Dermal Fibroblasts as the Main Target for Skin Anti-Age Correction Using a Combination of Regenerative Medicine Methods
Abstract
1. Introduction
2. Skin Cells and ECM Components Playing a Key Role in Skin Regeneration
2.1. Keratinocytes as the Main Cells of the Epidermis
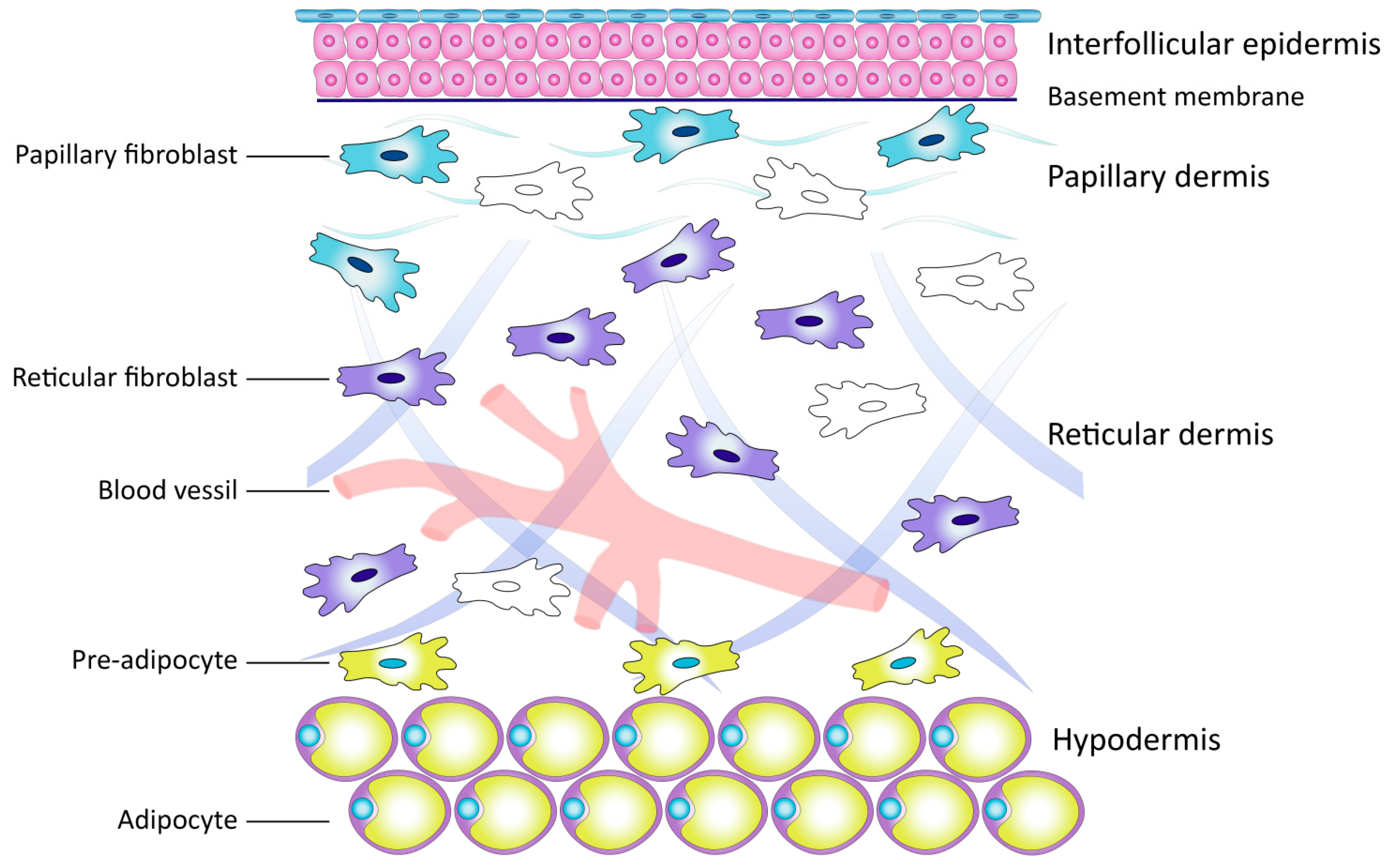
2.2. Dermal Fibroblasts as a Key Link in Skin Biology
- fibroblast progenitor cells (mesenchymal stem (stromal) and progenitor cells) possessing high proliferative potential and maintaining the number of fibroblasts in the dermis;
- mature (differentiated) and postmitotic fibroblasts make up the majority of cells in the dermis; they no longer divide in the skin in vivo but have high biosynthetic activity, producing and organizing all ECM components, which determines their main role in the fibroblastic differon;
- specialized fibroblasts (such as fibroblasts that resorb ECM, myofibroblasts that possess contractility, and fibrocytes), which are represented by the finally differentiated cells having minimal producing activity and maintaining cellular homeostasis in the skin.
