The Insulin Receptor Substrate 2 Mediates the Action of Insulin on HeLa Cell Migration via the PI3K/Akt Signaling Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Cell Isolation and Culture
2.3. Cell Proliferation Assays by MTS
2.4. RT-PCR
2.5. Immunoprecipitation and Western Blot
2.6. Cell Migration Assays
2.7. Statistical Analysis
3. Results
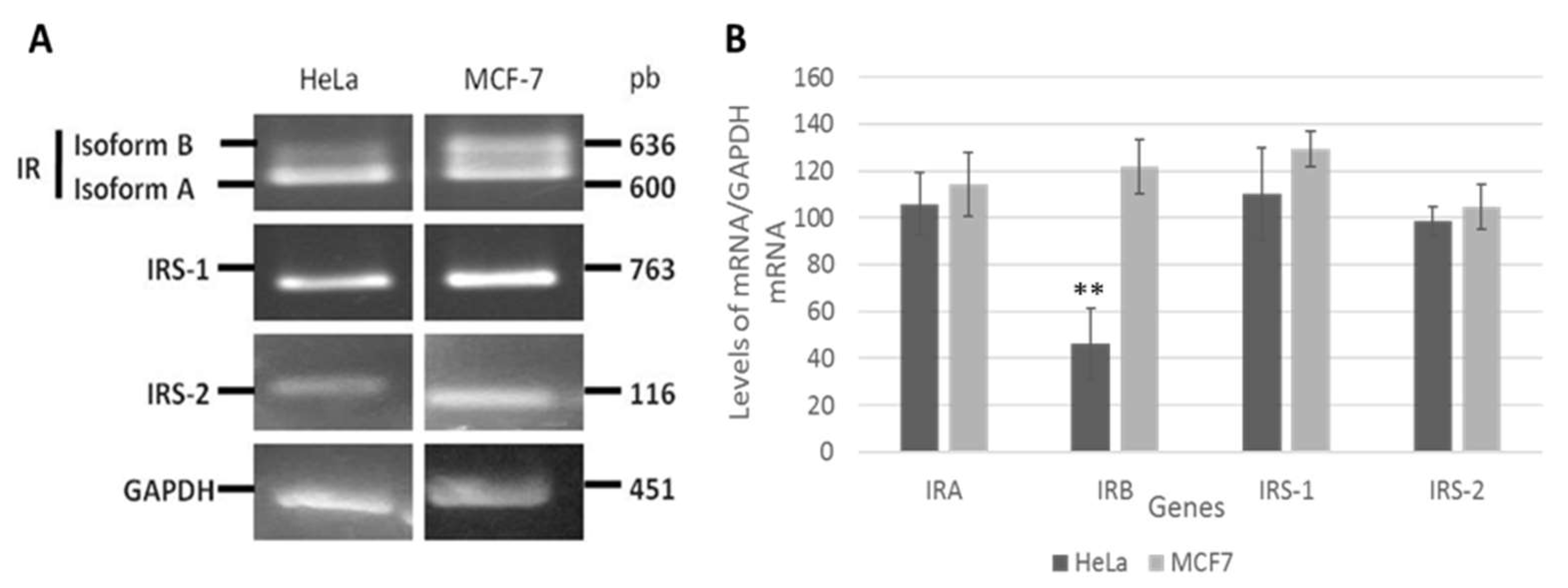
3.1. Insulin Receptor (IR) Isoforms Are Differentially Expressed in HeLa Cells
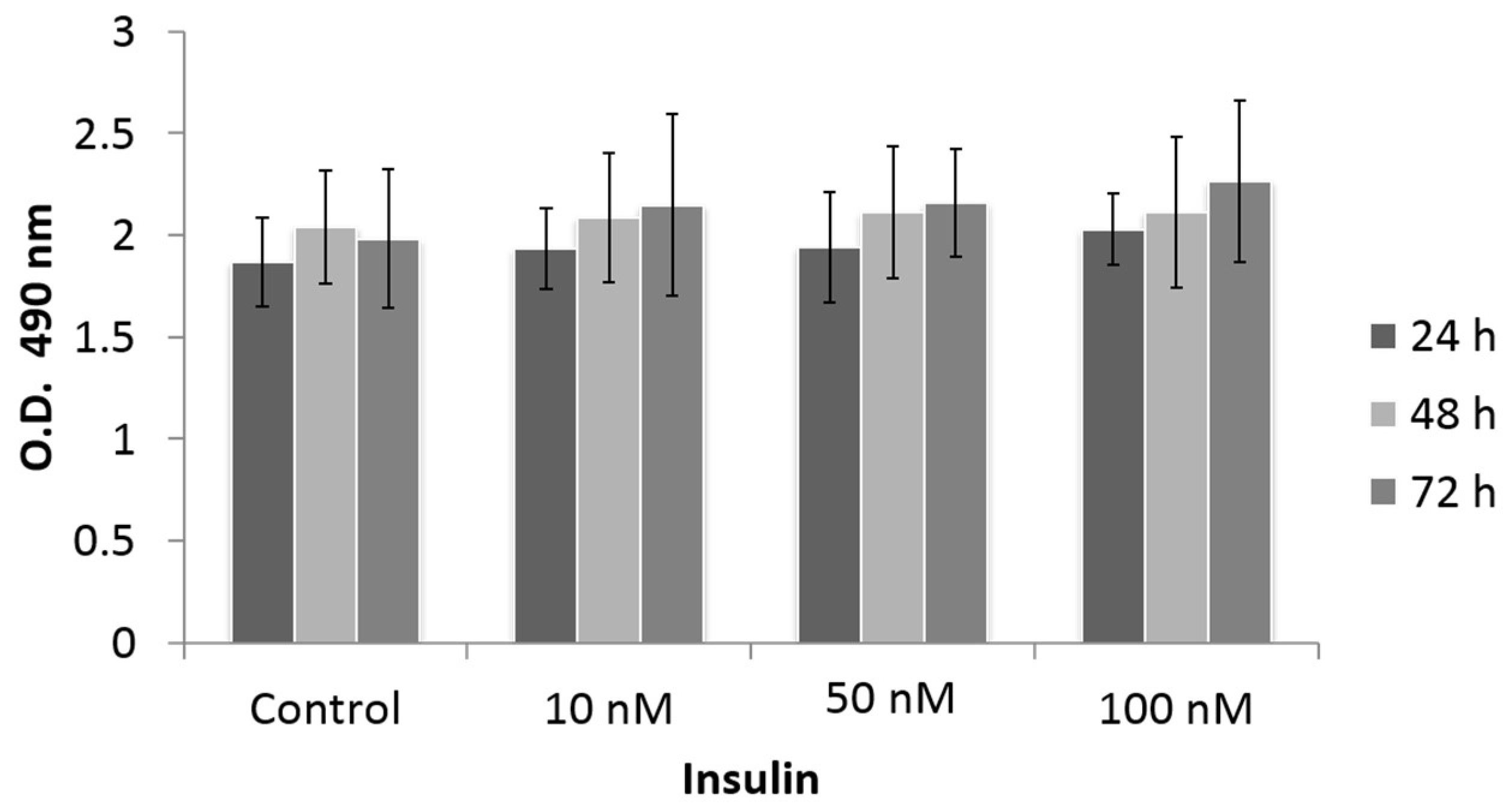
3.2. Effect of Insulin Treatment on Cell Proliferation
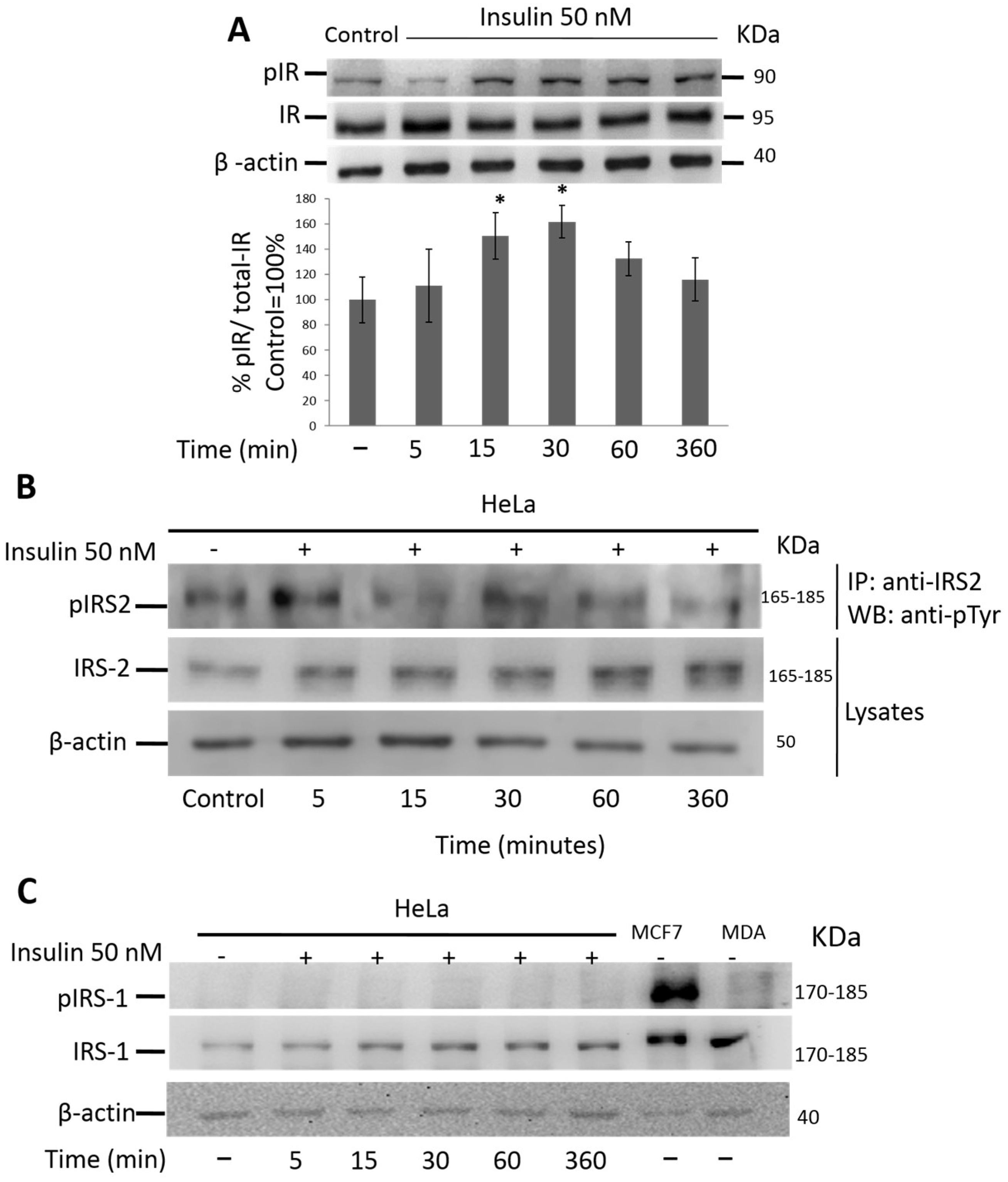
3.3. Insulin Activates IR and IRS-2 but Not IRS-1 in HeLa Cells
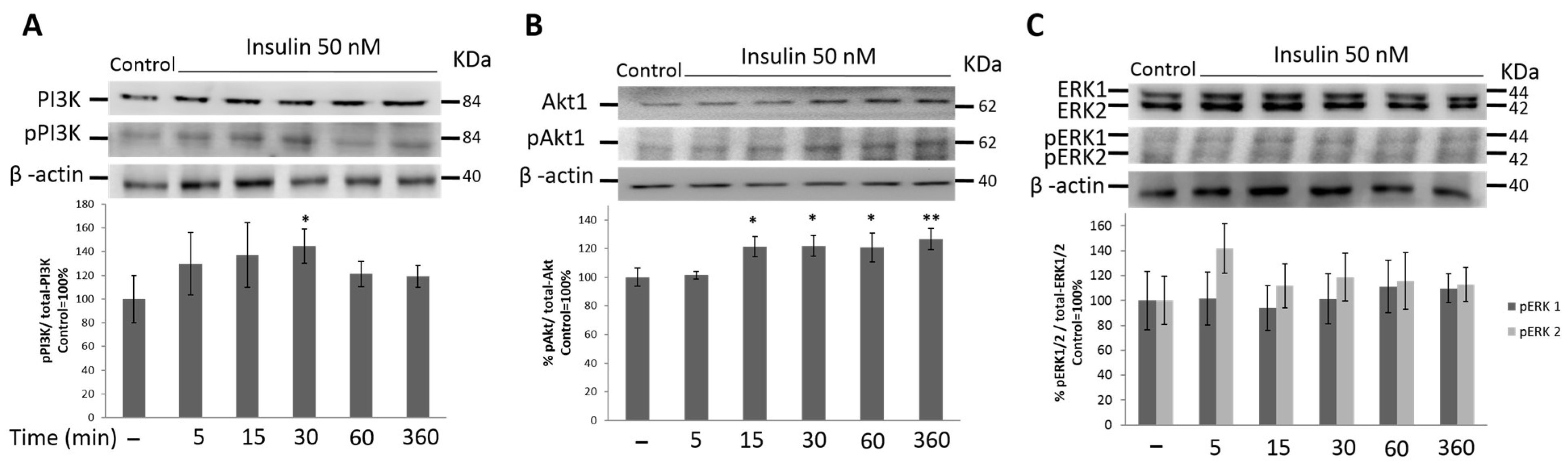
3.4. PI3K/Akt1 Pathway Is Up-Regulated by Insulin in HeLa Cells
3.5. MAPK Signaling Pathway Is Not Activated by Insulin Treatment in HeLa Cells
3.6. Insulin Induces Migration of HeLa Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Najjar, S. Insulin Action: Molecular Basis of Diabetes. In eLS; Najjar, S., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2003; pp. 1–10. [Google Scholar]
- Vella, V.; Milluzzo, A.; Scalisi, N.M.; Vigneri, P.; Sciacca, L. Insulin Receptor Isoforms in Cancer. Int. J. Mol. Sci. 2018, 19, 3615. [Google Scholar] [CrossRef]
- Gorgisen, G.; Gulacar, I.M.; Ozes, O.N. The role of insulin receptor substrate (IRS) proteins in oncogenic transformation. Cell. Mol. Biol. 2017, 63, 1–5. [Google Scholar] [CrossRef]
- Ma, Z.; Gibson, S.L.; Byrne, M.A.; Zhang, J.; White, M.F.; Shaw, L.M. Suppression of insulin receptor substrate 1 (IRS-1) promotes mammary tumor metastasis. Mol. Cell. Biol. 2006, 26, 9338–9351. [Google Scholar] [CrossRef]
- Dearth, R.K.; Cui, X.; Kim, H.J.; Kuiatse, I.; Lawrence, N.A.; Zhang, X.; Divisova, J.; Britton, O.L.; Mohsin, S.; Allred, D.C.; et al. Mammary tumorigenesis and metastasis caused by overexpression of insulin receptor substrate 1 (IRS-1) or IRS-2. Mol. Cell. Biol. 2006, 26, 9302–9314. [Google Scholar] [CrossRef]
- Koda, M.; Sulkowska, M.; Kanczuga-Koda, L.; Sulkowski, S. Expression of insulin receptor substrate 1 in primary breast cancer and lymph node metastases. J. Clin. Pathol. 2005, 58, 645–649. [Google Scholar] [CrossRef]
