Maximal Exercise Improves the Levels of Endothelial Progenitor Cells in Heart Failure Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Design of the Study
2.2. Cardiopulmonary Exercise Test
2.3. Quantification of Circulating Number of EPCs and CECs
2.4. Statistical Analysis
3. Results
3.1. Participants’ Characteristics
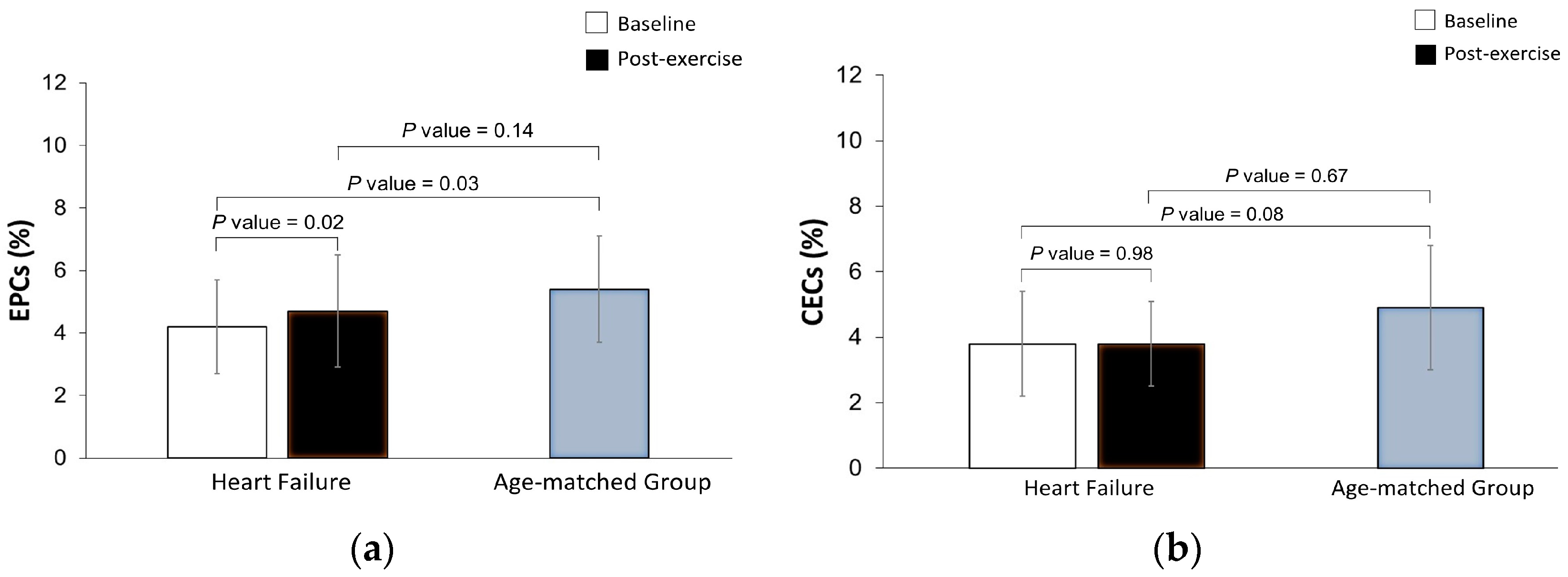
3.2. Effects of Maximal Exercise in the Levels of EPCs and CECs
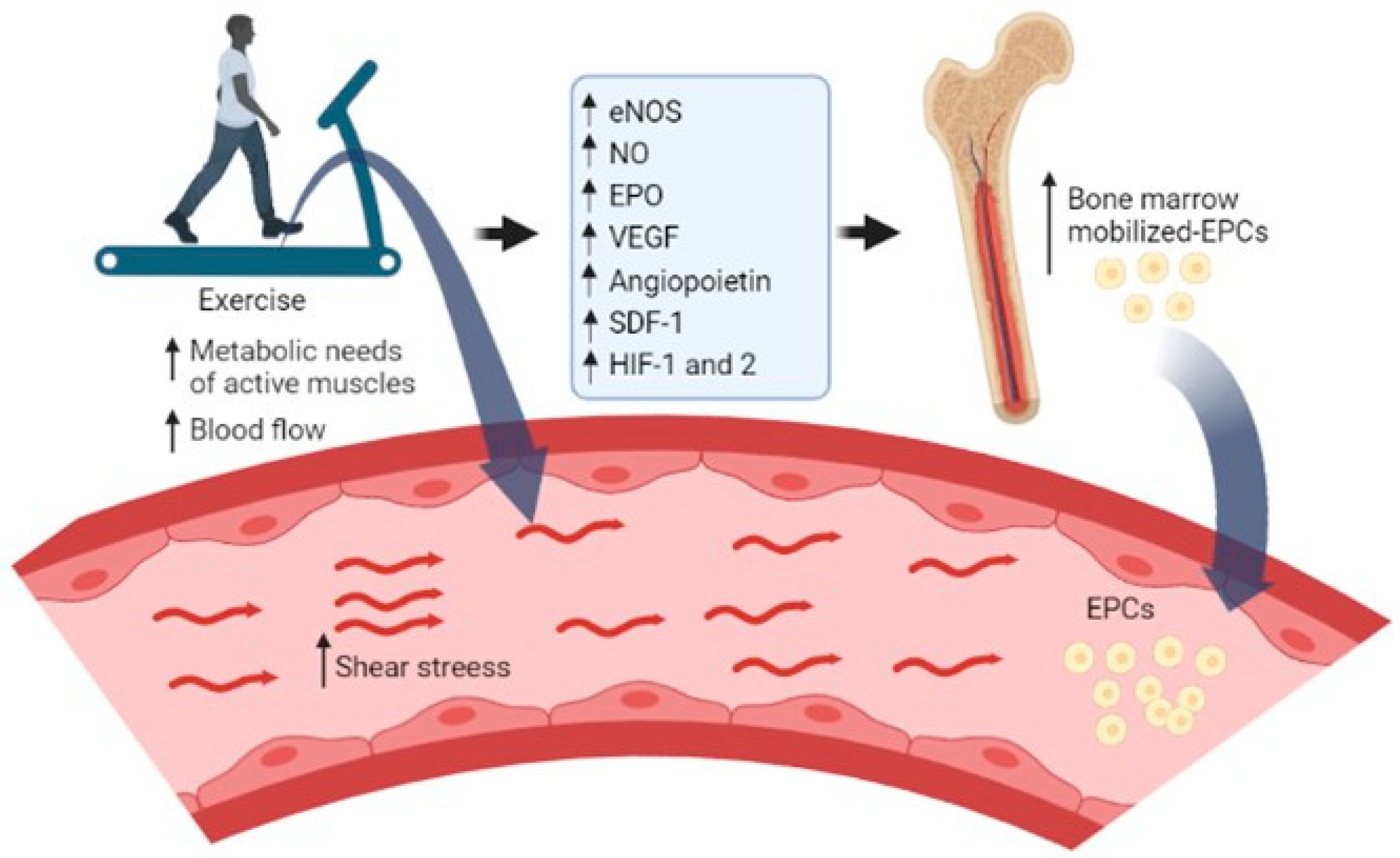
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Godo, S.; Shimokawa, H. Endothelial Functions. Arterioscler. Thromb. Vasc. Biol. 2017, 37, e108–e114. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, F.; Alves, A.J.; Teixeira, M.; Ribeiro, V.; Duarte, J.A.; Oliveira, J. Endothelial function and atherosclerosis: Circulatory markers with clinical usefulness. Rev. Port. Cardiol. 2009, 28, 1121–1151. [Google Scholar] [PubMed]
- Giannitsi, S.; Bougiakli, M.; Bechlioulis, A.; Naka, K. Endothelial dysfunction and heart failure: A review of the existing bibliography with emphasis on flow mediated dilation. JRSM Cardiovasc. Dis. 2019, 8, 2048004019843047. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, E.; Sugiyama, S.; Matsuzawa, Y.; Konishi, M.; Suzuki, H.; Nozaki, T.; Ohba, K.; Matsubara, J.; Maeda, H.; Horibata, Y.; et al. Incremental prognostic significance of peripheral endothelial dysfunction in patients with heart failure with normal left ventricular ejection fraction. J. Am. Coll. Cardiol. 2012, 60, 1778–1786. [Google Scholar] [CrossRef] [PubMed]
- Ambrosino, P.; Papa, A.; Buonauro, A.; Mosella, M.; Calcaterra, I.; Spedicato, G.A.; Maniscalco, M.; Di Minno, M.N.D. Clinical assessment of endothelial function in heart failure with preserved ejection fraction: A meta-analysis with meta-regressions. Eur. J. Clin. Investig. 2021, 51, e13552. [Google Scholar] [CrossRef]
- Sabatier, F.; Camoin-Jau, L.; Anfosso, F.; Sampol, J.; Dignat-George, F. Circulating endothelial cells, microparticles and progenitors: Key players towards the definition of vascular competence. J. Cell. Mol. Med. 2009, 13, 454–471. [Google Scholar] [CrossRef] [PubMed]
- Hristov, M.; Erl, W.; Weber, P.C. Endothelial progenitor cells: Mobilization, differentiation, and homing. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1185–1189. [Google Scholar] [CrossRef]