Subpopulations of Dermal Fibroblasts
2.3. Extracellular Matrix of the Dermis
2.3.1. Collagen
2.3.2. Elastic Network of the Dermis
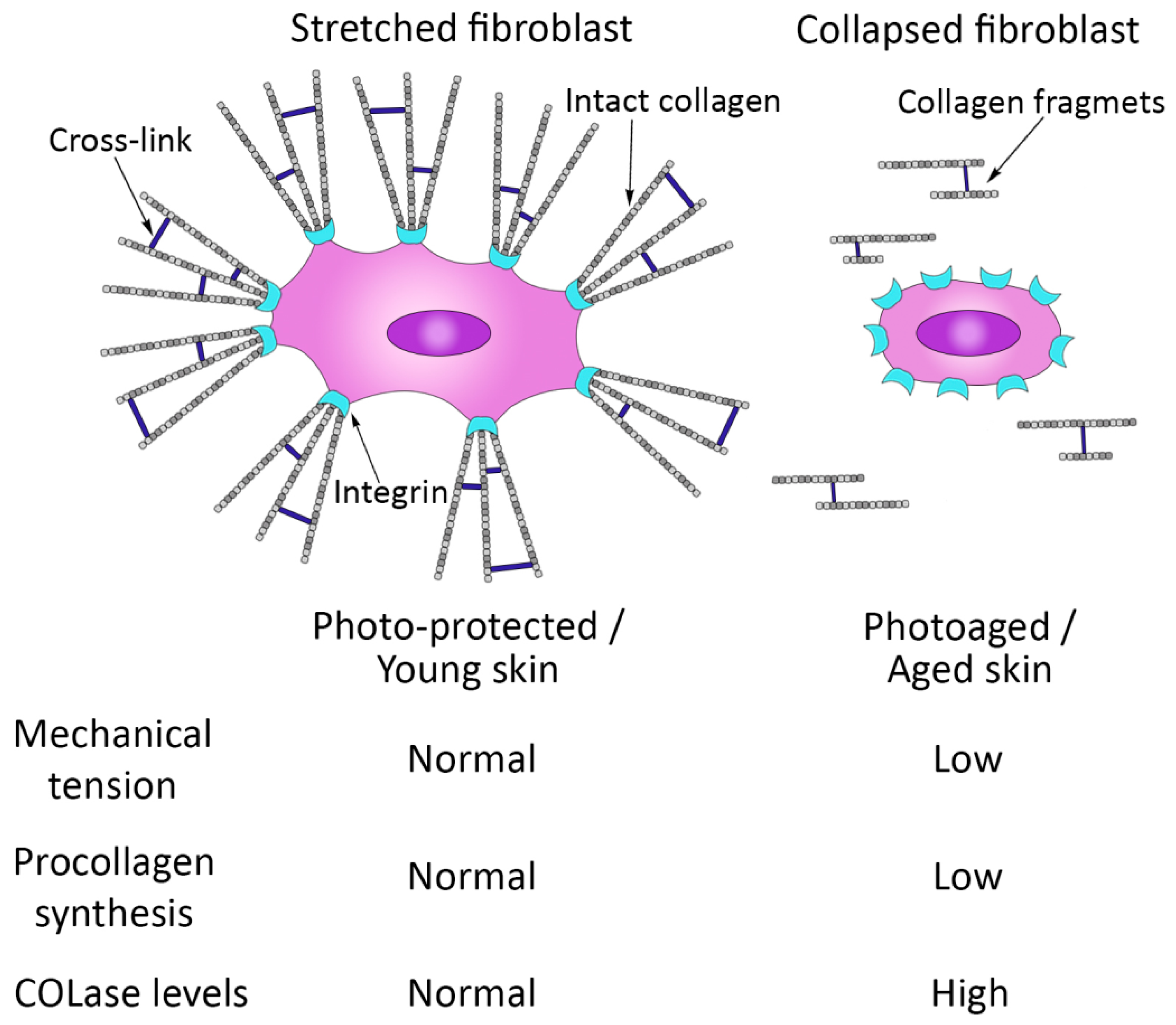
3. Molecular and Cellular Changes in the Skin during the Aging Process
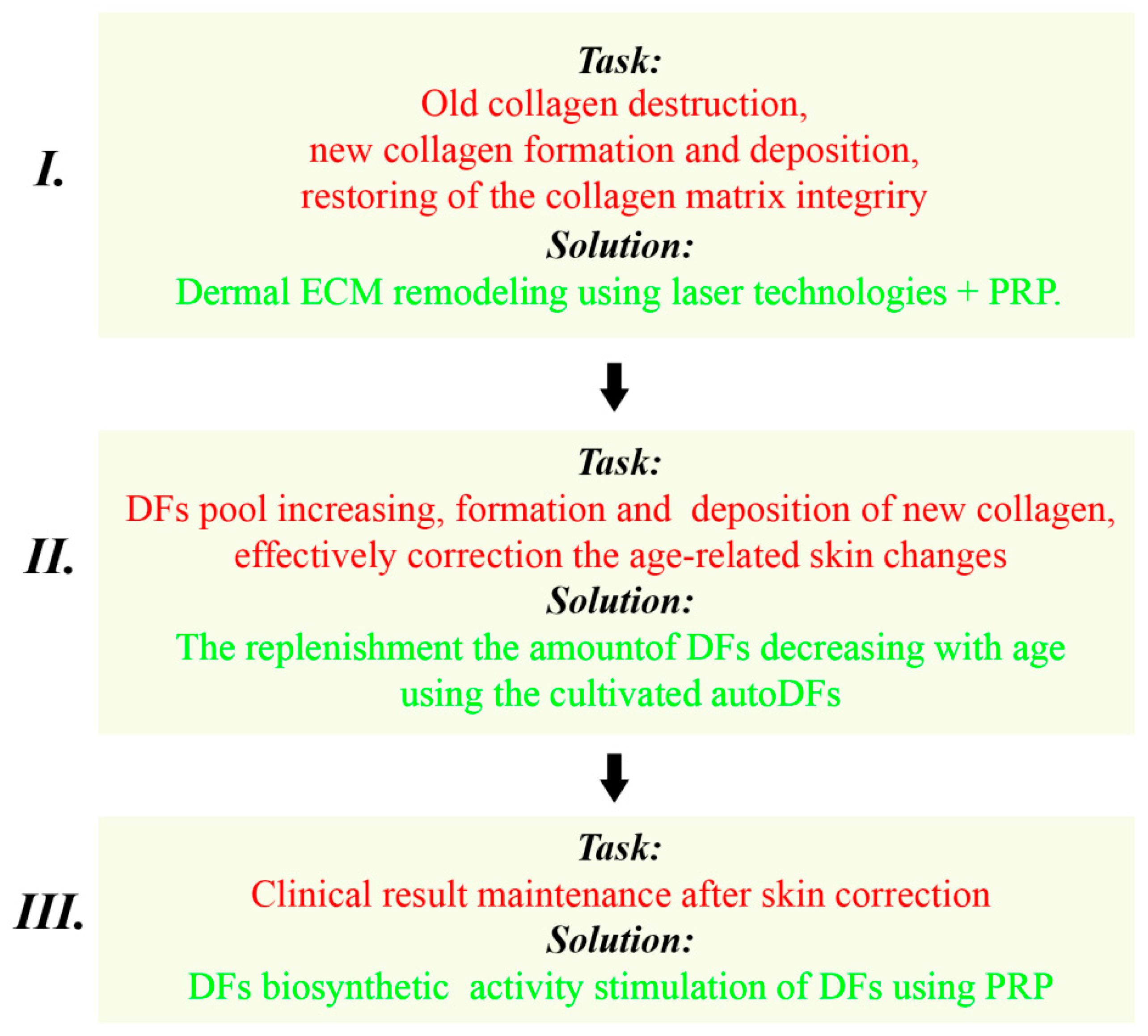
4. A Variant of the Skin Anti-Age Correction Program Using a Combination of Laser and Cellular Methods
4.1. Program Stages: The Solved Tasks and Recommended Methods
4.1.1. The First Stage
Dermal ECM Remodeling Using Laser Technologies
PRP Therapy in Combination with Laser Technologies
4.1.2. The Second Stage
4.1.3. The Third Stage
4.2. Approximate Step-by-Step Scheme of Anti-Age Correction of the Facial Skin Using a Combination of Regenerative Methods
- Stage I
- Application of the fractional laser photothermolysis (FL): one to three procedures with intervals of 3–4 weeks, depending on the characteristics of the patient’s skin and the used FL type (ablative or non-ablative).
- Conducting the PRP therapy of the skin: one procedure immediately after each FL procedure;
- (a)
- Intradermal PRP injections in the area exposed to FL after the non-ablative FL procedure;
- (b)
- Intradermal PRP injections or local PRP application throughout the area exposed to FL after the ablative FL procedure.
- Stage II (3 months after the end of stage I)
- Stage III (skin maintenance therapy, 8–12 months after stage II)
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rognoni, E.; Watt, F. Skin Cell Heterogeneity in Development, Wound Healing, and Cancer. Trends Cell Biol. 2018, 1433, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Simpson, C.L.; Patel, D. Deconstructing the skin: Cytoarchitectural determinants of epidermal morphogenesis. Nat. Rev. Mol. Cell Biol. 2011, 12, 565–580. [Google Scholar] [CrossRef] [PubMed]
- Arda, O.; Goksugur, N. Basic histological structure and functions of facial skin. Clin. Dermatol. 2014, 32, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Zorina, A.; Zorin, V. Age-Related Changes in the Fibroblastic Differon of the Dermis: Role in Skin Aging. Int. J. Mol. Sci. 2022, 23, 6135. [Google Scholar] [CrossRef]
- Zorina, A.; Zorin, V. Molecular Mechanisms of Changes in Homeostasis of the Dermal Extracellular Matrix: Both Involutional and Mediated by Ultraviolet Radiation. Int. J. Mol. Sci. 2022, 23, 6655. [Google Scholar] [CrossRef]
- Katoh, N.; Tennstedt, D. Gerontodermatology: The fragility of the epidermis in older adults. JEADV 2018, 32, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Bregegere, F. Cellular senescence in human keratinocytes: Unchanged proteolytic capacity and increased protein load. Exp. Geront. 2003, 38, 619–629. [Google Scholar] [CrossRef]
- Kanitakis, J. Anatomy, histology and immunohistochemistry of normal human skin. Eur. J. Dermatol. 2002, 12, 390–399. [Google Scholar]
- Zouboulis, C.; Adjaye, J. Human skin stem cells and the ageing process. Exp. Gerontol. 2008, 43, 986–997. [Google Scholar] [CrossRef]
- Liu, N.; Matsumura, H. Stem cell competition orchestrates skin homeostasis and ageing. Nature 2019, 568, 344–350. [Google Scholar] [CrossRef]
- Giancotti, F.G.; Tarone, G. Positional control of cell fate through joint integrin/receptor protein kinase signaling. Annu. Rev. Cell Dev. Biol. 2003, 19, 173–206. [Google Scholar] [CrossRef]
- Moore, K.A.; Lemischka, I.R. Stem cells and their niches. Science 2006, 311, 1880–1885. [Google Scholar] [CrossRef]
- Sorrell, J.M.; Caplan, A.I. Fibroblast heterogeneity: More than skin deep. J. Cell Sci. 2004, 117, 667–675. [Google Scholar] [CrossRef] [PubMed]
- El Ghalbzouri, A.; Lamme, E. Crucial role of fibroblasts in regulating epidermal morphogenesis. Cell Tissue Res. 2002, 310, 189–199. [Google Scholar] [CrossRef]