- Rocha, R.L.; Hilsenbeck, S.G.; Jackson, J.G.; VanDenBerg, C.L.; Weng, C.; Lee, A.V.; Yee, D. Insulin-like growth factor binding protein-3 and insulin receptor substrate-1 in breast cancer: Correlation with clinical parameters and disease-free survival. Clin. Cancer Res. 1997, 3, 103–109. [Google Scholar]
- Schnarr, B.; Strunz, K.; Ohsam, J.; Benner, A.; Wacker, J.; Mayer, D. Down-regulation of insulin-like growth factor-I receptor and insulin receptor substrate-1 expression in advanced human breast cancer. Int. J. Cancer 2000, 89, 506–513. [Google Scholar] [CrossRef]
- Han, C.H.; Cho, J.Y.; Moon, J.T.; Kim, H.J.; Kim, S.K.; Shin, D.H.; Chang, J.; Ahn, C.M.; Kim, S.K.; Chang, Y.S. Clinical significance of insulin receptor substrate-I down-regulation in non-small cell lung cancer. Oncol. Rep. 2006, 16, 1205–1210. [Google Scholar] [CrossRef]
- Reiss, K.; Wang, J.Y.; Romano, G.; Furnari, F.B.; Cavenee, W.K.; Morrione, A.; Tu, X.; Baserga, R. IGF-I receptor signaling in a prostatic cancer cell line with a PTEN mutation. Oncogene 2000, 19, 2687–2694. [Google Scholar] [CrossRef]
- Nishiyama, M.; Wands, J.R. Cloning and increased expression of an insulin receptor substrate-1-like gene in human hepatocellular carcinoma. Biochem. Biophys. Res. Commun. 1992, 183, 280–285. [Google Scholar] [CrossRef]
- Jeong, S.H.; Kim, H.B.; Kim, M.C.; Lee, J.M.; Lee, J.H.; Kim, J.H.; Kim, J.W.; Park, W.Y.; Kim, S.Y.; Kim, J.B.; et al. Hippo-mediated suppression of IRS2/AKT signaling prevents hepatic steatosis and liver cancer. J. Clin. Investig. 2018, 128, 1010–1025. [Google Scholar] [CrossRef]
- Gao, C.; Zhang, H.; Zhang, W.S.; Fang, L. Expression and Significance of Insulin Receptor Substrate 1 in Human Hepatocellular Carcinoma. Dis. Markers 2020, 2020, 7174062. [Google Scholar] [CrossRef]
- Gibson, S.L.; Ma, Z.; Shaw, L.M. Divergent roles for IRS-1 and IRS-2 in breast cancer metastasis. Cell Cycle 2007, 6, 631–637. [Google Scholar] [CrossRef]
