Anti-Inflammatory and Anti-Quorum Sensing Effect of Camellia sinensis Callus Lysate for Treatment of Acne
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Total Terpene Quantification
2.2.2. Catechin Quantification by Liquid Chromatography-Mass Spectrometry (LC-MS)
2.2.3. HaCaT Cells and C. acnes Stimuli
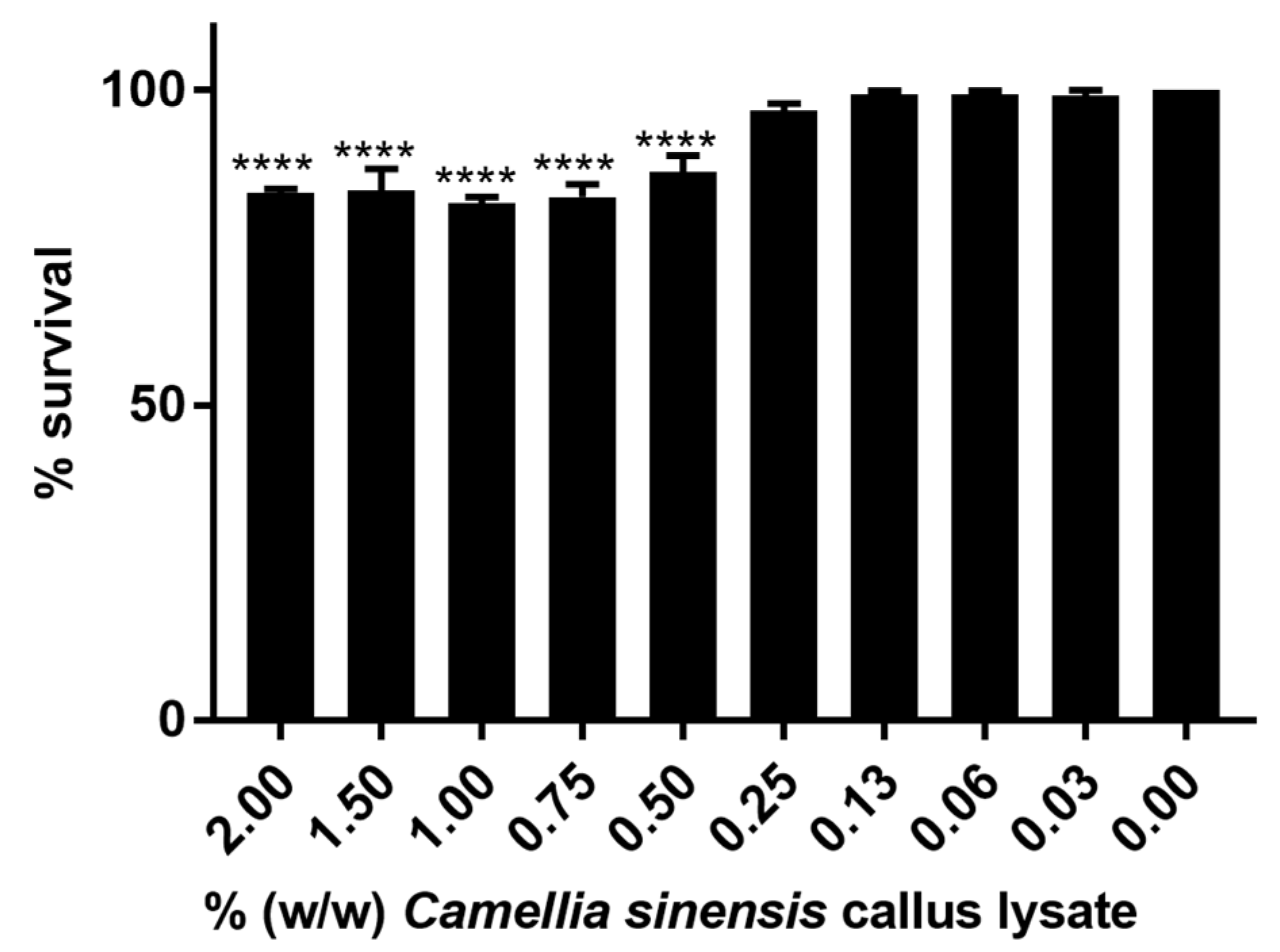
2.2.4. In Vitro Cytotoxicity Evaluation
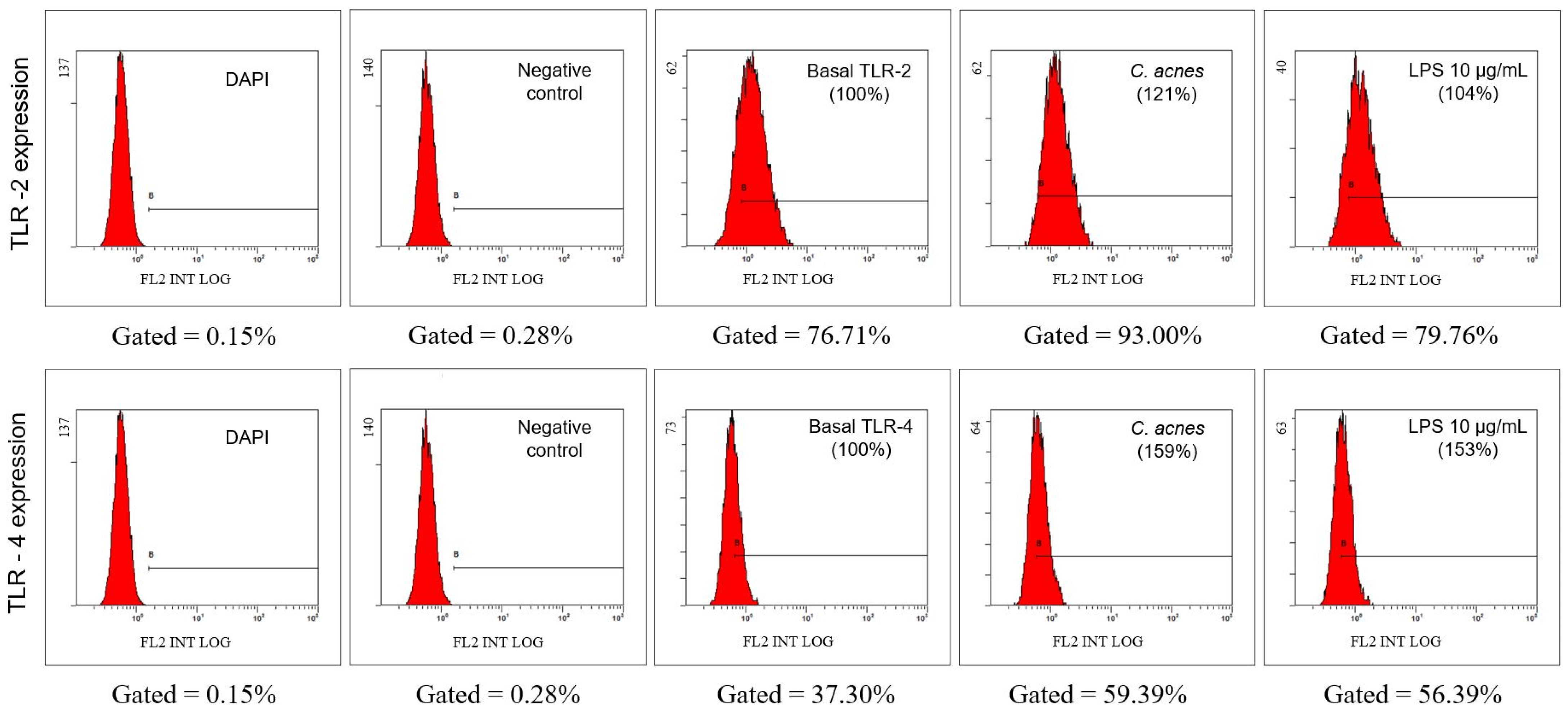
2.2.5. Evaluation of the Expression of Toll-Like Receptors (TLR) TLR2 and TLR4 in the Cell Membrane by Flow Cytometry
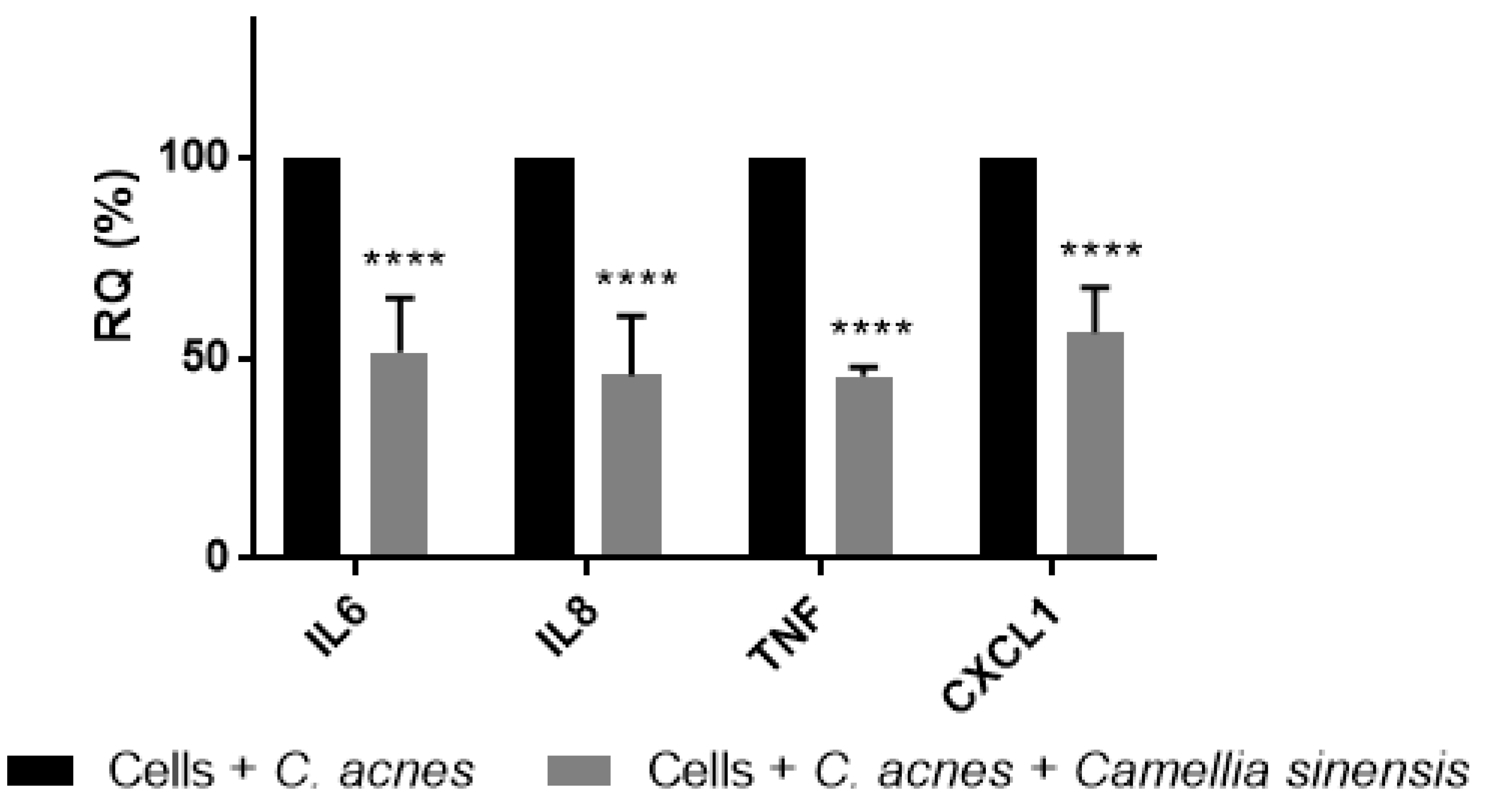
2.2.6. Camellia sinensis Anti-Inflammatory Activity Evaluation
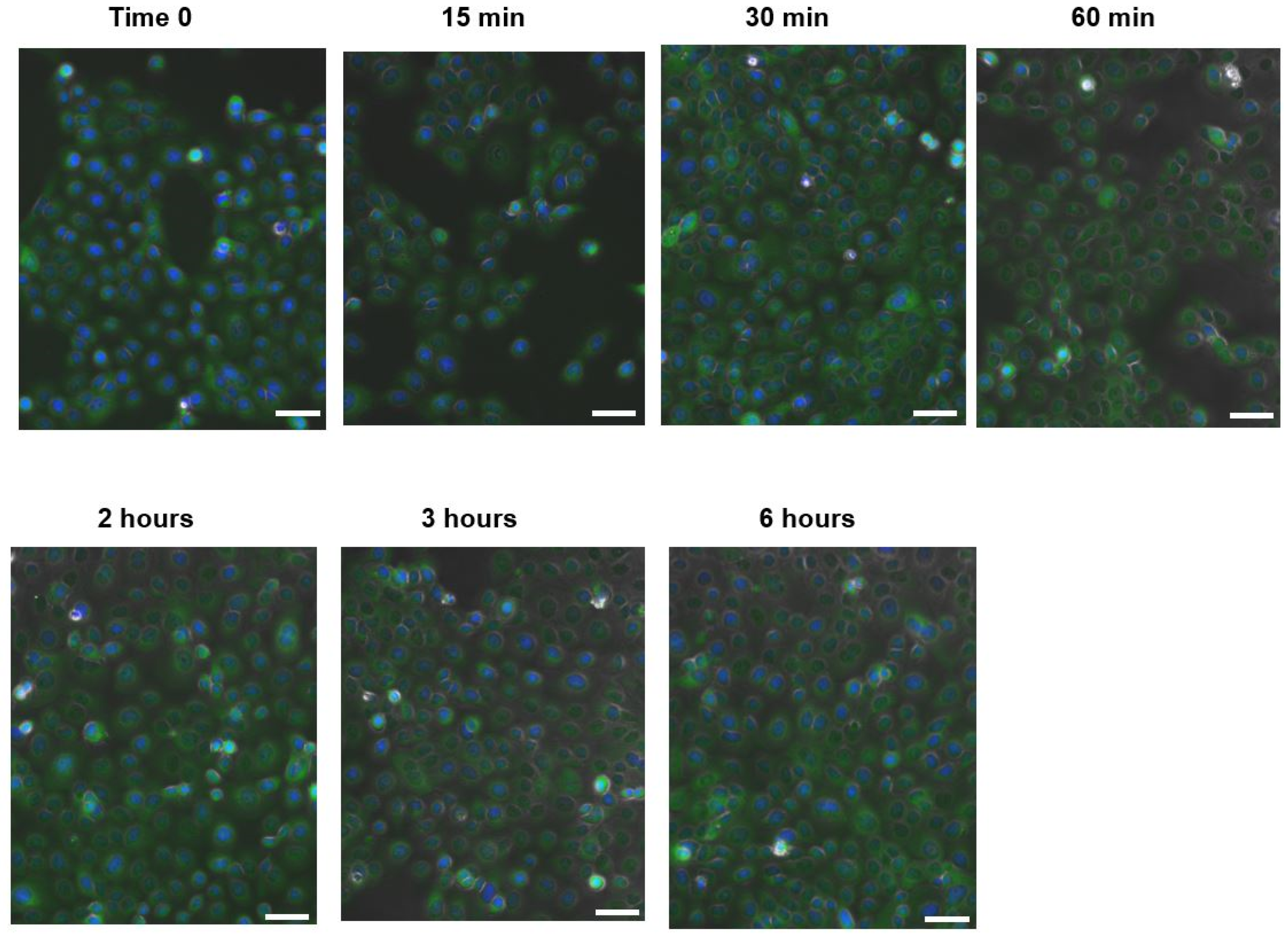
2.2.7. NF-κB Nuclear Translocation Evaluation
2.2.8. C. acnes Anti-Biofilm Activity of Camellia sinensis Callus Lysate
2.2.9. Antilipase Activity
2.2.10. Quantification of AI-2
2.2.11. Statistical Analysis of the Results
3. Results
3.1. Chemical Characterization of the Camellia sinensis Callus Extract
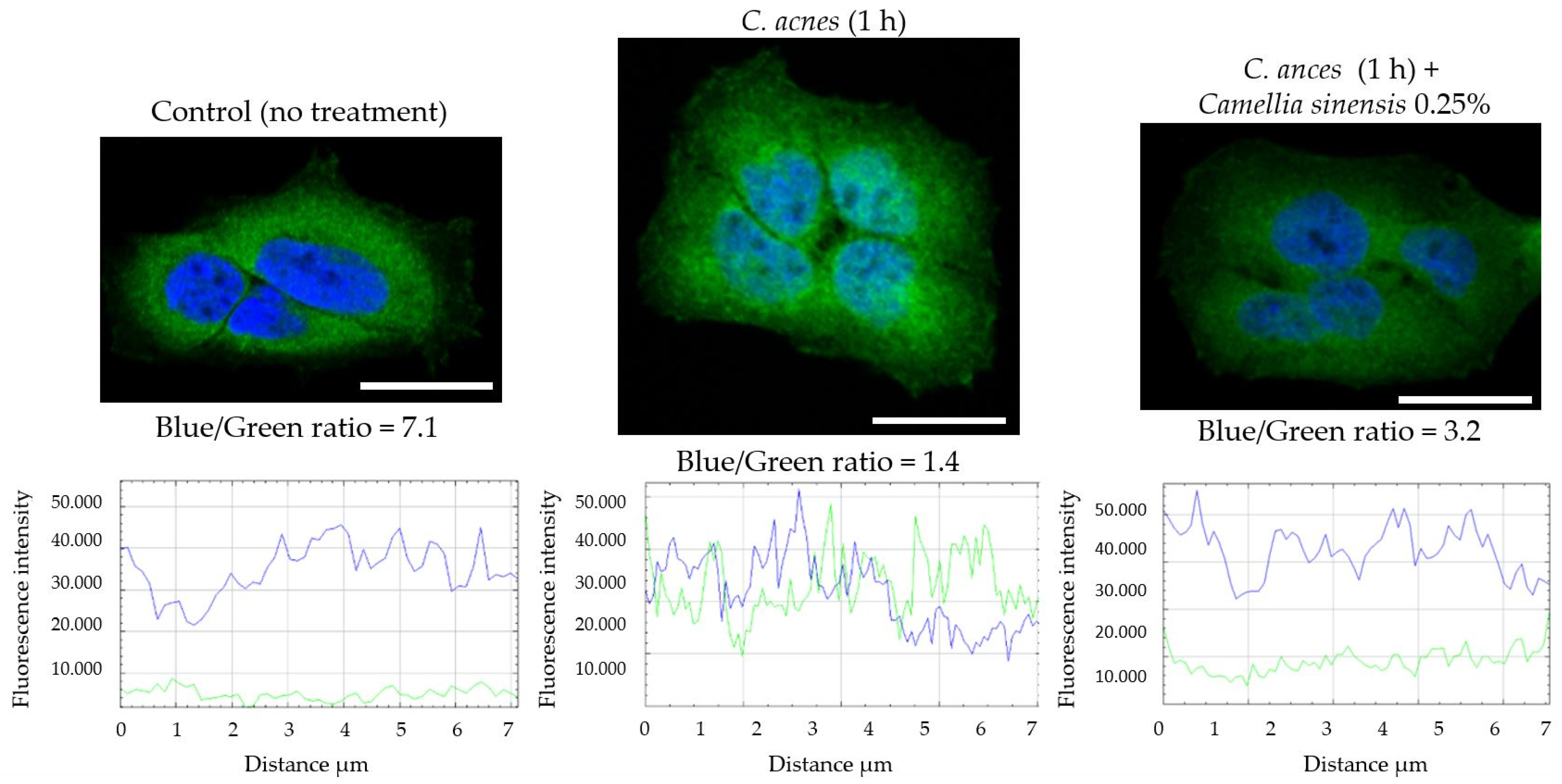
3.2. In Vitro Anti-Inflammatory Effect of Camellia sinensis Callus Cells Extract
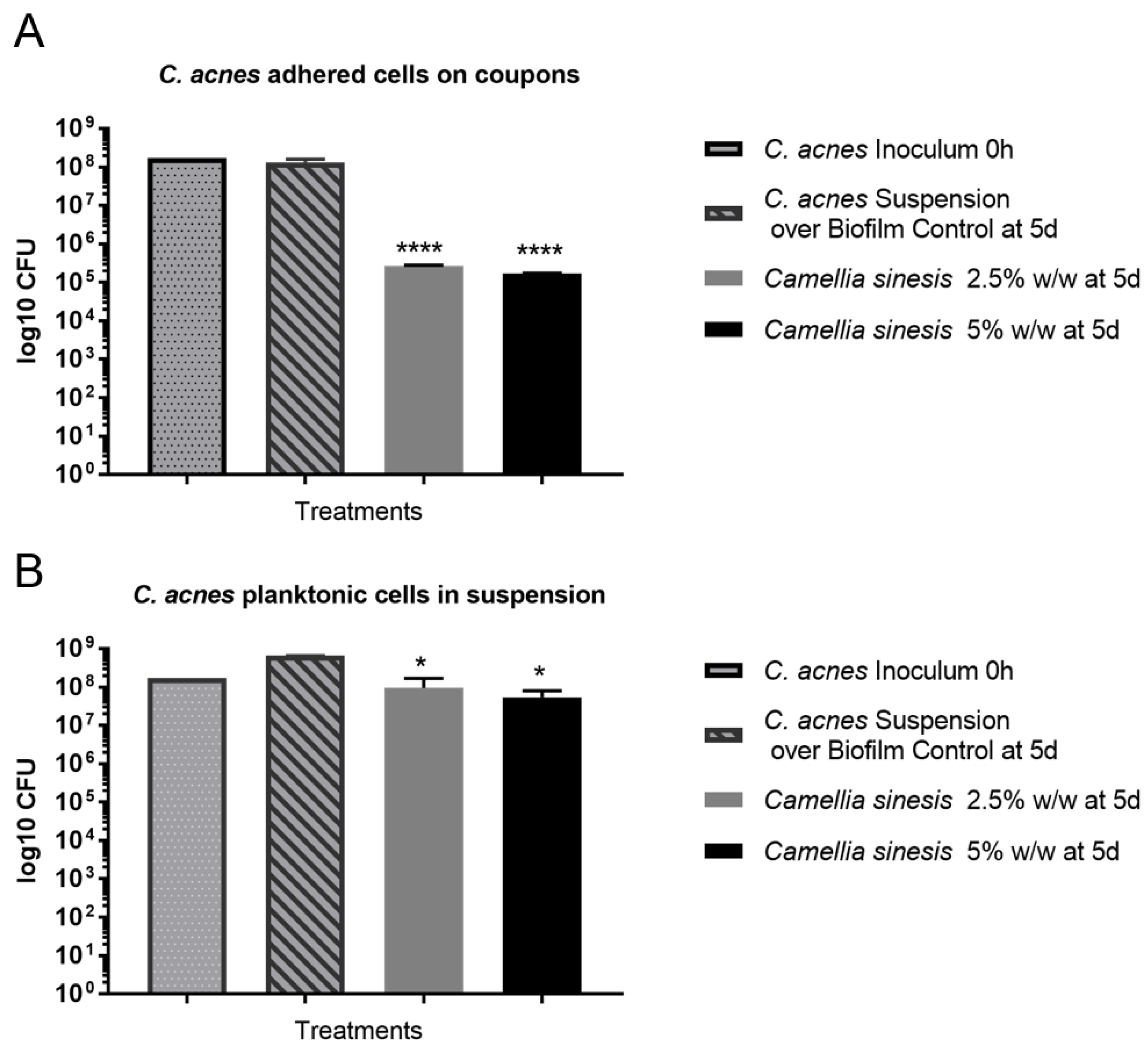
3.3. In Vitro Anti-Biofilm Activity
3.4. In Vitro Anti-Lipase Activity
3.5. Quantification of Autoinducer 2 (AI-2)
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Greydanus, D.E.; Azmeh, R.; Cabral, M.D.; Dickson, C.A.; Patel, D.R. Acne in the first three decades of life: An update of a disorder with profound implications for all decades of life. Dis. Mon. 2021, 67, 101103. [Google Scholar] [CrossRef] [PubMed]
- Lynn, D.; Umari, T.; Dellavalle, R.; Dunnick, C. The Epidemiology of Acne Vulgaris in Late Adolescence. Adolesc. Health Med. Ther. 2016, 7, 13. [Google Scholar] [CrossRef]
