Effects of Mindfulness-Based Therapy on Clinical Symptoms and DNA Methylation in Patients with Polycystic Ovary Syndrome and High Metabolic Risk
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
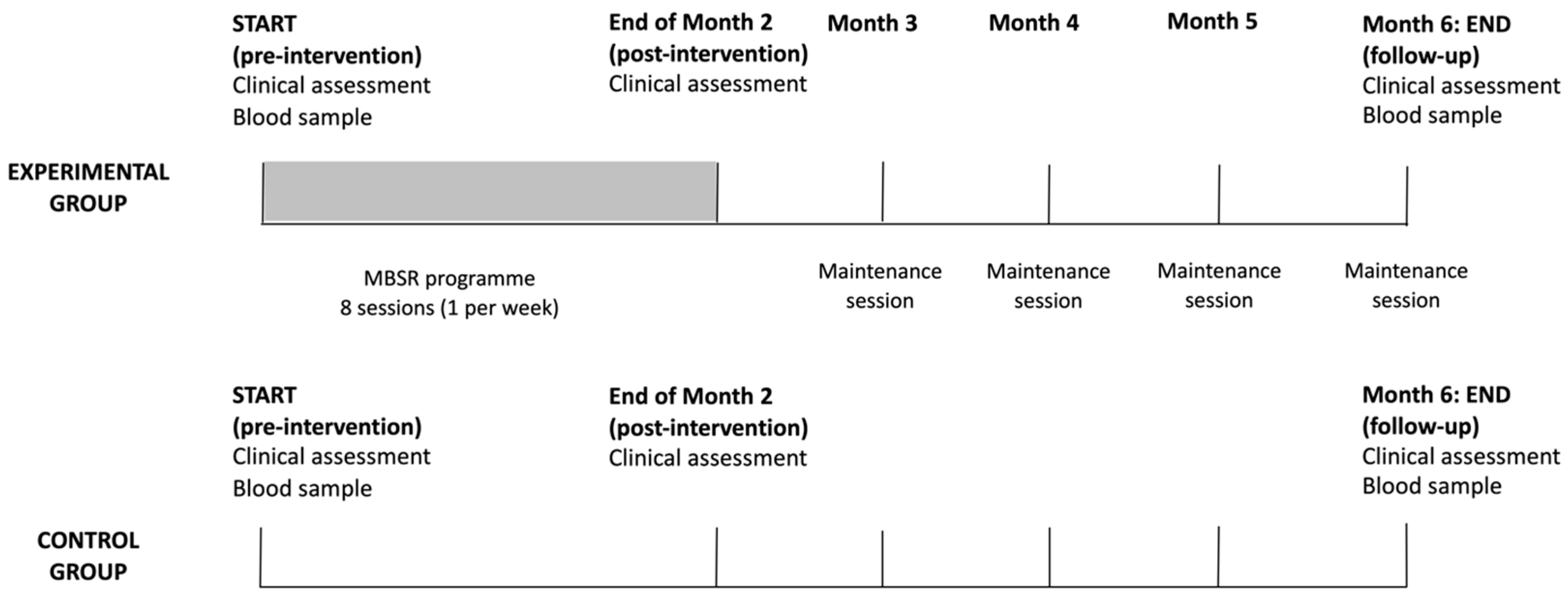
2.2. Study Design
2.3. Mindfulness-Based Stress Reduction Program
2.4. DNA Methylation Analysis
2.4.1. Selection of Candidate Genes and Target Sequences
| Amplicon | Position (Human Genome Build 19) and Length of Target Sequence | Number of CpG Islands | Functional Significance |
|---|---|---|---|
| BDNF-81_1 | chr11:27744260-27744605 (−), 346 | 22 | Brain-derived neurotrophic factor gene; regulates growth, differentiation, maintenance, death/survival and plasticity of neurons [54] |
| BDNF-81_2 | chr11:27743702-27743960 (−), 259 | 10 | |
| BDNF-81_3 | chr11:27743454-27743762 (−), 309 | 20 | |
| BDNF-14_1 | chr11:27741988-27742250 (−), 263 | 13 | |
| BDNF-58_1 | chr11:27740916-27741131 (−), 216 | 16 | |
| BDNF-58_2 | chr11:27740607-27740901 (−), 295 | 30 | |
| BDNF-95_1 | chr11:27721638-27721854 (−), 217 | 19 | |
| BDNF-95_2 | chr11:27722466-27722696 (−), 231 | 13 | |
| BDNF-95_5 | chr11:27722209-27722487 (−), 279 | 23 | |
| CEBPB_1 | chr20:48807584-48807968 (+), 385 | 52 | Transcription factor gene; participates in the ovarian follicle development and insulin signaling [55] |
| CEBPB_2 | chr20:48807389-48807650 (+), 262 | 23 | |
| COMT_1 | chr22:19951071-19951343, 273 | 14 | Catechol-O-methyl transferase (COMT) gene, determines prefrontal dopaminergic availability [56] |
| COMT_2 | chr22:19929042-19929349, 308 | 36 | |
| COMT_4 | chr22:19950002-19950320, 319 | 13 | |
| EPHX1_2 | chr1:225998005-225998262 (+), 258 | 25 | Epoxide hydrolase-1 gene; regulates steroid synthesis pathways associated with PCOS [57] |
| EPM2A_1 | chr6:146056330-146056621 (−), 292 | 36 | Dual-specificity phosphatase gene; involved in the regulation of glycogen metabolism [55] |
| FKBP51_1 | chr6:35656629-35656978 (−), 350 | 38 | FK506 binding protein 5 gene; regulates glucocorticoid activity and acute stress response [58] |
| FST_1 | chr5:52775484-52775780 (+), 297 | 23 | Follistatin, an activin-binding protein gene; associated with PCOS [59] |
| FST_2 | chr5:52776111-52776302 (+), 192 | 13 | |
| FST_3 | chr5:52776279-52776668 (+), 390 | 54 | |
| HTR1A_2 | chr5:63257662-63257938 (−),277 | 15 | Serotonin receptor 1A gene; regulates serotonin system function [60] |
| HTR1A_3 | chr5:63256777-63257065 (−), 289 | 20 | |
| IGFBP1_1 | chr7:45928046-45928301 (+), 256 | 21 | Insulin-like growth factor binding protein gene; regulates cell migration and metabolism [55] |
| IGFBP1_2 | chr7:45928280-45928533 (+), 254 | 35 | |
| INSR_1 | chr19:7293426-7293764 (−), 339 | 36 | Insulin receptor gene; associated with insulin resistance in PCOS [61] |
| LHCGR_1 | chr2:48982757-48983019 (−), 263 | 16 | Luteinizing hormone/choriogonadotropin receptor gene; involved in human gonadal maturation and function [62] |
| MAOA_2 | chrX:43513981-43514236, 256 | 18 | Monoamine oxidase A gene; involved in serotonin degradation [60] |
| MAOA_3 | chrX:43515510-43515787, 278 | 13 | |
| NR3C1_1 | chr5:142783586-142783906 (−), 321 | 39 | Glucocorticoid receptor gene; associated with regulation of HPA axis and stress response [63] |
| PPARG1A_1 | chr4:23890471-23890804 (−), 334 | 9 | Peroxisome proliferator-activated receptor gamma 1 gene; regulates ovarian function [64] |
| SLC6A4_3 | chr17:28562753-28563050 (−), 298 | 29 | Serotonin transporter gene; associated with depression treatment outcomes [58] |
| SLC6A4_5 | chr17:28563277-28563552 (−), 276 | 7 | |
| TBKBP1_1 | chr17:45772538-45772753 (+), 216 | 9 | TBKBP1 gene; involved in the TNF-α/NF-κB pathway activated under conditions of acute and chronic psychological stress [40] |
| TPH2_1 | chr12:72332514-72332805 (+), 292 | 9 | Tryptophan hydroxylase 2 gene; serotonin synthesis rate limiting enzyme [60] |
2.4.2. DNA Isolation and Bisulfite Conversion
2.4.3. Primer Design
2.4.4. Amplicon Generation and Sequencing
2.4.5. Library Preparation and Sequencing
2.5. Bioinformatic and Statistical Analysis
3. Results
3.1. Effects of the MBSR Program on Physical Health and Quality of Life
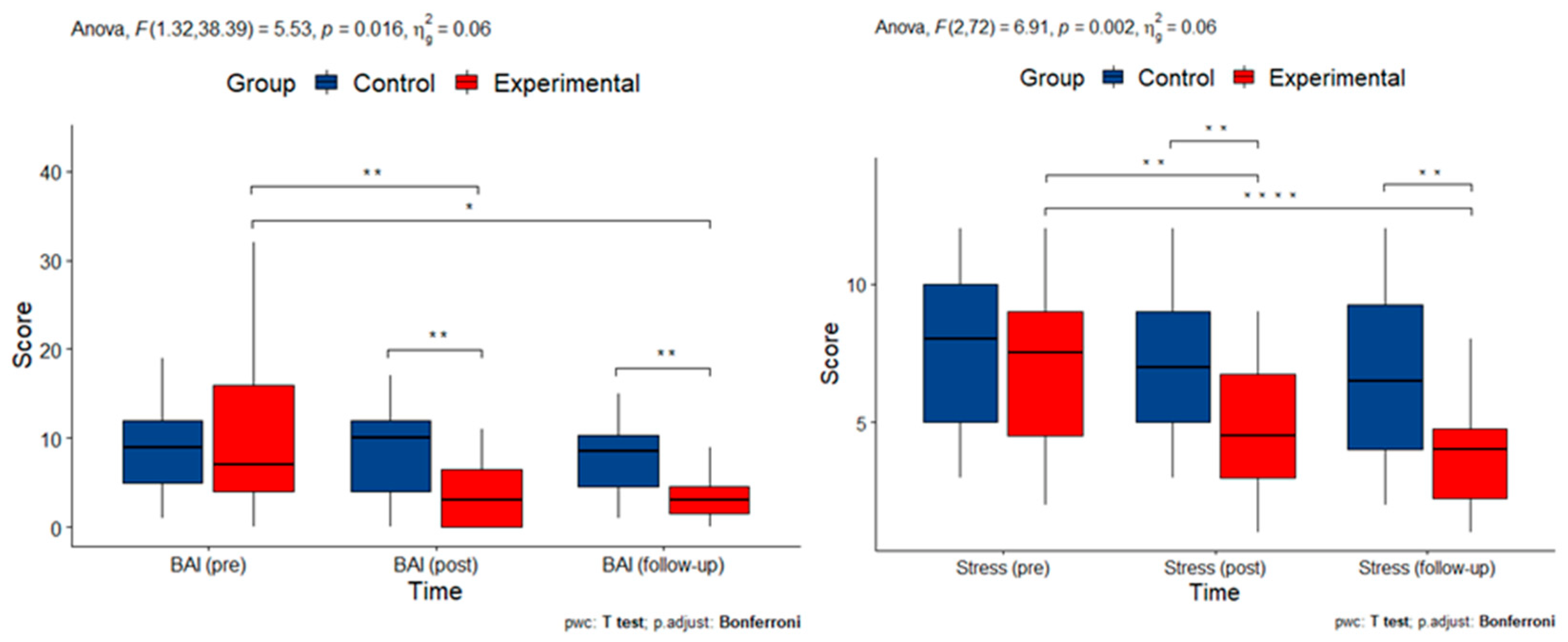