- Cheng, C.C.; Chang, S.J.; Chueh, Y.N.; Huang, T.S.; Huang, P.H.; Cheng, S.M.; Tsai, T.N.; Chen, J.W.; Wang, H.W. Distinct angiogenesis roles and surface markers of early and late endothelial progenitor cells revealed by functional group analyses. BMC Genom. 2013, 14, 182. [Google Scholar] [CrossRef]
- Hill, J.M.; Zalos, G.; Halcox, J.P.; Schenke, W.H.; Waclawiw, M.A.; Quyyumi, A.A.; Finkel, T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N. Engl. J. Med. 2003, 348, 593–600. [Google Scholar] [CrossRef]
- Lopes, J.; Teixeira, M.; Cavalcante, S.; Gouveia, M.; Duarte, A.; Ferreira, M.; Simões, M.I.; Conceição, M.; Ribeiro, I.P.; Gonçalves, A.C.; et al. Reduced Levels of Circulating Endothelial Cells and Endothelial Progenitor Cells in Patients with Heart Failure with Reduced Ejection Fraction. Arch. Med. Res. 2022, 53, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Kakzanov, Y.; Sevilya, Z.; Veturi, M.; Goldman, A.; Lev, E.I. Circulating Endothelial Progenitor Cells in Patients with Heart Failure with Preserved versus Reduced Ejection Fraction. Isr. Med. Assoc. J. 2021, 23, 364–368. [Google Scholar] [PubMed]
- Ross, M.D. Endothelial Regenerative Capacity and Aging: Influence of Diet, Exercise and Obesity. Curr. Cardiol. Rev. 2018, 14, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Djohan, A.H.; Sia, C.H.; Lee, P.S.; Poh, K.K. Endothelial Progenitor Cells in Heart Failure: An Authentic Expectation for Potential Future Use and a Lack of Universal Definition. J. Cardiovasc. Transl. Res. 2018, 11, 393–402. [Google Scholar] [CrossRef]
- Koller, L.; Hohensinner, P.; Sulzgruber, P.; Blum, S.; Maurer, G.; Wojta, J.; Hülsmann, M.; Niessner, A. Prognostic relevance of circulating endothelial progenitor cells in patients with chronic heart failure. Thromb. Haemost. 2016, 116, 309–316. [Google Scholar] [CrossRef]
- Samman Tahhan, A.; Hammadah, M.; Sandesara, P.B.; Hayek, S.S.; Kalogeropoulos, A.P.; Alkhoder, A.; Mohamed Kelli, H.; Topel, M.; Ghasemzadeh, N.; Chivukula, K.; et al. Progenitor Cells and Clinical Outcomes in Patients with Heart Failure. Circ. Heart Fail. 2017, 10, e004106. [Google Scholar] [CrossRef]
- Dignat-George, F.; Sampol, J. Circulating endothelial cells in vascular disorders: New insights into an old concept. Eur. J. Haematol. 2000, 65, 215–220. [Google Scholar] [CrossRef]
