The Case of an Endometrial Cancer Patient with Breast Cancer Who Has Achieved Long-Term Survival via Letrozole Monotherapy
Abstract
:1. Introduction
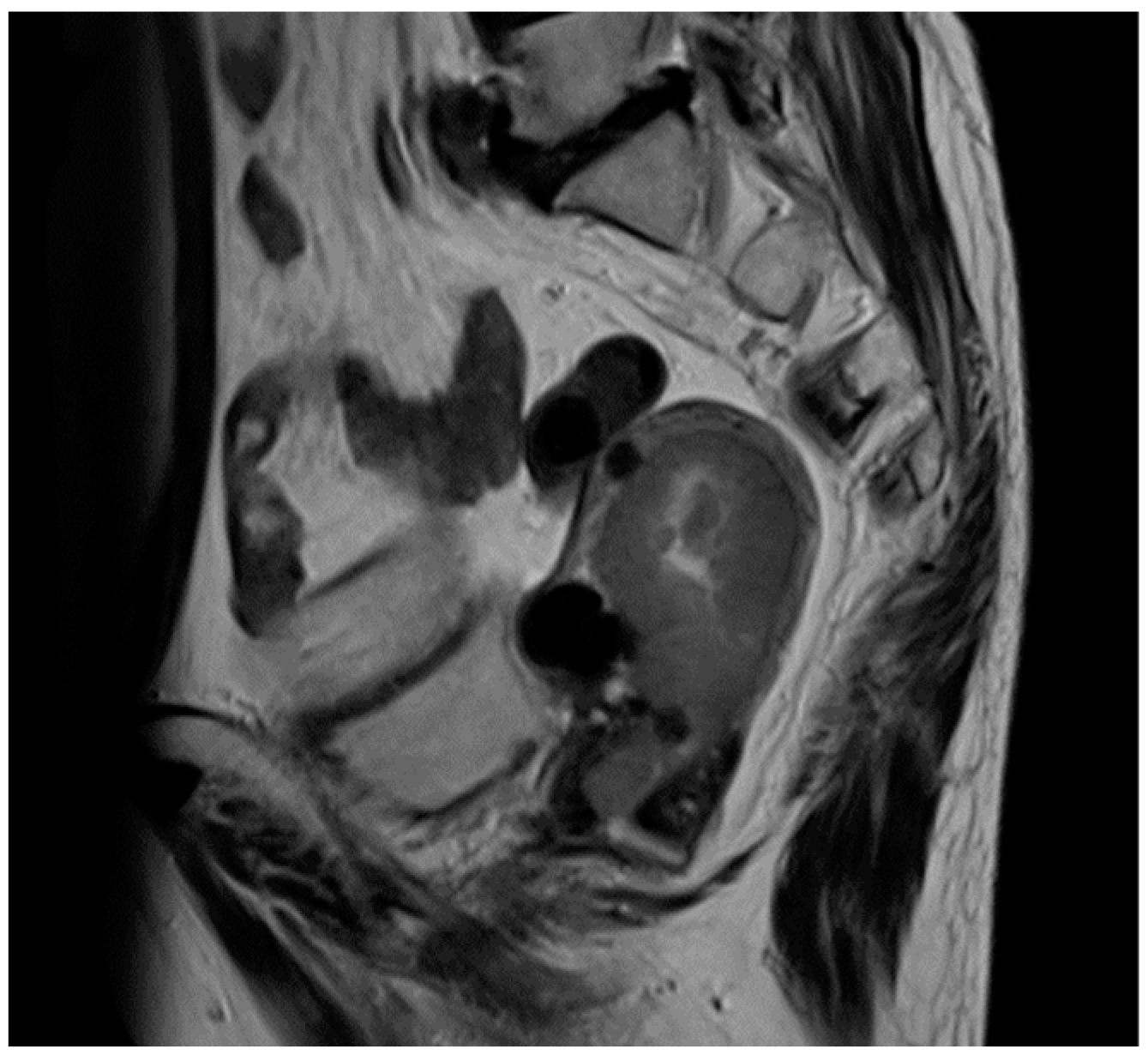
2. Case Presentation
3. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Poupon, C.; Bendifallah, S.; Ouldamer, L.; Canlorbe, G.; Raimond, E.; Hudry, N.; Coutant, C.; Graesslin, O.; Touboul, C.; Collinet, P.; et al. Management and survival of elderly and very elderly patients with of endometrial cancer: An age-stratified study of 1228 women from the FRANCOGYN Group. Ann. Surg. Oncol. 2017, 24, 1667–1676. [Google Scholar] [CrossRef]
- Rauh-Hain, J.A.; Pepin, K.J.; Meyer, L.A.; Clemmer, J.T.; Lu, K.H.; Rice, L.W.; Uppal, S.; Schorge, J.O.; Del Carmen, M.G. Management for elderly women with advanced-stage, high-grade endometrial cancer. Obstet. Gynecol. 2015, 126, 1198–1206. [Google Scholar] [CrossRef]
- Bourgin, C.; Saidani, M.; Poupon, C.; Cauchois, A.; Foucher, F.; Leveque, J.; Lavoue, V. Endometrial cancer in elderly women: Which disease, which surgical management? A systematic review of the literature. Eur. J. Surg. Oncol. 2016, 42, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Cancer Statistics 2020, GLOBOCAN estimates of Incidence and Mortality Worldwide for 36 cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Lortet-Tieulent, J.; Ferlay, J.; Bray, F.; Jemal, A. International patterns and trends in endometrial cancer incidence, 1978–2013. J. Natl. Cancer Inst. 2018, 110, 354–361. [Google Scholar] [CrossRef] [Green Version]
- Onstad, M.A.; Schmandt, R.E.; Lu, K.H. Addressing the Role of Obesity in Endometrial Cancer Risk, Prevention, and Treatment. J. Clin. Oncol. 2016, 34, 4225–4230. [Google Scholar] [CrossRef]
- Mundt, A.J.; Waggoner, S.; Yamada, D.; Rotmensch, J.; Connell, P.P. Age as a prognostic factor for recurrence in patients with endometrial carcinoma. Gynecol. Oncol. 2000, 79, 79–85. [Google Scholar] [CrossRef]
- Goodwin, J.S.; Samet, J.M.; Key, C.R.; Humble, C.; Kutvirt, D.; Hunt, C. Stage at diagnosis of cancer varies with the age of the patient. J. Am. Geriatr. Soc. 1986, 34, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Samet, J.; Hunt, W.C.; Key, C.; Humble, C.G.; Goodwin, J.S. Choice of cancer therapy varies with age of patient. JAMA 1986, 255, 3385–3390. [Google Scholar] [CrossRef]
