Altering Stomatal Density for Manipulating Transpiration and Photosynthetic Traits in Rice through CRISPR/Cas9 Mutagenesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Construction of CRISPR/Cas9 Expression Vectors
2.3. Agrobacterium-Mediated Transformation
2.4. Molecular Characterization of Transgenic Plants
2.5. Measuring Stomatal Density and Stomatal Size
2.6. Measurement of Gas Exchange Parameters
2.7. Statistical Analysis
3. Results
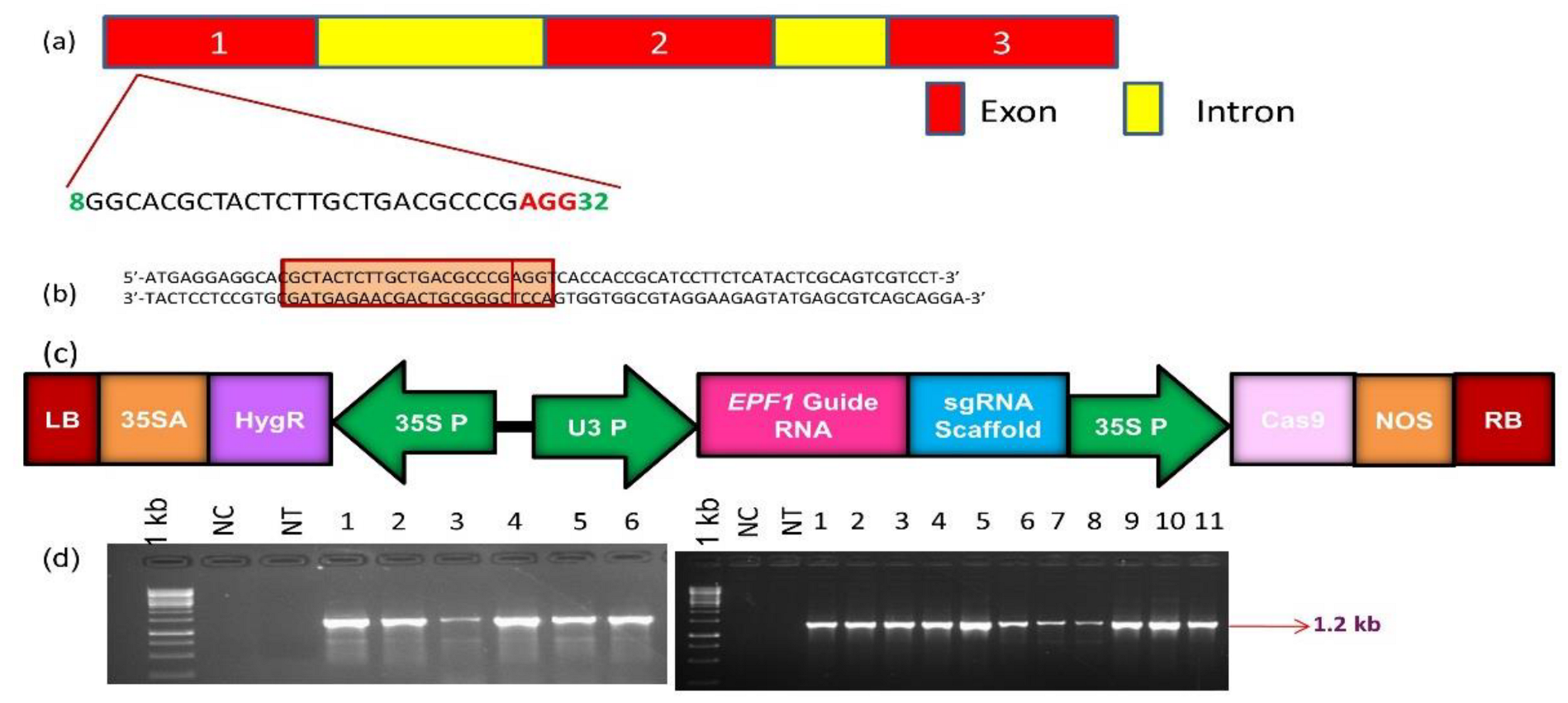
3.1. Cloning of gRNA Targeting OsEPF1 and the Generation of Gene-Edited Rice Lines
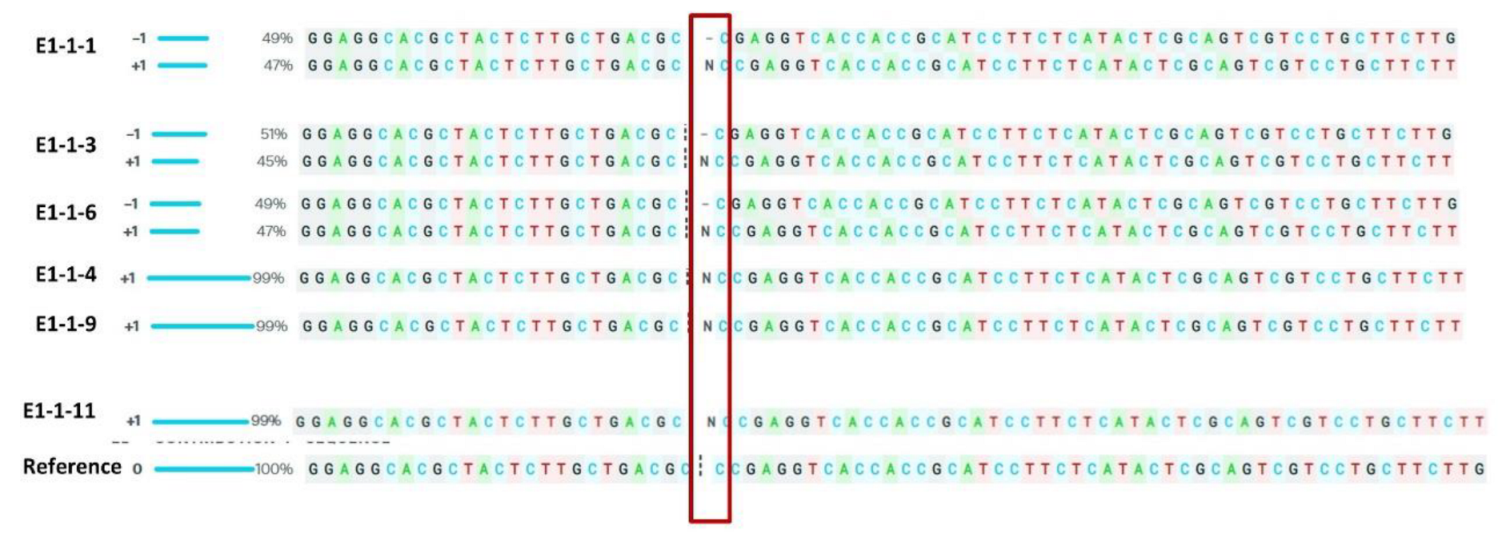
3.2. Characterization of T0 Lines
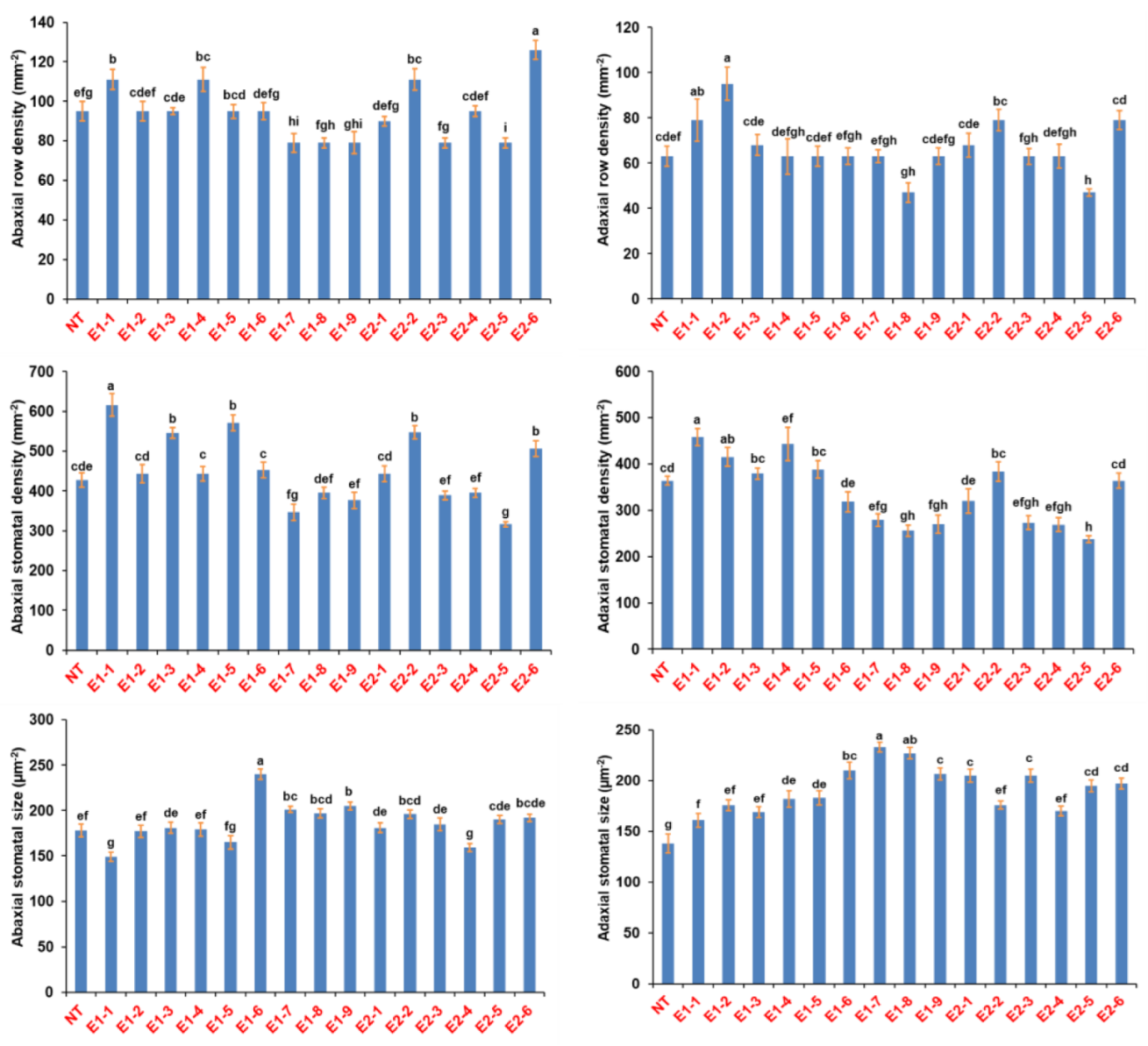
3.3. Phenotypic Evaluation of T0 Mutant Lines for Stomatal Density
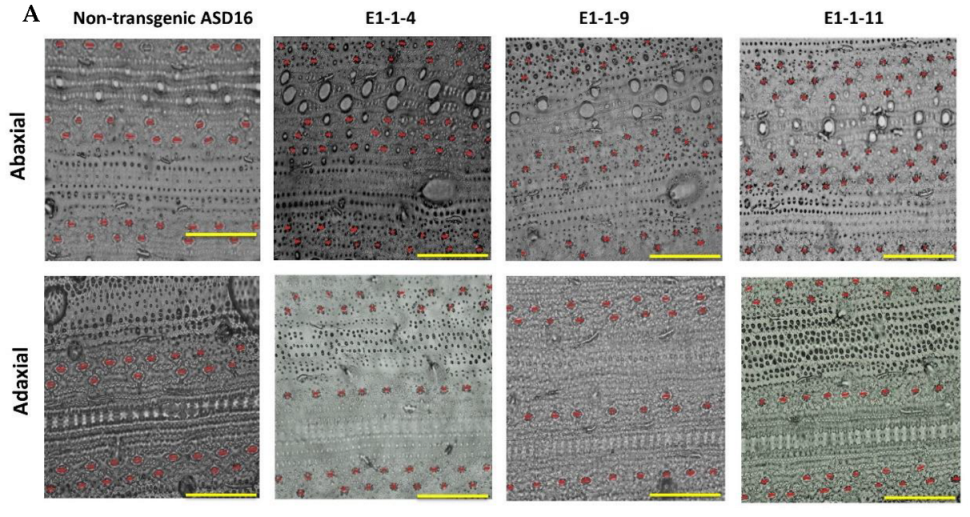
3.4. Phenotypic Evaluation of T1 Progenies
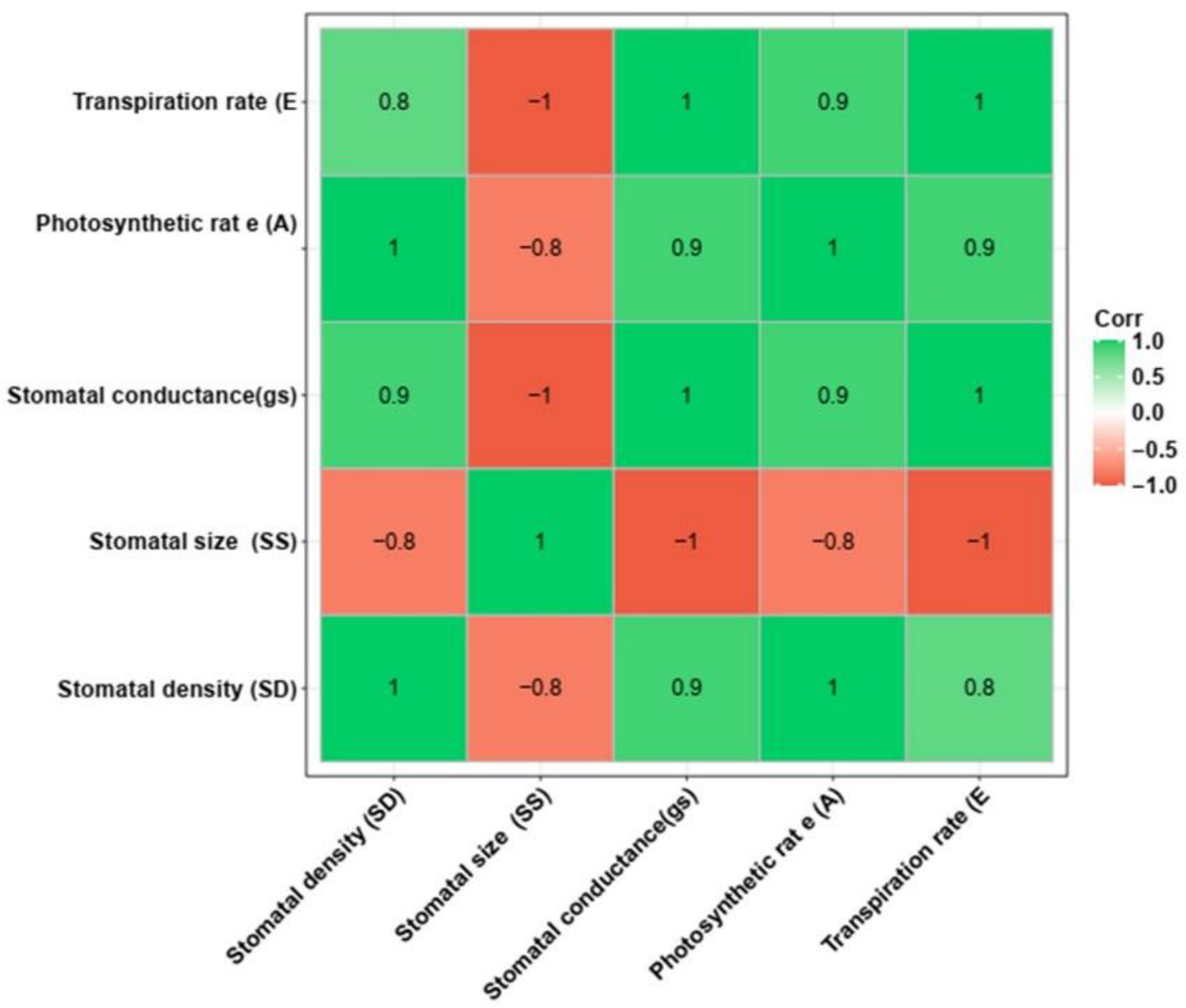
3.5. Physiological Evaluation of the T1 Mutant Lines
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gadal, N.; Shrestha, J.; Poudel, M.N.; Pokharel, B. A review on production status and growing environments of rice in Nepal and in the world. Arch. Agric. Environ. Sci. 2019, 4, 83–87. [Google Scholar] [CrossRef]
- Yadav, S.; Anand, S. Green Revolution and Food Security in India: A Review. Natl. Geogr. J. India 2022, 65, 312–323. [Google Scholar]
- Singh, R. Environmental consequences of agricultural development: A case study from the Green Revolution state of Haryana, India. Agric. Ecosyst. Environ. 2000, 82, 97–103. [Google Scholar] [CrossRef]
- Challinor, A.J.; Watson, J.; Lobell, D.B.; Howden, S.; Smith, D.; Chhetri, N. A meta-analysis of crop yield under climate change and adaptation. Nat. Clim. Chang. 2014, 4, 287–291. [Google Scholar] [CrossRef]
- Calanca, P.P. Effects of abiotic stress in crop production. In Quantification of Climate Variability, Adaptation and Mitigation for Agricultural Sustainability; Springer: Berlin/Heidelberg, Germany, 2017; pp. 165–180. [Google Scholar]
