Effects of Internal Exposure of Radioactive 56MnO2 Particles on the Lung in C57BL Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Irradiation and Dosimetry
2.3. Pathology
2.4. Measurement of mRNA Levels by Quantitative Reverse Transcription Polymerase Chain Reaction (RT-PCR)
2.5. Statistical Analysis
3. Results
3.1. Estimated Doses of Internal Irradiation in the Lung
3.2. Body Weight Changes
3.3. Organ Weights
3.4. Histology of the Lung
3.5. Expression of Biodosimetry Marker Genes, Ccng1, and Bax, in the Lung on Day 3
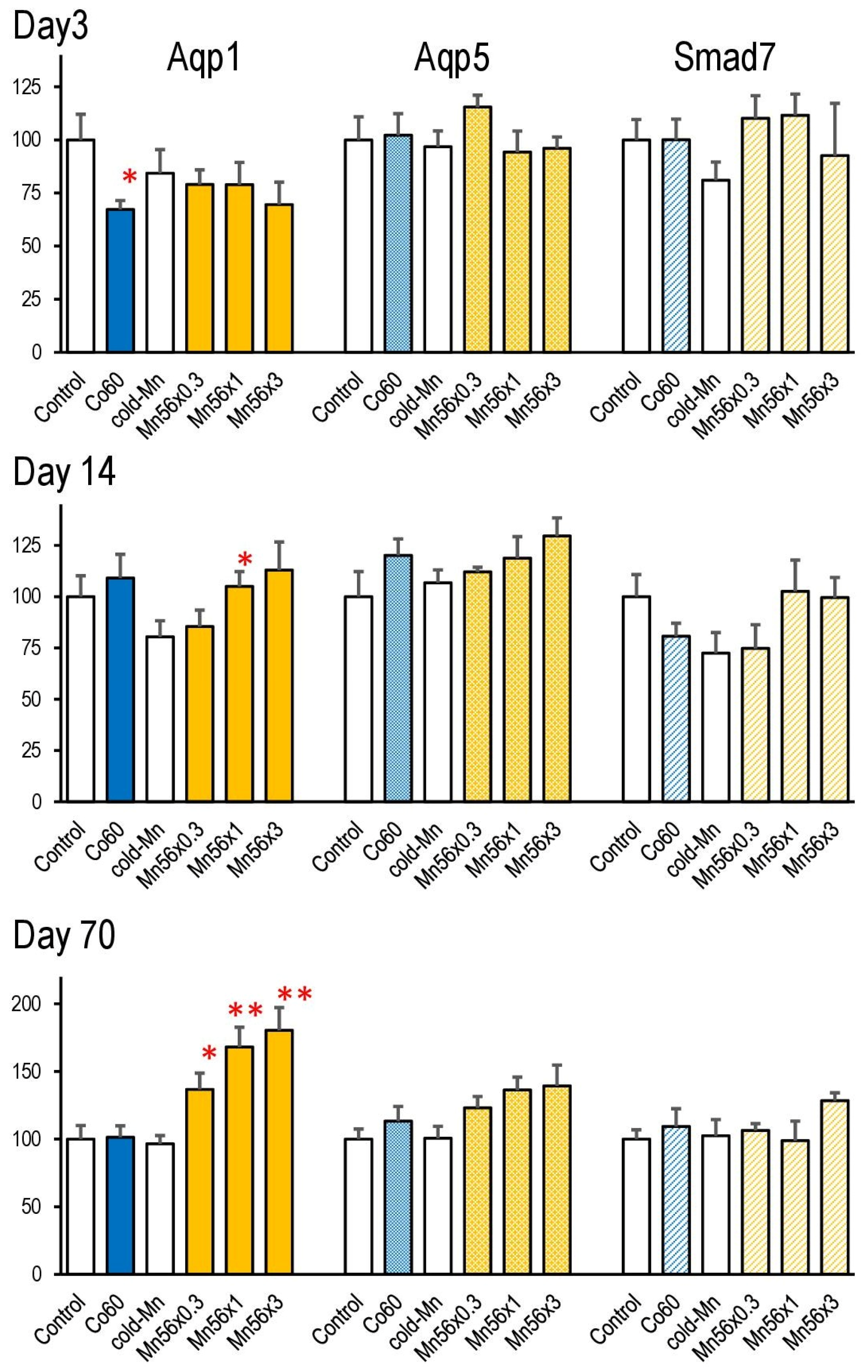
3.6. Gene Expressions of Aqp1, Aqp5, and Smad7 in the Lung
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Imanaka, T.; Endo, S.; Tanaka, K.; Shizuma, K. Gamma-ray exposure from neutron-induced radionuclides in soil in Hiroshima and Nagasaki based on DS02 calculations. Radiat. Environ. Biophys. 2008, 47, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Otani, K.; Ohtaki, M.; Yasuda, H. Solid cancer mortality risk among a cohort of Hiroshima early entrants after the atomic bombing, 1970–2010: Implications regarding health effects of residual radiation. J. Radiat. Res. 2022, 63 (Suppl. S1), i45–i53. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Endo, S.; Imanaka, T.; Shizuma, K.; Hasai, H.; Hoshi, M. Skin dose from neutron-activated soil for early entrants following the A-bomb detonation in Hiroshima: Contribution from β and γ rays. Radiat. Environ. Biophys. 2008, 47, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, N.; Baurzhan, A.; Chaizhunusova, N.; Amantayeva, G.; Kairkhanova, Y.; Shabdarbaeva, D.; Zhunussov, Y.; Zhumadilov, K.; Stepanenko, V.; Gnyrya, V.; et al. Effects of Internal Exposure to 56MnO2 Powder on Blood Parameters in Rats. Eurasian J. Med. 2020, 52, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Stepanenko, V.; Kaprin, A.; Ivanov, S.; Shegay, P.; Zhumadilov, K.; Petukhov, A.; Kolyzhenkov, T.; Bogacheva, V.; Zharova, E.; Iaskova, E.; et al. Internal doses in experimental mice and rats following exposure to neutron-activated 56MnO2 powder: Results of an international, multicenter study. Radiat. Environ. Biophys. 2020, 59, 683–692. [Google Scholar] [CrossRef]
- Fujimoto, N.; Amantayeva, G.; Chaizhunussova, N.; Shabdarbayeva, D.; Abishev, Z.; Ruslanova, B.; Zhunussov, Y.; Azhimkhanov, A.; Zhumadilov, K.; Petukhov, A.; et al. Low-Dose Radiation Exposure with 56MnO2 Powder Changes Gene Expressions in the Testes and the Prostate in Rats. Int. J. Mol. Sci. 2020, 21, 4989. [Google Scholar] [CrossRef]