- Xu, Y.; Burmeister, C.; Hanna, R.K.; Munkarah, A.; Elshaikh, M.A. Predictors of survival after recurrence in women with early-stage endometrial carcinoma. Int. J. Gynecol. Cancer 2016, 26, 1137–1142. [Google Scholar] [CrossRef]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Torgeson, A.; Boothe, D.; Poppe, M.M.; Suneja, G.; Gaffney, D.K. Disparities in care for elderly women with endometrial cancer adversely effects survival. Gynecol. Oncol. 2017, 147, 320–328. [Google Scholar] [CrossRef]
- Duska, L.; Shahrokni, A.; Powell, M. Treatment of Older Women with Endometrial Cancer: Improving Outcomes with Personalized Care. Am. Soc. Clin. Oncol. Educ. Book 2016, 35, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Van Der Putten, L.J.; Visser, N.; Van De Vijver, K.; Santacana, M.; Bronsert, P.; Bulten, J.; Hirschfeld, M.; Colas, E.; Gil-Moreno, A.; Garcia, A.; et al. L1CAM expression in endometrial carcinomas: An ENITEC collaboration study. Br. J. Cancer 2016, 115, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Trovik, J.; Wik, E.; Werner, H.M.; Krakstad, C.; Helland, H.; Vandenput, I.; Njolstad, T.S.; Stefansson, I.M.; Marcickiewicz, J.; Tingulstad, S.; et al. Hormone receptor loss in endometrial carcinoma curettage predicts lymph node metastasis and poor outcome in prospective multicentre trial. Eur. J. Cancer 2013, 49, 3431–3441. [Google Scholar] [CrossRef] [Green Version]
- Creutzberg, C.L.; Nout, R.A.; Lybeert, M.L.; Wárlám-Rodenhuis, C.C.; Jobsen, J.J.; Mens, J.W.; Lutgens, L.C.; Pras, E.; van de Poll-Franse, L.V.; van Putten, W.L.; et al. Fifteen-year radiotherapy outcomes of the randomized PORTEC-1 trial for endometrial carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, e631–e638. [Google Scholar] [CrossRef] [Green Version]
- Velho, S.; Oliveira, C.; Ferreira, A.; Ferreira, A.C.; Suriano, G.; Schwartz, S.; Duval, A., Jr.; Carneiro, F.; Machado, J.C.; Hamelin, R.; et al. The prevalence of PIK3CA mutations in gastric and colon cancer. Eur. J. Cancer 2005, 41, 1649–1654. [Google Scholar] [CrossRef]
- Lee, J.; van Hummelen, P.; Go, C.; Palescandolo, E.; Jang, J.; Park, H.Y.; Kang, S.Y.; Park, J.O.; Kang, W.K.; MacConaill, L.; et al. High-throughput mutation profiling identifies frequent somatic mutations in advanced gastric adenocarcinoma. PLoS ONE 2012, 7, e38892. [Google Scholar] [CrossRef]