- Kim, B.; van Golen, C.M.; Feldman, E.L. Insulin-like growth factor-I signaling in human neuroblastoma cells. Oncogene 2004, 23, 130–141. [Google Scholar] [CrossRef]
- Luo, X.; Fan, S.; Huang, W.; Zhai, S.; Ma, Z.; Li, P.; Sun, S.Y.; Wang, X. Downregulation of IRS-1 promotes metastasis of head and neck squamous cell carcinoma. Oncol. Rep. 2012, 28, 659–667. [Google Scholar] [CrossRef]
- Lu, C.C.; Chu, P.Y.; Hsia, S.M.; Wu, C.H.; Tung, Y.T.; Yen, G.C. Insulin induction instigates cell proliferation and metastasis in human colorectal cancer cells. Int. J. Oncol. 2017, 50, 736–744. [Google Scholar] [CrossRef]
- Lomperta, K.; Jakubowska, K.; Grudzinska, M.; Kanczuga-Koda, L.; Wincewicz, A.; Surmacz, E.; Sulkowski, S.; Koda, M. Insulin receptor substrate 1 may play divergent roles in human colorectal cancer development and progression. World J. Gastroenterol. 2020, 26, 4140–4150. [Google Scholar] [CrossRef]
- Lei, Y.; Jamal, M.; Zeng, X.; He, H.; Xiao, D.; Zhang, C.; Zhang, X.; Tan, H.; Xie, S.; Zhang, Q. Insulin receptor substrate 1(IRS1) is related with lymph node metastases and prognosis in esophageal squamous cell carcinoma. Gene 2022, 835, 146651. [Google Scholar] [CrossRef]
- Hao, S.; Li, Q.; Liu, Y.; Li, F.; Yang, Q.; Wang, J.; Wang, C. Insulin Receptor Substrate 1 Is Involved in the Phycocyanin-Mediated Antineoplastic Function of Non-Small Cell Lung Cancer Cells. Molecules 2021, 26, 4711. [Google Scholar] [CrossRef]
- Gorgisen, G.; Yaren, Z. Insulin receptor substrate 1 overexpression promotes survival of glioblastoma cells through AKT1 activation. Folia Neuropathol. 2020, 58, 38–44. [Google Scholar] [CrossRef]
- Zhang, X.; Kamaraju, S.; Hakuno, F.; Kabuta, T.; Takahashi, S.; Sachdev, D.; Yee, D. Motility response to insulin-like growth factor-I (IGF-I) in MCF-7 cells is associated with IRS-2 activation and integrin expression. Breast Cancer Res. Treat. 2004, 83, 161–170. [Google Scholar] [CrossRef]
- Cui, X.; Kim, H.J.; Kuiatse, I.; Kim, H.; Brown, P.H.; Lee, A.V. Epidermal growth factor induces insulin receptor substrate-2 in breast cancer cells via c-Jun NH(2)-terminal kinase/activator protein-1 signaling to regulate cell migration. Cancer Res. 2006, 66, 5304–5313. [Google Scholar] [CrossRef]