- Sorrell, M.; Caplan, A.I. Fibroblasts—A diverse population at the center of it all. Int. Rev. Cell Mol. Biol. 2009, 276, 161–214. [Google Scholar] [CrossRef]
- Rheinwald, J.G.; Green, H. Formation of a keratinizing epithelium in culture by a cloned cell line derived form a teratoma. Cell 1975, 6, 317–330. [Google Scholar] [CrossRef]
- Brauchle, M.; Angermeyer, K. Large induction of keratinocyte growth factor expression by serum growth factors and pro-inflammatory cytokines in cultured fibroblasts. Oncogene 1994, 9, 3199–3204. [Google Scholar]
- Marchese, C.; Felici, A. Fibroblast growth factor 10 induces proliferation and differentiation of human primary cultured keratinocytes. J. Investig. Dermatol. 2001, 116, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Werner, S.; Smola, H. Paracrine regulation of keratinocyte proliferation and differentiation. Trends Cell Biol. 2001, 11, 143–146. [Google Scholar] [CrossRef]
- Maas-Szabowski, N.; Szabowski, A. Organotypic cocultures with genetically modified mouse fibroblasts as a tool to dissect molecular mechanisms regulating keratinocyte growth and differentiation. J. Investig. Dermatol. 2001, 116, 816–820. [Google Scholar] [CrossRef] [PubMed]
- Philippeos, C.; Telerman, S. Spatial and single-cell transcriptional profiling identifies function ally distinct human dermal fibroblast subpopulations. J. Investig. Dermatol. 2018, 138, 811–825. [Google Scholar] [CrossRef] [PubMed]
- Marinkovich, M.P.; Keene, D.R. Cellular origin of the dermal-epidermal basement membrane. Dev. Dyn. 1993, 197, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Fleischmajer, R.; Schechter, A. Skin fibroblasts are the only source of nidogen during early basal lamina formation in vitro. J Investig. Dermatol. 1995, 105, 597–601. [Google Scholar] [CrossRef]
- Driskell, R.R.; Lichtenberger, B.M. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature 2013, 504, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Sorrel, J.; Baber, M. Clonal characterization of fibroblasts in the superficial layer of the adult human dermis. Cell Tissue Res. 2003, 327, 499–510. [Google Scholar] [CrossRef]
- Tabib, T.; Morse, C. SFRP2/DPP4andFMO1/LSP1define major fibroblast populations in human skin. J. Investig. Dermatol. 2018, 138, 802–810. [Google Scholar] [CrossRef]
- Driskell, R.R.; Watt, F.M. Understanding fibroblast heterogeneity in the skin. Trend Cell Biol. 2015, 25, 92–99. [Google Scholar] [CrossRef]
- Janson, D.G.; Saintigny, G. Different gene expression patterns in human papillary and reticular fibroblasts. J. Investig. Dermatol. 2012, 132, 2565–2572. [Google Scholar] [CrossRef]
- Wang, J.; Dodd, C. Deep dermal fibroblasts contribute to hypertrophic scarring. Lab. Investig. 2008, 88, 1278–1290. [Google Scholar] [CrossRef]
- Mine, S.; Fortunel, N.O. Aging Alters Functionally Human Dermal Papillary Fibroblasts but Not Reticular Fibroblasts: A New View of Skin Morphogenesis and Aging. PLoS ONE 2008, 3, e4066. [Google Scholar] [CrossRef]
- Haydont, V.; Bernard, B.A. Age-related evolutions of the dermis: Clinical signs, fibroblast and extracellular matrix dynamics. Mech. Ageing Dev. 2019, 177, 150–156. [Google Scholar] [CrossRef]
- Tracy, L.E.; Minasian, R.A. Extracellular Matrix and Dermal Fibroblast Function in the Healing Wound. Adv. Wound Care 2016, 5, 119–136. [Google Scholar] [CrossRef]
- Waller, J.M.; Maibach, H.I. Age and skin structure and function, a quantitative approach (II): Protein, glycosaminoglycan, water, and lipid content and structure. Skin Res. Technol. 2006, 12, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Graham, H.K.; Holmes, D.F. Identification of collagen fibril fusion during vertebrate tendon morphogenesis. The process relies on unipolar fibrils and is regulated by collagen–proteoglycan interaction. J. Mol. Biol. 2000, 295, 891–902. [Google Scholar] [CrossRef]