- Gannesen, A.V.; Zdorovenko, E.L.; Botchkova, E.A.; Hardouin, J.; Massier, S.; Kopitsyn, D.S.; Gorbachevskii, M.V.; Kadykova, A.A.; Shashkov, A.S.; Zhurina, M.V.; et al. Composition of the Biofilm Matrix of Cutibacterium acnes Acneic Strain RT5. Front. Microbiol. 2019, 10, 1284. [Google Scholar] [CrossRef]
- Parsek, M.R.; Greenberg, E.P. Sociomicrobiology: The Connections between Quorum Sensing and Biofilms. Trends Microbiol. 2005, 13, 27–33. [Google Scholar] [CrossRef]
- Bandara, H.M.H.N.; Lam, O.L.T.; Jin, L.J.; Samaranayake, L. Microbial Chemical Signaling: A Current Perspective. Crit. Rev. Microbiol. 2012, 38, 217–249. [Google Scholar] [CrossRef] [PubMed]
- Lwin, S.M.; Kimber, I.; McFadden, J.P. Acne, Quorum Sensing and Danger. Clin. Exp. Dermatol. 2014, 39, 162–167. [Google Scholar] [CrossRef]
- Zaenglein, A.L.; Pathy, A.L.; Schlosser, B.J.; Alikhan, A.; Baldwin, H.E.; Berson, D.S.; Bowe, W.P.; Graber, E.M.; Harper, J.C.; Kang, S.; et al. Guidelines of Care for the Management of Acne Vulgaris. J. Am. Acad. Dermatol. 2016, 74, 945–973.e33. [Google Scholar] [CrossRef] [PubMed]
- Leyden, J.; Stein-Gold, L.; Weiss, J. Why Topical Retinoids Are Mainstay of Therapy for Acne. Dermatol. Ther. 2017, 7, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Zasada, M.; Budzisz, E. Retinoids: Active Molecules Influencing Skin Structure Formation in Cosmetic and Dermatological Treatments. Postep. Dermatol. I Alergol. 2019, 36, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Sagransky, M.; Yentzer, B.A.; Feldman, S.R. Benzoyl Peroxide: A Review of Its Current Use in the Treatment of Acne Vulgaris. Expert. Opin. Pharmacother. 2009, 10, 2555–2562. [Google Scholar] [CrossRef]