- Jackson, J.G.; Zhang, X.; Yoneda, T.; Yee, D. Regulation of breast cancer cell motility by insulin receptor substrate-2 (IRS-2) in metastatic variants of human breast cancer cell lines. Oncogene 2001, 20, 7318–7325. [Google Scholar] [CrossRef]
- Piper, A.J.; Clark, J.L.; Mercado-Matos, J.; Matthew-Onabanjo, A.N.; Hsieh, C.C.; Akalin, A.; Shaw, L.M. Insulin Receptor Substrate-1 (IRS-1) and IRS-2 expression levels are associated with prognosis in non-small cell lung cancer (NSCLC). PLoS ONE 2019, 14, e0220567. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Y.; Ding, Y. Circular RNA circPRKDC promotes tumorigenesis of gastric cancer via modulating insulin receptor substrate 2 (IRS2) and mediating microRNA-493-5p. Bioengineered 2021, 12, 7631–7643. [Google Scholar] [CrossRef]
- Saltiel, A.R. Insulin signaling in health and disease. J. Clin. Investig. 2021, 131, e142241. [Google Scholar] [CrossRef]
- Serrano, M.L.; Sanchez-Gomez, M.; Bravo, M.M.; Yakar, S.; LeRoith, D. Differential expression of IGF-I and insulin receptor isoforms in HPV positive and negative human cervical cancer cell lines. Horm. Metab. Res. 2008, 40, 661–667. [Google Scholar] [CrossRef]
- Diaz-Barriga, F.; Carrizales, L.; Yanez, L.; Hernandez, J.M.; Dominguez Robles, M.C.; Palmer, E.; Saborio, J.L. Interaction of cadmium with actin microfilaments in vitro. Toxicol. Vitr. 1989, 3, 277–284. [Google Scholar] [CrossRef]
- Geback, T.; Schulz, M.M.; Koumoutsakos, P.; Detmar, M. TScratch: A novel and simple software tool for automated analysis of monolayer wound healing assays. Biotechniques 2009, 46, 265–274. [Google Scholar] [CrossRef]
- Cowey, S.; Hardy, R.W. The metabolic syndrome: A high-risk state for cancer? Am. J. Pathol. 2006, 169, 1505–1522. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, L.; Hamada, T.; Nowak, J.A.; Giannakis, M.; Ma, Y.; Song, M.; Nevo, D.; Kosumi, K.; Gu, M.; et al. Night-Shift Work Duration and Risk of Colorectal Cancer According to IRS1 and IRS2 Expression. Cancer Epidemiol. Biomark. Prev. 2020, 29, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Vassen, L.; Wegrzyn, W.; Klein-Hitpass, L. Human insulin receptor substrate-2 (IRS-2) is a primary progesterone response gene. Mol. Endocrinol. 1999, 13, 485–494. [Google Scholar] [CrossRef]