- Serraj, R.; McNally, K.L.; Slamet-Loedin, I.; Kohli, A.; Haefele, S.M.; Atlin, G.; Kumar, A. Drought resistance improvement in rice: An integrated genetic and resource management strategy. Plant Prod. Sci. 2011, 14, 1–14. [Google Scholar] [CrossRef]
- Reay, D. Climate-Smart Rice. In Climate-Smart Food; Springer: Berlin/Heidelberg, Germany, 2019; pp. 121–133. [Google Scholar]
- Singh, A.; Choudhury, B.; Bouman, B. Effects of rice establishment methods on crop performance, water use, and mineral nitrogen. In Water-Wise Rice Production; International Rice Research Institute: Los Baños, Philippines, 2002; pp. 237–246. [Google Scholar]
- Elert, E. Rice by the numbers: A good grain. Nature 2014, 514, S50. [Google Scholar] [CrossRef]
- Swain, P.; Raman, A.; Singh, S.; Kumar, A. Breeding drought tolerant rice for shallow rainfed ecosystem of eastern India. Field Crops Res. 2017, 209, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Quan, R.; Wang, J.; Hui, J.; Bai, H.; Lyu, X.; Zhu, Y.; Zhang, H.; Zhang, Z.; Li, S.; Huang, R. Improvement of salt tolerance using wild rice genes. Front. Plant Sci. 2018, 8, 2269. [Google Scholar] [CrossRef] [PubMed]
- Chinnusamy, V.; Jagendorf, A.; Zhu, J.K. Understanding and improving salt tolerance in plants. Crop Sci. 2005, 45, 437–448. [Google Scholar] [CrossRef]
- Radanielson, A.; Gaydon, D.; Li, T.; Angeles, O.; Roth, C. Modeling salinity effect on rice growth and grain yield with ORYZA v3 and APSIM-Oryza. Eur. J. Agron. 2018, 100, 44–55. [Google Scholar] [CrossRef]
- Niroula, R.K.; Pucciariello, C.; Ho, V.T.; Novi, G.; Fukao, T.; Perata, P. SUB1A-dependent and-independent mechanisms are involved in the flooding tolerance of wild rice species. Plant J. 2012, 72, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Hendrawan, V.S.A.; Komori, D. Developing flood vulnerability curve for rice crop using remote sensing and hydrodynamic modeling. Int. J. Disaster Risk Reduct. 2021, 54, 102058. [Google Scholar] [CrossRef]
