Neurobiology and Applications of Inositol in Psychiatry: A Narrative Review
Abstract
:1. Introduction
1.1. Background
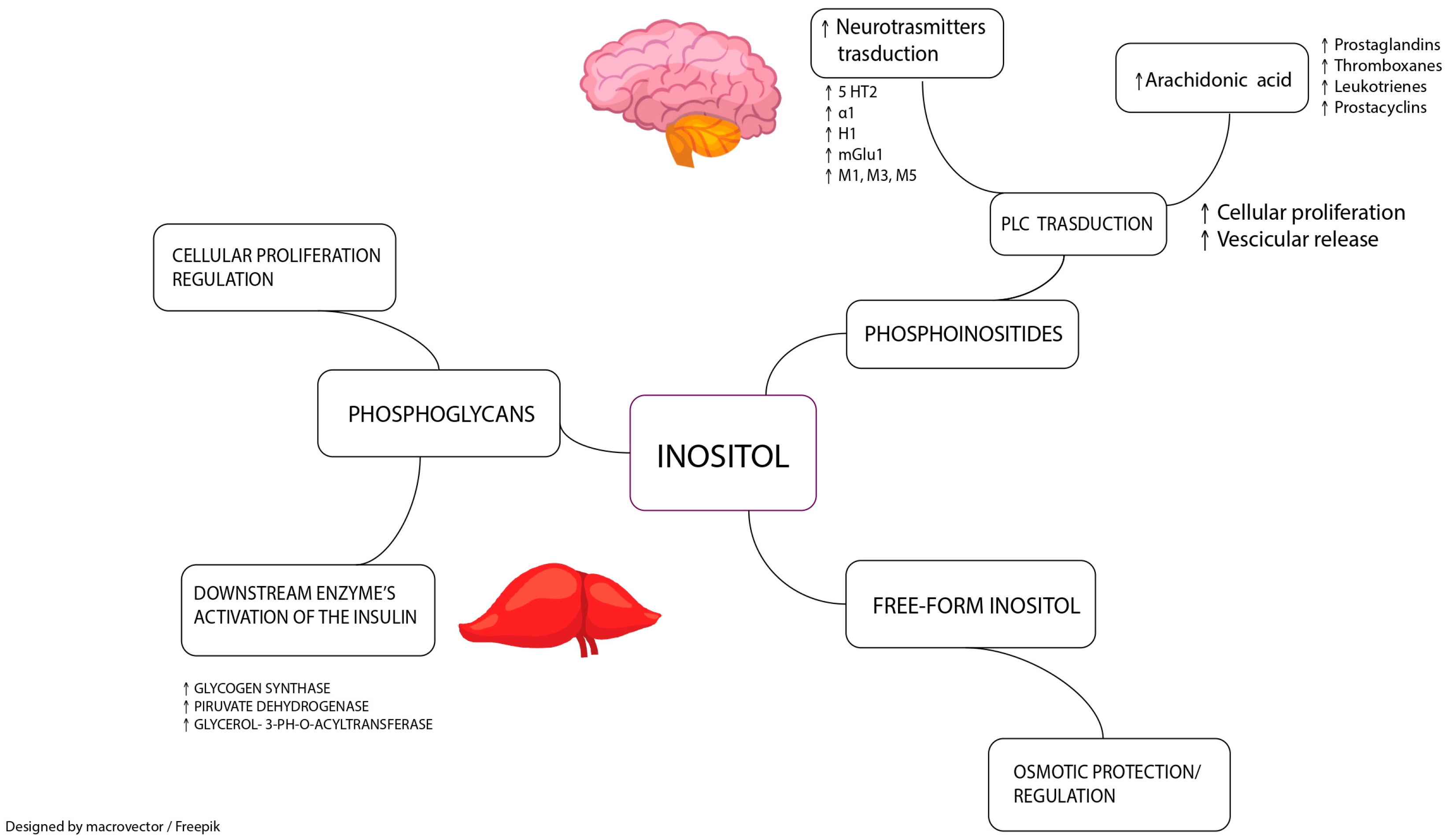
1.2. Biochemistry and Functions
1.3. Role in Metabolic Disorders
2. Inositol and Mood Disorders
3. Inositol and Psychotic Disorders
4. Anxiety Disorders
5. Other Psychiatric Disorders
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siracusa, L.; Napoli, E.; Ruberto, G. Novel Chemical and Biological Insights of Inositol Derivatives in Mediterranean Plants. Molecules 2022, 27, 1525. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.P.; Mills, S.J.; Potter, B.V. The “Other” Inositols and Their Phosphates: Synthesis, Biology, and Medicine (with Recent Advances in myo-Inositol Chemistry). Angew. Chem. Int. Ed. Engl. 2016, 55, 1614–1650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Downes, C.P.; Gray, A.; Lucocq, J.M. Probing phosphoinositide functions in signaling and membrane trafficking. Trends Cell Biol. 2005, 15, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Tsui, M.M.; York, J.D. Roles of inositol phosphates and inositol pyrophosphates in development, cell signaling and nuclear processes. Adv. Enzym. Regul. 2010, 50, 324–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vucenik, I. Anticancer Properties of Inositol Hexaphosphate and Inositol: An Overview. J. Nutr. Sci. Vitaminol. (Tokyo) 2019, 65, S18–S22. [Google Scholar] [CrossRef] [Green Version]
- Yancey, P.H. Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses. J. Exp. Biol. 2005, 208, 2819–2830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Claxson, A.; Morris, C.; Blake, D.; Sirén, M.; Halliwell, B.; Gustafsson, T.; Löfkvist, B.; Bergelin, I. The anti-inflammatory effects of D-myo-inositol-1.2.6-trisphosphate (PP56) on animal models of inflammation. Agents Actions 1990, 29, 68–70. [Google Scholar] [CrossRef] [PubMed]
- Owczarczyk-Saczonek, A.; Lahuta, L.B.; Ligor, M.; Placek, W.; Górecki, R.J.; Buszewski, B. The Healing-Promoting Properties of Selected Cyclitols-A Review. Nutrients 2018, 10, 1891. [Google Scholar] [CrossRef] [Green Version]
- Sarris, J.; Camfield, D.; Berk, M. Complementary medicine, self-help, and lifestyle interventions for obsessive compulsive disorder (OCD) and the OCD spectrum: A systematic review. J. Affect. Disord. 2012, 138, 213–221. [Google Scholar] [CrossRef]
- Palatnik, A.; Frolov, K.; Fux, M.; Benjamin, J. Double-blind, controlled, crossover trial of inositol versus fluvoxamine for the treatment of panic disorder. J. Clin. Psychopharmacol. 2001, 21, 335–339. [Google Scholar] [CrossRef]
- Levine, J.; Barak, Y.; Gonzalves, M.; Szor, H.; Elizur, A.; Kofman, O.; Belmaker, R.H. Double-blind, controlled trial of inositol treatment of depression. Am. J. Psychiatry 1995, 152, 792–794. [Google Scholar] [CrossRef] [PubMed]
- Sani, G.; Gualtieri, I.; Paolini, M.; Bonanni, L.; Spinazzola, E.; Maggiora, M.; Pinzone, V.; Brugnoli, R.; Angeletti, G.; Girardi, P.; et al. Drug Treatment of Trichotillomania (Hair-Pulling Disorder), Excoriation (Skin-picking) Disorder, and Nail-biting (Onychophagia). Curr. Neuropharmacol. 2019, 17, 775–786. [Google Scholar] [CrossRef] [PubMed]
- Nemets, B.; Mishory, A.; Levine, J.; Belmaker, R.H. Inositol addition does not improve depression in SSRI treatment failures. J. Neural. Transm. (Vienna) 1999, 106, 795–798. [Google Scholar] [CrossRef] [PubMed]
- Levine, J.; Mishori, A.; Susnosky, M.; Martin, M.; Belmaker, R.H. Combination of inositol and serotonin reuptake inhibitors in the treatment of depression. Biol. Psychiatry 1999, 45, 270–273. [Google Scholar] [CrossRef]
- Chengappa, K.N.; Levine, J.; Gershon, S.; Mallinger, A.G.; Hardan, A.; Vagnucci, A.; Pollock, B.; Luther, J.; Buttenfield, J.; Verfaille, S.; et al. Inositol as an add-on treatment for bipolar depression. Bipolar. Disord. 2000, 2, 47–55. [Google Scholar] [CrossRef]
- Gianfranco, C.; Vittorio, U.; Silvia, B.; Francesco, D. Myo-inositol in the treatment of premenstrual dysphoric disorder. Hum. Psychopharmacol. 2011, 26, 526–530. [Google Scholar] [CrossRef]
- Nemets, B.; Talesnick, B.; Belmaker, R.H.; Levine, J. Myo-inositol has no beneficial effect on premenstrual dysphoric disorder. World J. Biol. Psychiatry 2002, 3, 147–149. [Google Scholar] [CrossRef]
- Levine, J.; Rapaport, A.; Lev, L.; Bersudsky, Y.; Kofman, O.; Belmaker, R.H.; Shapiro, J.; Agam, G. Inositol treatment raises CSF inositol levels. Brain Res. 1993, 627, 168–170. [Google Scholar] [CrossRef]
- Levine, J.; Umansky, R.; Ezrielev, G.; Belmaker, R.H. Lack of effect of inositol treatment in chronic schizophrenia. Biol. Psychiatry 1993, 33, 673–675. [Google Scholar] [CrossRef]
- Levine, J.; Goldberger, I.; Rapaport, A.; Schwartz, M.; Schield, C.; Elizur, A.; Belmaker, R.; Shapiro, J.; Agam, G. CSF inositol in schizophrenia and high-dose inositol treatment of schizophrenia. Eur. Neuropsychopharmacol. 1994, 4, 487–490. [Google Scholar] [CrossRef]
- Fux, M.; Levine, J.; Aviv, A.; Belmaker, R.H. Inositol treatment of obsessive-compulsive disorder. Am. J. Psychiatry 1996, 153, 1219–1221. [Google Scholar] [CrossRef]
- Fux, M.; Benjamin, J.; Belmaker, R.H. Inositol versus placebo augmentation of serotonin reuptake inhibitors in the treatment of obsessive-compulsive disorder: A double-blind cross-over study. Int. J. Neuropsychopharmacol. 1999, 2, 193–195. [Google Scholar] [CrossRef] [Green Version]