- Vella, V.; Nicolosi, M.L.; Giuliano, M.; Morrione, A.; Malaguarnera, R.; Belfiore, A. Insulin Receptor Isoform A Modulates Metabolic Reprogramming of Breast Cancer Cells in Response to IGF2 and Insulin Stimulation. Cells 2019, 8, 1017. [Google Scholar] [CrossRef]
- Malaguarnera, R.; Frasca, F.; Garozzo, A.; Giani, F.; Pandini, G.; Vella, V.; Vigneri, R.; Belfiore, A. Insulin receptor isoforms and insulin-like growth factor receptor in human follicular cell precursors from papillary thyroid cancer and normal thyroid. J. Clin. Endocrinol. Metab. 2011, 96, 766–774. [Google Scholar] [CrossRef]
- Benabou, E.; Salame, Z.; Wendum, D.; Lequoy, M.; Tahraoui, S.; Merabtene, F.; Chretien, Y.; Scatton, O.; Rosmorduc, O.; Fouassier, L.; et al. Insulin receptor isoform A favors tumor progression in human hepatocellular carcinoma by increasing stem/progenitor cell features. Cancer Lett. 2019, 450, 155–168. [Google Scholar] [CrossRef]
- Vella, V.; Giuliano, M.; La Ferlita, A.; Pellegrino, M.; Gaudenzi, G.; Alaimo, S.; Massimino, M.; Pulvirenti, A.; Dicitore, A.; Vigneri, P.; et al. Novel Mechanisms of Tumor Promotion by the Insulin Receptor Isoform A in Triple-Negative Breast Cancer Cells. Cells 2021, 10, 3145. [Google Scholar] [CrossRef]
- Zhang, X.; Sheng, X.; Miao, T.; Yao, K.; Yao, D. Effect of insulin on thyroid cell proliferation, tumor cell migration, and potentially related mechanisms. Endocr. Res. 2019, 44, 55–70. [Google Scholar] [CrossRef]
- Heni, M.; Hennige, A.M.; Peter, A.; Siegel-Axel, D.; Ordelheide, A.M.; Krebs, N.; Machicao, F.; Fritsche, A.; Haring, H.U.; Staiger, H. Insulin promotes glycogen storage and cell proliferation in primary human astrocytes. PLoS ONE 2011, 6, e21594. [Google Scholar] [CrossRef]
- Nagle, J.A.; Ma, Z.; Byrne, M.A.; White, M.F.; Shaw, L.M. Involvement of insulin receptor substrate 2 in mammary tumor metastasis. Mol. Cell. Biol. 2004, 24, 9726–9735. [Google Scholar] [CrossRef]