- Lin, C.-J.; Li, C.-Y.; Lin, S.-K.; Yang, F.-H.; Huang, J.-J.; Liu, Y.-H.; Lur, H.-S. Influence of high temperature during grain filling on the accumulation of storage proteins and grain quality in rice (Oryza sativa L.). J. Agric. Food Chem. 2010, 58, 10545–10552. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Sugano, S.S.; Shimada, T.; Hara-Nishimura, I. Enhancement of leaf photosynthetic capacity through increased stomatal density in Arabidopsis. New Phytol. 2013, 198, 757–764. [Google Scholar] [CrossRef] [PubMed]
- SchluÈter, U.; Muschak, M.; Berger, D.; Altmann, T. Photosynthetic performance of an Arabidopsis mutant with elevated stomatal density (sdd1-1) under different light regimes. J. Exp. Bot. 2003, 54, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xue, X.; Zhu, J.-K.; Dong, J. Demethylation of ERECTA receptor genes by IBM1 histone demethylase affects stomatal development. Development 2016, 143, 4452–4461. [Google Scholar] [CrossRef]
- Hara, K.; Kajita, R.; Torii, K.U.; Bergmann, D.C.; Kakimoto, T. The secretory peptide gene EPF1 enforces the stomatal one-cell-spacing rule. Genes Dev. 2007, 21, 1720–1725. [Google Scholar] [CrossRef]
- Hunt, L.; Gray, J.E. The signaling peptide EPF2 controls asymmetric cell divisions during stomatal development. Curr. Biol. 2009, 19, 864–869. [Google Scholar] [CrossRef]
- Doheny-Adams, T.; Hunt, L.; Franks, P.J.; Beerling, D.J.; Gray, J.E. Genetic manipulation of stomatal density influences stomatal size, plant growth and tolerance to restricted water supply across a growth carbon dioxide gradient. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 547–555. [Google Scholar] [CrossRef]
- Bergmann, D.C.; Sack, F.D. Stomatal development. Annu. Rev. Plant Biol. 2007, 58, 163–181. [Google Scholar] [CrossRef]