- Woywodt, A.; Blann, A.D.; Kirsch, T.; Erdbruegger, U.; Banzet, N.; Haubitz, M.; Dignat-George, F. Isolation and enumeration of circulating endothelial cells by immunomagnetic isolation: Proposal of a definition and a consensus protocol. J. Thromb. Haemost. 2006, 4, 671–677. [Google Scholar] [CrossRef]
- Boos, C.J.; Balakrishnan, B.; Lip, G.Y. The effects of exercise stress testing on soluble E-selectin, von Willebrand factor, and circulating endothelial cells as indices of endothelial damage/dysfunction. Ann. Med. 2008, 40, 66–73. [Google Scholar] [CrossRef]
- Sapp, R.M.; Hagberg, J.M. CrossTalk opposing view: Acute exercise does not elicit damage to the endothelial layer of systemic blood vessels in healthy individuals. J. Physiol. 2018, 596, 541–544. [Google Scholar] [CrossRef]
- Martínez-Sales, V.; Sánchez-Lázaro, I.; Vila, V.; Almenar, L.; Contreras, T.; Reganon, E. Circulating endothelial cells in patients with heart failure and left ventricular dysfunction. Dis. Markers 2011, 31, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Erdbruegger, U.; Haubitz, M.; Woywodt, A. Circulating endothelial cells: A novel marker of endothelial damage. Clin. Chim. Acta 2006, 373, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Farinacci, M.; Krahn, T.; Dinh, W.; Volk, H.-D.; Düngen, H.-D.; Wagner, J.; Konen, T.; von Ahsen, O. Circulating endothelial cells as biomarker for cardiovascular diseases. Res. Pract. Thromb. Haemost. 2018, 3, 49–58. [Google Scholar] [CrossRef]
- Cavalcante, S.L.; Lopes, S.; Bohn, L.; Cavero-Redondo, I.; Álvarez-Bueno, C.; Viamonte, S.; Santos, M.; Oliveira, J.; Ribeiro, F. Effects of exercise on endothelial progenitor cells in patients with cardiovascular disease: A systematic review and meta-analysis of randomized controlled trials. Rev. Port. Cardiol. 2019, 38, 817–827. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, F.; Ribeiro, I.P.; Alves, A.J.; do Céu Monteiro, M.; Oliveira, N.L.; Oliveira, J.; Amado, F.; Remião, F.; Duarte, J.A. Effects of exercise training on endothelial progenitor cells in cardiovascular disease: A systematic review. Am. J. Phys. Med. Rehabil. 2013, 92, 1020–1030. [Google Scholar] [CrossRef]
- Recchioni, R.; Marcheselli, F.; Antonicelli, R.; Lazzarini, R.; Mensà, E.; Testa, R.; Procopio, A.D.; Olivieri, F. Physical activity and progenitor cell-mediated endothelial repair in chronic heart failure: Is there a role for epigenetics? Mech. Ageing Dev. 2016, 159, 71–80. [Google Scholar] [CrossRef]
