Network Pharmacological Analysis of a New Herbal Combination Targeting Hyperlipidemia and Efficacy Validation In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Preparation
Data Acquisition of Herbs from the Online Database
2.2. Hyperlipidemia-Associated Targets Prediction
2.3. Protein–Protein Interaction (PPI) Network Construction
2.4. Gene Ontology (GO) Terms and KEGG Pathway Enrichment Analysis
2.5. Chemicals and Antibodies
2.6. Preparation of Samples
2.7. Cell Culture and Treatment
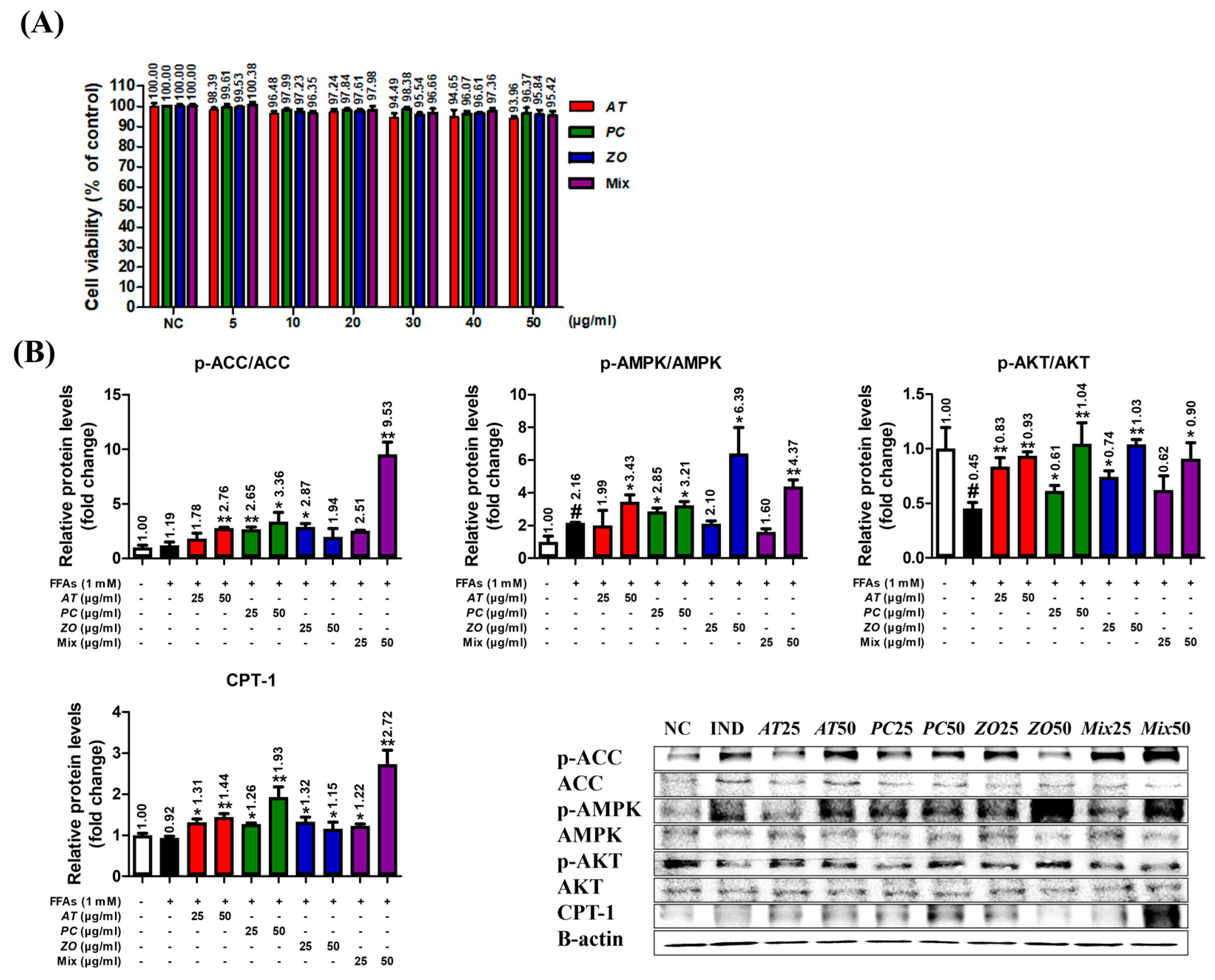
2.8. Cell Viability Assay
2.9. Western Blot Analysis
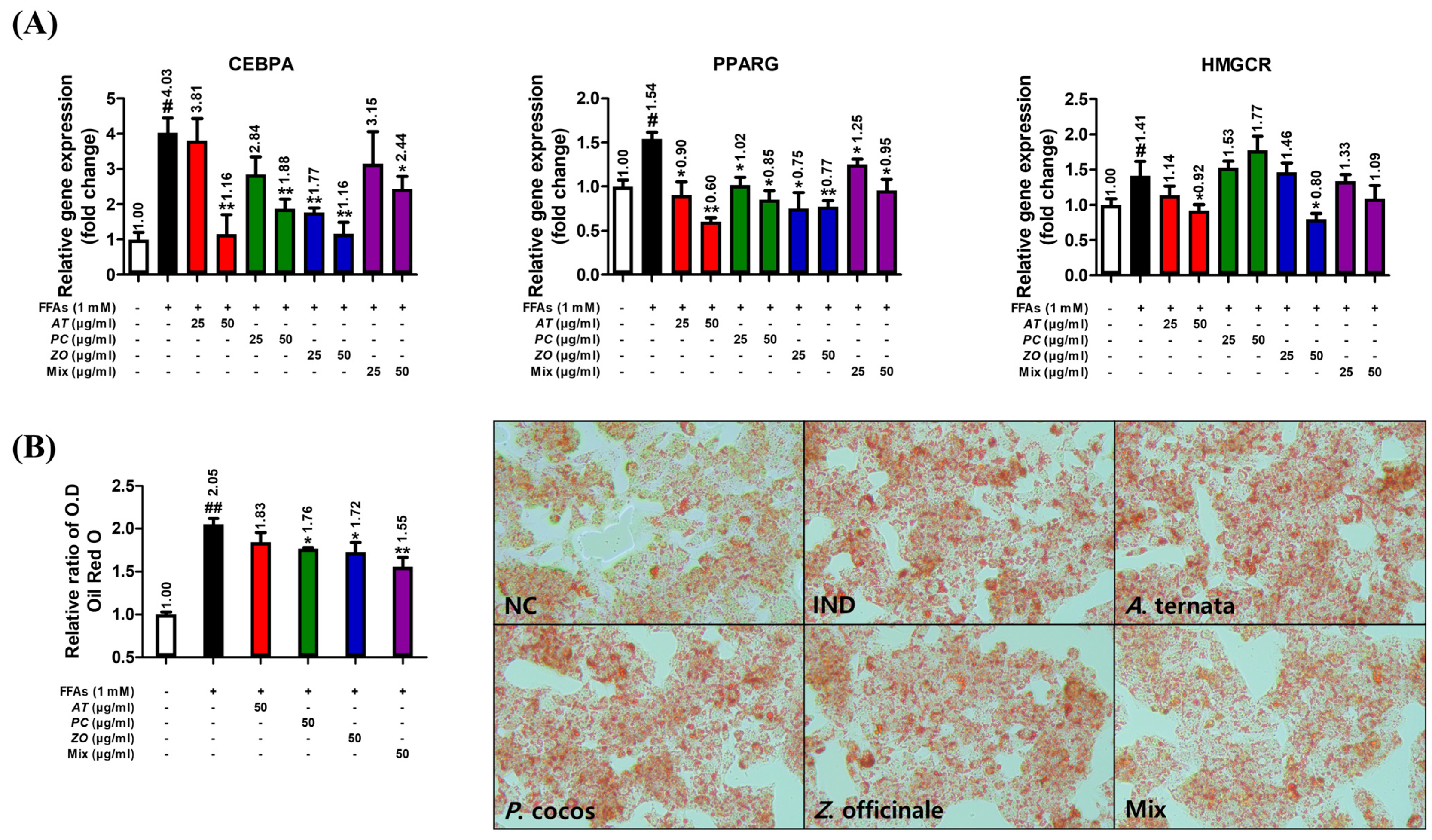
2.10. Quantitative Real-Time Polymerase Chain Reaction
2.11. Oil Red O Staining
2.12. Statistical Analysis
3. Results
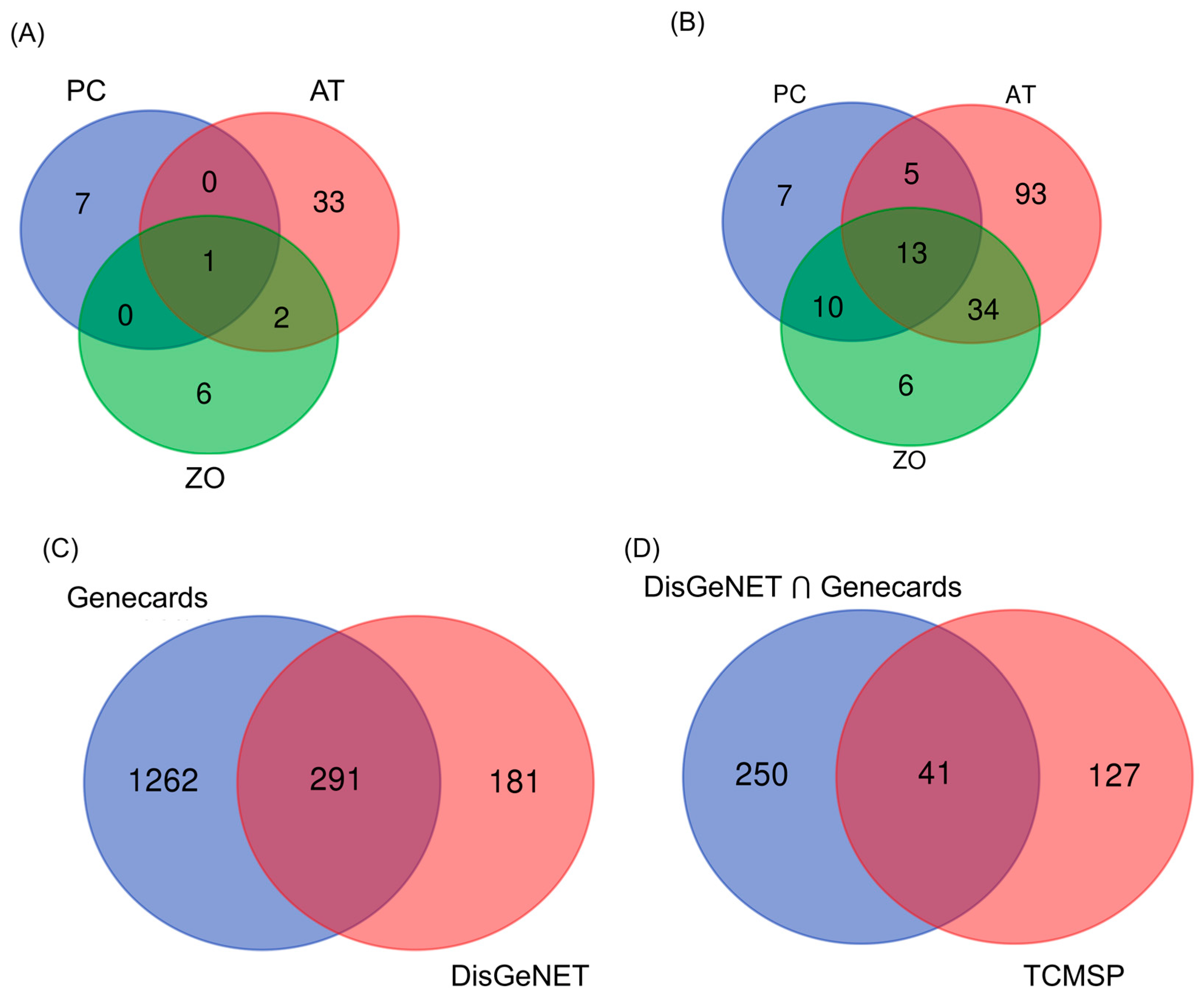
3.1. Selection of Potential Compounds from AT, PC, and ZO
3.2. Target Prediction
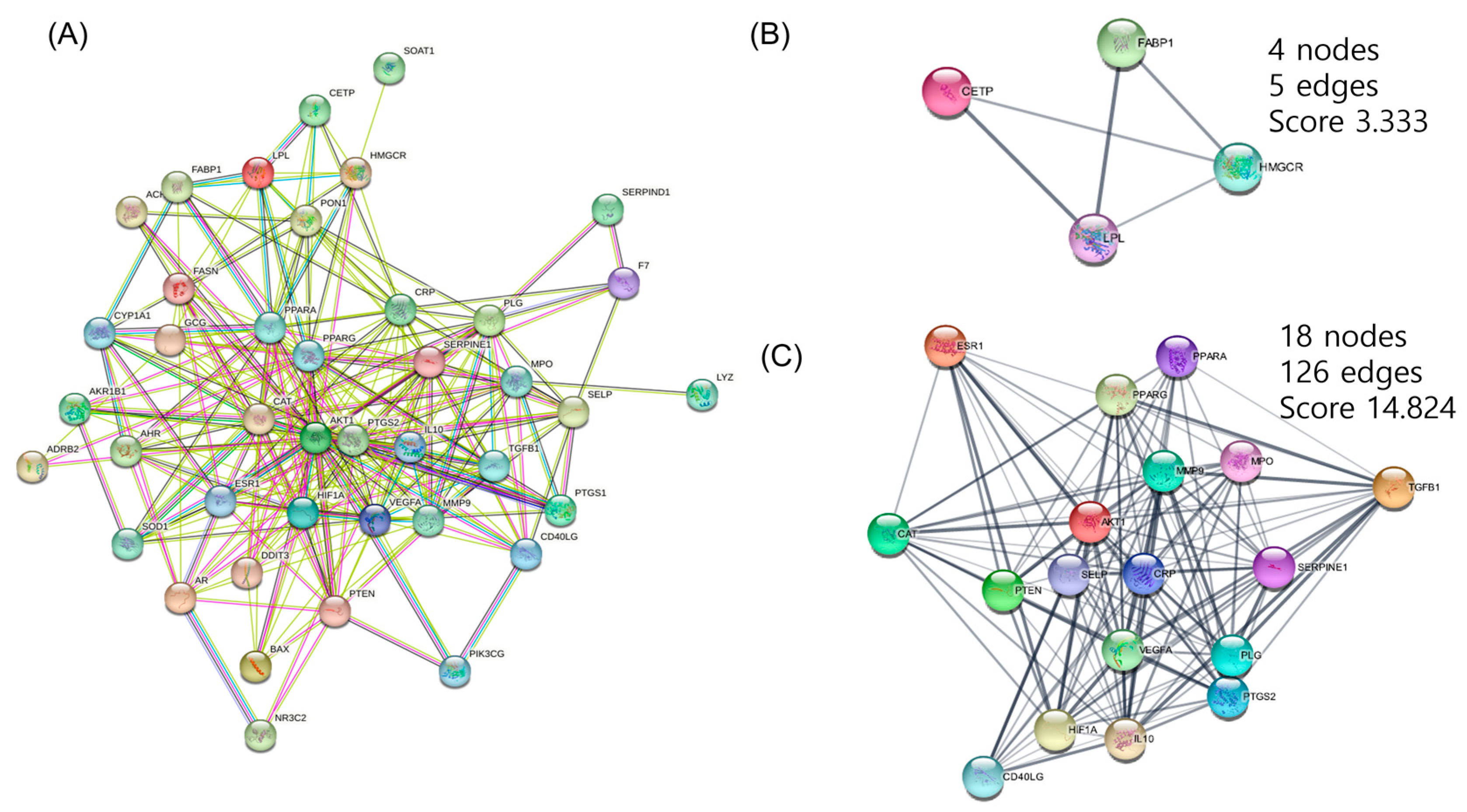
3.3. PPI Networks Construction and Analysis
3.4. CTP Visualization of Cytoscape
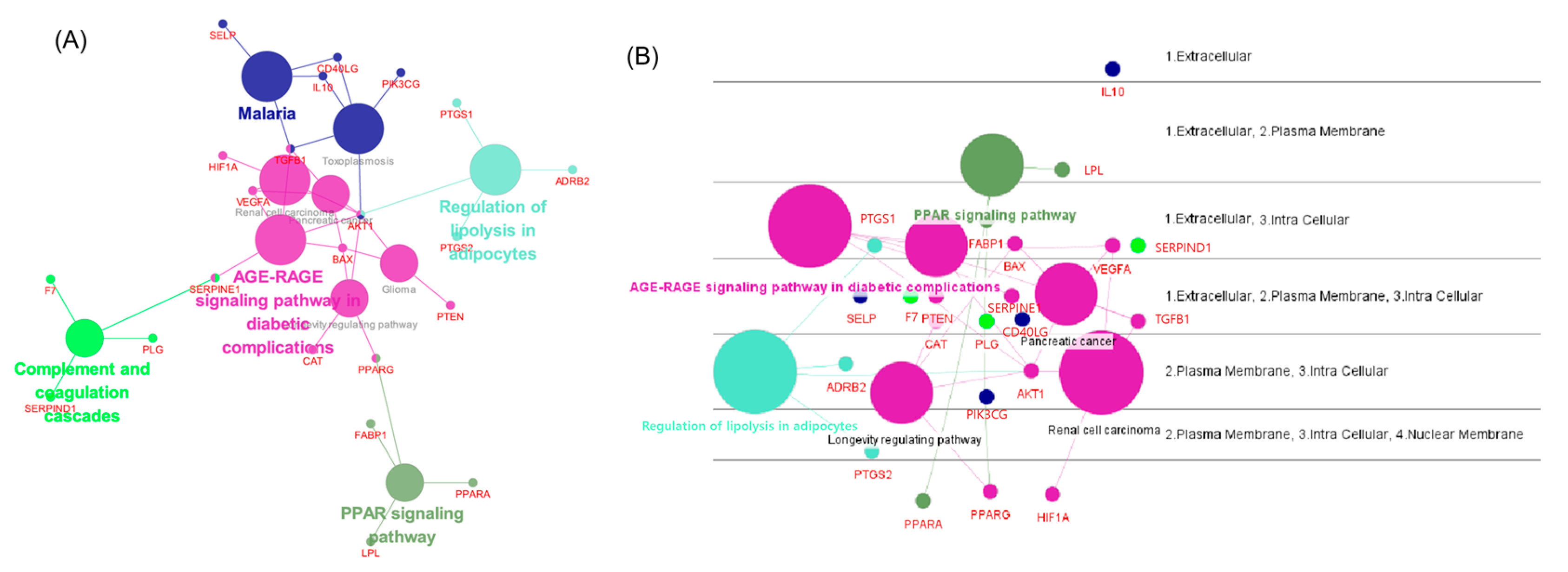
3.5. Network Analysis Using ClueGO, CluePedia
3.6. BP and KEGG Enrichment Analysis
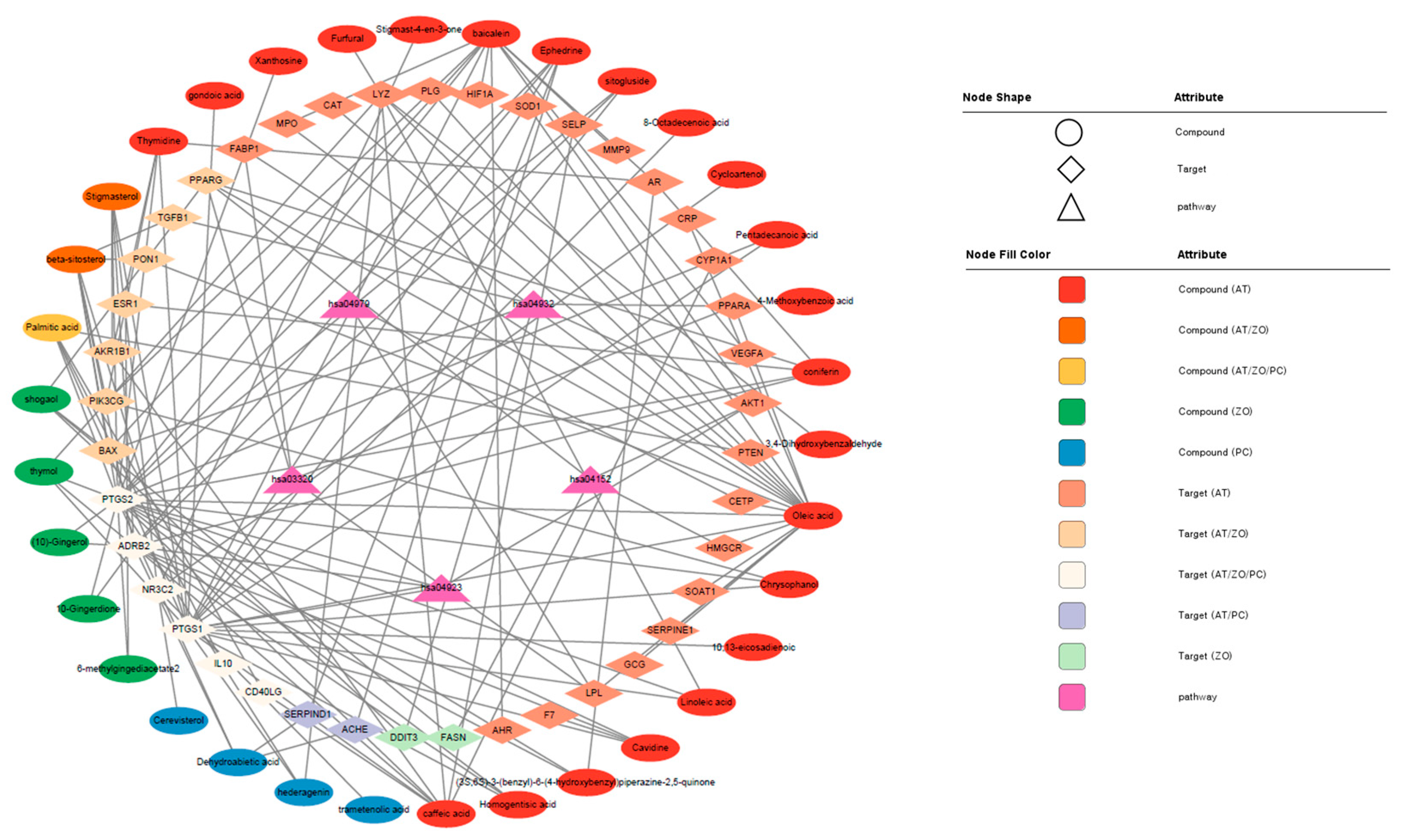
3.7. Visualization of the Target–Chemical Interaction Using STITCH
3.8. AT, PC, ZO, and Mixed Extract (MIX) Improved the Energy Metabolism-Related Proteins in the Hepatic Steatosis Model
3.9. AT, PC, and ZO Regulated the Expression of Genes Related to Lipogenesis and Reduced FFA-Induced Intracellular Lipid Accumulation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, N.; Ye, H. Exercise and hyperlipidemia. Phys. Exerc. Hum. Health 2020, 1228, 79–90. [Google Scholar]
- World Health Organization. The World Health Report 2002: Reducing Risks, Promoting Healthy Life; World Health Organization: Geneva, Switzerland, 2002. [Google Scholar]
- Song, D.-X.; Jiang, J.-G. Hypolipidemic components from medicine food homology species used in China: Pharmacological and health effects. Arch. Med. Res. 2017, 48, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Jain, K.S.; Kathiravan, M.; Somani, R.S.; Shishoo, C.J. The biology and chemistry of hyperlipidemia. Bioorganic Med. Chem. 2007, 15, 4674–4699. [Google Scholar] [CrossRef] [PubMed]
- Ruixing, Y.; Jinzhen, W.; Weixiong, L.; Yuming, C.; Dezhai, Y.; Shangling, P. The environmental and genetic evidence for the association of hyperlipidemia and hypertension. J. Hypertens. 2009, 27, 251–258. [Google Scholar] [CrossRef]
- Naukkarinen, J.; Ehnholm, C.; Peltonen, L. Genetics of familial combined hyperlipidemia. Curr. Opin. Lipidol. 2006, 17, 285–290. [Google Scholar] [CrossRef]
- Lee, J.; Hoang, T.; Lee, S.; Kim, J. Association Between Dietary Patterns and Dyslipidemia in Korean Women. Front. Nutr. 2021, 8, 756257. [Google Scholar] [CrossRef]