3.2. Effects and Interactions of the MBSR Program on Psychological Well-Being
3.3. Effects and Interactions of the MBSR Program on Mindfulness-Like Traits
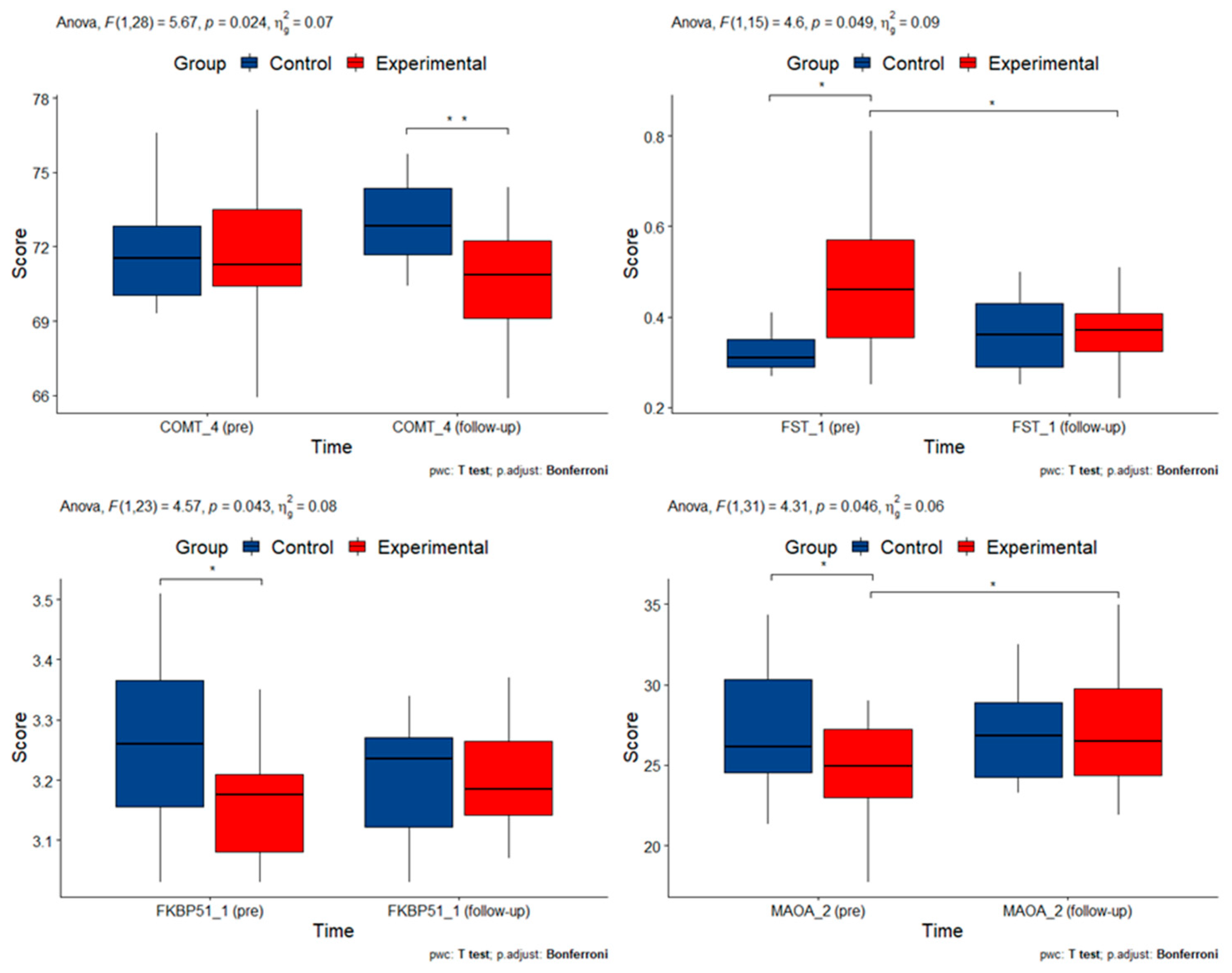
3.4. DNA Methylation Alterations in Candidate Genes
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Escobar-Morreale, H.F. Polycystic ovary syndrome: Definition, aetiology, diagnosis and treatment. Nat. Rev. Endocrinol. 2018, 14, 270–284. [Google Scholar] [CrossRef]
- Joham, A.E.; Norman, R.J.; Stener-Victorin, E.; Legro, R.S.; Franks, S.; Moran, L.J.; Boyle, J.; Teede, H.J. Polycystic ovary syndrome. Lancet Diabetes Endocrinol. 2022, 10, 668–680. [Google Scholar] [CrossRef] [PubMed]
- Witchel, S.F.; Oberfield, S.E.; Peña, A.S. Polycystic Ovary Syndrome: Pathophysiology, Presentation, and Treatment with Emphasis on Adolescent Girls. J. Endocr. Soc. 2019, 3, 1545–1573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasquali, R. Metabolic Syndrome in Polycystic Ovary Syndrome. In Frontiers of Hormone Research; Popovic, V., Korbonits, M., Eds.; S. Karger AG: Basel, Switzerland, 2018; Volume 49, pp. 114–130. ISBN 978-3-318-06334-9. [Google Scholar]
- Wang, Z.; Groen, H.; Cantineau, A.E.P.; Van Elten, T.M.; Karsten, M.D.A.; Van Oers, A.M.; Mol, B.W.J.; Roseboom, T.J.; Hoek, A. Dietary Intake, Eating Behavior, Physical Activity, and Quality of Life in Infertile Women with PCOS and Obesity Compared with Non-PCOS Obese Controls. Nutrients 2021, 13, 3526. [Google Scholar] [CrossRef]
- Herman, R.; Sikonja, J.; Jensterle, M.; Janez, A.; Dolzan, V. Insulin Metabolism in Polycystic Ovary Syndrome: Secretion, Signaling, and Clearance. Int. J. Mol. Sci. 2023, 24, 3140. [Google Scholar] [CrossRef]
- Zhu, T.; Cui, J.; Goodarzi, M.O. Polycystic Ovary Syndrome and Risk of Type 2 Diabetes, Coronary Heart Disease, and Stroke. Diabetes 2021, 70, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Meczekalski, B.; Pérez-Roncero, G.R.; López-Baena, M.T.; Chedraui, P.; Pérez-López, F.R. The polycystic ovary syndrome and gynecological cancer risk. Gynecol. Endocrinol. 2020, 36, 289–293. [Google Scholar] [CrossRef]
- Dokras, A.; Stener-Victorin, E.; Yildiz, B.O.; Li, R.; Ottey, S.; Shah, D.; Epperson, N.; Teede, H. Androgen Excess-Polycystic Ovary Syndrome Society: Position statement on depression, anxiety, quality of life, and eating disorders in polycystic ovary syndrome. Fertil. Steril. 2018, 109, 888–899. [Google Scholar] [CrossRef]
- Castelo-Branco, C.; Naumova, I. Quality of life and sexual function in women with polycystic ovary syndrome: A comprehensive review. Gynecol. Endocrinol. 2020, 36, 96–103. [Google Scholar] [CrossRef]
- Xing, L.; Xu, J.; Wei, Y.; Chen, Y.; Zhuang, H.; Tang, W.; Yu, S.; Zhang, J.; Yin, G.; Wang, R.; et al. Depression in polycystic ovary syndrome: Focusing on pathogenesis and treatment. Front. Psychiatry 2022, 13, 1001484. [Google Scholar] [CrossRef]
- Walters, K.A.; Gilchrist, R.B.; Ledger, W.L.; Teede, H.J.; Handelsman, D.J.; Campbell, R.E. New Perspectives on the Pathogenesis of PCOS: Neuroendocrine Origins. Trends Endocrinol. Metab. 2018, 29, 841–852. [Google Scholar] [CrossRef] [PubMed]
- Visser, J.A. The importance of metabolic dysfunction in polycystic ovary syndrome. Nat. Rev. Endocrinol. 2021, 17, 77–78. [Google Scholar] [CrossRef] [PubMed]
- Stepto, N.K.; Moreno-Asso, A.; McIlvenna, L.C.; Walters, K.A.; Rodgers, R.J. Molecular Mechanisms of Insulin Resistance in Polycystic Ovary Syndrome: Unraveling the Conundrum in Skeletal Muscle? J. Clin. Endocrinol. Metab. 2019, 104, 5372–5381. [Google Scholar] [CrossRef] [Green Version]
- Rudnicka, E.; Suchta, K.; Grymowicz, M.; Calik-Ksepka, A.; Smolarczyk, K.; Duszewska, A.M.; Smolarczyk, R.; Meczekalski, B. Chronic Low Grade Inflammation in Pathogenesis of PCOS. Int. J. Mol. Sci. 2021, 22, 3789. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.-X.; Li, X.-L. The Disorders of Endometrial Receptivity in PCOS and Its Mechanisms. Reprod. Sci. 2022, 29, 2465–2476. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.; O’Brien, C.; Hawrelak, J.; Gersh, F.L. Polycystic Ovary Syndrome: An Evolutionary Adaptation to Lifestyle and the Environment. Int. J. Environ. Res. Public Health 2022, 19, 1336. [Google Scholar] [CrossRef]