- Caine, R.S.; Yin, X.; Sloan, J.; Harrison, E.L.; Mohammed, U.; Fulton, T.; Biswal, A.K.; Dionora, J.; Chater, C.C.; Coe, R.A. Rice with reduced stomatal density conserves water and has improved drought tolerance under future climate conditions. New Phytol. 2019, 221, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Biswal, A.K.; Dionora, J.; Perdigon, K.M.; Balahadia, C.P.; Mazumdar, S.; Chater, C.; Lin, H.-C.; Coe, R.A.; Kretzschmar, T. CRISPR-Cas9 and CRISPR-Cpf1 mediated targeting of a stomatal developmental gene EPFL9 in rice. Plant Cell Rep. 2017, 36, 745–757. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, U.; Caine, R.; Atkinson, J.; Harrison, E.; Wells, D.; Chater, C.; Gray, J.; Swarup, R.; Murchie, E. Rice plants overexpressing OsEPF1 show reduced stomatal density and increased root cortical aerenchyma formation. Sci. Rep. 2019, 9, 5584. [Google Scholar]
- Karavolias, N.; Patel, D.; Seong, K.; Tjahjadi, M.; Gueorguieva, G.-A.; Tanaka, J.; Dahlbeck, D.; Cho, M.-J.; Niyogi, K.K.; Staskawicz, B.J. CRISPR/Cas9 knockout of EPFL10 reduces stomatal density while maintaining photosynthesis and enhancing water conservation in rice. bioRxiv 2021. [Google Scholar] [CrossRef]
- Hunt, L.; Bailey, K.J.; Gray, J.E. The signalling peptide EPFL9 is a positive regulator of stomatal development. New Phytol. 2010, 186, 609–614. [Google Scholar] [CrossRef]
- Concordet, J.-P.; Haeussler, M. CRISPOR: Intuitive guide selection for CRISPR/Cas9 genome editing experiments and screens. Nucleic Acids Res. 2018, 46, W242–W245. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Yang, Y. RNA-guided genome editing in plants using a CRISPR–Cas system. Mol. Plant 2013, 6, 1975–1983. [Google Scholar] [CrossRef]
- Weigel, D.; Glazebrook, J. Vectors and Agrobacterium hosts for Arabidopsis transformation. Cold Spring Harb. Protoc. 2006, 2006, pdb-ip29. [Google Scholar] [CrossRef]
- Hiei, Y.; Komari, T. Agrobacterium-mediated transformation of rice using immature embryos or calli induced from mature seed. Nat. Protoc. 2008, 3, 824–834. [Google Scholar] [CrossRef]
- Porebski, S.; Bailey, L.G.; Baum, B.R. Modification of a CTAB DNA extraction protocol for plants containing high polysaccharide and polyphenol components. Plant Mol. Biol. Report. 1997, 15, 8–15. [Google Scholar] [CrossRef]