- Bandyopadhyay, D.; Qureshi, A.; Ghosh, S.; Ashish, K.; Heise, L.R.; Hajra, A.; Ghosh, R.K. Safety and efficacy of extremely low LDL-cholesterol levels and its prospects in hyperlipidemia management. J. Lipids 2018, 2018, 8598054. [Google Scholar] [CrossRef]
- Shattat, G.F. A review article on hyperlipidemia: Types, treatments and new drug targets. Biomed. Pharmacol. J. 2015, 7, 399–409. [Google Scholar] [CrossRef]
- Thompson, P.D.; Panza, G.; Zaleski, A.; Taylor, B. Statin-associated side effects. J. Am. Coll. Cardiol. 2016, 67, 2395–2410. [Google Scholar] [CrossRef]
- Brault, M.; Ray, J.; Gomez, Y.-H.; Mantzoros, C.S.; Daskalopoulou, S.S. Statin treatment and new-onset diabetes: A review of proposed mechanisms. Metabolism 2014, 63, 735–745. [Google Scholar] [CrossRef]
- Yu, Q.; Chen, Y.; Xu, C.-B. Statins and new-onset diabetes mellitus: LDL receptor may provide a key link. Front. Pharmacol. 2017, 8, 372. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Zhu, X.; Bai, H.; Ning, K. Network pharmacology databases for traditional Chinese medicine: Review and assessment. Front. Pharmacol. 2019, 10, 123. [Google Scholar] [CrossRef]
- Zhao, S.; Li, S. Network-based relating pharmacological and genomic spaces for drug target identification. PLoS ONE 2010, 5, e11764. [Google Scholar] [CrossRef]
- Banerjee, S.; Bhattacharjee, P.; Kar, A.; Mukherjee, P.K. LC–MS/MS analysis and network pharmacology of Trigonella foenum-graecum–A plant from Ayurveda against hyperlipidemia and hyperglycemia with combination synergy. Phytomedicine 2019, 60, 152944. [Google Scholar] [CrossRef]
- Huang, Y.; Guo, S.; Yang, J.; Tang, Y.; Zhu, X.; Ren, S. An Objective Diagnosis Model with Integrated Metabolic and Immunity Parameters for Phlegm-Dampness Constitution. Evid. Based Complement. Altern. Med. 2022, 2022, 3353549. [Google Scholar] [CrossRef]
- Jiang, Y.-H.; Zhang, P.; Tao, Y.; Liu, Y.; Cao, G.; Zhou, L.; Yang, C.-H. Banxia Baizhu Tianma decoction attenuates obesity-related hypertension. J. Ethnopharmacol. 2021, 266, 113453. [Google Scholar] [CrossRef]
- Lee, A.Y.; Park, W.; Kang, T.-W.; Cha, M.H.; Chun, J.M. Network pharmacology-based prediction of active compounds and molecular targets in Yijin-Tang acting on hyperlipidaemia and atherosclerosis. J. Ethnopharmacol. 2018, 221, 151–159. [Google Scholar] [CrossRef]
- Lee, S.M.; Lee, J.; Kang, E.; Kim, H.-L.; Hwang, G.-S.; Jung, J. Lipidomic analysis reveals therapeutic effects of Yijin-Tang on high-fat/high-cholesterol diet-induced obese mice. Phytomedicine 2020, 74, 152936. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Zhang, Y.; Tong, H.; Zhang, Y.; Lu, T. Potential mechanisms for traditional Chinese medicine in treating airway mucus hypersecretion associated with coronavirus disease 2019. Front. Mol. Biosci. 2020, 7, 577285. [Google Scholar] [CrossRef]