- Seedat, S.; Stein, D.J. Inositol augmentation of serotonin reuptake inhibitors in treatment-refractory obsessive-compulsive disorder: An open trial. Int. Clin. Psychopharmacol. 1999, 14, 353–356. [Google Scholar] [CrossRef] [Green Version]
- Leppink, E.W.; Redden, S.A.; Grant, J.E. A double-blind, placebo-controlled study of inositol in trichotillomania. Int. Clin. Psychopharmacol. 2017, 32, 107–114. [Google Scholar] [CrossRef]
- Kaplan, Z.; Amir, M.; Swartz, M.; Levine, J. Inositol treatment of post-traumatic stress disorder. Anxiety 1996, 2, 51–52. [Google Scholar] [CrossRef]
- Benjamin, J.; Levine, J.; Fux, M.; Aviv, A.; Levy, D.; Belmaker, R.H. Double-blind, placebo-controlled, crossover trial of inositol treatment for panic disorder. Am. J. Psychiatry 1995, 152, 1084–1086. [Google Scholar] [CrossRef] [PubMed]
- Levine, J.; Aviram, A.; Holan, A.; Ring, A.; Barak, Y.; Belmaker, R.H. Inositol treatment of autism. J. Neural Transm. (Vienna) 1997, 104, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Gelber, D.; Levine, J.; Belmaker, R.H. Effect of inositol on bulimia nervosa and binge eating. Int. J. Eat. Disord. 2001, 29, 345–348. [Google Scholar] [CrossRef]
- Levine, J.; Pomerantz, T.; Stier, S.; Belmaker, R.H. Lack of effect of 6 g inositol treatment of post-ECT cognitive function in humans. J. Psychiatr. Res. 1995, 29, 487–489. [Google Scholar] [CrossRef]
- Dinicola, S.; Minini, M.; Unfer, V.; Verna, R.; Cucina, A.; Bizzarri, M. Nutritional and Acquired Deficiencies in Inositol Bioavailability. Correlations with Metabolic Disorders. Int. J. Mol. Sci. 2017, 18, 2187. [Google Scholar] [CrossRef] [Green Version]
- Troyer, D.A.; Schwertz, D.W.; Kreisberg, J.I.; Venkatachalam, M.A. Inositol phospholipid metabolism in the kidney. Annu. Rev. Physiol. 1986, 48, 51–71. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, R.; Eisenberg, F., Jr. Selective hormonal control of myo-inositol biosynthesis in reproductive organs and liver of the male rat. Proc. Natl. Acad. Sci. USA 1981, 78, 4863–4866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gambioli, R.; Montanino Oliva, M.; Nordio, M.; Chiefari, A.; Puliani, G.; Unfer, V. New Insights into the Activities of D-Chiro-Inositol: A Narrative Review. Biomedicines 2021, 9, 1378. [Google Scholar] [CrossRef] [PubMed]
- Loewus, M.W.; Loewus, F.A.; Brillinger, G.U.; Otsuka, H.; Floss, H.G. Stereochemistry of the myo-inositol-1-phosphate synthase reaction. J. Biol. Chem. 1980, 255, 11710–11712. [Google Scholar] [CrossRef]
- Caputo, M.; Bona, E.; Leone, I.; Samà, M.T.; Nuzzo, A.; Ferrero, A.; Aimaretti, G.; Marzullo, P.; Prodam, F. Inositols and metabolic disorders: From farm to bedside. J. Tradit. Complement. Med. 2020, 10, 252–259. [Google Scholar] [CrossRef]
- Clements, R.S., Jr.; Darnell, B. Myo-inositol content of common foods: Development of a high-myo-inositol diet. Am. J. Clin. Nutr. 1980, 33, 1954–1967. [Google Scholar] [CrossRef] [Green Version]
- Caspary, W.F.; Crane, R.K. Active transport of myo-inositol and its relation to the sugar transport system in hamster small intestine. Biochim. Biophys. Acta 1970, 203, 308–316. [Google Scholar] [CrossRef]
- Lewin, L.M.; Beer, R. Prostatic secretion as the source of myo-inositol in human seminal fluid. Fertil. Steril. 1973, 24, 666–670. [Google Scholar] [CrossRef]
- Hinton, B.T.; White, R.W.; Setchell, B.P. Concentrations of myo-inositol in the luminal fluid of the mammalian testis and epididymis. J. Reprod Fertil 1980, 58, 395–399. [Google Scholar] [CrossRef] [Green Version]
- Spector, R.; Lorenzo, A.V. The origin of myo-inositol in brain, cerebrospinal fluid and choroid plexus. J. Neurochem. 1975, 25, 353–354. [Google Scholar] [CrossRef]
- Combs, G.F., Jr.; McClung, J.P. The Vitamins: Fundamental Aspects in Nutrition and Health; Academic press: London, UK, 2016. [Google Scholar]
- Spector, R. Myo-inositol transport through the blood-brain barrier. Neurochem. Res. 1988, 13, 785–787. [Google Scholar] [CrossRef] [PubMed]
- Gambioli, R.; Forte, G.; Aragona, C.; Bevilacqua, A.; Bizzarri, M.; Unfer, V. The use of D-chiro-Inositol in clinical practice. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Lewin, L.M.; Wagenknecht, A.C. An inositol phosphatide of peas. Arch. Biochem. Biophys. 1960, 87, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Rameh, L.E.; Tolias, K.F.; Duckworth, B.C.; Cantley, L.C. A new pathway for synthesis of phosphatidylinositol-4,5-bisphosphate. Nature 1997, 390, 192–196. [Google Scholar] [CrossRef]
- Nishizuka, Y. Studies and perspectives of protein kinase C. Science 1986, 233, 305–312. [Google Scholar] [CrossRef]
- Berridge, M.J. Inositol trisphosphate and calcium signalling mechanisms. Biochim. Biophys. Acta 2009, 1793, 933–940. [Google Scholar] [CrossRef] [Green Version]
- Jones, D.R.; Varela-Nieto, I. Diabetes and the role of inositol-containing lipids in insulin signaling. Mol. Med. 1999, 5, 505–514. [Google Scholar] [CrossRef] [Green Version]
- Penninx, B.; Lange, S.M.M. Metabolic syndrome in psychiatric patients: Overview, mechanisms, and implications. Dialogues Clin. Neurosci. 2018, 20, 63–73. [Google Scholar] [CrossRef]
- Croze, M.L.; Soulage, C.O. Potential role and therapeutic interests of myo-inositol in metabolic diseases. Biochimie 2013, 95, 1811–1827. [Google Scholar] [CrossRef]
- Pintaudi, B.; Di Vieste, G.; Bonomo, M. The Effectiveness of Myo-Inositol and D-Chiro Inositol Treatment in Type 2 Diabetes. Int. J. Endocrinol. 2016, 2016, 9132052. [Google Scholar] [CrossRef] [Green Version]