- Nast, A.; Dreno, B.; Bettoli, V.; Degitz, K.; Erdmann, R.; Finlay, A.Y.; Ganceviciene, R.; Haedersdal, M.; Layton, A.; López-Estebaranz, J.L.; et al. European Evidence-Based (S3) Guidelines for the Treatment of Acne. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 1–29. [Google Scholar] [CrossRef]
- Azimi, H.; Fallah-Tafti, M.; Khakshur, A.A.; Abdollahi, M. A review of phytotherapy of acne vulgaris: Perspective of new pharmacological treatments. Fitoterapia 2012, 83, 1306–1317. [Google Scholar] [CrossRef]
- Fisk, W.A.; Lev-Tov, H.A.; Sivamani, R.K. Botanical and phytochemical therapy of acne: A systematic review. Phytother. Res. 2014, 28, 1137–1152. [Google Scholar] [CrossRef]
- Feuillolay, C.; Pecastaings, S.; Le Gac, C.; Fiorini-Puybaret, C.; Luc, J.; Joulia, P.; Roques, C. A Myrtus Communis Extract Enriched in Myrtucummulones and Ursolic Acid Reduces Resistance of Propionibacterium acnes Biofilms to Antibiotics Used in Acne Vulgaris. Phytomedicine 2016, 23, 307–315. [Google Scholar] [CrossRef] [PubMed]
- de Canha, M.N.; Komarnytsky, S.; Langhansova, L.; Lall, N. Exploring the Anti-Acne Potential of Impepho [Helichrysum odoratissimum (L.) Sweet] to Combat Cutibacterium acnes Virulence. Front. Pharmacol. 2020, 10, 1559. [Google Scholar] [CrossRef]
- Abozeid, D.; Fawzy, G.; Issa, M.; Abdeltawab, N.; Soliman, F. Medicinal Plants and Their Constituents in the Treatment of Acne Vulgaris. Biointerface Res. Appl. Chem. 2023, 13, 189. [Google Scholar] [CrossRef]
- Vuong, Q.V. Epidemiological evidence linking tea consumption to human health: A review. Crit. Rev. Food Sci. Nutr. 2014, 54, 523–536. [Google Scholar] [CrossRef] [PubMed]
- Hagiu, A.; Attin, T.; Schmidlin, P.R.; Ramenzoni, L.L. Dose-Dependent Green Tea Effect on Decrease of Inflammation in Human Oral Gingival Epithelial Keratinocytes: In Vitro Study. Clin. Oral. Investig. 2020, 24, 2375–2383. [Google Scholar] [CrossRef]
