxidase A and monoamine oxidase B cell populations in human brainstem. Neuroscience 1988, 25, 439–456. [Google Scholar] [CrossRef]
- Muñoz, P.; Huenchuguala, S.; Paris, I.; Segura-Aguilar, J. Dopamine oxidation and autophagy. Park. Dis. 2012, 2012, 920953. [Google Scholar] [CrossRef]
- Hare, D.J.; Double, K.L. Iron and dopamine: A toxic couple. Brain 2016, 139, 1026–1035. [Google Scholar] [CrossRef]
- Trist, B.G.; Hare, D.J.; Double, K.L. Oxidative stress in the aging substantia nigra and the etiology of Parkinson’s disease. Aging Cell 2019, 18, e13031. [Google Scholar] [CrossRef] [PubMed]
- Dias, V.; Junn, E.; Mouradian, M.M. The role of oxidative stress in Parkinson’s disease. J. Park. Dis. 2013, 3, 461–491. [Google Scholar] [CrossRef] [PubMed]
- Dean, E.D.; Li, Y.; Torres, G.E.; Miller, G.W. Identification of a novel interaction between α-synuclein and VMAT2. FASEB J. 2008, 22, 715.6. [Google Scholar] [CrossRef]
- Butler, B.; Goodwin, S.; Saha, K.; Becker, J.P.; Sambo, D.; Davari, P.; Goodwin, J.S.; Khoshbouei, H. Dopamine transporter activity is modulated by α-synuclein. J. Biol. Chem. 2015, 290, 19009. [Google Scholar]
- Pereira, J.B.; Kumar, A.; Hall, S.; Palmqvist, S.; Stomrud, E.; Bali, D.; Parchi, P.; Mattsson-Carlgren, N.; Janelidze, S.; Hansson, O. DOPA decarboxylase is an emerging biomarker for Parkinsonian disorders including preclinical Lewy body disease. Nat. Aging 2023, 3, 1201–1209. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.U. Role of PI3K/Akt/mTOR signaling pathway in hepatocellular carcinoma. Lin. Chuang Gan Dan. Bing. ZaZhi 2014, 30, 954–957. [Google Scholar]
- Glaviano, A.; Foo, A.S.C.; Lam, H.Y.; Yap, K.C.H.; Jacot, W.; Jones, R.H.; Eng, H.; Nair, M.G.; Makvandi, P.; Geoerger, B.; et al. PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. Mol. Cancer 2023, 22, 138. [Google Scholar] [CrossRef]
- Kalantzis, E.D.; Scorilas, A.; Vassilacopoulou, D. Evidence for L-Dopa Decarboxylase Involvement in Cancer Cell Cytotoxicity Induced by Docetaxel and Mitoxantrone. Curr. Pharm. Biotechnol. 2018, 19, 1087–1096. [Google Scholar] [CrossRef]
- Araki, W.; Wurtman, R.J. Increased expression of amyloid precursor protein and amyloid precursor-like protein 2 during trophic factor withdrawal-induced death of neuronal PC12 cells. Brain Res. Mol. Brain Res. 1998, 56, 169–177. [Google Scholar] [CrossRef]
- Quinn, C.M.; Kågedal, K.; Terman, A.; Stroikin, U.; Brunk, U.T.; Jessup, W.; Garner, B. Induction of fibroblast apolipoprotein E expression during apoptosis, starvation-induced growth arrest and mitosis. Neuropharmacology 2001, 41, 464–471. [Google Scholar] [CrossRef]
- Strope, T.A.; Wilkins, H.M. Amyloid precursor protein and mitochondria. Curr. Opin. Neurobiol. 2023, 78, 102651. [Google Scholar] [CrossRef] [PubMed]
- Loch, R.A.; Wang, H.; Perálvarez-Marín, A.; Berger, P.; Nielsen, H.; Chroni, A.; Luo, J. Cross interactions between Apolipoprotein E and amyloid proteins in neurodegenerative diseases. Comput. Struct. Biotechnol. J. 2023, 21, 1189–1204. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lotsios, N.S.; Arvanitis, N.; Charonitakis, A.G.; Mpekoulis, G.; Frakolaki, E.; Vassilaki, N.; Sideris, D.C.; Vassilacopoulou, D. Expression of Human L-Dopa Decarboxylase (DDC) under Conditions of Oxidative Stress. Curr. Issues Mol. Biol. 2023, 45, 10179-10192. https://doi.org/10.3390/cimb45120635
Lotsios NS, Arvanitis N, Charonitakis AG, Mpekoulis G, Frakolaki E, Vassilaki N, Sideris DC, Vassilacopoulou D. Expression of Human L-Dopa Decarboxylase (DDC) under Conditions of Oxidative Stress. Current Issues in Molecular Biology. 2023; 45(12):10179-10192. https://doi.org/10.3390/cimb45120635
Chicago/Turabian StyleLotsios, Nikolaos S., Nikolaos Arvanitis, Alexandros G. Charonitakis, George Mpekoulis, Efseveia Frakolaki, Niki Vassilaki, Diamantis C. Sideris, and Dido Vassilacopoulou. 2023. "Expression of Human L-Dopa Decarboxylase (DDC) under Conditions of Oxidative Stress" Current Issues in Molecular Biology 45, no. 12: 10179-10192. https://doi.org/10.3390/cimb45120635
APA StyleLotsios, N. S., Arvanitis, N., Charonitakis, A. G., Mpekoulis, G., Frakolaki, E., Vassilaki, N., Sideris, D. C., & Vassilacopoulou, D. (2023). Expression of Human L-Dopa Decarboxylase (DDC) under Conditions of Oxidative Stress. Current Issues in Molecular Biology, 45(12), 10179-10192. https://doi.org/10.3390/cimb45120635