- Morley, L.C.; Tang, T.; Yasmin, E.; Norman, R.J.; Balen, A.H. Insulin-sensitising drugs (metformin, rosiglitazone, pioglitazone, D-chiro-inositol) for women with polycystic ovary syndrome, oligo amenorrhoea and subfertility. Cochrane Database Syst. Rev. 2017, 11, Cd003053. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xing, C.; Zhao, H.; He, B. The effectiveness of coenzyme Q10, vitamin E, inositols, and vitamin D in improving the endocrine and metabolic profiles in women with polycystic ovary syndrome: A network Meta-analysis. Gynecol. Endocrinol. 2021, 37, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Giordano, D.; Corrado, F.; Santamaria, A.; Quattrone, S.; Pintaudi, B.; Di Benedetto, A.; D’Anna, R. Effects of myo-inositol supplementation in postmenopausal women with metabolic syndrome: A perspective, randomized, placebo-controlled study. Menopause 2011, 18, 102–104. [Google Scholar] [CrossRef] [PubMed]
- Capasso, I.; Esposito, E.; Maurea, N.; Montella, M.; Crispo, A.; De Laurentiis, M.; D’Aiuto, M.; Frasci, G.; Botti, G.; Grimaldi, M.; et al. Combination of inositol and alpha lipoic acid in metabolic syndrome-affected women: A randomized placebo-controlled trial. Trials 2013, 14, 273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maurizi, A.R.; Menduni, M.; Del Toro, R.; Kyanvash, S.; Maggi, D.; Guglielmi, C.; Pantano, A.L.; Defeudis, G.; Fioriti, E.; Manfrini, S.; et al. A pilot study of D-chiro-inositol plus folic acid in overweight patients with type 1 diabetes. Acta Diabetol. 2017, 54, 361–365. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, K.S.; Lee, S.K.; Min, K.W.; Han, K.A.; Kim, Y.K.; Ku, B.J. Effects of pinitol on glycemic control, insulin resistance and adipocytokine levels in patients with type 2 diabetes mellitus. Ann. Nutr. Metab. 2012, 60, 1–5. [Google Scholar] [CrossRef]
- Hong, J.H.; Jang, H.W.; Kang, Y.E.; Lee, J.H.; Kim, K.S.; Kim, H.J.; Park, K.R.; Ku, B.J. Urinary chiro- and myo-inositol levels as a biological marker for type 2 diabetes mellitus. Dis. Markers 2012, 33, 193–199. [Google Scholar] [CrossRef]
- Daughaday, W.H.; Larner, J. The renal excretion of inositol in normal and diabetic human beings. J. Clin. Investig. 1954, 33, 326–332. [Google Scholar] [CrossRef]
- Kennington, A.S.; Hill, C.R.; Craig, J.; Bogardus, C.; Raz, I.; Ortmeyer, H.K.; Hansen, B.C.; Romero, G.; Larner, J. Low urinary chiro-inositol excretion in non-insulin-dependent diabetes mellitus. N. Engl. J. Med. 1990, 323, 373–378. [Google Scholar] [CrossRef]
- Asplin, I.; Galasko, G.; Larner, J. chiro-inositol deficiency and insulin resistance: A comparison of the chiro-inositol- and the myo-inositol-containing insulin mediators isolated from urine, hemodialysate, and muscle of control and type II diabetic subjects. Proc. Natl. Acad. Sci. USA 1993, 90, 5924–5928. [Google Scholar] [CrossRef] [Green Version]
- McCall, A.L. The impact of diabetes on the CNS. Diabetes 1992, 41, 557–570. [Google Scholar] [CrossRef] [PubMed]
- Brands, A.M.; Biessels, G.J.; de Haan, E.H.; Kappelle, L.J.; Kessels, R.P. The effects of type 1 diabetes on cognitive performance: A meta-analysis. Diabetes Care 2005, 28, 726–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wessels, A.M.; Scheltens, P.; Barkhof, F.; Heine, R.J. Hyperglycaemia as a determinant of cognitive decline in patients with type 1 diabetes. Eur. J. Pharmacol. 2008, 585, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Griffith, R.J.; Alsweiler, J.; Moore, A.E.; Brown, S.; Middleton, P.; Shepherd, E.; Crowther, C.A. Interventions to prevent women from developing gestational diabetes mellitus: An overview of Cochrane Reviews. Cochrane Database Syst. Rev. 2020, 6, Cd012394. [Google Scholar] [CrossRef]
- Vitagliano, A.; Saccone, G.; Cosmi, E.; Visentin, S.; Dessole, F.; Ambrosini, G.; Berghella, V. Inositol for the prevention of gestational diabetes: A systematic review and meta-analysis of randomized controlled trials. Arch. Gynecol. Obstet. 2019, 299, 55–68. [Google Scholar] [CrossRef]
- Farren, M.; Daly, N.; McKeating, A.; Kinsley, B.; Turner, M.J.; Daly, S. The Prevention of Gestational Diabetes Mellitus with Antenatal Oral Inositol Supplementation: A Randomized Controlled Trial. Diabetes Care 2017, 40, 759–763. [Google Scholar] [CrossRef] [Green Version]
- D’Anna, R.; Scilipoti, A.; Giordano, D.; Caruso, C.; Cannata, M.L.; Interdonato, M.L.; Corrado, F.; Di Benedetto, A. myo-Inositol supplementation and onset of gestational diabetes mellitus in pregnant women with a family history of type 2 diabetes: A prospective, randomized, placebo-controlled study. Diabetes Care 2013, 36, 854–857. [Google Scholar] [CrossRef] [Green Version]
- Malvasi, A.; Casciaro, F.; Minervini, M.M.; Kosmas, I.; Mynbaev, O.A.; Pacella, E.; Monti Condesnitt, V.; Creanza, A.; Di Renzo, G.C.; Tinelli, A. Myo-inositol, D-chiro-inositol, folic acid and manganese in second trimester of pregnancy: A preliminary investigation. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 270–274. [Google Scholar]
- Santamaria, A.; Di Benedetto, A.; Petrella, E.; Pintaudi, B.; Corrado, F.; D’Anna, R.; Neri, I.; Facchinetti, F. Myo-inositol may prevent gestational diabetes onset in overweight women: A randomized, controlled trial. J. Matern. Fetal. Neonatal. Med. 2016, 29, 3234–3237. [Google Scholar] [CrossRef]