- Abe, K.; Ijiri, M.; Suzuki, T.; Taguchi, K.; Koyama, Y.; Isemura, M. Green tea with a high catechin content suppresses inflammatory cytokine expression in the galactosamine-injured rat liver. Biomed. Res. 2005, 26, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Miyoshi, N.; Isemura, M. Health-promoting effects of green tea. Proc. Jpn. Acad. Ser. B 2012, 88, 88–101. [Google Scholar] [CrossRef]
- Hamilton-Miller, J.M.T. Antimicrobial properties of tea (Camellia sinensis L.). Antimicrob. Agents Chemother. 1995, 39, 2375–2377. [Google Scholar] [CrossRef]
- Cox-Georgian, D.; Ramadoss, N.; Dona, C.; Basu, C. Therapeutic and Medicinal Uses of Terpenes. Med. Plants 2019, 12, 333–359. [Google Scholar] [CrossRef]
- Afzal, M.; Safer, A.M.; Menon, M. Green tea polyphenols and their potential role in health and disease. Inflammopharmacology 2015, 23, 151–161. [Google Scholar] [CrossRef]
- Wawrosch, C.; Zotchev, S.B. Production of bioactive plan secondary metabolites through in vitro technologies-status and outlook. Appl. Microbiol. Biotechnol. 2021, 18, 6649–6668. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.; Chiruvella, K.K.; Rao, Y.K.; Geethangili, M.; Raghavan, S.C.; Ghanta, R.G. In Vitro Production of Echioidinin, 7-O-Methywogonin from Callus Cultures of Andrographis lineata and Their Cytotoxicity on Cancer Cells. PLoS ONE 2015, 10, e0141154. [Google Scholar] [CrossRef]
- Georgiev, V.; Slavov, A.; Vasileva, I.; Pavlov, A. Plant Cell Culture as Emerging Technology for Production of Active Cosmetic Ingredients. Eng. Life Sci. 2018, 18, 779–798. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T. Biotechnology Applications of Plant Callus Cultures. Engineering 2019, 5, 50–59. [Google Scholar] [CrossRef]