- Burgeson, R.E.; Nimni, M.E. Collagen types. Molecular structure and tissue distribution. Clin. Orthop. 1992, 282, 250–272. [Google Scholar] [CrossRef]
- Kadler, K.E.; Holmes, D.F. Collagen fibril formation. Biochem. J. 1996, 316, 1–11. [Google Scholar] [CrossRef]
- Ruggiero, F.; Roulet, M. Dermis collagens: Beyond their structural properties. J. Soc. Biol. 2005, 199, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Birk, D.E. Type V collagen: Heterotypic I/V collagen interactions in the regulation of fibril assembly. Micron 2001, 32, 223–237. [Google Scholar] [CrossRef]
- Hansen, U.; Bruckner, P. Macromolecular specificity of collagen fibrillogenesis. Fibrils of collagens I and XI contain a heterotypic alloyed core and a collagen I sheath. J. Biol. Chem. 2003, 278, 37352–37359. [Google Scholar] [CrossRef]
- Fitzgerald, J.; Bateman, J.F. A new FACIT of collagen family: COL21A1. FEBS Lett. 2001, 505, 275–280. [Google Scholar] [CrossRef]
- Kadler, K.E.; Hill, A. Collagen fibrillogenesis: Fibronectin, integrins, and minor collagens as organizers and nucleators. Curr. Opin. Cell Biol. 2008, 20, 495–501. [Google Scholar] [CrossRef]
- Baldock, C.; Sherratt, M.J. The supramolecular organization of collagen VI microfibrils. J. Mol. Biol. 2003, 330, 297–307. [Google Scholar] [CrossRef]
- Knupp, C.; Pinall, C. Structural correlation between cjllagen VI micrifibrills and collagen VI banded aggregates. J. Struct. Biol. 2006, 154, 312–326. [Google Scholar] [CrossRef]
- Ricard-Blum, S. The Collagen Family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef] [PubMed]
- Ricard-Blum, S.; Ruggiero, F. The collagen superfamily: From the extracellular matrix to the cell membrane. Pathol. Biol. 2005, 53, 430–442. [Google Scholar] [CrossRef] [PubMed]
- Naylor, E.; Watson, R. Molecular aspects of skin ageing. Maturitas 2011, 69, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Kim, Y.K. Loss of elastic fibers caus es skin wrinkles in sun-damaged human skin. J. Dermatol. Sci. 2008, 50, 99–107. [Google Scholar] [CrossRef]
- Marionnet, C.; Pierrard, C. Interactions between fibroblasts and keratinocytes in morphogenesis of dermal epidermal junction in a model of reconstructed skin. J. Investig. Dermatol. 2006, 126, 971–979. [Google Scholar] [CrossRef]
- Haniffa, M.; Collin, M. Mesenchymal stem cells: The fibroblasts new clothes? Haemotologica 2009, 94, 258–263. [Google Scholar] [CrossRef]
- Lavker, R. Structural alterations in exposed and unexposed aged skin. J. Investig. Dermatol. 1979, 73, 559–566. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Makrantonaki, E. Clinical aspects and molecular diagnostics of skin aging. Clin. Dermatol. 2011, 29, 3–14. [Google Scholar] [CrossRef]
- Yaar, M.; Gilcherest, B.A. Photoageing: Mechanism, prevention and therapy. Br. J. Dermatol. 2007, 157, 874–887. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.; Kang, S. Mechanism of photoaging and chronological skin aging. Arch. Dermatol. 2002, 138, 1462–1467. [Google Scholar] [CrossRef] [PubMed]
- Miyamura, Y.; Coelho, S. Regulation of human skin pigmentation and responses to ultraviolet radiation. Pigment. Cell Res. 2007, 20, 2–13. [Google Scholar] [CrossRef]
- Varani, J.; Schuger, L. Reduced fibroblast interaction with intact collagen as a mechanism for depressed collagen synthesis in photodamaged skin. J. Investig. Dermatol. 2004, 122, 1471–1479. [Google Scholar] [CrossRef]