- D’Anna, R.; Di Benedetto, A.; Scilipoti, A.; Santamaria, A.; Interdonato, M.L.; Petrella, E.; Neri, I.; Pintaudi, B.; Corrado, F.; Facchinetti, F. Myo-inositol Supplementation for Prevention of Gestational Diabetes in Obese Pregnant Women: A Randomized Controlled Trial. Obstet. Gynecol. 2015, 126, 310–315. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.J.; Ullah, A.; Basit, S. Genetic Basis of Polycystic Ovary Syndrome (PCOS): Current Perspectives. Appl. Clin. Genet. 2019, 12, 249–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiani, A.K.; Donato, K.; Dhuli, K.; Stuppia, L.; Bertelli, M. Dietary supplements for polycystic ovary syndrome. J. Prev. Med. Hyg. 2022, 63, E206–E213. [Google Scholar] [CrossRef] [PubMed]
- Facchinetti, F.; Orrù, B.; Grandi, G.; Unfer, V. Short-term effects of metformin and myo-inositol in women with polycystic ovarian syndrome (PCOS): A meta-analysis of randomized clinical trials. Gynecol. Endocrinol. 2019, 35, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Yang, K. Effectiveness of myoinositol for polycystic ovary syndrome: A systematic review and meta-analysis. Endocrine 2018, 59, 30–38. [Google Scholar] [CrossRef]
- Fleming, R.; Hopkinson, Z.E.; Wallace, A.M.; Greer, I.A.; Sattar, N. Ovarian function and metabolic factors in women with oligomenorrhea treated with metformin in a randomized double blind placebo-controlled trial. J. Clin. Endocrinol. Metab. 2002, 87, 569–574. [Google Scholar] [CrossRef]
- Regidor, P.A.; Schindler, A.E.; Lesoine, B.; Druckman, R. Management of women with PCOS using myo-inositol and folic acid. New clinical data and review of the literature. Horm. Mol. Biol. Clin. Investig. 2018, 34, 1–10. [Google Scholar] [CrossRef]
- Papaleo, E.; Unfer, V.; Baillargeon, J.P.; Fusi, F.; Occhi, F.; De Santis, L. Myo-inositol may improve oocyte quality in intracytoplasmic sperm injection cycles. A prospective, controlled, randomized trial. Fertil. Steril. 2009, 91, 1750–1754. [Google Scholar] [CrossRef]
- Raffone, E.; Rizzo, P.; Benedetto, V. Insulin sensitiser agents alone and in co-treatment with r-FSH for ovulation induction in PCOS women. Gynecol. Endocrinol. 2010, 26, 275–280. [Google Scholar] [CrossRef]
- Paul, C.; Laganà, A.S.; Maniglio, P.; Triolo, O.; Brady, D.M. Inositol’s and other nutraceuticals’ synergistic actions counteract insulin resistance in polycystic ovarian syndrome and metabolic syndrome: State-of-the-art and future perspectives. Gynecol. Endocrinol. 2016, 32, 431–438. [Google Scholar] [CrossRef]
- Isabella, R.; Raffone, E. CONCERN: Does ovary need D-chiro-inositol? J. Ovarian Res. 2012, 5, 14. [Google Scholar] [CrossRef] [Green Version]
- Bevilacqua, A.; Carlomagno, G.; Gerli, S.; Montanino Oliva, M.; Devroey, P.; Lanzone, A.; Soulange, C.; Facchinetti, F.; Carlo Di Renzo, G.; Bizzarri, M.; et al. Results from the International Consensus Conference on myo-inositol and D-chiro-inositol in Obstetrics and Gynecology--assisted reproduction technology. Gynecol. Endocrinol. 2015, 31, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Barkai, A.I.; Dunner, D.L.; Gross, H.A.; Mayo, P.; Fieve, R.R. Reduced myo-inositol levels in cerebrospinal fluid from patients with affective disorder. Biol. Psychiatry 1978, 13, 65–72. [Google Scholar]
- Shimon, H.; Agam, G.; Belmaker, R.H.; Hyde, T.M.; Kleinman, J.E. Reduced frontal cortex inositol levels in postmortem brain of suicide victims and patients with bipolar disorder. Am. J. Psychiatry 1997, 154, 1148–1150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gruber, S.; Frey, R.; Mlynárik, V.; Stadlbauer, A.; Heiden, A.; Kasper, S.; Kemp, G.J.; Moser, E. Quantification of metabolic differences in the frontal brain of depressive patients and controls obtained by 1H-MRS at 3 Tesla. Investig. Radiol. 2003, 38, 403–408. [Google Scholar] [CrossRef]
- Coupland, N.J.; Ogilvie, C.J.; Hegadoren, K.M.; Seres, P.; Hanstock, C.C.; Allen, P.S. Decreased prefrontal Myo-inositol in major depressive disorder. Biol. Psychiatry 2005, 57, 1526–1534. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Zhang, L.; Li, L.; Liu, P.; Gao, J.; Liu, X.; Zou, J.; Zhang, Y.; Liu, J.; Zhang, Z.; et al. High-frequency rTMS treatment increases left prefrontal myo-inositol in young patients with treatment-resistant depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2010, 34, 1189–1195. [Google Scholar] [CrossRef] [PubMed]
- Frey, R.; Metzler, D.; Fischer, P.; Heiden, A.; Scharfetter, J.; Moser, E.; Kasper, S. Myo-inositol in depressive and healthy subjects determined by frontal 1H-magnetic resonance spectroscopy at 1.5 tesla. J. Psychiatr. Res. 1998, 32, 411–420. [Google Scholar] [CrossRef]
- Davanzo, P.; Thomas, M.A.; Yue, K.; Oshiro, T.; Belin, T.; Strober, M.; McCracken, J. Decreased anterior cingulate myo-inositol/creatine spectroscopy resonance with lithium treatment in children with bipolar disorder. Neuropsychopharmacology 2001, 24, 359–369. [Google Scholar] [CrossRef] [Green Version]
- Davanzo, P.; Yue, K.; Thomas, M.A.; Belin, T.; Mintz, J.; Venkatraman, T.N.; Santoro, E.; Barnett, S.; McCracken, J. Proton magnetic resonance spectroscopy of bipolar disorder versus intermittent explosive disorder in children and adolescents. Am. J. Psychiatry 2003, 160, 1442–1452. [Google Scholar] [CrossRef]
- Silverstone, P.H.; McGrath, B.M. Lithium and valproate and their possible effects on themyo-inositol second messenger system in healthy volunteers and bipolar patients. Int. Rev. Psychiatry 2009, 21, 414–423. [Google Scholar] [CrossRef]
- Kato, T.; Takahashi, S.; Shioiri, T.; Inubushi, T. Alterations in brain phosphorous metabolism in bipolar disorder detected by in vivo 31P and 7Li magnetic resonance spectroscopy. J. Affect. Disord. 1993, 27, 53–59. [Google Scholar] [CrossRef]