- Fujimoto, N.; Ruslanova, B.; Abishev, Z.; Chaizhunussova, N.; Shabdarbayeva, D.; Amantayeva, G.; Farida, R.; Sandybayev, M.; Nagano, K.; Zhumadilov, K.; et al. Biological impacts on the lungs in rats internally exposed to radioactive 56MnO2 particle. Sci. Rep. 2021, 11, 11055. [Google Scholar] [CrossRef]
- Coggle, J.E.; Lambert, B.E.; Moores, S.R. Radiation effects in the lung. Environ. Health Perspect. 1986, 70, 261–291. [Google Scholar] [CrossRef]
- Down, J.D. The nature and relevance of late lung pathology following localised irradiation of the thorax in mice and rats. Br. J. Cancer Suppl. 1986, 7, 330–332. [Google Scholar]
- Flanders, K.C. Smad3 as a mediator of the fibrotic response. Int. J. Exp. Pathol. 2004, 85, 47–64. [Google Scholar] [CrossRef]
- Travis, E.L.; Harley, R.A.; Fenn, J.O.; Klobukowski, C.J.; Hargrove, H.B. Pathologic changes in the lung following single and multi-fraction irradiation. Int. J. Radiat. Oncol. Biol. Phys. 1977, 2, 475–490. [Google Scholar] [CrossRef]
- Paun, A.; Lemay, A.-M.; Haston, C.K. Gene expression profiling distinguishes radiation-induced fibrosing alveolitis from alveolitis in mice. Radiat. Res. 2010, 173, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Zhou, J.; Zhang, S.; Chen, Q.; Lai, R.; Ding, W.; Song, C.; Meng, X.; Wu, J. Integrating microRNA and mRNA expression profiles in response to radiation-induced injury in rat lung. Radiat. Oncol. 2014, 9, 111. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A.S. Role of aquaporins in lung liquid physiology. Respir. Physiol. Neurobiol. 2007, 159, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Yadav, E.; Yadav, N.; Hus, A.; Yadav, J.S. Aquaporins in lung health and disease: Emerging roles, regulation, and clinical implications. Respir. Med. 2020, 174, 106193. [Google Scholar] [CrossRef]
- Su, X.; Song, Y.; Jiang, J.; Bai, C. The role of aquaporin-1 (AQP1) expression in a murine model of lipopolysaccharide-induced acute lung injury. Respir. Physiol. Neurobiol. 2004, 142, 1–11. [Google Scholar] [CrossRef]
- Sun, C.Y.; Zhao, Y.X.; Zhong, W.; Liu, D.W.; Chen, Y.Z.; Qin, L.L.; Bai, L.; Liu, D. The expression of aquaporins 1 and 5 in rat lung after thoracic irradiation. J. Radiat. Res. 2014, 55, 683–689. [Google Scholar] [CrossRef]
- Kabacik, S.; MacKay, A.; Tamber, N.; Manning, G.; Finnon, P.; Paillier, F.; Ashworth, A.; Bouffler, S.; Badie, C. Gene expression following ionising radiation: Identification of biomarkers for dose estimation and prediction of individual response. Int. J. Radiat. Biol. 2011, 87, 115–129. [Google Scholar] [CrossRef]
- Li, M.J.; Wang, W.W.; Chen, S.W.; Shen, Q.; Min, R. Radiation dose effect of DNA repair-related gene expression in mouse white blood cells. Med. Sci. Monit. 2011, 17, 290–297. [Google Scholar] [CrossRef]