- Solé-Boldo, L.; Raddatz, G. Single-cell transcriptomes of the human skin reveal age-related loss of fibroblast priming. Commun. Biol. 2020, 3, 188. [Google Scholar] [CrossRef]
- Varani, J.; Dame, M. Decreased collagen production in chronologically aged skin: Roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Am. J. Pathol. 2006, 168, 1861–1868. [Google Scholar] [CrossRef]
- Rittié, L.; Fisher, G.J. UV-light-induced signal cascades and skin aging. Ageing Res. Rev. 2002, 1, 705–720. [Google Scholar] [CrossRef]
- Cole, M.A.; Quan, T. Extracellular matrix regulation of fibroblast function: Redefining our perspective on skin aging. J. Cell Commun. Signal. 2018, 12, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Brack, A.S. Cellular mechanisms of somatic stem cell aging. Curr. Topics Dev. Biol. 2014, 107, 405–438. [Google Scholar] [CrossRef]
- Young, H.E.; Black, A.C., Jr. Adult stem cells. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 2004, 276, 75–102. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Kerr, C. The Epigenetics of Stem Cell Aging Comes of Age. Trends Cell Biol. 2019, 29, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Tümpel, S.; Rudolph, K.L. Quiescence: Good and Bad of Stem Cell Aging. Trends Cell Biol. 2019, 29, 672–685. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, G. Molecular mechanisms of skin ageing. Mechanisms of Ageing and Development. Mech. Ageing Dev. 2002, 123, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Wlaschek, M.; Maity, P. Connective tissue and fibroblast senescence in skin aging. J. Investig. Dermatol. 2021, 141, 985–992. [Google Scholar] [CrossRef]
- Demaria, M.; Desprez, P. Cell Autonomous and Non-autonomous Effects of Senescent Cells in the Skin. J. Investig. Dermatol. 2015, 35, 1722–1726. [Google Scholar] [CrossRef]
- Herranz, N.; Gil, J. Mechanisms and functions of cellular senescence. J. Clin. Investig. 2018, 128, 1238–1246. [Google Scholar] [CrossRef]
- Gorgoulis, V.; Adams, P.D. Cellular Senescence: Defining a Path Forward. Cell 2019, 179, 814–827. [Google Scholar] [CrossRef]
- Fisher, G.; Varani, J. Looking older: Fibroblast Collapse and Therapeutic Implications. Arch. Dermatol. 2008, 144, 666–672. [Google Scholar] [CrossRef]
- Kim, S.H.; Turnbull, J. Extracellular matrix and cell signalling: The dynamic cooperation of integrin, proteoglycan and growth factor receptor. J. Endocrinol. 2011, 209, 139–151. [Google Scholar] [CrossRef]
- Silver, F.; DeVore, D. Role of mechanophysiology in aging of ECM: Effects in mechanochemical transduction. J. Appl. Physiol. 2003, 95, 2134–2141. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.; Nash, A. Combining nano-physical and computational investigations to understand the nature of “aging” in dermal collagen. Int. J. Nanomed. 2017, 12, 3303–3314. [Google Scholar] [CrossRef] [PubMed]
- Brennan, M.; Bhatti, H. Matrixmetalloproteinase-1 is the major collagenolytic enzyme responsible for collagen damage in UV irradiated human skin. Photochem. Photobiol. 2003, 78, 43–48. [Google Scholar] [CrossRef]
- Rittié, L.; Fisher, G.J. Natural and Sun-Induced Aging of Human Skin. Cold Spring Harb. Perspect. Med. 2015, 5, a015370. [Google Scholar] [CrossRef] [PubMed]
- Kohl, E.; Steinbauer, J. Skin ageing. J. Eur. Acad. Dermatol. Venereol. (JEADV) 2011, 25, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Han, K.-H.; Choi, H.R. Alteration of the TGF-b/SMAD pathway in intrinsicallyand UV-induced skin aging. Mech. Ageing Dev. 2005, 126, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Reigle, K.; Di Lullo, G. Non-enzymatic glycation of type I collagen diminishes collagen-proteoglycan binding and weakens cell adhesion. J. Cell Biochem. 2008, 104, 1684–1698. [Google Scholar] [CrossRef]