- Kourek, C.; Alshamari, M.; Mitsiou, G.; Psarra, K.; Delis, D.; Linardatou, V.; Pittaras, T.; Ntalianis, A.; Papadopoulos, C.; Panagopoulou, N.; et al. The acute and long-term effects of a cardiac rehabilitation program on endothelial progenitor cells in chronic heart failure patients: Comparing two different exercise training protocols. Int. J. Cardiol. Heart Vasc. 2021, 32, 100702. [Google Scholar] [CrossRef]
- Kourek, C.; Karatzanos, E.; Psarra, K.; Georgiopoulos, G.; Delis, D.; Linardatou, V.; Gavrielatos, G.; Papadopoulos, C.; Nanas, S.; Dimopoulos, S. Endothelial progenitor cells mobilization after maximal exercise according to heart failure severity. World J. Cardiol. 2020, 12, 526–539. [Google Scholar] [CrossRef]
- Kourek, C.; Karatzanos, E.; Psarra, K.; Ntalianis, A.; Mitsiou, G.; Delis, D.; Linardatou, V.; Pittaras, T.; Vasileiadis, I.; Dimopoulos, S.; et al. Endothelial progenitor cells mobilization after maximal exercise in patients with chronic heart failure. Hell. J. Cardiol. 2021, 62, 70–72. [Google Scholar] [CrossRef]
- Van Craenenbroeck, E.M.; Beckers, P.J.; Possemiers, N.M.; Wuyts, K.; Frederix, G.; Hoymans, V.Y.; Wuyts, F.; Paelinck, B.P.; Vrints, C.J.; Conraads, V.M. Exercise acutely reverses dysfunction of circulating angiogenic cells in chronic heart failure. Eur. Heart J. 2010, 31, 1924–1934. [Google Scholar] [CrossRef]
- Van Craenenbroeck, E.M.; Bruyndonckx, L.; Van Berckelaer, C.; Hoymans, V.Y.; Vrints, C.J.; Conraads, V.M. The effect of acute exercise on endothelial progenitor cells is attenuated in chronic heart failure. Eur. J. Appl. Physiol. 2011, 111, 2375–2379. [Google Scholar] [CrossRef]
- Van Craenenbroeck, E.M.; Hoymans, V.Y.; Beckers, P.J.; Possemiers, N.M.; Wuyts, K.; Paelinck, B.P.; Vrints, C.J.; Conraads, V.M. Exercise training improves function of circulating angiogenic cells in patients with chronic heart failure. Basic Res. Cardiol. 2010, 105, 665–676. [Google Scholar] [CrossRef]
- García-Conesa, M.-T.; Philippou, E.; Pafilas, C.; Massaro, M.; Quarta, S.; Andrade, V.; Jorge, R.; Chervenkov, M.; Ivanova, T.; Dimitrova, D.; et al. Exploring the Validity of the 14-Item Mediterranean Diet Adherence Screener (MEDAS): A Cross-National Study in Seven European Countries around the Mediterranean Region. Nutrients 2020, 12, 2960. [Google Scholar] [CrossRef] [PubMed]