- Kato, T.; Shioiri, T.; Murashita, J.; Hamakawa, H.; Inubushi, T.; Takahashi, S. Phosphorus-31 magnetic resonance spectroscopy and ventricular enlargement in bipolar disorder. Psychiatry Res. 1994, 55, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Q.; Wang, Z.Z.; Chen, N.H. The receptor hypothesis and the pathogenesis of depression: Genetic bases and biological correlates. Pharmacol. Res. 2021, 167, 105542. [Google Scholar] [CrossRef] [PubMed]
- Einat, H.; Belmaker, R.H.; Kopilov, M.; Klein, E.; Gazawi, H.; Ben-Shachar, D. Rat brain monoamines after acute and chronic myo-inositol treatment. Eur. Neuropsychopharmacol. 1999, 10, 27–30. [Google Scholar] [CrossRef]
- Undie, A.S.; Friedman, E. Selective dopaminergic mechanism of dopamine and SKF38393 stimulation of inositol phosphate formation in rat brain. Eur. J. Pharmacol. 1992, 226, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Todd, R.D.; Heller, A.; O’Malley, K.L. Pharmacological and functional characterization of D2, D3 and D4 dopamine receptors in fibroblast and dopaminergic cell lines. J. Pharmacol. Exp. Ther. 1994, 268, 495–502. [Google Scholar] [PubMed]
- Einat, H.; Clenet, F.; Shaldubina, A.; Belmaker, R.H.; Bourin, M. The antidepressant activity of inositol in the forced swim test involves 5-HT(2) receptors. Behav. Brain Res. 2001, 118, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Eden Evins, A.; Demopulos, C.; Yovel, I.; Culhane, M.; Ogutha, J.; Grandin, L.D.; Nierenberg, A.A.; Sachs, G.S. Inositol augmentation of lithium or valproate for bipolar depression. Bipolar. Disord. 2006, 8, 168–174. [Google Scholar] [CrossRef]
- Mukai, T.; Kishi, T.; Matsuda, Y.; Iwata, N. A meta-analysis of inositol for depression and anxiety disorders. Hum. Psychopharmacol. 2014, 29, 55–63. [Google Scholar] [CrossRef]
- Schefft, C.; Kilarski, L.L.; Bschor, T.; Köhler, S. Efficacy of adding nutritional supplements in unipolar depression: A systematic review and meta-analysis. Eur. Neuropsychopharmacol. 2017, 27, 1090–1109. [Google Scholar] [CrossRef]
- Harwood, A.J. Lithium and bipolar mood disorder: The inositol-depletion hypothesis revisited. Mol. Psychiatry 2005, 10, 117–126. [Google Scholar] [CrossRef] [Green Version]
- McGrath, B.M.; Greenshaw, A.J.; McKay, R.; Slupsky, C.M.; Silverstone, P.H. Unlike lithium, anticonvulsants and antidepressants do not alter rat brain myo-inositol. Neuroreport 2007, 18, 1595–1598. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Venkatasubramanian, P.N.; Bárány, M.; Davis, J.M. Proton magnetic resonance spectroscopy of the brain in schizophrenic and affective patients. Schizophr. Res. 1992, 8, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Shaltiel, G.; Shamir, A.; Shapiro, J.; Ding, D.; Dalton, E.; Bialer, M.; Harwood, A.J.; Belmaker, R.H.; Greenberg, M.L.; Agam, G. Valproate decreases inositol biosynthesis. Biol. Psychiatry 2004, 56, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.C.; DelBello, M.P.; Cecil, K.M.; Adler, C.M.; Bryan, H.S.; Stanford, K.E.; Strakowski, S.M. Lithium treatment effects on Myo-inositol in adolescents with bipolar depression. Biol. Psychiatry 2006, 60, 998–1004. [Google Scholar] [CrossRef] [Green Version]
- Silverstone, P.H.; McGrath, B.M.; Kim, H. Bipolar disorder and myo-inositol: A review of the magnetic resonance spectroscopy findings. Bipolar Disord. 2005, 7, 1–10. [Google Scholar] [CrossRef]
- Sherman, W.R.; Munsell, L.Y.; Gish, B.G.; Honchar, M.P. Effects of systemically administered lithium on phosphoinositide metabolism in rat brain, kidney, and testis. J. Neurochem. 1985, 44, 798–807. [Google Scholar] [CrossRef]
- Lepore, E.; Lauretta, R.; Bianchini, M.; Mormando, M.; Di Lorenzo, C.; Unfer, V. Inositols Depletion and Resistance: Principal Mechanisms and Therapeutic Strategies. Int. J. Mol. Sci. 2021, 22, 6796. [Google Scholar] [CrossRef]
- Janiri, L.; D’Ambrosio, F.; Di Lorenzo, C. Combined treatment of myo-inositol and d-chiro-inositol (80:1) as a therapeutic approach to restore inositol eumetabolism in patients with bipolar disorder taking lithium and valproic acid. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 5483–5489. [Google Scholar] [CrossRef]
- Das, T.K.; Dey, A.; Sabesan, P.; Javadzadeh, A.; Théberge, J.; Radua, J.; Palaniyappan, L. Putative Astroglial Dysfunction in Schizophrenia: A Meta-Analysis of (1)H-MRS Studies of Medial Prefrontal Myo-Inositol. Front. Psychiatry 2018, 9, 438. [Google Scholar] [CrossRef] [Green Version]
- Brand, A.; Richter-Landsberg, C.; Leibfritz, D. Multinuclear NMR studies on the energy metabolism of glial and neuronal cells. Dev. Neurosci. 1993, 15, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Filibian, M.; Frasca, A.; Maggioni, D.; Micotti, E.; Vezzani, A.; Ravizza, T. In vivo imaging of glia activation using 1H-magnetic resonance spectroscopy to detect putative biomarkers of tissue epileptogenicity. Epilepsia 2012, 53, 1907–1916. [Google Scholar] [CrossRef] [PubMed]
- Jeon, P.; Mackinley, M.; Théberge, J.; Palaniyappan, L. The trajectory of putative astroglial dysfunction in first episode schizophrenia: A longitudinal 7-Tesla MRS study. Sci. Rep. 2021, 11, 22333. [Google Scholar] [CrossRef] [PubMed]
- Kubota, M.; Moriguchi, S.; Takahata, K.; Nakajima, S.; Horita, N. Treatment effects on neurometabolite levels in schizophrenia: A systematic review and meta-analysis of proton magnetic resonance spectroscopy studies. Schizophr. Res. 2020, 222, 122–132. [Google Scholar] [CrossRef]