- Ghorai, N.; Ghorai, N.; Chakraborty, S.; Gucchait, S.; Saha, S.K.; Biswas, S. Estimation of Total Terpenoids Concentration in Plant Tissues Using a Monoterpene, Linalool as Standard Reagent. Protoc. Exch. 2012, 1–7. [Google Scholar] [CrossRef]
- Fischer, K.; Tschismarov, R.; Pilz, A.; Straubinger, S.; Carotta, S.; McDowell, A.; Decker, T. Cutibacterium acnes Infection Induces Type I Interferon Synthesis Through the CGAS-STING Pathway. Front. Immunol. 2020, 11, 2630. [Google Scholar] [CrossRef]
- Barnard, E.; Liu, J.; Yankova, E.; Cavalcanti, S.M.; Magalhães, M.; Li, H.; Patrick, S.; McDowell, A. Strains of the Propionibacterium acnes Type III Lineage Are Associated with the Skin Condition Progressive Macular Hypomelanosis. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Champer, J.; Kim, J. Analysis of the Surface, Secreted, and Intracellular Proteome of Propionibacterium acnes. EuPA Open Proteom. 2015, 9, 1–7. [Google Scholar] [CrossRef]
- Denhez, B.; Rousseau, M.; Spino, C.; Dancosst, D.A.; Dumas, M.È.; Guay, A.; Lizotte, F.; Geraldes, P. Saturated fatty acids induce Insulin resistance in podocytes through inhibition of IRS1 via activation of both IKKβ and mTORC1. Sci. Rep. 2020, 10, 21628. [Google Scholar] [CrossRef]
- Coenye, T.; Peeters, E.; Nelis, H.J. Biofilm Formation by Propionibacterium acnes Is Associated with Increased Resistance to Antimicrobial Agents and Increased Production of Putative Virulence Factors. Res. Microbiol. 2007, 158, 386–392. [Google Scholar] [CrossRef]
- Humpton, T.J.; Nomura, K.; Weber, J.; Magnussen, H.M.; Hock, A.K.; Nixon, C.; Dhayade, S.; Stevenson, D.; Huang, D.T.; Strathdee, D.; et al. Differential requirements for MDM2 E3 activity during embryogenesis and in adult mice. Genes Dev. 2021, 35, 117–132. [Google Scholar] [CrossRef] [PubMed]