- Porter, H.A.; Perry, A.; Kingsley, C.; Tran, N.L.; Keegan, A.D. IRS1 is highly expressed in localized breast tumors and regulates the sensitivity of breast cancer cells to chemotherapy, while IRS2 is highly expressed in invasive breast tumors. Cancer Lett. 2013, 338, 239–248. [Google Scholar] [CrossRef]
- Takatani, T.; Shirakawa, J.; Shibue, K.; Gupta, M.K.; Kim, H.; Lu, S.; Hu, J.; White, M.F.; Kennedy, R.T.; Kulkarni, R.N. Insulin receptor substrate 1, but not IRS2, plays a dominant role in regulating pancreatic alpha cell function in mice. J. Biol. Chem. 2021, 296, 100646. [Google Scholar] [CrossRef]
- Dalmizrak, O.; Wu, A.; Chen, J.; Sun, H.; Utama, F.E.; Zambelli, D.; Tran, T.H.; Rui, H.; Baserga, R. Insulin receptor substrate-1 regulates the transformed phenotype of BT-20 human mammary cancer cells. Cancer Res. 2007, 67, 2124–2130. [Google Scholar] [CrossRef]
- Shaw, L.M. Identification of insulin receptor substrate 1 (IRS-1) and IRS-2 as signaling intermediates in the alpha6beta4 integrin-dependent activation of phosphoinositide 3-OH kinase and promotion of invasion. Mol. Cell. Biol. 2001, 21, 5082–5093. [Google Scholar] [CrossRef]
- Kim, S.J.; Khadka, D.; Seo, J.H. Interplay between Solid Tumors and Tumor Microenvironment. Front. Immunol. 2022, 13, 882718. [Google Scholar] [CrossRef]
- Vaupel, P.; Kallinowski, F.; Okunieff, P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: A review. Cancer Res. 1989, 49, 6449–6465. [Google Scholar]
- Ancey, P.B.; Contat, C.; Meylan, E. Glucose transporters in cancer—From tumor cells to the tumor microenvironment. FEBS J. 2018, 285, 2926–2943. [Google Scholar] [CrossRef]
- Ishida, T.; Shimamoto, T.; Ozaki, N.; Takaki, S.; Kuchimaru, T.; Kizaka-Kondoh, S.; Omata, T. Investigation of the Influence of Glucose Concentration on Cancer Cells by Using a Microfluidic Gradient Generator without the Induction of Large Shear Stress. Micromachines 2016, 7, 155. [Google Scholar] [CrossRef]
- Guvakova, M.A. Insulin-like growth factors control cell migration in health and disease. Int. J. Biochem. Cell Biol. 2007, 39, 890–909. [Google Scholar] [CrossRef]