- Hattingen, E.; Raab, P.; Franz, K.; Zanella, F.E.; Lanfermann, H.; Pilatus, U. Myo-inositol: A marker of reactive astrogliosis in glial tumors? NMR Biomed. 2008, 21, 233–241. [Google Scholar] [CrossRef]
- Romeo, B.; Petillion, A.; Martelli, C.; Benyamina, A. Magnetic resonance spectroscopy studies in subjects with high risk for psychosis: A meta-analysis and review. J. Psychiatr. Res. 2020, 125, 52–65. [Google Scholar] [CrossRef]
- Wang, Y.M.; Xiao, Y.H.; Xie, W.L. Metabolite abnormalities in psychosis risk: A meta-analysis of proton magnetic resonance spectroscopy studies. Asian J. Psychiatr. 2020, 54, 102220. [Google Scholar] [CrossRef]
- Levine, J.; Gonsalves, M.; Babur, I.; Stier, S.; Elizur, A.; Kofman, O.; Belmaker, R. Inositol 6 g daily may be effective in depression but not in schizophrenia. Hum. Psychopharmacol. Clin. Exp. 1993, 8, 49–53. [Google Scholar] [CrossRef]
- Firth, J.; Stubbs, B.; Sarris, J.; Rosenbaum, S.; Teasdale, S.; Berk, M.; Yung, A.R. The effects of vitamin and mineral supplementation on symptoms of schizophrenia: A systematic review and meta-analysis. Psychol. Med. 2017, 47, 1515–1527. [Google Scholar] [CrossRef] [Green Version]
- Levine, J. Controlled trials of inositol in psychiatry. Eur. Neuropsychopharmacol. 1997, 7, 147–155. [Google Scholar] [CrossRef]
- Benjamin, J.; Nemetz, H.; Fux, M.; Bleichman, I.; Agam, G. Acute inositol does not attenuate m-CPP-induced anxiety, mydriasis and endocrine effects in panic disorder. J. Psychiatr. Res. 1997, 31, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Jorm, A.F.; Christensen, H.; Griffiths, K.M.; Parslow, R.A.; Rodgers, B.; Blewitt, K.A. Effectiveness of complementary and self-help treatments for anxiety disorders. Med. J. Aust. 2004, 181, S29-46. [Google Scholar] [CrossRef] [PubMed]
- Freire, R.C.; Machado, S.; Arias-Carrión, O.; Nardi, A.E. Current pharmacological interventions in panic disorder. CNS Neurol. Disord. Drug Targets 2014, 13, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Zulfarina, M.S.; Syarifah-Noratiqah, S.B.; Nazrun, S.A.; Sharif, R.; Naina-Mohamed, I. Pharmacological Therapy in Panic Disorder: Current Guidelines and Novel Drugs Discovery for Treatment-resistant Patient. Clin. Psychopharmacol. Neurosci. 2019, 17, 145–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, H.; Kotler, M.; Kaplan, Z.; Matar, M.A.; Kofman, O.; Belmaker, R.H. Inositol has behavioral effects with adaptation after chronic administration. J. Neural Transm. (Vienna) 1997, 104, 299–305. [Google Scholar] [CrossRef]
- Kofman, O.; Einat, H.; Cohen, H.; Tenne, H.; Shoshana, C. The anxiolytic effect of chronic inositol depends on the baseline level of anxiety. J. Neural Transm. (Vienna) 2000, 107, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Harvey, B.H.; Scheepers, A.; Brand, L.; Stein, D.J. Chronic inositol increases striatal D(2) receptors but does not modify dexamphetamine-induced motor behavior. Relevance to obsessive-compulsive disorder. Pharmacol. Biochem. Behav. 2001, 68, 245–253. [Google Scholar] [CrossRef]
- Gogou, M.; Kolios, G. The effect of dietary supplements on clinical aspects of autism spectrum disorder: A systematic review of the literature. Brain Dev. 2017, 39, 656–664. [Google Scholar] [CrossRef]
| Authors, Year Study Design Type of Disorder Treatment Duration Follow-Up Characteristics of the Population Studied | Interventions | Measures | Study Aim (s) Efficacy |
|---|---|---|---|
| Mood Disorders | |||
| Levine et al. 1995a [11] Design: Randomized Clinical Trial (RCT) Treatment duration: 4 weeks Participants: Major Depressive Disorder (MDD), Bipolar Disorder (BD) (28) Male/female: 12/16 Mean age (range): inositol group 63.7 (35–80) Control group 50.5 (20–71) Diagnosis: MDD | Intervention group: Inositol 12 g/day Control group: Glucose | Hamilton Depression Rating Scale (HDRS) | Study aim: to evaluate the efficacy of administration of inositol for the treatment of depression. Results: Inositol group showed an improvement in the HDRS scores. |
| Nemets et al. 1999 [13] Design: RCT Treatment duration: 4 weeks Participants: MDD (36) Male/female: 14/22 Mean age (range): Selective Serotonin Reuptake Inhibitors (SSRI) + Inositol group 49.5 ± 11 SSRI + control group 51.5 ± 11 Diagnosis: MDD in patients who have failed to respond to SSRI | Intervention group: Inositol 12 g/day + SSRI Control group: Glucose + SSRI | HDRS | Study aim: to evaluate the efficacy of administration of inositol in augmentation with SSRI for the treatment of depression. Results: inositol group did not improve depression in SSRI treatment failures. |
| Levine et al. 1999 [14] Design: RCT Treatment duration: 4 weeks Participants: MDD (27) Male/female: 8/19 Mean age (range): SSRI + Inositol group 45.9 ± 5 SSRI + control group 49.6 ± 5 Diagnosis: MDD | Intervention group: Inositol 12 g/day + SSRI Control group: placebo + SSRI | HDRS | Study aim: to evaluate the clinical response to the addition of inositol to SSRIs in the treatment of depression. Results: inositol group did not show any significant improvement in depression. |