- Spoerke, J.M.; O’Brien, C.; Huw, L.; Koeppen, H.; Fridlyand, J.; Brachmann, R.K.; Haverty, P.M.; Pandita, A.; Mohan, S.; Sampath, D.; et al. Phosphoinositide 3-kinase (PI3K) pathway alterations are associated with histologic subtypes and are predictive of sensitivity to PI3K inhibitors in lung cancer preclinical models. Clin. Cancer Res. 2012, 18, 6771–6783. [Google Scholar] [CrossRef] [Green Version]
- Gallia, G.L.; Rand, V.; Siu, I.M.; Eberhart, C.G.; James, C.D.; Marie, S.K.; Oba-Shinjo, S.M.; Carlotti, C.G.; Caballero, O.L.; Simpson, A.J.; et al. PIK3CA gene mutations in pediatric and adult glioblastoma multiforme. Mol. Cancer Res. 2006, 4, 709–714. [Google Scholar] [CrossRef] [Green Version]
- Krakstad, C.; Birkeland, E.; Seidel, D.; Kusonmano, K.; Petersen, K.; Mjøs, S.; Hoivik, E.A.; Wik, E.; Halle, M.K.; Øyan, A.M.; et al. High-throughput mutation profiling of primary and metastatic endometrial cancers identifies KRAS, FGFR2 and PIK3CA to be frequently mutated. PLoS ONE 2012, 7, e52795. [Google Scholar] [CrossRef] [Green Version]
- Kuo, K.T.; Mao, T.L.; Jones, S.; Veras, E.; Ayhan, A.; Wang, T.L.; Glas, R.; Slamon, D.; Velculescu, V.E.; Kuman, R.J.; et al. Frequent activating mutations of PIK3CA in ovarian clear cell carcinoma. Am. J. Pathol. 2009, 174, 1597–1601. [Google Scholar] [CrossRef] [Green Version]
- Slomovitz, B.M.; Coleman, R.L. The PI3K/AKT/mTOR pathway as a therapeutic target in endometrial cancer. Clin. Cancer Res. 2012, 18, 5856–5864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez, A.C.; Blanchard, Z.; Maurer, K.A.; Gertz, J. Estrogen Signaling in Endometrial Cancer: A Key Oncogenic Pathway with Several Open Questions. Horm. Cancer 2019, 10, 51–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boulay, A.; Rudloff, J.; Ye, J.; Zumstein-Mecker, S.; O’Reilly, T.; Evans, D.B.; Chen, S.; Lane, H.A. Dual inhibition of mTOR and estrogen receptor signaling in vitro induces cell death in models of breast cancer. Clin. Cancer Res. 2005, 11, 5319–5328. [Google Scholar] [CrossRef] [Green Version]
- Slomovitz, B.M.; Jiang, Y.; Yates, M.S.; Soliman, P.T.; Johnston, T.; Nowakowski, M.; Levenback, C.; Zhang, Q.; Ring, K.; Munsell, M.F.; et al. Phase II study of everolimus and letrozole in patients with recurrent endometrial carcinoma. J. Clin. Oncol. 2015, 33, 930–936. [Google Scholar] [CrossRef]