- Wang, Y.; Lei, L.; Su, Q.; Qin, S.; Zhong, J.; Ni, Y.; Yang, J. Resveratrol Inhibits Insulin-Induced Vascular Smooth Muscle Cell Proliferation and Migration by Activating SIRT1. Evid. Based Complement. Alternat. Med. 2022, 2022, 8537881. [Google Scholar] [CrossRef]
- Sarkar, P.L.; Lee, W.; Williams, E.D.; Lubik, A.A.; Stylianou, N.; Shokoohmand, A.; Lehman, M.L.; Hollier, B.G.; Gunter, J.H.; Nelson, C.C. Insulin Enhances Migration and Invasion in Prostate Cancer Cells by Up-Regulation of FOXC2. Front. Endocrinol. 2019, 10, 481. [Google Scholar] [CrossRef]
- Mercado-Matos, J.; Janusis, J.; Zhu, S.; Chen, S.S.; Shaw, L.M. Identification of a Novel Invasion-Promoting Region in Insulin Receptor Substrate 2. Mol. Cell. Biol. 2018, 38, e00590–e00617. [Google Scholar] [CrossRef] [PubMed]

| Target | Primer Sequence 5′-3′ | Position | Size (bp) |
|---|---|---|---|
| IR | 5′-AACCAGAGTGAGTATGAGGAT-3′ | nt 2201-2221 | Isoform B: 636 |
| 5′-CCGTTCCAGAGCGAAGTGCTT-3′ | nt 2780-2800 | Isoform A: 600 | |
| IRS1 | 5′-TCCACTGTGACACCAGAATAAT-3 | nt 4011-4032 | 763 |
| 5′-CGCCAACATTGTTCATTCCAA-3′ | nt 4753-4773 | ||
| IRS2 | 5′-TAGGCATCAATGGGTGGTATTT-3′ | nt 6358-6380 | 116 |
| 5′-CTACGGATAGAGGGCGAGTTA-3′ | nt 6453-6474 | ||
| GAPDH | 5′-ACCACAGTCCATGCCATCAC-3′ | nt 602-621 | 451 |
| 5′-TCCACCACCCTGTTGCTGTA-3′ | nt 1031-1053 |
| Primary Antibody | Epitope/ Specificity | Host Species | Type | Dilution | Source |
|---|---|---|---|---|---|
| Insulin Receptor β (C19) | C-terminus/IgG | Rabbit | Polyclonal | 1:500 | Santa Cruz SC-711 |
| IRS1 (C-20) | C-terminus/IgG | Rabbit | Polyclonal | 1:500 | Santa Cruz SC-559 |
| IRS2 (H-205) | aa 926-1130/IgG | Rabbit | Polyclonal | 1:500 | Santa Cruz SC-8299 |
| PI 3-kinase (Z-8) | p85α/IgG | Rabbit | Polyclonal | 1:500 | Santa Cruz SC-423 |
| ERK 1 (C-16) | C-terminus/IgG | Rabbit | Polyclonal | 1:500 | Santa Cruz SC-93 |
| Akt1 (B1) | aa 345-480/IgG1 | Mouse | Monoclonal | 1:500 | Santa Cruz SC-5298 |
| Phospho insulin receptor | Phospho Tyr/1361/IgG | Rabbit | Polyclonal | 1:500 | Abcam ab60946 |