| Chengappa et al. 2000 [15] Design: RCT Treatment duration: 6 weeks Participants: BD depression (22) Male/female: 8/14 Mean age (range): inositol group 38 ± 8 Control group: 47 ± 13 Diagnosis: BD depression | Intervention group: inositol 12 g/day + mood disorders drugs Control group: placebo + mood disorders drugs | HDRS; Clinical Global Improvement (CGI-I); Montgomery–Asberg Depression Rating Scale (MADRS); Udvalg for Kliniske Undersøgelser Side Effects Scale (UKU); Young Mania Rating Scale (YMRS) | Study aim: to evaluate the inositol’s potential efficacy and safety in BD. Results: 6 patients of the inositol group showed a decrease in the baseline HDRS score and in the CGI-I scale compared to 3 subjects assigned to placebo. On the MADRS, 8 patients of the inositol group showed decreased scores compared to 4 subjects assigned to the placebo group. |
| Premenstrual Dysphoric Disorder (PMDD) | |||
| Gianfranco et al. 2011 [16] Design: RCT Treatment duration: 6 menstrual cycles Participants: PMDD (71) Male/female: 71 females Mean age (range): 18–45 years Diagnosis: PMDD | Intervention group: myo-inositol powder 12 g OR myo-inositol capsules soft gel 3.6 g/day Control group: powder or soft gel capsule placebo | Penn Daily Symptoms Records scale (DSR), HDRS, Clinical Global Impression-Severity of Illness scale (CGI-S) | Study aim: to evaluate the effect of myo-inositol on the treatment of PMDD Results: the inositol group showed significant improvement in DSR, HDRS and CGI-S scales. |
| Nemets et al. 2002 [17] Design: RCT Treatment duration: 6 menstrual cycles Participants: PMDD (12) Male/female: 12 females Mean age (range): 35.9 ± 5 (30–43) Diagnosis: PMDD | Intervention group: myo-inositol 12 g/day Control group: Placebo | Tension Analog Scale, Irritability Analog, Sadness Analog, Headache Analog, Bloating Analog, Breast Tenderness, HDRS, CGI-S, Self-rating for premenstrual tension syndrome (PMTS) | Study aim: to evaluate the inositol’s efficacy in PMDD Results: inositol was not found to be superior to placebo in any of the scales used. |
| Psychotic Disorders | |||
| Levine et al. 1993a [18] Design: RCT crossover Treatment duration: 4 weeks Participants: 10 Male/female: 6/4 Mean age (range): 36.8 (32–48) Diagnosis: schizophrenia | Intervention group: myo-Inositol 6 g/day + antipsychotic therapy Control group: Mannitol + antipsychotic therapy | Brief Psychiatric Rating Scale (BPRS) | Study aim: to evaluate the efficacy of the administration of inositol for the treatment of schizophrenia. Results: no overall effect of 6 g daily inositol on total symptoms scores was found. |
| Levine et al. 1993b [19] Design: RCT crossover Treatment duration: 10 days Participants: 11 Male/female: 7/4 Mean age (range): 53.2 (33–60) Diagnosis: schizophrenia | Intervention group: Inositol 6 g/day + antipsychotic therapy Control group: Lactose + antipsychotic therapy | BPRS | Study aim: to evaluate the efficacy of the administration of inositol for the treatment of schizophrenia. Results: no overall effect of 6 g daily inositol on total symptoms scores was found. |
| Levine et al. 1994 [20] Design: RCT crossover Treatment duration: 4 weeks Participants: 12 Male/female: 4/8 Mean age (range): 44.7 (26–63) Diagnosis: schizophrenia | Intervention group: Inositol 12 g/day + antipsychotic therapy Control group: dextrose + antipsychotic therapy | Positive and Negative Syndrome Scale (PANSS) | Study aim: to evaluate the efficacy of inositol supplements and the level of inositol in cerebrospinal fluid of schizophrenic patients. Results: no overall effect of 12 g daily inositol on total symptoms scores was found. |
| Obsessive-Compulsive Disorder (OCD) | |||
| Fux et al. 1996 [21] Design: RCT crossover Treatment duration: 6 weeks Participants: OCD with lack of response to previous treatments with SSRIs or clomipramine or with reported side effects to previous treatment (13) Male/female: 5/8 Mean age (range): 33.7 years (23–56) Diagnosis: OCD | Intervention group: Inositol 18 g/day Control group: glucose | Yale-Brown Obsessive-Compulsive Scale (Y-BOCS), HDRS, Hamilton Anxiety Rating Scale (HARS) | Study aim: to evaluate the efficacy of inositol in the treatment of OCD. Results: no statistical significance was observed between inositol and placebo. |
| Fux et al. 1999 [22] Design: RCT double-blind crossover Treatment duration: 6 weeks Participants: OCD (10) with inadequate response to serotonin reuptake inhibitors (SRI) therapy Male/female: 2/8 Mean age (SD): 30.3 (9) Diagnosis: OCD | Intervention group: Inositol 18 g/day as an add-on to SRI treatment Control group: glucose | Y-BOCS, HDRS, HARS | Study aim: to confirm the efficacy of inositol in OCD Results: there were no significant differences between inositol and placebo treatment. |
| Seedat et al. 1999 [23] Design: open-label trial Treatment duration: 6 weeks Participants: OCD (10) with lack of adequate response to SRI treatments Male/female: 3/7 Mean age (range): 33.3 ± 14.2 years Diagnosis: OCD | Intervention group: inositol 18 g/day as add-on to SRI treatments | Y-BOCS, CGI-S, MADRS | Study aim: to evaluate the efficacy of inositol in patients with lack of adequate response to SRI treatments. Results: there were significant improvements in CGI-S and Y-BOCS scales in the inositol group but not in MADRS scores. |
| Trichotillomania | |||
| Leppink et al. 2016 [24] Design: RCT Treatment duration: 10 weeks Participants: Tri (38) Male/female: 3/35 Mean age (range): 28.9 ± 11.4 Diagnosis: trichotillomania | Intervention group: inositol 6 g/day, after two weeks 12 g/day, after two weeks 18 g/day Control group: placebo powder | Massachusetts General Hospital Hair Pulling Scale, NIMH Trichotillomania Severity Scale, CGI-S, Sheehan Disability Scale (SDS), HARS, HDRS, Quality of life Inventory (QoLI) | Study aim: to investigate the efficacy of inositol in trichotillomania Results: no improvement in symptoms was reported in the inositol group. |