- Kerr, G.D.; Egbert, S.D.; Al-Nabulsi, I.; Bailiff, I.K.; Beck, H.L.; Belukha, I.G.; Cockayne, J.E.; Cullings, H.M.; Eckerman, K.F.; Granovskaya, E.; et al. Workshop Report on Atomic Bomb Dosimetry—Review of Dose Related Factors for the Evaluation of Exposures to Residual Radiation at Hiroshima and Nagasaki. Health Phys. 2015, 109, 582–600. [Google Scholar] [CrossRef]
- Stepanenko, V.; Rakhypbekov, T.; Otani, K.; Endo, S.; Satoh, K.; Kawano, N.; Shichijo, K.; Nakashima, M.; Takatsuji, T.; Sakaguchi, A.; et al. Internal exposure to neutron-activated 56Mn dioxide powder in Wistar rats: Part 1: Dosimetry. Radiat. Environ. Biophys. 2017, 56, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Kallman, R.F.; Kohn, H.I. The reaction of the mouse thymus to x-rays measured by changes in organ weight. Radiat. Res. 1955, 2, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, H.; Tanaka, I.; Yakumaru, H.; Tanaka, M.; Yokochi, K.; Fukutsu, K.; Tajima, K.; Nishimura, M.; Shimada, Y.; Akashi, M. Quantification of damage due to low-dose radiation exposure in mice: Construction and application of a biodosimetric model using mRNA indicators in circulating white blood cells. J. Radiat. Res. 2016, 57, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Ghandhi, S.A.; Weber, W.; Doyle-Eisele, M.; Melo, D.; Guilmette, R.; Amundson, S.A. Gene expression response of mice after a single dose of (137)cs as an internal emitter. Radiat. Res. 2014, 182, 380–389. [Google Scholar] [CrossRef]
- Paul, S.; Smilenov, L.B.; Elliston, C.D.; Amundson, S.A. Radiation Dose-Rate Effects on Gene Expression in a Mouse Biodosimetry Model. Radiat. Res. 2015, 184, 24–32. [Google Scholar] [CrossRef]
- Zhang, Z.; Cai, Z.; Li, K.; Fang, Y.; An, L.; Hu, Z.; Wang, S.; Hang, H. The Effect of Ionizing Radiation on mRNA Levels of the DNA Damage Response Genes Rad9, Rad1 and Hus1 in Various Mouse Tissues. Radiat. Res. 2015, 183, 94–104. [Google Scholar] [CrossRef]
- Jangiam, W.; Udomtanakunchai, C.; Reungpatthanaphong, P.; Tungjai, M.; Honikel, L.; Gordon, C.R.; Rithidech, K.N. Late Effects of Low-Dose Radiation on the Bone Marrow, Lung, and Testis Collected From the Same Exposed BALB/cJ Mice. Dose-Response 2018, 16, 1–13. [Google Scholar] [CrossRef]
- Shin, E.; Lee, S.; Kang, H.; Kim, J.; Kim, K.; Youn, H.S.; Jin, Y.W.; Seo, S.; Youn, B.H. Organ-Specific Effects of Low Dose Radiation Exposure: A Comprehensive Review. Front. Genet. 2020, 11, 566244. [Google Scholar] [CrossRef]
- Ding, N.-H.; Li, J.J.; Sun, L.-Q. Molecular mechanisms and treatment of radiation-induced lung fibrosis. Curr. Drug Targets 2013, 14, 1347–1356. [Google Scholar] [CrossRef]