- Skriver, S.K.; Jensen, M.B.; Eriksen, J.O.; Ahlborn, L.B.; Knoop, A.S.; Rossing, M.; Ejlertsen, B.; Laenkholm, A.V. Induction of PIK3CA alterations during neoadjuvant letrozole may improve outcome in postmenopausal breast cancer patients. Breast Cancer Res. Treat. 2020, 184, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Konstantinopoulos, P.A.; Lee, E.K.; Xiong, N.; Krasner, C.; Campos, S.; Kolin, D.L.; Liu, J.F.; Horowitz, N.; Wright, A.A.; Bouberhan, S.; et al. A Phase II, Two-Stage Study of Letrozole and Abemaciclib in Estrogen Receptor–Positive Recurrent Endometrial Cancer. J. Clin. Oncol. 2023, 41, 599–608. [Google Scholar] [CrossRef]





| Function | Actionable Gene Variants | Variant Allele Frequency (%) | Copy Number | CNV |
|---|---|---|---|---|
| OG | CTNNB1 p.Ser37Phe (ClinVar: Pathogenic) | 10.2 | 2.96 | neutral |
| TSG | PIK3R1 p.Met582Ile fs*10 | 45 | 1.9 | UPD |
| TSG | TP53 c.375+5G>T (ClinVar: Likely Pathogenic) | 10.3 | 2.45 | neutral |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishikawa, M.; Nakayama, K.; Razia, S.; Yamashita, H.; Ishibashi, T.; Haraga, H.; Kanno, K.; Ishikawa, N.; Kyo, S. The Case of an Endometrial Cancer Patient with Breast Cancer Who Has Achieved Long-Term Survival via Letrozole Monotherapy. Curr. Issues Mol. Biol. 2023, 45, 2908-2916. https://doi.org/10.3390/cimb45040190
Ishikawa M, Nakayama K, Razia S, Yamashita H, Ishibashi T, Haraga H, Kanno K, Ishikawa N, Kyo S. The Case of an Endometrial Cancer Patient with Breast Cancer Who Has Achieved Long-Term Survival via Letrozole Monotherapy. Current Issues in Molecular Biology. 2023; 45(4):2908-2916. https://doi.org/10.3390/cimb45040190
Chicago/Turabian StyleIshikawa, Masako, Kentaro Nakayama, Sultana Razia, Hitomi Yamashita, Tomoka Ishibashi, Hikaru Haraga, Kosuke Kanno, Noriyoshi Ishikawa, and Satoru Kyo. 2023. "The Case of an Endometrial Cancer Patient with Breast Cancer Who Has Achieved Long-Term Survival via Letrozole Monotherapy" Current Issues in Molecular Biology 45, no. 4: 2908-2916. https://doi.org/10.3390/cimb45040190
APA StyleIshikawa, M., Nakayama, K., Razia, S., Yamashita, H., Ishibashi, T., Haraga, H., Kanno, K., Ishikawa, N., & Kyo, S. (2023). The Case of an Endometrial Cancer Patient with Breast Cancer Who Has Achieved Long-Term Survival via Letrozole Monotherapy. Current Issues in Molecular Biology, 45(4), 2908-2916. https://doi.org/10.3390/cimb45040190