- Vázquez-Martínez, E.R.; Gómez-Viais, Y.I.; García-Gómez, E.; Reyes-Mayoral, C.; Reyes-Muñoz, E.; Camacho-Arroyo, I.; Cerbón, M. DNA methylation in the pathogenesis of polycystic ovary syndrome. Reproduction 2019, 158, R27–R40. [Google Scholar] [CrossRef]
- Rawat, K.; Sandhu, A.; Gautam, V.; Saha, P.K.; Saha, L. Role of genomic DNA methylation in PCOS pathogenesis: A systematic review and meta-analysis involving case-controlled clinical studies. Mol. Hum. Reprod. 2022, 28, gaac024. [Google Scholar] [CrossRef]
- Nilsson, E.; Benrick, A.; Kokosar, M.; Krook, A.; Lindgren, E.; Källman, T.; Martis, M.M.; Højlund, K.; Ling, C.; Stener-Victorin, E. Transcriptional and Epigenetic Changes Influencing Skeletal Muscle Metabolism in Women With Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2018, 103, 4465–4477. [Google Scholar] [CrossRef] [Green Version]
- Echiburú, B.; Milagro, F.; Crisosto, N.; Pérez-Bravo, F.; Flores, C.; Arpón, A.; Salas-Pérez, F.; Recabarren, S.E.; Sir-Petermann, T.; Maliqueo, M. DNA methylation in promoter regions of genes involved in the reproductive and metabolic function of children born to women with PCOS. Epigenetics 2020, 15, 1178–1194. [Google Scholar] [CrossRef]
- Abbott, D.H.; Kraynak, M.; Dumesic, D.A.; Levine, J.E. In utero Androgen Excess: A Developmental Commonality Preceding Polycystic Ovary Syndrome? In Frontiers of Hormone Research; Pasquali, R., Pignatelli, D., Eds.; S. Karger AG: Basel, Switzerland, 2019; Volume 53, pp. 1–17. ISBN 978-3-318-06470-4. [Google Scholar]
- Kaliman, P. Epigenetics and meditation. Curr. Opin. Psychol. 2019, 28, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Venditti, S.; Verdone, L.; Reale, A.; Vetriani, V.; Caserta, M.; Zampieri, M. Molecules of Silence: Effects of Meditation on Gene Expression and Epigenetics. Front. Psychol. 2020, 11, 1767. [Google Scholar] [CrossRef] [PubMed]
- Andersen, G.B.; Tost, J. A Summary of the Biological Processes, Disease-Associated Changes, and Clinical Applications of DNA Methylation. In DNA Methylation Protocols; Tost, J., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2018; Volume 1708, pp. 3–30. ISBN 978-1-4939-7479-5. [Google Scholar]
- Cui, P.; Ma, T.; Tamadon, A.; Han, S.; Li, B.; Chen, Z.; An, X.; Shao, L.R.; Wang, Y.; Feng, Y. Hypothalamic DNA methylation in rats with dihydrotestosterone-induced polycystic ovary syndrome: Effects of low-frequency electro-acupuncture. Exp. Physiol. 2018, 103, 1618–1632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kokosar, M. Polycystic Ovary Syndrome. Androgen Excess and Insulin Resistance in Women: Identification of Molecular Targets to Improve Glucose Homeostasis; Göteborgs Universitet: Göteborg, Sweden, 2018. [Google Scholar]
- Tran, B.X.; Harijanto, C.; Vu, G.T.; Ho, R.C.M. Global mapping of interventions to improve quality of life using mind-body therapies during 1990–2018. Complement. Ther. Med. 2020, 49, 102350. [Google Scholar] [CrossRef]
- Bandealy, S.S.; Sheth, N.C.; Matuella, S.K.; Chaikind, J.R.; Oliva, I.A.; Philip, S.R.; Jones, P.M.; Hoge, E.A. Mind-Body Interventions for Anxiety Disorders: A Review of the Evidence Base for Mental Health Practitioners. Focus 2021, 19, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Ebner, S.A.; Meikis, L.; Morat, M.; Held, S.; Morat, T.; Donath, L. Effects of Movement-Based Mind-Body Interventions on Physical Fitness in Healthy Older Adults: A Meta-Analytical Review. Gerontology 2021, 67, 125–143. [Google Scholar] [CrossRef]
- Buric, I.; Farias, M.; Jong, J.; Mee, C.; Brazil, I.A. What Is the Molecular Signature of Mind–Body Interventions? A Systematic Review of Gene Expression Changes Induced by Meditation and Related Practices. Front. Immunol. 2017, 8, 670. [Google Scholar] [CrossRef] [Green Version]
- Kohls, N.; Esch, T.; Gerber, L.; Adrian, L.; Wittmann, M. Mindfulness Meditation and Fantasy Relaxation in a Group Setting Leads to a Diminished Sense of Self and an Increased Present Orientation. Behav. Sci. 2019, 9, 87. [Google Scholar] [CrossRef] [Green Version]
- Kropp, A.; Sedlmeier, P. What Makes Mindfulness-Based Interventions Effective? An Examination of Common Components. Mindfulness 2019, 10, 2060–2072. [Google Scholar] [CrossRef]