- Liu, W.; Xie, X.; Ma, X.; Li, J.; Chen, J.; Liu, Y.-G. DSDecode: A web-based tool for decoding of sequencing chromatograms for genotyping of targeted mutations. Mol. Plant 2015, 8, 1431–1433. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, C. Intracellular signalling: Sphingosine-1-phosphate branches out. Curr. Biol. 2001, 11, R535–R538. [Google Scholar] [CrossRef] [PubMed]
- McAusland, L.; Vialet-Chabrand, S.; Davey, P.; Baker, N.R.; Brendel, O.; Lawson, T. Effects of kinetics of light-induced stomatal responses on photosynthesis and water-use efficiency. New Phytol. 2016, 211, 1209–1220. [Google Scholar] [CrossRef] [PubMed]
- Kostaki, K.-I.; Coupel-Ledru, A.; Bonnell, V.C.; Gustavsson, M.; Sun, P.; McLaughlin, F.J.; Fraser, D.P.; McLachlan, D.H.; Hetherington, A.M.; Dodd, A.N. Guard cells integrate light and temperature signals to control stomatal aperture. Plant Physiol. 2020, 182, 1404–1419. [Google Scholar] [CrossRef] [PubMed]
- Abrash, E.; Anleu Gil, M.X.; Matos, J.L.; Bergmann, D.C. Conservation and divergence of YODA MAPKKK function in regulation of grass epidermal patterning. Development 2018, 145, dev165860. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; He, J.; Zhou, X.; Zhong, J.; Li, J.; Liang, Y.-K. Homologous genes of epidermal patterning factor regulate stomatal development in rice. J. Plant Physiol. 2019, 234, 18–27. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, L.; Yu, Q.; Zhou, W.; Gou, X.; Li, J.; Hou, S. Multiple transcriptional factors control stomata development in rice. New Phytol. 2019, 223, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Sugano, S.S.; Shimada, T.; Imai, Y.; Okawa, K.; Tamai, A.; Mori, M.; Hara-Nishimura, I. Stomagen positively regulates stomatal density in Arabidopsis. Nature 2010, 463, 241–244. [Google Scholar] [CrossRef]