- Taga, M.E.; Xavier, K.B. Methods for Analysis of Bacterial Autoinducer-2 Production. Curr. Protoc. Microbiol. 2011, 23, 1C.1.1–1C.1.15. [Google Scholar] [CrossRef]
- Bae, J.; Kim, N.; Shin, Y.; Kim, S.-Y.; Kim, Y.-J. Activity of Catechins and Their Applications. Biomed. Dermatol. 2020, 4, 8. [Google Scholar] [CrossRef]
- Zhao, T.; Li, C.; Wang, S.; Song, X. Green Tea (Camellia sinensis): A Review of Its Phytochemistry, Pharmacology, and Toxicology. Molecules 2022, 27, 3909. [Google Scholar] [CrossRef]
- Dréno, B.; Dagnelie, M.A.; Khammari, A.; Corvec, S. The Skin Microbiome: A New Actor in Inflammatory Acne. Am. J. Clin. Dermatol. 2020, 21, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Ochoa, M.T.; Krutzik, S.R.; Takeuchi, O.; Uematsu, S.; Legaspi, A.J.; Brightbill, H.D.; Holland, D.; Cunliffe, W.J.; Akira, S.; et al. Activation of Toll-Like receptor 2 in acne triggers inflammatory cytokine responses. J. Immunol. 2002, 169, 1535–1541. [Google Scholar] [CrossRef]
- Pivarcsi, A.; Bodai, L.; Réthi, B.; Kenderessy-Szabó, A.; Koreck, A.; Széll, M.; Beer, Z.; Bata-Csörgoő, Z.; Magócsi, M.; Rajnavölgyi, É.; et al. Expression and function of Toll-like receptors 2 and 4 in human keratinocytes. Int. Immunol. 2003, 15, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Olaru, F.; Jensen, L.E. Chemokine expression by human keratinocyte cell lines after activation of Toll-like receptors. Exp. Dermatol. 2010, 19, e314–e316. [Google Scholar] [CrossRef]