- Lutz, A.; Jha, A.P.; Dunne, J.D.; Saron, C.D. Investigating the phenomenological matrix of mindfulness-related practices from a neurocognitive perspective. Am. Psychol. 2015, 70, 632–658. [Google Scholar] [CrossRef]
- Querstret, D.; Morison, L.; Dickinson, S.; Cropley, M.; John, M. Mindfulness-based stress reduction and mindfulness-based cognitive therapy for psychological health and well-being in nonclinical samples: A systematic review and meta-analysis. Int. J. Stress Manag. 2020, 27, 394–411. [Google Scholar] [CrossRef]
- Wersebe, H.; Lieb, R.; Meyer, A.H.; Hofer, P.; Gloster, A.T. The link between stress, well-being, and psychological flexibility during an Acceptance and Commitment Therapy self-help intervention. Int. J. Clin. Health Psychol. 2018, 18, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Kraines, M.A.; Peterson, S.K.; Tremont, G.N.; Beard, C.; Brewer, J.A.; Uebelacker, L.A. Mindfulness-Based Stress Reduction and Mindfulness-Based Cognitive Therapy for Depression: A Systematic Review of Cognitive Outcomes. Mindfulness 2022, 13, 1126–1135. [Google Scholar] [CrossRef]
- Rogers, J.M.; Ferrari, M.; Mosely, K.; Lang, C.P.; Brennan, L. Mindfulness-based interventions for adults who are overweight or obese: A meta-analysis of physical and psychological health outcomes: Mindfulness for adults who are overweight or obese. Obes. Rev. 2017, 18, 51–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Black, D.S.; Christodoulou, G.; Cole, S. Mindfulness meditation and gene expression: A hypothesis-generating framework. Curr. Opin. Psychol. 2019, 28, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Chaix, R.; Fagny, M.; Cosin-Tomás, M.; Alvarez-López, M.; Lemee, L.; Regnault, B.; Davidson, R.J.; Lutz, A.; Kaliman, P. Differential DNA methylation in experienced meditators after an intensive day of mindfulness-based practice: Implications for immune-related pathways. Brain Behav. Immun. 2020, 84, 36–44. [Google Scholar] [CrossRef] [Green Version]
- Kaliman, P.; Álvarez-López, M.J.; Cosín-Tomás, M.; Rosenkranz, M.A.; Lutz, A.; Davidson, R.J. Rapid changes in histone deacetylases and inflammatory gene expression in expert meditators. Psychoneuroendocrinology 2014, 40, 96–107. [Google Scholar] [CrossRef] [Green Version]
- Cowan, S.; Lim, S.; Alycia, C.; Pirotta, S.; Thomson, R.; Gibson-Helm, M.; Blackmore, R.; Naderpoor, N.; Bennett, C.; Ee, C.; et al. Lifestyle management in polycystic ovary syndrome—Beyond diet and physical activity. BMC Endocr. Disord. 2023, 23, 14. [Google Scholar] [CrossRef]
- Moradi, F.; Ghadiri-Anari, A.; Dehghani, A.; Reza Vaziri, S.; Enjezab, B. The effectiveness of counseling based on acceptance and commitment therapy on body image and self-esteem in polycystic ovary syndrome: An RCT. Int. J. Reprod. Biomed. 2020, 18, 243–252. [Google Scholar] [CrossRef]
- Phimphasone-Brady, P.; Palmer, B.; Vela, A.; Johnson, R.L.; Harnke, B.; Hoffecker, L.; Coons, H.L.; Epperson, C.N. Psychosocial interventions for women with polycystic ovary syndrome: A systematic review of randomized controlled trials. FS Rev. 2022, 3, 42–56. [Google Scholar] [CrossRef]
- Young, C.C.; Monge, M.; Minami, H.; Rew, L.; Conroy, H.; Peretz, C.; Tan, L. Outcomes of a Mindfulness-Based Healthy Lifestyle Intervention for Adolescents and Young Adults with Polycystic Ovary Syndrome. J. Pediatr. Adolesc. Gynecol. 2022, 35, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C.; et al. Diagnosis and Management of the Metabolic Syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beck, A.T.; Steer, R.A.; Brown, G. Beck Depression Inventory–II. Psychol. Assess. 2011. [CrossRef]
- Beck, A.T.; Epstein, N.; Brown, G.; Steer, R.A. An inventory for measuring clinical anxiety: Psychometric properties. J. Consult. Clin. Psychol. 1988, 56, 893–897. [Google Scholar] [CrossRef]
- Dernovšek, M.Z.; Gorenc, M.; Jeriček Klanšček, H. Ko te Strese Stres: Kako Prepoznati in Zdraviti Stresne, Anksiozne in Depresivne Motnje; Ponatis.; Inštitut za Varovanje Zdravja Republike Slovenije: Ljubljana, Slovenia, 2012; ISBN 978-961-6202-85-5. [Google Scholar]
- Hays, R.D.; Morales, L.S. The RAND-36 measure of health-related quality of life. Ann. Med. 2001, 33, 350–357. [Google Scholar] [CrossRef]