- Hoymans, V.Y.; Van Craenenbroeck, A.H.; Bruyndonckx, L.; van Ierssel, S.H.; Vrints, C.J.; Conraads, V.M.; Van Craenenbroeck, E.M. TransFix® for delayed flow cytometry of endothelial progenitor cells and angiogenic T cells. Microvasc. Res. 2012, 84, 384–386. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.W.; Rider, R.; Glanville, M.; Narayanan, K.; Razvi, S.; Weaver, J.U. Metformin improves circulating endothelial cells and endothelial progenitor cells in type 1 diabetes: MERIT study. Cardiovasc. Diabetol. 2016, 15, 116. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Lucke, C.; Fichtlscherer, S.; Aicher, A.; Tschöpe, C.; Schultheiss, H.P.; Zeiher, A.M.; Dimmeler, S. Quantification of circulating endothelial progenitor cells using the modified ISHAGE protocol. PLoS ONE 2010, 5, e13790. [Google Scholar] [CrossRef]
- Kraan, J.; Strijbos, M.H.; Sieuwerts, A.M.; Foekens, J.A.; den Bakker, M.A.; Verhoef, C.; Sleijfer, S.; Gratama, J.W. A new approach for rapid and reliable enumeration of circulating endothelial cells in patients. J. Thromb. Haemost. 2012, 10, 931–939. [Google Scholar] [CrossRef]
- Van Craenenbroeck, E.M.; Denollet, J.; Paelinck, B.P.; Beckers, P.; Possemiers, N.; Hoymans, V.Y.; Vrints, C.J.; Conraads, V.M. Circulating CD34+/KDR+ endothelial progenitor cells are reduced in chronic heart failure patients as a function of Type D personality. Clin. Sci. 2009, 117, 165–172. [Google Scholar] [CrossRef]
- Gevaert, A.B.; Beckers, P.J.; Van Craenenbroeck, A.H.; Lemmens, K.; Van De Heyning, C.M.; Heidbuchel, H.; Vrints, C.J.; Van Craenenbroeck, E.M. Endothelial dysfunction and cellular repair in heart failure with preserved ejection fraction: Response to a single maximal exercise bout. Eur. J. Heart Fail. 2019, 21, 125–127. [Google Scholar] [CrossRef]
- Rehman, J.; Li, J.L.; Parvathaneni, L.; Karlsson, G.; Panchal, V.R.; Temm, C.J.; Mahenthiran, J.; March, K.L. Exercise acutely increases circulating endothelial progenitor cells and monocyte-/macrophage-derived angiogenic cells. J. Am. Coll. Cardiol. 2004, 43, 2314–2318. [Google Scholar] [CrossRef]
- West, D.J.; Campbell, M.D.; Gonzalez, J.T.; Walker, M.; Stevenson, E.J.; Ahmed, F.W.; Wijaya, S.; Shaw, J.A.; Weaver, J.U. The inflammation, vascular repair and injury responses to exercise in fit males with and without Type 1 diabetes: An observational study. Cardiovasc. Diabetol. 2015, 14, 71. [Google Scholar] [CrossRef] [PubMed]