- Sakoda, K.; Yamori, W.; Shimada, T.; Sugano, S.S.; Hara-Nishimura, I.; Tanaka, Y. Higher stomatal density improves photosynthetic induction and biomass production in Arabidopsis under fluctuating light. Front. Plant Sci. 2020, 11, 589603. [Google Scholar] [CrossRef]
- Schuler, M.L.; Sedelnikova, O.V.; Walker, B.J.; Westhoff, P.; Langdale, J.A. SHORTROOT-mediated increase in stomatal density has no impact on photosynthetic efficiency. Plant Physiol. 2018, 176, 757–772. [Google Scholar] [CrossRef]
- Drake, P.L.; Froend, R.H.; Franks, P.J. Smaller, faster stomata: Scaling of stomatal size, rate of response, and stomatal conductance. J. Exp. Bot. 2013, 64, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Papanatsiou, M.; Amtmann, A.; Blatt, M.R. Stomatal spacing safeguards stomatal dynamics by facilitating guard cell ion transport independent of the epidermal solute reservoir. Plant Physiol. 2016, 172, 254–263. [Google Scholar] [CrossRef]
- Franks, P.J.; W. Doheny-Adams, T.; Britton-Harper, Z.J.; Gray, J.E. Increasing water-use efficiency directly through genetic manipulation of stomatal density. New Phytol. 2015, 207, 188–195. [Google Scholar] [CrossRef] [PubMed]
- McElwain, J.C.; Yiotis, C.; Lawson, T. Using modern plant trait relationships between observed and theoretical maximum stomatal conductance and vein density to examine patterns of plant macroevolution. New Phytol. 2016, 209, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H.; Hashimoto-Sugimoto, M.; Iba, K.; Terashima, I.; Yamori, W. Improved stomatal opening enhances photosynthetic rate and biomass production in fluctuating light. J. Exp. Bot. 2020, 71, 2339–2350. [Google Scholar] [CrossRef] [PubMed]
- Lawson, T.; Vialet-Chabrand, S. Chlorophyll fluorescence imaging. In Photosynthesis; Springer: Berlin/Heidelberg, Germany, 2018; pp. 121–140. [Google Scholar]
- Pitaloka, M.K.; Caine, R.S.; Hepworth, C.; Harrison, E.L.; Sloan, J.; Chutteang, C.; Phuntong, C.; Nongngok, R.; Toojinda, T.; Ruengpayak, S. Induced Genetic Variations in Stomatal Density and Size of Rice Strongly Affect Water-Use Efficiency, Drought Tolerance, and Responses to Abiotic Stresses. Front. Plant Sci. 2021, 13, 801706. [Google Scholar] [CrossRef]