- Logar Zakrajšek, B.; Bren, A.; Sočan, G.; Pajek, J. Pilotna raziskava psihometričnih lastnosti vprašalnikov SF-36v2 in ESRD-SCL-TM za merjenje z zdravjem povezane kakovosti življenja bolnikov po presaditvi ledvice. Psihol. Obz. Horiz. Psychol. 2018, 12, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Baer, R.A.; Smith, G.T.; Hopkins, J.; Krietemeyer, J.; Toney, L. Using self-report assessment methods to explore facets of mindfulness. Assessment 2006, 13, 27–45. [Google Scholar] [CrossRef] [Green Version]
- Kent, W.J.; Sugnet, C.W.; Furey, T.S.; Roskin, K.M.; Pringle, T.H.; Zahler, A.M.; Haussler, A.D. The Human Genome Browser at UCSC. Genome Res. 2002, 12, 996–1006. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Meng, L.; Pei, F.; Zheng, Y.; Leng, J. A review of DNA methylation in depression. J. Clin. Neurosci. 2017, 43, 39–46. [Google Scholar] [CrossRef]
- Shen, H.; Qiu, L.; Zhang, Z.; Qin, Y.; Cao, C.; Di, W. Genome-Wide Methylated DNA Immunoprecipitation Analysis of Patients with Polycystic Ovary Syndrome. PLoS ONE 2013, 8, e64801. [Google Scholar] [CrossRef]
- Na, K.-S.; Won, E.; Kang, J.; Kim, A.; Choi, S.; Tae, W.-S.; Kim, Y.-K.; Lee, M.-S.; Joe, S.-H.; Ham, B.-J. Differential effect of COMT gene methylation on the prefrontal connectivity in subjects with depression versus healthy subjects. Neuropharmacology 2018, 137, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Sang, Q.; Li, X.; Wang, H.; Wang, H.; Zhang, S.; Feng, R.; Xu, Y.; Li, Q.; Zhao, X.; Xing, Q.; et al. Quantitative Methylation Level of the EPHX1 Promoter in Peripheral Blood DNA Is Associated with Polycystic Ovary Syndrome. PLoS ONE 2014, 9, e88013. [Google Scholar] [CrossRef] [PubMed]
- Bishop, J.R.; Lee, A.M.; Mills, L.J.; Thuras, P.D.; Eum, S.; Clancy, D.; Erbes, C.R.; Polusny, M.A.; Lamberty, G.J.; Lim, K.O. Methylation of FKBP5 and SLC6A4 in Relation to Treatment Response to Mindfulness Based Stress Reduction for Posttraumatic Stress Disorder. Front. Psychiatry 2018, 9, 418. [Google Scholar] [CrossRef] [PubMed]
- Sang, Q.; Zhang, S.; Zou, S.; Wang, H.; Feng, R.; Li, Q.; Jin, L.; He, L.; Xing, Q.; Wang, L. Quantitative analysis of follistatin (FST) promoter methylation in peripheral blood of patients with polycystic ovary syndrome. Reprod. BioMedicine Online 2013, 26, 157–163. [Google Scholar] [CrossRef] [Green Version]
- Kouter, K.; Zupanc, T.; Videtič Paska, A. Targeted sequencing approach: Comprehensive analysis of DNA methylation and gene expression across blood and brain regions in suicide victims. World J. Biol. Psychiatry 2022, 24, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Jin, F.; Huang, C.; Du, M.; Gao, M.; Wei, X. DNA methylation of AMHRII and INSR gene is associated with the pathogenesis of Polycystic Ovary Syndrome (PCOS). Technol. Health Care 2021, 29, 11–25. [Google Scholar] [CrossRef]
- Selig, J.; Troppmann, B.; Gromoll, J. Impact of DNA methylation on the regulation of the luteinizing hormone/choriogonadotropin receptor expression. Exp. Clin. Endocrinol. Diabetes 2013, 121, OP5_28. [Google Scholar] [CrossRef]
- Yehuda, R.; Daskalakis, N.P.; Desarnaud, F.; Makotkine, I.; Lehrner, A.L.; Koch, E.; Flory, J.D.; Buxbaum, J.D.; Meaney, M.J.; Bierer, L.M. Epigenetic Biomarkers as Predictors and Correlates of Symptom Improvement Following Psychotherapy in Combat Veterans with PTSD. Front. Psychiatry 2013, 4, 118. [Google Scholar] [CrossRef] [Green Version]
- Qu, F.; Wang, F.-F.; Yin, R.; Ding, G.-L.; El-prince, M.; Gao, Q.; Shi, B.-W.; Pan, H.-H.; Huang, Y.-T.; Jin, M.; et al. A molecular mechanism underlying ovarian dysfunction of polycystic ovary syndrome: Hyperandrogenism induces epigenetic alterations in the granulosa cells. J. Mol. Med. 2012, 90, 911–923. [Google Scholar] [CrossRef]
- ThermoFisher Methyl Primer Express v1.0. Available online: https://resource.thermofisher.com/page/WE28396_2/ (accessed on 24 November 2021).
- Institute of Enzymology BiSearch v2.63. Available online: http://bisearch.enzim.hu/ (accessed on 24 November 2021).
- Integrated DNA Technologies IDT Oligo Analyzer. Available online: https://eu.idtdna.com/pages/tools/oligoanalyzer (accessed on 24 November 2021).