- Rübe, C.E.; Uthe, D.; Schmid, K.W.; Richter, K.D.; Wessel, J.; Schuck, A.; Willich, N.; Rübe, C. Dose-dependent induction of transforming growth factor β (TGF-β) in the lung tissue of fibrosis-prone mice after thoracic irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2000, 47, 1033–1042. [Google Scholar] [CrossRef]
- Straub, J.M.; New, J.; Hamilton, C.D.; Lominska, C.; Shnayder, Y.; Thomas, S.M. Radiation-induced fibrosis: Mechanisms and implications for therapy. J. Cancer Res. Clin. Oncol. 2015, 141, 1985–1994. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.H.; Chen, D.Q.; Wang, Y.N.; Feng, Y.L.; Cao, G.; Vaziri, N.D.; Zhao, Y.Y. New insights into TGF-β/Smad signaling in tissue fibrosis. Chem. Biol. Interact. 2018, 292, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, T.; Hata, H.; Ozawa, H.; Takata, K. Immunohistochemical localization of the aquaporins AQP1, AQP3, AQP4, and AQP5 in the mouse respiratory system. Acta Histochem. Cytochem. 2009, 42, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Bai, C.X.; Fukuda, N.; Song, Y.L.; Ma, T.H.; Matthay, M.A.; Verkman, A.S. Lung fluid transport in aquaporin-1 and aquaporin-4 knockout mice. J. Clin. Investig. 1999, 103, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Wang, G.; Zhang, W.; Peng, Q.; Xue, M.; Jinhong, H. Expression of pulmonary aquaporin 1 is dramatically upregulated in mice with pulmonary fibrosis induced by bleomycin. Arch. Med. Sci. 2013, 9, 916–921. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, K.; Wang, Y.; Zhang, R.; Shang, J.; Jiang, W.; Wang, A. The effects of aquaporin-1 in pulmonary edema induced by fat embolism syndrome. Int. J. Mol. Sci. 2016, 17, 1183. [Google Scholar] [CrossRef]
- Stepanenko, V.; Kaprin, A.; Ivanov, S.; Shegay, P.; Bogacheva, V.; Hoshi, M. Overview and analysis of internal radiation dose estimates in experimental animals in a framework of international studies of the sprayed neutron-induced 56Mn radioactive microparticles effects. J. Radiat. Res. 2022, 63 (Suppl. S1), i8–i15. [Google Scholar] [CrossRef]
- Stepanenko, V.; Kaprin, A.; Ivanov, S.; Shegay, P.; Bogacheva, V.; Sato, H.; Shichijo, K.; Toyoda, S.; Kawano, N.; Ohtaki, M.; et al. Microdistribution of internal radiation dose in biological tissues exposed to 56Mn dioxide microparticles. J. Radiat. Res. 2022, 63 (Suppl. S1), i21–i25. [Google Scholar] [CrossRef]
- Towne, J.E.; Harrod, K.S.; Krane, C.M.; Menon, A.G. Decreased expression of aquaporin (AQP)1 and AQP5 in mouse lung after acute viral infection. Am. J. Respir. Cell Mol. Biol. 2000, 22, 34–44. [Google Scholar] [CrossRef]