- Liu, N.; Huo, G.; Zhang, L.; Zhang, X. Effect of Zingiber OfficinaleRosc on lipid peroxidation in hyperlipidemia rats. J. Hyg. Res. 2003, 32, 22–23. [Google Scholar]
- Chen, H.; Chen, L.; Tang, D.-D.; Chen, D.-Q.; Miao, H.; Zhao, Y.-Y.; Ma, S.-C. Metabolomics reveals hyperlipidemic biomarkers and antihyperlipidemic effect of Poria cocos. Curr. Metab. 2016, 4, 104–115. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Shin, Y.-O.; Ha, Y.-W.; Lee, S.; Oh, J.-K.; Kim, Y.S. Anti-obesity effect of Pinellia ternata extract in Zucker rats. Biol. Pharm. Bull. 2006, 29, 1278–1281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nemes, K.; Åberg, F. Interpreting lipoproteins in non-alcoholic fatty liver disease. Curr. Opin. Lipidol. 2017, 28, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Hydes, T.; Alam, U.; Cuthbertson, D.J. The impact of macronutrient intake on non-alcoholic fatty liver disease (NAFLD): Too much fat, too much carbohydrate, or just too many calories? Front. Nutr. 2021, 8, 640557. [Google Scholar] [CrossRef]
- Chen, M.; Guo, W.-L.; Li, Q.-Y.; Xu, J.-X.; Cao, Y.-J.; Liu, B.; Yu, X.-D.; Rao, P.-F.; Ni, L.; Lv, X.-C. The protective mechanism of Lactobacillus plantarum FZU3013 against non-alcoholic fatty liver associated with hyperlipidemia in mice fed a high-fat diet. Food Funct. 2020, 11, 3316–3331. [Google Scholar] [CrossRef]
- Deprince, A.; Haas, J.T.; Staels, B. Dysregulated lipid metabolism links NAFLD to cardiovascular disease. Mol. Metab. 2020, 42, 101092. [Google Scholar] [CrossRef]
- Świderska, E.; Strycharz, J.; Wróblewski, A.; Szemraj, J.; Drzewoski, J.; Śliwińska, A. Role of PI3K/AKT pathway in insulin-mediated glucose uptake. Blood Glucose Levels 2018, 1, 1–18. [Google Scholar]
- Ferreira, D.; Castro, R.; Machado, M.; Evangelista, T.; Silvestre, A.; Costa, A.; Coutinho, J.; Carepa, F.; Cortez-Pinto, H.; Rodrigues, C. Apoptosis and insulin resistance in liver and peripheral tissues of morbidly obese patients is associated with different stages of non-alcoholic fatty liver disease. Diabetologia 2011, 54, 1788–1798. [Google Scholar] [CrossRef]
- Niu, Y.; Li, S.; Na, L.; Feng, R.; Liu, L.; Li, Y.; Sun, C. Mangiferin decreases plasma free fatty acids through promoting its catabolism in liver by activation of AMPK. PLoS ONE 2012, 7, e30782. [Google Scholar] [CrossRef]
- Lee, W.H.; Kim, S.G. AMPK-dependent metabolic regulation by PPAR agonists. PPAR Res. 2010, 2010, 549101. [Google Scholar] [CrossRef]