- Illumina PCR, Amplicon PCR and Index PCR: 16S Metagenomic Sequencing Library Preparation. Available online: https://www.illumina.com/content/dam/illumina-support/documents/documentation/chemistry_documentation/16s/16s-metagenomic-library-prep-guide-15044223-b.pdf (accessed on 21 February 2023).
- Andrews, S. Fastqc: A Quality Control Tool for High Throughput Sequence Data. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 20 March 2022).
- Krueger, F. Trimgalore. Available online: https://github.com/FelixKrueger/TrimGalore (accessed on 20 March 2022).
- Krueger, F.; Andrews, S.R. Bismark: A flexible aligner and methylation caller for Bisulfite-Seq applications. Bioinformatics 2011, 27, 1571–1572. [Google Scholar] [CrossRef] [Green Version]
- R Core Team R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. Available online: https://www.R-project.org/ (accessed on 21 February 2023).
- Altuna Akalin MethylKit. Available online: https://bioconductor.org/packages/methylKit (accessed on 22 February 2023).
- Park, Y.; Cavalcante, R. MethylSig. Available online: https://bioconductor.org/packages/methylSig (accessed on 22 February 2023).
- Allaire, J. RStudio: Integrated Development Environment for R; RStudio: Boston, MA, USA, 2012; Volume 770, pp. 165–171. [Google Scholar]
- Mair, P.; Wilcox, R. Robust statistical methods in R using the WRS2 package. Behav. Res. Methods 2020, 52, 464–488. [Google Scholar] [CrossRef] [PubMed]
- Grahn Kronhed, A.-C.; Enthoven, P.; Spångeus, A.; Willerton, C. Mindfulness and Modified Medical Yoga as Intervention in Older Women with Osteoporotic Vertebral Fracture. J. Altern. Complement. Med. 2020, 26, 610–619. [Google Scholar] [CrossRef]
- Morledge, T.J.; Allexandre, D.; Fox, E.; Fu, A.Z.; Higashi, M.K.; Kruzikas, D.T.; Pham, S.V.; Reese, P.R. Feasibility of an Online Mindfulness Program for Stress Management—A Randomized, Controlled Trial. Ann. Behav. Med. 2013, 46, 137–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Berkel, J.; Boot, C.R.L.; Proper, K.I.; Bongers, P.M.; Van Der Beek, A.J. Effectiveness of a Worksite Mindfulness-Related Multi-Component Health Promotion Intervention on Work Engagement and Mental Health: Results of a Randomized Controlled Trial. PLoS ONE 2014, 9, e84118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooperman, N.A.; Hanley, A.W.; Kline, A.; Garland, E.L. A pilot randomized clinical trial of mindfulness-oriented recovery enhancement as an adjunct to methadone treatment for people with opioid use disorder and chronic pain: Impact on illicit drug use, health, and well-being. J. Subst. Abus. Treat. 2021, 127, 108468. [Google Scholar] [CrossRef]
- Allexandre, D.; Bernstein, A.M.; Walker, E.; Hunter, J.; Roizen, M.F.; Morledge, T.J. A Web-Based Mindfulness Stress Management Program in a Corporate Call Center: A Randomized Clinical Trial to Evaluate the Added Benefit of Onsite Group Support. J. Occup. Environ. Med. 2016, 58, 254–264. [Google Scholar] [CrossRef] [Green Version]
- Lucas, A.R.; Klepin, H.D.; Porges, S.W.; Rejeski, W.J. Mindfulness-Based Movement: A Polyvagal Perspective. Integr. Cancer Ther. 2018, 17, 5–15. [Google Scholar] [CrossRef] [Green Version]
- Chambers, R.; Gullone, E.; Allen, N.B. Mindful emotion regulation: An integrative review. Clin. Psychol. Rev. 2009, 29, 560–572. [Google Scholar] [CrossRef]
- Chiesa, A.; Serretti, A.; Jakobsen, J.C. Mindfulness: Top–down or bottom–up emotion regulation strategy? Clin. Psychol. Rev. 2013, 33, 82–96. [Google Scholar] [CrossRef]
- Guendelman, S.; Medeiros, S.; Rampes, H. Mindfulness and Emotion Regulation: Insights from Neurobiological, Psychological, and Clinical Studies. Front. Psychol. 2017, 8, 220. [Google Scholar] [CrossRef] [Green Version]
- Roemer, L.; Williston, S.K.; Rollins, L.G. Mindfulness and emotion regulation. Curr. Opin. Psychol. 2015, 3, 52–57. [Google Scholar] [CrossRef]
- Hill, C.L.M.; Updegraff, J.A. Mindfulness and its relationship to emotional regulation. Emotion 2012, 12, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Hempel, S.; Taylor, S.L.; Marshall, N.J.; Miake-Lye, I.M.; Beroes, J.M.; Shanman, R.; Solloway, M.R.; Shekelle, P.G. Evidence Map of Mindfulness; VA Evidence-based Synthesis Program Reports; Department of Veterans Affairs (US): Washington, DC, USA, 2014.