- Fournet, M.; Bonte, F. Glycation damage: A possible hub for major pathophysiological disorders and aging. Aging Dis. 2018, 9, 880–900. [Google Scholar] [CrossRef]
- Fisher, G.J.; Voorhees, J.J. Molecular mechanisms of retinoid actions in skin. FASEB J. 1996, 10, 1002–1013. [Google Scholar] [CrossRef]
- Griffiths, C.E.; Kang, S. Two concentrations of topical tretinoin (retinoic acid) cause similar improvement of photoaging but different degrees of irritation. A double-blind, vehicle-controlled comparison of 0.1% and 0.025% tretinoin creams. Arch. Dermatol. 1995, 131, 1037–1044. [Google Scholar] [CrossRef]
- Kang, S.; Leyden, J.J. Tazarotene cream for the treatment of facial photodamage: A multicenter, investigator-masked, randomized, vehicle-controlled, parallel comparison of 0.01%, 0.025%, 0.05%, and 0.1% tazarotene creams with 0.05% tretinoin emollient cream applied once daily for 24 weeks. Arch. Dermatol. 2001, 137, 1597–1604. [Google Scholar] [CrossRef] [PubMed]
- Kafi, R.; Kwak, H.S.R. Improvement of Naturally Aged Skin With Vitamin A (Retinol). Arch. Dermatol. 2007, 143, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Han, K.-H.; Chien, A.L. Photoaging. Dermatol. Clin. 2014, 32, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Ratner, D.; Tse, Y. Cutaneous laser resurfacing. J. Am. Acad. Dermatol. 1999, 41, 365–389. [Google Scholar] [CrossRef]
- Riggs, K.; Keller, M. Ablative laser resurfacing: High-energy pulsed carbon dioxide and erbium:yttrium-aluminum-garnet. Clin. Dermatol. 2007, 25, 462–473. [Google Scholar] [CrossRef]
- Orringer, J.S.; Kang, S. Connective tissue remodeling induced by carbon dioxide laser resurfacing of photodamaged human skin. Arch. Dermatol. 2004, 140, 1326–1332. [Google Scholar] [CrossRef]
- Orringer, J.; Rittie, L. Intraepidermal erbium: YAG laser resurfacing: Impact on the dermal matrix. J. Am. Acad. Dermatol. 2011, 64, 119–128. [Google Scholar] [CrossRef]
- Tomasek, J.; Gabbiani, G. Miofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Mol. Cell Biol. 2002, 3, 349–363. [Google Scholar] [CrossRef]
- Midwood, K.S.; Williams, L.V. Tissue repair and the dynamics of the extracellular matrix. Int. J. Biochem. Cell Biol. 2004, 36, 1031–1037. [Google Scholar] [CrossRef]
- Manstein, D.; Herron, G.S. Fractional photothermolysis: A new concept for cutaneus remodeling using microscopic patterns of thermal injury. Lasers Surg. Med. 2004, 34, 426–438. [Google Scholar] [CrossRef]
- Lopez-Rivera, E.; Lizarbe, T.R. Matrix metalloproteinase 13 mediated nitric oxide activation of endothelial cell migration. Proc. Natl. Acad. Sci. USA 2005, 102, 3685–3690. [Google Scholar] [CrossRef] [PubMed]
- Ming, H.; Kimyai-Asadi, A. Fractional photothermolysis: A review and update. Semin. Cutan. Med. Surg. 2008, 27, 63–71. [Google Scholar] [CrossRef]
- Bodendorf, M.O.; Grunewald, S. Fractional laser skin therapy. J. Dtsch. Dermatol. Ges. 2009, 7, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Saedi, N.; Petelin, A. Fractionation: A new era in laser resurfacing. Clin. Plast. Surg. 2011, 38, 449–461. [Google Scholar] [CrossRef]
- Hantash, B.M.; Bedi, V.P. In vivo histological evaluation of a novel ablative fractional resurfacing device. Lasers Surg. Med. 2007, 39, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Archer, K.A.; Carniol, P. Diode Laser and Fractional Laser Innovations. Facial. Plast. Surg. 2019, 35, 248–255. [Google Scholar] [CrossRef]
- Laubach, H.J.; Tannous, Z. Skin responses to fractional photothermolysis. Lasers Surg. Med. 2006, 38, 142–149. [Google Scholar] [CrossRef]
- Geronemus, R.G. Fractional photothermolysis: Current and future applications. Lasers Surg. Med. 2006, 38, 169–176. [Google Scholar] [CrossRef]