- Liu, S.; Jing, F.; Yu, C.; Gao, L.; Qin, Y.; Zhao, J. AICAR-induced activation of AMPK inhibits TSH/SREBP-2/HMGCR pathway in liver. PLoS ONE 2015, 10, e0124951. [Google Scholar] [CrossRef]
- Vecchione, G.; Grasselli, E.; Compalati, A.D.; Ragazzoni, M.; Cortese, K.; Gallo, G.; Voci, A.; Vergani, L. Ethanol and fatty acids impair lipid homeostasis in an in vitro model of hepatic steatosis. Food Chem. Toxicol. 2016, 90, 84–94. [Google Scholar] [CrossRef]
- Ru, J.; Li, P.; Wang, J.; Zhou, W.; Li, B.; Huang, C.; Li, P.; Guo, Z.; Tao, W.; Yang, Y. TCMSP: A database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform. 2014, 6, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Zhang, W.; Huang, C.; Li, Y.; Yu, H.; Wang, Y.; Duan, J.; Ling, Y. A novel chemometric method for the prediction of human oral bioavailability. Int. J. Mol. Sci. 2012, 13, 6964–6982. [Google Scholar] [CrossRef]
- Tao, W.; Xu, X.; Wang, X.; Li, B.; Wang, Y.; Li, Y.; Yang, L. Network pharmacology-based prediction of the active ingredients and potential targets of Chinese herbal Radix Curcumae formula for application to cardiovascular disease. J. Ethnopharmacol. 2013, 145, 1–10. [Google Scholar] [CrossRef]
- Yue, S.-J.; Liu, J.; Feng, W.-W.; Zhang, F.-L.; Chen, J.-X.; Xin, L.-T.; Peng, C.; Guan, H.-S.; Wang, C.-Y.; Yan, D. System pharmacology-based dissection of the synergistic mechanism of Huangqi and Huanglian for diabetes mellitus. Front. Pharmacol. 2017, 8, 694. [Google Scholar] [CrossRef]
- Safran, M.; Dalah, I.; Alexander, J.; Rosen, N.; Iny Stein, T.; Shmoish, M.; Nativ, N.; Bahir, I.; Doniger, T.; Krug, H. GeneCards Version 3: The human gene integrator. Database 2010, 2010, baq020. [Google Scholar] [CrossRef]
- Piñero, J.; Queralt-Rosinach, N.; Bravo, A.; Deu-Pons, J.; Bauer-Mehren, A.; Baron, M.; Sanz, F.; Furlong, L.I. DisGeNET: A discovery platform for the dynamical exploration of human diseases and their genes. Database 2015, 2015, bav028. [Google Scholar] [CrossRef]
- Bardou, P.; Mariette, J.; Escudié, F.; Djemiel, C.; Klopp, C. jvenn: An interactive Venn diagram viewer. BMC Bioinform. 2014, 15, 293. [Google Scholar] [CrossRef]
- Cline, M.S.; Smoot, M.; Cerami, E.; Kuchinsky, A.; Landys, N.; Workman, C.; Christmas, R.; Avila-Campilo, I.; Creech, M.; Gross, B. Integration of biological networks and gene expression data using Cytoscape. Nat. Protoc. 2007, 2, 2366–2382. [Google Scholar] [CrossRef]
- Bindea, G.; Galon, J.; Mlecnik, B. CluePedia Cytoscape plugin: Pathway insights using integrated experimental and in silico data. Bioinformatics 2013, 29, 661–663. [Google Scholar] [CrossRef] [PubMed]