- Takeuchi, O.; Hoshino, K.; Kawai, T.; Sanjo, H.; Takada, H.; Ogawa, T.; Takeda, K.; Akira, S. Differential Roles of TLR2 and TLR4 in Recognition of Gram-Negative and Gram-Positive Bacterial Cell Wall Components. Immunity 1999, 11, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.C.; Shamala, L.F.; Yi, X.K.; Yan, Z.; Wei, S. Analysis of Terpene Synthase Family Genes in Camellia sinensis with an Emphasis on Abiotic Stress Conditions. Sci. Rep. 2020, 10, 933. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Moon, D.G.; Ma, J.; Chen, L. Characteristics of non-volatile metabolites in fresh shoots from tea plant (Camellia sinensis) and its closely related species and varieties. Beverage Plant Res. 2022, 2, 9. [Google Scholar] [CrossRef]
- Menezes, J.C.; Borges, G.B.; Gomes, F.D.; Vieira, M.D.; Marques, A.R.; Machado, A.M. Volatile compounds and quality analysis in commercial medicinal plants of Camellia sinensis. Cienc. Rural 2019, 49, 12. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. Signaling to NF-kB by Toll-like Receptors. Trends Mol. Med. 2007, 13, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Firlej, E.; Kowalska, W.; Szymaszek, K.; Roliński, J.; Bartosińska, J. The Role of Skin Immune System in Acne. J. Clin. Med. 2022, 11, 1579. [Google Scholar] [CrossRef]
- Kelhälä, H.L.; Palatsi, R.; Fyhrquist, N.; Lehtimäki, S.; Väyrynen, J.P.; Kallioinen, M.; Kubin, M.E.; Greco, D.; Tasanen, K.; Alenius, H.; et al. IL-17/Th17 Pathway Is Activated in Acne Lesions. PLoS ONE 2014, 9, e105238. [Google Scholar] [CrossRef]
- Laclaverie, M.; Rouaud-Tinguely, P.; Grimaldi, C.; Jugé, R.; Marchand, L.; Aymard, E.; Closs, B. Development and Characterization of a 3D in Vitro Model Mimicking Acneic Skin. Exp. Dermatol. 2021, 30, 347–357. [Google Scholar] [CrossRef]
- Nguyen, A.T.; Kim, K.Y. Inhibition of Proinflammatory Cytokines in Cutibacterium acnes-Induced Inflammation in HaCaT Cells by Using Buddleja Davidii Aqueous Extract. Int. J. Inflam. 2020. [Google Scholar] [CrossRef]