- NA, J.; CHOI, M. Rapid Healing and Reduced Erythema after Ablative Fractional Carbon Dioxide Laser Resurfacing Combined with the Application of Autologous Platelet-Rich Plasma. Dermatol. Surg. 2011, 37, 463–468. [Google Scholar] [CrossRef]
- SHIN, M.; LEE, J. Platelet-Rich Plasma Combined with Fractional Laser Therapy for Skin Rejuvenation. Dermatol. Surg. 2012, 38, 623–630. [Google Scholar] [CrossRef]
- Hui, Q.; Chang, P. The clinical efficacy of autologous platelet-rich plasma combined with ultra-pulsed fractional CO2 laser therapy for facial rejuvenation. Rejuvenation Res. 2017, 20, 25–31. [Google Scholar] [CrossRef]
- Marx, R. Platelet–Rich plasma (PRP): What is PRP and what is not PRP? Implant. Dent. 2001, 10, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Kumaran, A.M.S. Platelet-rich plasma in dermatology: Boon or a bane? Indian J. Derm. Venereol. Leprol. 2014, 80, 5–14. [Google Scholar] [CrossRef]
- Sommeling, C.; Heyneman, A. The use of platelet-rich plasma in plastic surgery: A systematic review. J. Plast. Reconstr. Aesthetic Surg. 2013, 66, 301–312. [Google Scholar] [CrossRef]
- Kim, D.H.; Je, Y.J. Can platelet-rich plasma be used for skin rejuvenation? Evaluation of effects of platelet-rich plasma on human dermal fibroblast. Ann. Dermatol. 2011, 23, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Marx, R. Platelet—Rich plasma: Evidence to support its use. J. Oral Maxillofac. Surg. 2004, 62, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Krasna, M.; Domanović, D. Platelet gel stimulates proliferation of human dermal fibroblasts in vitro. Act. Dermatovenerol. Alp. Panonica Adriat. 2007, 16, 105–110. [Google Scholar]
- Hom, D.; Maisel, R. Angiogenic growth factors: Their effects and potential in soft tissue wound healing. Ann. Otol. Rhinol. Laryngol. 1992, 101, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Roman, P.; Bolta, Z. Use platelet- growth factors in treating wounds and soft-tissue injuries. Acta Dermatoven. APA 2007, 16, 156–165. [Google Scholar]
- Burd, A.; Zhu, N. A study of Q-switched Nd:YAG laser irradiation and paracrine function in human skin cells. Photodermatol. Photoimmunol. Photomed. 2005, 21, 131–137. [Google Scholar] [CrossRef]
- Ehrenfest, D.M.; Bielecki, T. In search of a consensus terminology in the field of platelet concentrates for surgical use: Platelet-rich plasma (PRP), platelet-rich fibrin (PRF), fibrin gel polymerization and leukocytes. Curr. Pharm. Biotechnol. 2012, 13, 1131–1137. [Google Scholar] [CrossRef] [PubMed]
- Gawdat, H.; Hegazy, R. Autologous platelet rich plasma: Topical versus intradermal after fractional ablative carbon dioxide laser treatment of atrophic acne scars. Dermatol. Surg. 2014, 40, 152–161. [Google Scholar] [CrossRef]
- Ramezankhani, R.; Torabi, S. Two Decades of Global Progress in Authorized Advanced Therapy Medicinal Products: An Emerging Revolution in Therapeutic Strategies. Front. Cell Dev. Biol. 2020, 8, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Zorin, V.; Zorina, A. Clinical-instrumental and morphological evaluation of the effect of autologous dermal fibroblasts Administration. J. Tissue Eng. Regen. Med. 2014, 11, 778–786. [Google Scholar] [CrossRef]
- Boss, W.K.; Usal, H. Autologous cultured fibroblasts as cellular therapy in plastic surgery. Clin. Plast. Surg. 2000, 27, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Boss, W.K.; Usal, H. Autologous cultured fibroblast: A protein repair system. Ann. Plast. Surg. 2000, 44, 536–542. [Google Scholar] [CrossRef]
- Weiss, R.A.; Weiss, M.A. Autologous cultured fibroblast injection for facial contour deformities: A prospective, placebo-controlled, Phase III clinical trial. Dermatol. Surg. 2007, 33, 263–268. [Google Scholar] [CrossRef]