- Bindea, G.; Mlecnik, B.; Hackl, H.; Charoentong, P.; Tosolini, M.; Kirilovsky, A.; Fridman, W.-H.; Pagès, F.; Trajanoski, Z.; Galon, J. ClueGO: A Cytoscape plugin to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 2009, 25, 1091–1093. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P. STRING v11: Protein–protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szklarczyk, D.; Santos, A.; Von Mering, C.; Jensen, L.J.; Bork, P.; Kuhn, M. STITCH 5: Augmenting protein–chemical interaction networks with tissue and affinity data. Nucleic Acids Res. 2016, 44, D380–D384. [Google Scholar] [CrossRef]
- Dennis, G.; Sherman, B.T.; Hosack, D.A.; Yang, J.; Gao, W.; Lane, H.C.; Lempicki, R.A. DAVID: Database for annotation, visualization, and integrated discovery. Genome Biol. 2003, 4, 1–11. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Bonnot, T.; Gillard, M.B.; Nagel, D.H. A simple protocol for informative visualization of enriched gene ontology terms. Bio-Protoc 2019, 9, e3429. [Google Scholar] [CrossRef]
- Lim, D.-W.; Kim, H.; Lee, S.-J.; Yu, G.-R.; Kim, J.-E.; Park, W.-H. Jwa Kum Whan attenuates non-alcoholic fatty liver disease by modulating glucose metabolism and the insulin signaling pathway. Evid. Based Complement. Altern. Med. 2019, 2019, 4589810. [Google Scholar] [CrossRef]
- Yu, G.R.; Lim, D.W.; Karunarathne, W.A.H.M.; Kim, G.Y.; Kim, H.; Kim, J.E.; Park, W.H. A non-polar fraction of Saponaria officinalis L. acted as a TLR4/MD2 complex antagonist and inhibited TLR4/MyD88 signaling in vitro and in vivo. FASEB J. 2022, 36, e22387. [Google Scholar] [CrossRef]
- Yu, G.-R.; Lee, S.-J.; Kim, D.-H.; Lim, D.-W.; Kim, H.; Park, W.-H.; Kim, J.-E. Literature-based drug repurposing in traditional Chinese medicine: Reduced inflammatory M1 macrophage polarization by Jisil Haebaek Gyeji-Tang alleviates cardiovascular disease in vitro and ex vivo. Evid. -Based Complement. Altern. Med. 2020, 2020, 8881683. [Google Scholar] [CrossRef]
- Lin, Y.; Ren, N.; Li, S.; Chen, M.; Pu, P. Novel anti-obesity effect of scutellarein and potential underlying mechanism of actions. Biomed. Pharmacother. 2019, 117, 109042. [Google Scholar] [CrossRef]
- Yao, H.-R.; Liu, J.; Plumeri, D.; Cao, Y.-B.; He, T.; Lin, L.; Li, Y.; Jiang, Y.-Y.; Li, J.; Shang, J. Lipotoxicity in HepG2 cells triggered by free fatty acids. Am. J. Transl. Res. 2011, 3, 284. [Google Scholar]
- Long, F.; Yang, H.; Xu, Y.; Hao, H.; Li, P. A strategy for the identification of combinatorial bioactive compounds contributing to the holistic effect of herbal medicines. Sci. Rep. 2015, 5, 12361. [Google Scholar] [CrossRef]
- Efferth, T.; Koch, E. Complex interactions between phytochemicals. The multi-target therapeutic concept of phytotherapy. Curr. Drug Targets 2011, 12, 122–132. [Google Scholar] [CrossRef]