| Phospho-IRS1 | Phospho Tyr/632/ IgG | Rabbit | Monoclonal | 1:500 | Abcam ab109543 |
| Anti- phospho Tyrosine | Tyrosine-phosphorylated proteins/IgG2b | Mouse | Monoclonal | 1:500 | Millipore 05-947 |
| Phospho-Akt1 | Phospho Thr 308/IgG | Rabbit | Monoclonal | 1:500 | Millipore 05-802R |
| Phospho-Erk1/2 | Phospho Thr 202/Tyr 204, Thr 185/Tyr 187/IgG | Rabbit | Monoclonal | 1:500 | Millipore 05-797R |
| Phospho-PI3K p85 | p85 Phospho Tyr 467, Tyr 199/IgG | Rabbit | Polyclonal | 1:500 | GeneTex GTX132597 |
| β-actin (4E8H3) | IgG1 | Mouse | Monoclonal | 1:500 | [29] |
| Secondary antibody | Epitope Specificity | Host species | Type | Dilution | Source |
| Rabbit anti-goat IgG HRP-coupled | Rabbit | Polyclonal | 1:5000 | Santa Cruz SC-2768 | |
| Mouse IgG HRP-coupled | Mouse | Polyclonal | 1:5000 | GeneTex GTX213111-01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez Báez, A.; Castro Romero, I.; Chihu Amparan, L.; Castañeda, J.R.; Ayala, G. The Insulin Receptor Substrate 2 Mediates the Action of Insulin on HeLa Cell Migration via the PI3K/Akt Signaling Pathway. Curr. Issues Mol. Biol. 2023, 45, 2296-2308. https://doi.org/10.3390/cimb45030148
Martínez Báez A, Castro Romero I, Chihu Amparan L, Castañeda JR, Ayala G. The Insulin Receptor Substrate 2 Mediates the Action of Insulin on HeLa Cell Migration via the PI3K/Akt Signaling Pathway. Current Issues in Molecular Biology. 2023; 45(3):2296-2308. https://doi.org/10.3390/cimb45030148
Chicago/Turabian StyleMartínez Báez, Anabel, Ivone Castro Romero, Lilia Chihu Amparan, Jose Ramos Castañeda, and Guadalupe Ayala. 2023. "The Insulin Receptor Substrate 2 Mediates the Action of Insulin on HeLa Cell Migration via the PI3K/Akt Signaling Pathway" Current Issues in Molecular Biology 45, no. 3: 2296-2308. https://doi.org/10.3390/cimb45030148
APA StyleMartínez Báez, A., Castro Romero, I., Chihu Amparan, L., Castañeda, J. R., & Ayala, G. (2023). The Insulin Receptor Substrate 2 Mediates the Action of Insulin on HeLa Cell Migration via the PI3K/Akt Signaling Pathway. Current Issues in Molecular Biology, 45(3), 2296-2308. https://doi.org/10.3390/cimb45030148