- Wang, F.; Huang, H.; Lu, F.; Chen, Y. Acute lung injury and change in expression of aquaporins 1 and 5 in a rat model of acute pancreatitis. Hepatogastroenterology 2010, 57, 1553–1562. [Google Scholar]
- Dagle, G.E.; Sanders, C.L. Radionuclide injury to the lung. Environ. Health Perspect. 1984, 55, 129–137. [Google Scholar] [CrossRef] [PubMed]
- O’Neal, S.L.; Zheng, W. Manganese Toxicity Upon Overexposure: A Decade in Review. Curr. Environ. Health Rep. 2015, 2, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Adkins, B.; Luginbuhl, G.H.; Gardner, D.E. Acute exposure of laboratory mice to manganese oxide. Am. Ind. Hyg. Assoc. J. 1980, 41, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Camner, P.; Curstedt, T.; Jarstrand, C.; Johannsson, A.; Robertson, B.; Wiernik, A. Rabbit lung after inhalation of manganese chloride: A comparison with the effects of chlorides of nickel, cadmium, cobalt, and copper. Environ. Res. 1985, 38, 301–309. [Google Scholar] [CrossRef] [PubMed]



| Gene | GenBank Accession# | Q-PCR Primer Sequences (5′ -> 3′) | |
|---|---|---|---|
| Forward | Reverse | ||
| Ccng1 | NM_009831 | CCTGCCACTGCAGGATCATA | AAGGTCAAATCTCGGCCACTT |
| Bax | NM_007527 | CGTGGACACGGACTCCCCCC | TGATCAGCTCGGGCACTTTA |
| Aqp1 | NM_007472 | ACCTGCTGGCGATTGACTACA | CATAGATGAGCACTGCCAGGG |
| Aqp5 | NM_009701 | CTCCCCAGCCTTATCCATTG | CACGATCGGTCCTACCCAGA |
| Smad7 | AF015260 | TTGCTGTGAATCTTACGGGAAG | GGTTTGAGAAAATCCATTGGGT |
| Actb | NM_007393.5 | CTGTCCCTGTATGCCTCTGGTC | TGAGGTAGTCCGTCAGGTCCC |
| Body Weights (g) | Thymus (g/kg bw) | Spleen (g/kg bw) | Lung (g/kg bw) | Heart (g/kg bw) | Liver (g/kg bw) | Kidney (g/kg bw) | Testis (g/kg bw) | |
|---|---|---|---|---|---|---|---|---|
| Day 3 | ||||||||
| Mn56 × 0.3 | 28.9 ± 1.44 | 1.5 ± 0.04 | 4 ± 0.56 | 10.4 ± 0.87 | 5.8 ± 0.19 | 52 ± 3.0 | 15 ± 0.55 | 6.6 ± 0.92 |
| Mn56 × 1 | 28.4 ± 1.18 | 1.4 ± 0.23 | 3.6 ± 0.2 | 9.2 ± 0.62 | 6.5 ± 0.33 | 52 ± 1.7 | 16 ± 0.71 | 7.1 ± 0.57 |
| Mn56 × 3 | 28.3 ± 1.33 | 1.6 ± 0.14 | 4.3 ± 0.59 | 9.7 ± 0.27 | 6 ± 0.29 | 50 ± 2.1 | 16 ± 0.60 | 6.2 ± 0.53 |
| Co60 | 27.9 ± 1.08 | 1.0 ± 0.13 * | 3.2 ± 0.6 | 10.2 ± 0.69 | 5.9 ± 0.24 | 51 ± 2.8 | 15 ± 0.45 | 7.4 ± 0.64 |
| Cold-Mn | 28.1 ± 1.3 | 1.5 ± 0.08 | 4.1 ± 0.48 | 10.6 ± 0.38 | 6.2 ± 0.22 | 53 ± 1.8 | 16 ± 0.66 | 7.3 ± 0.66 |
| Control | 27.8 ± 0.86 | 1.4 ± 0.15 | 3.8 ± 0.38 | 9.7 ± 0.4 | 6.1 ± 0.27 | 52 ± 1.7 | 15 ± 0.27 | 7.1 ± 0.69 |
| Day 14 | ||||||||
| Mn56 × 0.3 | 28.5 ± 1.04 | 1.5 ± 0.15 | 3.2 ± 0.25 | 8.9 ± 0.40 | 5.5 ± 0.14 | 51 ± 2.7 | 15 ± 0.35 | 7.5 ± 0.51 |
| Mn56 × 1 | 29.4 ± 0.75 | 1.8 ± 0.1 | 3.7 ± 0.18 | 9.2 ± 0.76 | 5.9 ± 0.18 | 52 ± 1.6 | 16 ± 0.96 | 5.6 ± 1.1 |