- Lahlou, M. Screening of natural products for drug discovery. Expert Opin. Drug Discov. 2007, 2, 697–705. [Google Scholar] [CrossRef]
- Jiao, X.; Jin, X.; Ma, Y.; Yang, Y.; Li, J.; Liang, L.; Liu, R.; Li, Z. A comprehensive application: Molecular docking and network pharmacology for the prediction of bioactive constituents and elucidation of mechanisms of action in component-based Chinese medicine. Comput. Biol. Chem. 2021, 90, 107402. [Google Scholar] [CrossRef]
- Poornima, P.; Kumar, J.D.; Zhao, Q.; Blunder, M.; Efferth, T. Network pharmacology of cancer: From understanding of complex interactomes to the design of multi-target specific therapeutics from nature. Pharmacol. Res. 2016, 111, 290–302. [Google Scholar] [CrossRef]
- Xiao, P.-T.; Liu, S.-Y.; Kuang, Y.-J.; Jiang, Z.-M.; Lin, Y.; Xie, Z.-S.; Liu, E.-H. Network pharmacology analysis and experimental validation to explore the mechanism of sea buckthorn flavonoids on hyperlipidemia. J. Ethnopharmacol. 2021, 264, 113380. [Google Scholar] [CrossRef]
- Ye, J.; Li, L.; Hu, Z. Exploring the molecular mechanism of action of Yinchen Wuling powder for the treatment of hyperlipidemia, using network pharmacology, molecular docking, and molecular dynamics simulation. BioMed Res. Int. 2021, 2021, 9965906. [Google Scholar] [CrossRef]
- Duncan, R.E.; Ahmadian, M.; Jaworski, K.; Sarkadi-Nagy, E.; Sul, H.S. Regulation of lipolysis in adipocytes. Annu. Rev. Nutr. 2007, 27, 79–101. [Google Scholar] [CrossRef]
- Kersten, S. Peroxisome proliferator activated receptors and lipoprotein metabolism. PPAR Res. 2008, 2008, 132960. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Vega, G.L. Fibric acids: Effects on lipids and lipoprotein metabolism. Am. J. Med. 1987, 83, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, H.N. Effects of statins on triglyceride metabolism. Am. J. Cardiol. 1998, 81, 32B–35B. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, B.; Aguilar, D.; Deswal, A.; Dunbar, S.B.; Francis, G.S.; Horwich, T.; Jessup, M.; Kosiborod, M.; Pritchett, A.M.; Ramasubbu, K. Contributory risk and management of comorbidities of hypertension, obesity, diabetes mellitus, hyperlipidemia, and metabolic syndrome in chronic heart failure: A scientific statement from the American Heart Association. Circulation 2016, 134, e535–e578. [Google Scholar] [CrossRef]
- Ul-Haq, Z.; Mackay, D.F.; Fenwick, E.; Pell, J.P. Impact of metabolic comorbidity on the association between body mass index and health-related quality of life: A Scotland-wide cross-sectional study of 5608 participants. BMC Public Health 2012, 12, 143. [Google Scholar] [CrossRef] [Green Version]


| Compound List | ||||
|---|---|---|---|---|
| Herb | Name | CID | OB | DL |