- Stefanaki, C.; Bacopoulou, F.; Livadas, S.; Kandaraki, A.; Karachalios, A.; Chrousos, G.P.; Diamanti-Kandarakis, E. Impact of a mindfulness stress management program on stress, anxiety, depression and quality of life in women with polycystic ovary syndrome: A randomized controlled trial. Stress 2015, 18, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Correa, J.B.; Sperry, S.L.; Darkes, J. A case report demonstrating the efficacy of a comprehensive cognitive-behavioral therapy approach for treating anxiety, depression, and problematic eating in polycystic ovarian syndrome. Arch. Women’s Ment. Health 2015, 18, 649–654. [Google Scholar] [CrossRef]
- Abdollahi, L.; Mirghafourvand, M.; Babapour Kheyradin, J.; Mohammadi, M. The Effect of Cognitive Behavioral Therapy on Depression and Obesity in Women with Polycystic Ovarian Syndrome: A Randomized Controlled Clinical Trial. Iran Red Crescent Med. J. 2018, 20, e62735. [Google Scholar] [CrossRef]
- Jiskoot, G.; Benneheij, S.H.; Beerthuizen, A.; de Niet, J.E.; de Klerk, C.; Timman, R.; Busschbach, J.J.; Laven, J.S.E. A three-component cognitive behavioural lifestyle program for preconceptional weight-loss in women with polycystic ovary syndrome (PCOS): A protocol for a randomized controlled trial. Reprod Health 2017, 14, 34. [Google Scholar] [CrossRef] [Green Version]
- Cooney, L.G.; Milman, L.W.; Hantsoo, L.; Kornfield, S.; Sammel, M.D.; Allison, K.C.; Epperson, C.N.; Dokras, A. Cognitive-behavioral therapy improves weight loss and quality of life in women with polycystic ovary syndrome: A pilot randomized clinical trial. Fertil. Steril. 2018, 110, 161–171.e1. [Google Scholar] [CrossRef]
- Patel, S. Polycystic ovary syndrome (PCOS), an inflammatory, systemic, lifestyle endocrinopathy. J. Steroid Biochem. Mol. Biol. 2018, 182, 27–36. [Google Scholar] [CrossRef]
- Wiegand, A.; Blickle, A.; Brückmann, C.; Weller, S.; Nieratschker, V.; Plewnia, C. Dynamic DNA Methylation Changes in the COMT Gene Promoter Region in Response to Mental Stress and Its Modulation by Transcranial Direct Current Stimulation. Biomolecules 2021, 11, 1726. [Google Scholar] [CrossRef]
- Peng, H.; Zhu, Y.; Strachan, E.; Fowler, E.; Bacus, T.; Roy-Byrne, P.; Goldberg, J.; Vaccarino, V.; Zhao, J. Childhood Trauma, DNA Methylation of Stress-Related Genes, and Depression: Findings From Two Monozygotic Twin Studies. Psychosom. Med. 2018, 80, 599–608. [Google Scholar] [CrossRef]
- Ziegler, C.; Domschke, K. Epigenetic signature of MAOA and MAOB genes in mental disorders. J. Neural Transm. 2018, 125, 1581–1588. [Google Scholar] [CrossRef] [PubMed]
- Norman, R.J.; Milner, C.R.; Groome, N.P.; Robertson, D.M. Circulating follistatin concentrations are higher and activin concentrations are lower in polycystic ovarian syndrome. Hum. Reprod. 2001, 16, 668–672. [Google Scholar] [CrossRef] [PubMed]
- Kouter, K.; Nedic, G.; Erjavec, T.; Milos, L.; Tudor, S.; Uzun, N.M.; Pivac, N.; Videtič Paska, A. Difference in methylation and expression of brain-derived neurotrophic factor in Alzheimer’s disease and mild cognitive impairment. Biomedicines 2023, 11, 235. [Google Scholar] [CrossRef] [PubMed]



| Participant Characteristics | Experimental Group | Control Group | p-Value |
|---|---|---|---|
| Age (mean (SD)) | 42.5 y (6.4) | 37.7 y (7.4) | 0.028 |
| Education (mean (SD)) | 14.1 y (1.8) | 14.1 y (1.6) | 0.687 |
| Currently unemployed (n) | 3 | 1 | |
| Metformin treatment (n) | 1 | 1 | |
| Infertility (n) | 6 | 4 | |
| Metabolic syndrome (n) | 16 | 12 | |
| Body weight (mean (SD)) | 103.8 kg (22.9) | 93.0 kg (15.4) | 0.084 |
| Body height (mean (SD)) | 167.8 cm (4.9) | 165.0 cm (6.2) | 0.105 |
| BMI (mean (SD)) | 36.2 (7.8) | 34.3 (6.3) | 0.381 |
| Waist circumference (mean (SD)) | 115.9 cm (13.4) | 108.7 cm (12.2) | 0.073 |
| Systolic blood pressure (mean (SD)) | 126.6 mm Hg (15.4) | 123.4 mm Hg (17.4) | 0.158 |
| Diastolic blood pressure (mean (SD)) | 87.1 mm Hg (12.8) | 83.3 mm Hg (14.3) | 0.364 |