| Post-traumatic Stress Disorder (PTSD) | |||
| Kaplan et al. 1996 [25] Design: RCT Treatment duration: 4 weeks Participants: PTSD (13) Male/female: 8/5 Mean age (range): 39.7 (25–56) Diagnosis: PTSD | Intervention group: inositol 12 g/day Control group: placebo powder (glucose) | Impact of Event Scale (IES), Symptom Check List (SCL-90), HDRS and HARS | Study aim: to assess the efficacy of inositol in PTSD Results: no improvement in PTSD core symptoms (avoidance and intrusion) was reported in the inositol group. |
| Anxiety Disorders | |||
| Benjamin et al. 1995 [26] Design: RCT Treatment duration: 4 weeks Participants: 21 Male/female: 9/12 Mean age (SD): 35.8 (7) Diagnosis: panic disorder with or without agoraphobia | Intervention group: Inositol 12 g/day and lorazepam in case of anxiety Control group: mannitol or glucose powder and lorazepam in case of anxiety | Marks-Matthews Phobia Scale, HARS, HDRS | Study aim: to evaluate the efficacy of inositol in anxiety and panic disorder. Results: frequency and severity of panic attacks, phobia scores and panic scores were significantly improved in the inositol group in comparison to the control group. |
| Palatnik et al. 2001 [10] Design: RCT crossover Treatment duration: 1 month Participants: 21 patients Male/female: 9/12 Mean age (SD): 39.2 (11) Diagnosis: panic disorder with or without agoraphobia | Intervention group: Inositol 18 g/day Control group: fluvoxamine up to 150 mg/day | Summation of entries in a daily panic diary, HARS, CGI-S, HDRS, Marks-Matthews Fear Questionnaire | Study aim: to evaluate the efficacy of inositol in comparison to fluvoxamine in the treatment of panic disorder. Results: inositol and fluvoxamine had approximately the same efficacy at reducing HARS, phobia and CGI-S scores and inositol was slightly more effective than fluvoxamine at reducing the number of panic attacks with fewer collateral effects in the inositol group. |
| Autism Spectrum Disorder (ASD) | |||
| Levine et al. 1997 [27] Design: RCT crossover Treatment duration: 4 weeks Participants: autistic children (10) Male/female: 9/1 Mean age (SD): 5.6 ± 3.2 Diagnosis: autism | Intervention group: myo-inositol 200 mg/kg/day, in one case in add-on with carbamazepine for epilepsy Control group: dextrose | Childhood Autism Rating Scale (CARS), CGI-S, Conners Parent-Teacher Questionnaire (CONNERS) | Study aim: to evaluate the efficacy of inositol in autism disorder. Results: no benefit showed in children with autism of inositol. |
| Bulimia Nervosa (BN) and Binge Eating Disorders (BED) | |||
| Gelber et al. 2001 [28] Design: RCT crossover Treatment duration: 6 weeks Participants: BN, BED (12) Male/female: 1/11 Mean age (range): 24 (20–39) Diagnosis: BN, BED | Intervention group: inositol 18 g/day Control group: grape sugar | Eating Attitude Test (EAT), Visual Analog Scale of severity of binge eating (VAS-B), CGI-S, Eating Disorders Inventory (EDI), HDRS, HARS, 14-item side effects inventory. | Study aim: to assess the efficacy of inositol in BN and BED. Results: inositol group showed significant improvements in CGI-S and VAS-B, with a borderline significant effect on the EDI in comparison to the control group. No statistical difference was reported for EAT. |
| Others | |||
| Levine et al. 1995b [29] Design: RCT crossover Treatment duration: 5 days before the 5th or 6th RCT Participants: in treatment with ECT (15) Male/female: 5/10 Mean age (range): 49 (27–72) Diagnosis: MDD, Schizoaffective disorder depressed, neuroleptic non-responsive schizophrenia | Intervention group: inositol 6 gr/day Control group: dextrose | HDRS, BPRS, Cognitive function tests | Study aim: to assess whether inositol might enhance cholinergic function and reverse ECT-induced memory impairment Results: no effects were found on post-ECT cognitive impairment. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Concerto, C.; Chiarenza, C.; Di Francesco, A.; Natale, A.; Privitera, I.; Rodolico, A.; Trovato, A.; Aguglia, A.; Fisicaro, F.; Pennisi, M.; et al. Neurobiology and Applications of Inositol in Psychiatry: A Narrative Review. Curr. Issues Mol. Biol. 2023, 45, 1762-1778. https://doi.org/10.3390/cimb45020113
Concerto C, Chiarenza C, Di Francesco A, Natale A, Privitera I, Rodolico A, Trovato A, Aguglia A, Fisicaro F, Pennisi M, et al. Neurobiology and Applications of Inositol in Psychiatry: A Narrative Review. Current Issues in Molecular Biology. 2023; 45(2):1762-1778. https://doi.org/10.3390/cimb45020113
Chicago/Turabian StyleConcerto, Carmen, Cecilia Chiarenza, Antonio Di Francesco, Antimo Natale, Ivan Privitera, Alessandro Rodolico, Antonio Trovato, Andrea Aguglia, Francesco Fisicaro, Manuela Pennisi, and et al. 2023. "Neurobiology and Applications of Inositol in Psychiatry: A Narrative Review" Current Issues in Molecular Biology 45, no. 2: 1762-1778. https://doi.org/10.3390/cimb45020113
APA StyleConcerto, C., Chiarenza, C., Di Francesco, A., Natale, A., Privitera, I., Rodolico, A., Trovato, A., Aguglia, A., Fisicaro, F., Pennisi, M., Bella, R., Petralia, A., Signorelli, M. S., & Lanza, G. (2023). Neurobiology and Applications of Inositol in Psychiatry: A Narrative Review. Current Issues in Molecular Biology, 45(2), 1762-1778. https://doi.org/10.3390/cimb45020113