| Zingiber officinale (ZO) | (10)-Gingerol | 168115 | 19.14 | 0.28 |
| 10-Gingerdione | 5317591 | 21.42 | 0.29 | |
| 6-methylgingediacetate2 | 53179662 | 48.73 | 0.32 | |
| shogaol | 5281794 | 31.00 | 0.14 | |
| poriferast-5-en-3beta-ol | 457801 | 36.91 | 0.75 | |
| thymol | 6989 | 41.47 | 0.03 | |
| Poria cocos (PC) | Cerevisterol | 10181133 | 37.96 | 0.77 |
| Dehydroabietic acid | 94391 | 14.93 | 0.28 | |
| (2R)-2-[(3S,5R,10S,13R,14R,16R,17R)-3,16-dihydroxy-4,4,10,13,14-pentamethyl-2,3,5,6,12,15,16,17-octahydro-1H-cyclopenta[a]phenanthren-17-yl]-6-methylhept-5-enoic acid | 10743008 | 30.93 | 0.81 | |
| Ergosterol peroxide | 5351516 | 40.36 | 0.81 | |
| ergosta-7,22E-dien-3beta-ol | 5283628 | 43.51 | 0.72 | |
| hederagenin | 73299 | 22.42 | 0.74 | |
| trametenolic acid | 12309443 | 38.71 | 0.80 | |
| Arum ternata (AT) | (3S,6S)-3-(benzyl)-6-(4-hydroxybenzyl)piperazine-2,5-quinone | 11438306 | 46.89 | 0.27 |
| 10,13-eicosadienoic | 549062 | 39.99 | 0.20 | |
| 3,4-Dihydroxybenzaldehyde | 8768 | 38.35 | 0.03 | |
| 4-Methoxybenzoic acid | 7478 | 29.69 | 0.03 | |
| 8-Octadecenoic acid | 5282758 | 33.13 | 0.14 | |
| Baicalein | 5281605 | 33.52 | 0.21 | |
| Xanthosine | 64959 | 44.72 | 0.21 | |
| caffeic acid | 689043 | 25.76 | 0.05 | |
| oct-1-ene | 8125 | 39.25 | 0.01 | |
| Baicalin | 64982 | 40.12 | 0.75 | |
| Choline | 305 | 0.47 | 0.01 | |
| 9-oxononanoic acid | 75704 | 19.60 | 0.03 | |
| docosanoic acid | 8125 | 15.69 | 0.26 | |
| 9-Heptadecanol | 136435 | 14.24 | 0.09 | |
| Palmitic acid | 985 | 19.30 | 0.10 | |
| Baicalein | 5281605 | 33.52 | 0.21 | |
| Hydroquinone | 785 | 29.26 | 0.02 | |
| Anethole | 637563 | 32.49 | 0.03 | |
| Adenine | 190 | 62.81 | 0.03 | |
| Cavidine | 193148 | 35.64 | 0.81 | |
| Chrysophanol | 10208 | 18.64 | 0.21 | |
| Coniferin | 5280372 | 10.28 | 0.27 | |
| Cycloartenol | 92110 | 38.69 | 0.78 | |
| Ephedrine | 9294 | 43.35 | 0.03 | |
| Furfural | 7362 | 34.35 | 0.01 | |
| gondoic acid | 5282768 | 30.70 | 0.20 | |
| Homogentisic acid | 780 | 92.44 | 0.04 | |
| Linoleic acid | 5280450 | 41.90 | 0.14 | |
| Oleic acid | 445639 | 33.13 | 0.14 | |
| Pentadecanoic acid | 13849 | 20.18 | 0.08 | |
| Sitogluside | 5742590 | 20.63 | 0.62 | |
| Stigmast-4-en-3-one | 5484202 | 36.08 | 0.76 | |
| Thymidine | 5789 | 11.34 | 0.11 | |
| AT ∩ ZO | beta-sitosterol | 222284 | 15.00 | 0.81 |
| Stigmasterol | 5280794 | 43.83 | 0.76 | |
| AT ∩ ZO ∩ PC | Palmitic acid | 985 | 19.30 | 0.10 |
| Degree | Stress | Betweenness Centrality | |
|---|---|---|---|
| AKT1 | 31 | 894 | 0.131579 |
| PPARG | 27 | 682 | 0.092949 |
| PTGS2 | 26 | 494 | 0.049889 |
| CAT | 26 | 484 | 0.049086 |
| VEGFA | 25 | 454 | 0.042859 |
| PPARA | 23 | 448 | 0.049087 |
| CRP | 23 | 542 | 0.066034 |