| Mn56 × 3 | 28.9 ± 1.02 | 2.1 ± 0.29 | 4.1 ± 0.35 | 9.7 ± 0.49 | 6.0 ± 0.45 | 47 ± 4.6 | 16 ± 0.32 | 7.4 ± 0.68 |
| Co60 | 29.4 ± 0.63 | 1.2 ± 0.22 | 2.8 ± 0.23 | 9.5 ± 0.51 | 5.6 ± 0.22 | 48 ± 1.9 | 17 ± 0.34 | 5.7 ± 0.32 |
| Cold-Mn | 28.9 ± 0.89 | 1.7 ± 0.17 | 3.9 ± 0.29 | 9 ± 0.49 | 5.5 ± 0.28 | 53 ± 2.5 | 16 ± 0.44 | 7.8 ± 0.83 |
| Control | 29 ± 0.94 | 1.6 ± 0.14 | 3.2 ± 0.10 | 9.9 ± 0.48 | 5.6 ± 0.1 | 49 ± 1.4 | 17 ± 1.02 | 6.8 ± 0.56 |
| Day 70 | ||||||||
| Mn56 × 0.3 | 32.0 ± 0.96 | 1.4 ± 0.14 | 2.9 ± 0.19 | 9.3 ± 0.30 | 5.4 ± 0.19 | 45 ± 1.0 | 14 ± 0.46 | 7.8 ± 0.74 |
| Mn56 × 1 | 32.6 ± 1.36 | 1.3 ± 0.31 | 3.3 ± 0.41 | 9.0 ± 0.65 | 5.5 ± 0.25 | 47 ± 1.3 | 15 ± 0.68 | 6.5 ± 0.39 |
| Mn56 × 3 | 30.1 ± 0.80 | 1.4 ± 0.12 | 3.4 ± 0.42 | 9.0 ± 0.59 | 5.9 ± 0.17 | 46 ± 2.6 | 15 ± 0.50 | 7.3 ± 0.90 |
| Co60 | 31.5 ± 1.16 | 1.3 ± 0.10 | 2.7 ± 0.22 | 8.0 ± 0.18 | 5.5 ± 0.18 | 49 ± 2.0 | 16 ± 0.34 | 6.5 ± 0.20 |
| Cold-Mn | 31.4 ± 1.14 | 1.4 ± 0.12 | 3.2 ± 0.37 | 8.8 ± 0.19 | 6.1 ± 0.27 | 45 ± 0.8 | 16 ± 0.15 | 7.4 ± 0.65 |
| Control | 32.5 ± 1.62 | 1.1 ± 0.11 | 2.7 ± 0.27 | 8.9 ± 0.21 | 5.4 ± 0.28 | 47 ± 0.8 | 16 ± 1.15 | 6.8 ± 0.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abishev, Z.; Ruslanova, B.; Apbassova, S.; Chaizhunussova, N.; Shabdarbayeva, D.; Azimkhanov, A.; Zhumadilov, K.; Stepanenko, V.; Ivanov, S.; Shegay, P.; et al. Effects of Internal Exposure of Radioactive 56MnO2 Particles on the Lung in C57BL Mice. Curr. Issues Mol. Biol. 2023, 45, 3208-3218. https://doi.org/10.3390/cimb45040209
Abishev Z, Ruslanova B, Apbassova S, Chaizhunussova N, Shabdarbayeva D, Azimkhanov A, Zhumadilov K, Stepanenko V, Ivanov S, Shegay P, et al. Effects of Internal Exposure of Radioactive 56MnO2 Particles on the Lung in C57BL Mice. Current Issues in Molecular Biology. 2023; 45(4):3208-3218. https://doi.org/10.3390/cimb45040209
Chicago/Turabian StyleAbishev, Zhaslan, Bakhyt Ruslanova, Saulesh Apbassova, Nailya Chaizhunussova, Dariya Shabdarbayeva, Almas Azimkhanov, Kassym Zhumadilov, Valeriy Stepanenko, Sergey Ivanov, Peter Shegay, and et al. 2023. "Effects of Internal Exposure of Radioactive 56MnO2 Particles on the Lung in C57BL Mice" Current Issues in Molecular Biology 45, no. 4: 3208-3218. https://doi.org/10.3390/cimb45040209
APA StyleAbishev, Z., Ruslanova, B., Apbassova, S., Chaizhunussova, N., Shabdarbayeva, D., Azimkhanov, A., Zhumadilov, K., Stepanenko, V., Ivanov, S., Shegay, P., Hoshi, M., & Fujimoto, N. (2023). Effects of Internal Exposure of Radioactive 56MnO2 Particles on the Lung in C57BL Mice. Current Issues in Molecular Biology, 45(4), 3208-3218. https://doi.org/10.3390/cimb45040209