- Volaklis, K.A.; Tokmakidis, S.P.; Halle, M. Acute and chronic effects of exercise on circulating endothelial progenitor cells in healthy and diseased patients. Clin. Res. Cardiol. 2013, 102, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Laufs, U.; Urhausen, A.; Werner, N.; Scharhag, J.; Heitz, A.; Kissner, G.; Böhm, M.; Kindermann, W.; Nickenig, G. Running exercise of different duration and intensity: Effect on endothelial progenitor cells in healthy subjects. Eur. J. Cardiovasc. Prev. Rehabil. 2005, 12, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Mitsiou, G.; Karatzanos, E.; Smilios, I.; Psarra, K.; Patsaki, I.; Douda, H.T.; Ntalianis, A.; Nanas, S.; Tokmakidis, S.P. Exercise promotes endothelial progenitor cell mobilization in patients with chronic heart failure. Eur. J. Prev. Cardiol. 2022, 28, e24–e27. [Google Scholar] [CrossRef]
- Ferentinos, P.; Tsakirides, C.; Swainson, M.; Davison, A.; Martyn-St James, M.; Ispoglou, T. The impact of different forms of exercise on circulating endothelial progenitor cells in cardiovascular and metabolic disease. Eur. J. Appl. Physiol. 2022, 122, 815–860. [Google Scholar] [CrossRef]
- Chiang, C.H.; Huang, P.H.; Leu, H.B.; Hsu, C.Y.; Wang, K.F.; Chen, J.W.; Lin, S.J. Decreased circulating endothelial progenitor cell levels in patients with heart failure with preserved ejection fraction. Cardiology 2013, 126, 191–201. [Google Scholar] [CrossRef]
- Aicher, A.; Heeschen, C.; Mildner-Rihm, C.; Urbich, C.; Ihling, C.; Technau-Ihling, K.; Zeiher, A.M.; Dimmeler, S. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat. Med. 2003, 9, 1370–1376. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, J.M.; Chen, L.; Luo, C.F.; Tang, A.L.; Tao, J. Acute exercise-induced nitric oxide production contributes to upregulation of circulating endothelial progenitor cells in healthy subjects. J. Hum. Hypertens 2007, 21, 452–460. [Google Scholar] [CrossRef]
- Heinisch, P.P.; Bello, C.; Emmert, M.Y.; Carrel, T.; Dreßen, M.; Hörer, J.; Winkler, B.; Luedi, M.M. Endothelial Progenitor Cells as Biomarkers of Cardiovascular Pathologies: A Narrative Review. Cells 2022, 11, 1678. [Google Scholar] [CrossRef]
- Chang, E.; Paterno, J.; Duscher, D.; Maan, Z.N.; Chen, J.S.; Januszyk, M.; Rodrigues, M.; Rennert, R.C.; Bishop, S.; Whitmore, A.J.; et al. Exercise induces stromal cell-derived factor-1α-mediated release of endothelial progenitor cells with increased vasculogenic function. Plast. Reconstr. Surg. 2015, 135, 340e–350e. [Google Scholar] [CrossRef]