| IL10 | 22 | 238 | 0.018832 |
| SERPINE1 | 21 | 476 | 0.049813 |
| MMP9 | 19 | 164 | 0.011888 |
| HIF1A | 19 | 178 | 0.012385 |
| PLG | 18 | 472 | 0.057540 |
| ESR1 | 17 | 154 | 0.010555 |
| PTEN | 16 | 228 | 0.023134 |
| MPO | 15 | 314 | 0.053684 |
| TGFB1 | 14 | 26 | 0.001131 |
| SELP | 12 | 98 | 0.007016 |
| FASN | 12 | 104 | 0.011726 |
| PON1 | 11 | 100 | 0.012281 |
| LPL | 11 | 72 | 0.006200 |
| CD40LG | 11 | 42 | 0.004880 |
| AR | 11 | 94 | 0.010259 |
| GCG | 11 | 42 | 0.005491 |
| AHR | 11 | 30 | 0.001772 |
| SOD1 | 10 | 12 | 0.000844 |
| HMGCR | 9 | 256 | 0.051766 |
| DDIT3 | 9 | 26 | 0.001189 |
| CYP1A1 | 9 | 50 | 0.005523 |
| BAX | 8 | 8 | 0.000513 |
| PTGS1 | 8 | 4 | 0.000256 |
| AKR1B1 | 7 | 2 | 0.000056 |
| FABP1 | 7 | 18 | 0.001893 |
| CETP | 6 | 6 | 0.000569 |
| F7 | 5 | 32 | 0.005104 |
| PIK3CG | 4 | 2 | 0.000214 |
| ACHE | 4 | 6 | 0.000341 |
| ADRB2 | 3 | 0 | 0.000000 |
| NR3C2 | 3 | 8 | 0.000383 |
| SERPIND1 | 2 | 0 | 0.000000 |
| LYZ | 1 | 0 | 0.000000 |
| SOAT1 | 1 | 0 | 0.000000 |
| KEGG Pathway | |||
|---|---|---|---|
| Entry | Pathway | FDR | Genes |
| hsa04923 | Regulation of lipolysis in adipocytes | 0.00039 | AKT1, PTGS1, PTGS2, ADRB2 |
| hsa04932 | Non-alcoholic fatty liver disease | 0.00054 | AKT1, BAX, DDIT3, PPARA, TGFB1 |
| hsa03320 | PPAR signaling pathway | 0.00062 | FABP1, LPL, PPARA, PPARG |
| hsa04152 | AMPK signaling pathway | 0.0019 | AKT1, FASN, HMGCR, PPARG |
| hsa04979 | Cholesterol metabolism | 0.0023 | CETP, LPL, SOAT1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, T.-H.; Yu, G.-R.; Kim, H.; Kim, J.-E.; Lim, D.-W.; Park, W.-H. Network Pharmacological Analysis of a New Herbal Combination Targeting Hyperlipidemia and Efficacy Validation In Vitro. Curr. Issues Mol. Biol. 2023, 45, 1314-1332. https://doi.org/10.3390/cimb45020086
Kim T-H, Yu G-R, Kim H, Kim J-E, Lim D-W, Park W-H. Network Pharmacological Analysis of a New Herbal Combination Targeting Hyperlipidemia and Efficacy Validation In Vitro. Current Issues in Molecular Biology. 2023; 45(2):1314-1332. https://doi.org/10.3390/cimb45020086
Chicago/Turabian StyleKim, Tae-Hyoung, Ga-Ram Yu, Hyuck Kim, Jai-Eun Kim, Dong-Woo Lim, and Won-Hwan Park. 2023. "Network Pharmacological Analysis of a New Herbal Combination Targeting Hyperlipidemia and Efficacy Validation In Vitro" Current Issues in Molecular Biology 45, no. 2: 1314-1332. https://doi.org/10.3390/cimb45020086
APA StyleKim, T.-H., Yu, G.-R., Kim, H., Kim, J.-E., Lim, D.-W., & Park, W.-H. (2023). Network Pharmacological Analysis of a New Herbal Combination Targeting Hyperlipidemia and Efficacy Validation In Vitro. Current Issues in Molecular Biology, 45(2), 1314-1332. https://doi.org/10.3390/cimb45020086