| Heart rate (mean (SD)) | 77.5 bpm (9.4) | 77.8 bpm (10.2) | 0.925 |
| Glucose—0 min (mean (SD)) | 6.2 mmol/L (2.6) | 5.6 mmol/L (1.7) | 0.279 |
| Glucose—120 min (mean (SD)) | 7.8 mmol/L (2.5) | 7.4 mmol/L (3.5) | 0.389 |
| Cholesterol (mean (SD)) | 5.1 mmol/L (0.9) | 5.1 mmol/L (1.1) | 0.904 |
| HDL (mean (SD)) | 1.3 mmol/L (0.3) | 1.2 mmol/L (0.2) | 0.989 |
| LDL (mean (SD)) | 3.1 mmol/L (0.9) | 3.2 mmol/L (0.9) | 0.638 |
| Triglycerides (mean (SD)) | 1.8 mmol/L (0.8) | 1.3 mmol/L (0.6) | 0.018 |
| BAI score (mean (SD)) | 15.3 (12.5) | 12.0 (8.8) | 0.579 |
| BDI-II score (mean (SD)) | 10.4 (9.2) | 8.9 (9.2) | 0.3650 |
| Perceived Stress score (mean (SD)) | 7.7 (3.1) | 7.4 (2.8) | 0.756 |
| RAND-36 (mean (SD)) | |||
| Physical functioning | 74.8 (23.7) | 83.1 (17.5) | 0.238 |
| Physical role limitations | 70.2 (35.0) | 76.2 (33.0) | 0.598 |
| Emotional role limitations | 57.1 (38.2) | 77.8 (32.2) | 0.067 |
| Energy/Fatigue | 50.2 (15.8) | 53.3 (14.9) | 0.519 |
| Emotional well-being | 62.9 (12.0) | 61.7 (12.3) | 0.762 |
| Social functioning | 76.2 (23.7) | 79.8 (19.1) | 0.786 |
| Pain | 65.0 (27.6) | 73.6 (22.6) | 0.416 |
| General health | 53.1 (23.2) | 60.7 (17.8) | 0.239 |
| FFMQ (mean (SD)) | |||
| Observing | 3.1 (0.8) | 2.8 (0.7) | 0.244 |
| Describing (R) | 3.0 (1.0) | 3.2 (0.9) | 0.510 |
| Acting (R) | 2.7 (0.9) | 2.7 (0.8) | 0.886 |
| Non-judging (R) | 2.4 (0.8) | 2.4 (0.7) | 0.961 |
| Non-reactivity | 2.8 (0.7) | 2.7 (0.6) | 0.658 |
| Participant Characteristics | p-Value (ANOVA) |
|---|---|
| BMI | 0.011 |
| Waist circumference | 0.024 |
| Mean arterial blood pressure | 0.581 |
| Heart rate | 0.631 |
| Glucose—0 min | 0.0460 |
| Glucose—120 min | 0.039 |
| Cholesterol | 0.811 |
| S-HDL | 0.461 |
| S-LDL | 0.693 |
| Triglycerides | 0.5387 |
| Candidate Gene Amplicon | p-Value (ANOVA) |
|---|---|
| BDNF_95_5 | 0.797 |
| BDNF_95_2 | 0.382 |
| BDNF_58_2 | 0.731 |
| BDNF_58_1 | 0.845 |
| BDNF_14_1 | 0.439 |
| BDNF_81_3 | 0.564 |
| BDNF_81_1 | 0.795 |
| CEBPB_2 | 0.696 |
| CEBPB_1 | 0.557 |
| COMT_2 | 0.532 |
| COMT_4 | 0.024 |
| COMT_1 | 0.717 |
| EPHX1_2 | 0.929 |
| EPM2A_1 | 0.63 |
| FKBP51_1 | 0.043 |
| FST_1 | 0.049 |
| FST_3 | 0.491 |
| HTR1A_3 | 0.115 |
| HTR1A_2 | 0.684 |
| IGFBP1_1 | 0.611 |
| IGFBP1_2 | 0.169 |
| INSR_1 | 0.91 |
| LHCGR_1 | 0.952 |
| MAOA_2 | 0.046 |
| MAOA_3 | 0.862 |
| PPRG1A_1 | 0.85 |
| SLC6A4_3 | 0.58 |
| SLC6A4_5 | 0.403 |
| TPH2_1 | 0.896 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dema, H.; Videtič Paska, A.; Kouter, K.; Katrašnik, M.; Jensterle, M.; Janež, A.; Oblak, A.; Škodlar, B.; Bon, J. Effects of Mindfulness-Based Therapy on Clinical Symptoms and DNA Methylation in Patients with Polycystic Ovary Syndrome and High Metabolic Risk. Curr. Issues Mol. Biol. 2023, 45, 2717-2737. https://doi.org/10.3390/cimb45040178
Dema H, Videtič Paska A, Kouter K, Katrašnik M, Jensterle M, Janež A, Oblak A, Škodlar B, Bon J. Effects of Mindfulness-Based Therapy on Clinical Symptoms and DNA Methylation in Patients with Polycystic Ovary Syndrome and High Metabolic Risk. Current Issues in Molecular Biology. 2023; 45(4):2717-2737. https://doi.org/10.3390/cimb45040178
Chicago/Turabian StyleDema, Hana, Alja Videtič Paska, Katarina Kouter, Mojca Katrašnik, Mojca Jensterle, Andrej Janež, Aleš Oblak, Borut Škodlar, and Jurij Bon. 2023. "Effects of Mindfulness-Based Therapy on Clinical Symptoms and DNA Methylation in Patients with Polycystic Ovary Syndrome and High Metabolic Risk" Current Issues in Molecular Biology 45, no. 4: 2717-2737. https://doi.org/10.3390/cimb45040178
APA StyleDema, H., Videtič Paska, A., Kouter, K., Katrašnik, M., Jensterle, M., Janež, A., Oblak, A., Škodlar, B., & Bon, J. (2023). Effects of Mindfulness-Based Therapy on Clinical Symptoms and DNA Methylation in Patients with Polycystic Ovary Syndrome and High Metabolic Risk. Current Issues in Molecular Biology, 45(4), 2717-2737. https://doi.org/10.3390/cimb45040178