- O’Sullivan, S.E. The effects of exercise training on markers of endothelial function in young healthy men. Int. J. Sports Med. 2003, 24, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Sapp, R.M.; Evans, W.S.; Eagan, L.E.; Chesney, C.A.; Zietowski, E.M.; Prior, S.J.; Ranadive, S.M.; Hagberg, J.M. The effects of moderate and high-intensity exercise on circulating markers of endothelial integrity and activation in young, healthy men. J. Appl. Physiol. (1985) 2019, 127, 1245–1256. [Google Scholar] [CrossRef] [PubMed]
- Mutin, M.; Canavy, I.; Blann, A.; Bory, M.; Sampol, J.; Dignat-George, F. Direct evidence of endothelial injury in acute myocardial infarction and unstable angina by demonstration of circulating endothelial cells. Blood 1999, 93, 2951–2958. [Google Scholar] [CrossRef] [PubMed]
- Adams, V. CrossTalk proposal: Acute exercise elicits damage to the endothelial layer of systemic blood vessels in healthy individuals. J. Physiol. 2018, 596, 537–539. [Google Scholar] [CrossRef] [PubMed]
- Dawson, E.A.; Green, D.J.; Cable, N.T.; Thijssen, D.H. Effects of acute exercise on flow-mediated dilatation in healthy humans. J. Appl. Physiol. (1985) 2013, 115, 1589–1598. [Google Scholar] [CrossRef]
| Heart Failure Group (n = 13) | Age-Matched Group (n = 13) | p | |
|---|---|---|---|
| Sex (males/females) | 13 (11/2) | 13 (2/11) | ≤0.001 |
| Age (years) | 67.8 ± 9.7 | 65.7 ± 7.1 | 0.52 |
| Anthropometric data | |||
| Weight (kg) | 75.6 ± 11.9 | 73.2 ± 16.7 | 0.68 |
| Height (cm) | 165.1 ± 9.6 | 156.7 ± 10 | 0.03 |
| Body mass index (kg/m2) | 27.7 ± 3.6 | 29.6 ± 5.6 | 0.30 |
| Cardiovascular risk factors, n (%) | |||
| Overweight | 5 (38.5) | 6 (46.2) | 0.69 |
| Obesity | 4 (30.8) | 5 (38.5) | 1.00 |
| Hypertension | 11 (84.6) | 7 (53.8) | 0.20 |
| Diabetes mellitus | 7 (53.8) | 4 (30.8) | 0.23 |
| Dyslipidaemia | 8 (61.5) | 8 (61.5) | 1.00 |
| Heart failure characteristics | |||
| HFrEF, n (%) | 8 (61.5) | - | - |
| HFmrEF, n (%) | 4 (30.8) | - | - |
| HFpEF, n (%) | 1 (7.7) | - | - |
| LVEF, % | 37.2 ± 12.0 | - | - |
| NYHA functional class, n (%) | |||
| I | 2 (15.4) | - | - |
| II | 11 (84.6) | - | - |
| Aetiology | |||
| Ischemic, n (%) | 7 (53.8) | - | - |
| Dilated, n (%) | 6 (46.2) | - | - |