- Sreeharsha, R.V.; Sekhar, K.M.; Reddy, A.R. Delayed flowering is associated with lack of photosynthetic acclimation in Pigeon pea (Cajanus cajan L.) grown under elevated CO2. Plant Sci. 2015, 231, 82–93. [Google Scholar] [CrossRef]



| Name of the Construct/Organism Name of the Gene/ | Selection Marker | Forward and Reverse Primers | Annealing Temperature | Size of the Amplicon |
|---|---|---|---|---|
| Agrobacterium tumefaciens harboring pRGEB31-EPF1-sgRNA | CaMV35S and hpt | hpt–F: 5′ TACACAGCCATCGGTCCA 3′ | 59 °C | 1.3 kb |
| CaMV35S -R: 5′ ACCTCCTCGGATTCCATTGC 3′ | ||||
| E. coli harboring pRGEB31-EPF1-sgRNA | M13 and EPFs gRNA | M13 -R: 5′ TCACACAGGAAACAGCTATG 3′ | 55 °C | 445 bp |
| EPF sgRNA R-AAACCGGGCGTCAGCAAGAGTAGCG | ||||
| Oryza sativa (ASD 16) | CaMV35S and hpt | hpt–F: 5′ TACACAGCCATCGGTCCA 3′ 59 °C 1.3 kb | 59 °C | 1.3 kb |
| CaMV35S -R: 5′ ACCTCCTCGGATTCCATTGC 3′ | ||||
| Gene specific primer EPF1 | EPF F- CAATGGCTGCACACACATATAC | 58 °C | 400 bp | |
| EPFR-CAAGCAAGCACATTCGGTAAG |
| Line No | Mutation Type | Mutation Allele Size |
|---|---|---|
| E1-1 | Biallelic | +1, −1 |
| E1-2 | Biallelic | +1, −1 |
| E1-3 | Multiallelic | +1, −7, −7 |
| E1-4 | Biallelic | +1, −1 |
| E1-5 | Biallelic | +1, −1 |
| E1-6 | Biallelic | +1, −1 |
| E1-7 | Multiallelic | −3, 0, −2, −3 |
| E1-8 | Multiallelic | −3, 0, −2, −3 |
| E1-9 | Triallelic | +1, −1, 0 |
| E2-1 | Monoallelic | +1 |
| E2-2 | Biallelic | +1, +2 |
| E2-3 | Monoallelic | +1 |
| E2-4 | Triallelic | +1, −1, 0 |
| E2-5 | Monoallelic | +1 |
| E2-6 | Biallelic | +1, −1 |
| E2-7 | Multiallelic | +1, −1, 0, −5 |
| E2-8 | Multiallelic | +1, −1, 0, −5 |
| Event | Abx_SD_mm | (%) Increase in Abaxial_SD (mm−2) | Adx_SD_mm | (%) Increase in Adaxial_SD (mm−2) | Abaxial Stomatal Size (µm2) | Adaxial Stomatal Size (µm2) |
|---|---|---|---|---|---|---|
| NTL | 456 ± 10.7 c | - | 391 ± 9.98 b | - | 219.8 ± 5.37 a | 197.2 ± 6.6 a |