| Collagen Types | Localization |
|---|---|
| Fiber-forming (fibrillar) collagens | |
| Large fiber-forming collagens | |
| Type I | Reticular dermis layer predominantly |
| Type III | Papillary dermis layer predominantly |
| Small (minor) fiber-forming collagens | |
| Type V | Both layers of the dermis |
| Non-fibrillar collagens | |
| FACIT collagens | |
| Type XII | Papillary dermis layer |
| Type XIV | Reticular dermis layer |
| Type XVI | Papillary dermis layer |
| Type XXII | Papillary dermis layer |
| Collagens forming network-like structures | |
| Basement membrane collagens | |
| Type IV | Basement membrane |
| Collagens of anchoring fibrils | |
| Type VII | Fibrils at the border of dermis and epidermis |
| Collagens of microfibrils | |
| Type VI | Microfibrils in both layers of the dermis |
| Transmembrane collagens | |
| Type XIII | Cellular membranes |
| Protein Profiling | |
|---|---|
| Mesenchymal markers/expression-cells+% | |
| CD73 (NT5E 5′-nucleotidase ecto) | Hi/>95 |
| CD90 (THY1 Thy-1 cell surface antigen) | Hi/>99 |
| CD105 (ENG endoglin) | Hi/>98 |
| Collagen I (COL1A1 collagen type I alpha 1 chain) | Hi/>95 |
| Collagen III (COL3A1 collagen type III alpha 1 chain) | Hi/>95 |
| Elastin (ELN elastin) | Hi/>95 |
| Vimentin (VIM vimentin) | Hi/>98 |
| Prolyl 4-hydroxylase (P4HB prolyl 4-hydroxylase subunit beta) | Hi/>99 |
| Endothelial and hematopoietic markers/expression-cells+% | |
| CD31 (PECAM1 platelet and endothelial cell adhesion molecule 1) | Low/<1 |
| CD34 (CD34 CD34 molecule) | Low/<1 |
| CD45 (PTPRC protein tyrosine phosphatase receptor type C) | Low/<1 |
| Epithelial markers/expression-cells+% | |
| CD324 (CDH1 Cadherin 1, Type 1, E-Cadherin (Epithelial)) | Low/<0.5 |
| pan-Cytokeratin 14,15,16,19 (KRT14, KRT15, KRT16, KRT19) | Low/<0.5 |
| Differentiation markers/expression-cells+% | |
| Aggrecan (ACAN aggrecan)—chondrogenic | Low/<1 |
| Osteocalcin (BGLAP bone gamma-carboxyglutamate protein)—osteogenic | Low/<1 |
| FABP4 (FABP4 fatty acid binding protein 4)—adipogenic | Low/<0.5 |
| a-SMA (ACTA2 actin alpha 2)—smooth muscle | Variable/1–30 |
| a-sk-Actin (ACTA1 Actin Alpha 1)—skeletal muscle | Low/<1.5 |
| sk-Myosin (MYH1 Myosin Heavy Chain 1)—skeletal muscle | Low/<0.5 |
| Myogenin (MYOG myogenin)—myogenic | Low/<0.5 |
| MyoD1 (MYOD1 myogenic differentiation 1)—myogenic | Low/<0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zorina, A.; Zorin, V.; Isaev, A.; Kudlay, D.; Vasileva, M.; Kopnin, P. Dermal Fibroblasts as the Main Target for Skin Anti-Age Correction Using a Combination of Regenerative Medicine Methods. Curr. Issues Mol. Biol. 2023, 45, 3829-3847. https://doi.org/10.3390/cimb45050247
Zorina A, Zorin V, Isaev A, Kudlay D, Vasileva M, Kopnin P. Dermal Fibroblasts as the Main Target for Skin Anti-Age Correction Using a Combination of Regenerative Medicine Methods. Current Issues in Molecular Biology. 2023; 45(5):3829-3847. https://doi.org/10.3390/cimb45050247
Chicago/Turabian StyleZorina, Alla, Vadim Zorin, Artur Isaev, Dmitry Kudlay, Maria Vasileva, and Pavel Kopnin. 2023. "Dermal Fibroblasts as the Main Target for Skin Anti-Age Correction Using a Combination of Regenerative Medicine Methods" Current Issues in Molecular Biology 45, no. 5: 3829-3847. https://doi.org/10.3390/cimb45050247
APA StyleZorina, A., Zorin, V., Isaev, A., Kudlay, D., Vasileva, M., & Kopnin, P. (2023). Dermal Fibroblasts as the Main Target for Skin Anti-Age Correction Using a Combination of Regenerative Medicine Methods. Current Issues in Molecular Biology, 45(5), 3829-3847. https://doi.org/10.3390/cimb45050247