| Current medication, n (%) | |||
| Statins | 10 (76.9) | 8 (61.5) | 0.67 |
| Antidiabetic medication | 6 (46.2) | 3 (23.1) | 0.41 |
| Diuretics | 9 (69.2) | 5 (38.5) | 0.11 |
| Angiotensin II receptor blockers | 1 (7.7) | 2 (15.4) | 1.00 |
| Angiotensin converting enzyme inhibitors | 10 (76.9) | 3 (23.1) | 0.006 |
| Calcium channel blockers | 2 (15.4) | 1 (7.7) | 1.00 |
| Beta-blockers | 8 (61.5) | 0 (0.0) | 0.002 |
| Mediterranean Diet Adherence | |||
| Total scoring | 8 ± 2 | 8 ± 2 | 0.47 |
| Weak adherence, n (%) | 0 (0.0) | 1 (7.7) | 1.00 |
| Moderate to fair adherence, n (%) | 11 (84.6) | 8 (61.5) | 0.37 |
| Good or very good adherence, n (%) | 2 (15.4) | 4 (30.8) | 0.64 |
| Circulating Cells Populations | |||
| Endothelial progenitor cells (%) | 4.2 × 10−3 ± 1.5 × 10−3 | 5.4 × 10−3 ± 1.7 × 10−3 | 0.03 |
| Endothelial cells (%) | 3.8 × 10−3 ± 1.6 × 10−3 | 4.9 × 10−3 ± 1.9 × 10−3 | 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavalcante, S.; Viamonte, S.; Cadilha, R.S.; Ribeiro, I.P.; Gonçalves, A.C.; Sousa-Venâncio, J.; Gouveia, M.; Teixeira, M.; Santos, M.; Oliveira, J.; et al. Maximal Exercise Improves the Levels of Endothelial Progenitor Cells in Heart Failure Patients. Curr. Issues Mol. Biol. 2023, 45, 1950-1960. https://doi.org/10.3390/cimb45030125
Cavalcante S, Viamonte S, Cadilha RS, Ribeiro IP, Gonçalves AC, Sousa-Venâncio J, Gouveia M, Teixeira M, Santos M, Oliveira J, et al. Maximal Exercise Improves the Levels of Endothelial Progenitor Cells in Heart Failure Patients. Current Issues in Molecular Biology. 2023; 45(3):1950-1960. https://doi.org/10.3390/cimb45030125
Chicago/Turabian StyleCavalcante, Suiane, Sofia Viamonte, Rui S. Cadilha, Ilda P. Ribeiro, Ana Cristina Gonçalves, João Sousa-Venâncio, Marisol Gouveia, Manuel Teixeira, Mário Santos, José Oliveira, and et al. 2023. "Maximal Exercise Improves the Levels of Endothelial Progenitor Cells in Heart Failure Patients" Current Issues in Molecular Biology 45, no. 3: 1950-1960. https://doi.org/10.3390/cimb45030125
APA StyleCavalcante, S., Viamonte, S., Cadilha, R. S., Ribeiro, I. P., Gonçalves, A. C., Sousa-Venâncio, J., Gouveia, M., Teixeira, M., Santos, M., Oliveira, J., & Ribeiro, F. (2023). Maximal Exercise Improves the Levels of Endothelial Progenitor Cells in Heart Failure Patients. Current Issues in Molecular Biology, 45(3), 1950-1960. https://doi.org/10.3390/cimb45030125