| E1-1-4 | 706 ± 16.11 b | 54 | 557 ± 19.14 a | 42.5 | 109.9 ± 2.53 b | 127.1 ± 2.8 b |
| E1-1-9 | 701 ± 19.82 b | 53.7 | 391 ± 17.02 b | 0 | 93.2 ± 2.18 c | 102.6 ± 2.78 c |
| E1-1-11 | 891 ± 21.26 a | 95 | 543 ± 14.29 a | 38.8 | 79.5 ± 2.47 c | 108.2 ± 2.98 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rathnasamy, S.A.; Kambale, R.; Elangovan, A.; Mohanavel, W.; Shanmugavel, P.; Ramasamy, G.; Alagarsamy, S.; Marimuthu, R.; Rajagopalan, V.R.; Manickam, S.; et al. Altering Stomatal Density for Manipulating Transpiration and Photosynthetic Traits in Rice through CRISPR/Cas9 Mutagenesis. Curr. Issues Mol. Biol. 2023, 45, 3801-3814. https://doi.org/10.3390/cimb45050245
Rathnasamy SA, Kambale R, Elangovan A, Mohanavel W, Shanmugavel P, Ramasamy G, Alagarsamy S, Marimuthu R, Rajagopalan VR, Manickam S, et al. Altering Stomatal Density for Manipulating Transpiration and Photosynthetic Traits in Rice through CRISPR/Cas9 Mutagenesis. Current Issues in Molecular Biology. 2023; 45(5):3801-3814. https://doi.org/10.3390/cimb45050245
Chicago/Turabian StyleRathnasamy, Sakthi Ambothi, Rohit Kambale, Allimuthu Elangovan, Williams Mohanavel, Priyanka Shanmugavel, Gowtham Ramasamy, Senthil Alagarsamy, Rajavel Marimuthu, Veera Ranjani Rajagopalan, Sudha Manickam, and et al. 2023. "Altering Stomatal Density for Manipulating Transpiration and Photosynthetic Traits in Rice through CRISPR/Cas9 Mutagenesis" Current Issues in Molecular Biology 45, no. 5: 3801-3814. https://doi.org/10.3390/cimb45050245
APA StyleRathnasamy, S. A., Kambale, R., Elangovan, A., Mohanavel, W., Shanmugavel, P., Ramasamy, G., Alagarsamy, S., Marimuthu, R., Rajagopalan, V. R., Manickam, S., Ramanathan, V., Muthurajan, R., & Vellingiri, G. (2023). Altering Stomatal Density for Manipulating Transpiration and Photosynthetic Traits in Rice through CRISPR/Cas9 Mutagenesis. Current Issues in Molecular Biology, 45(5), 3801-3814. https://doi.org/10.3390/cimb45050245



_Kim.png)