- Hwang, D.H.; Lee, D.Y.; Koh, P.O.; Yang, H.R.; Kang, C.; Kim, E. Rosa davurica Pall. improves Propionibacterium acnes-Induced Inflammatory Responses in Mouse Ear Edema Model and Suppresses pro-Inflammatory Chemokine Production via MAPK and NF-kB Pathways in HaCaT Cells. Int. J. Mol. Sci. 2020, 21, 1717. [Google Scholar] [CrossRef]
- Bandyopadhyay, D.; Chatterjee, T.K.; Dasgupta, A.; Lourduraja, J.; Dastidar, S.G. In Vitro and in Vivo Antimicrobial Action of Tea: The Commonest Beverage of Asia. Biol. Pharm. Bull. 2005, 28, 2125–2127. [Google Scholar] [CrossRef]
- Sowjanya, N.S.; Ramya, S.R.; Kanungo, R. In Vitro Antibacterial Activity of Green Tea (Camellia sinensis) Extract against Staphylococcus Aureus and MRSA. IP Int. J. Med. Microbiol. Trop. Dis. 2020, 4, 214–217. [Google Scholar] [CrossRef]
- Anita, P.; Sivasamy, S.; Kumar, P.M.; Balan, I.N.; Ethiraj, S. In Vitro Antibacterial Activity of Camellia sinensis Extract against Cariogenic Microorganisms. J. Basic Clin. Pharm. 2015, 6, 35. [Google Scholar] [CrossRef] [PubMed]
- Fournière, M.; Latire, T.; Souak, D.; Feuilloley, M.G.J.; Bedoux, G. Staphylococcus epidermidis and cutibacterium Acnes: Two Major Sentinels of Skin Microbiota and the Influence of Cosmetics. Microorganisms 2020, 8, 1752. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Cheng, J.W.; Zhang, Q.; Hua, Z.X.; Miao, X. Comparison of the Skin Microbiota of Patients with Acne Vulgaris and Healthy Controls. Ann. Palliat. Med. 2021, 10, 7933–7941. [Google Scholar] [CrossRef] [PubMed]
- Coenye, T.; Spittaels, K.J.; Achermann, Y. The Role of Biofilm Formation in the Pathogenesis and Antimicrobial Susceptibility of Cutibacterium acnes. Biofilm 2022, 4, 100063. [Google Scholar] [CrossRef]
- Pena, R.T.; Blasco, L.; Ambroa, A.; González-Pedrajo, B.; Fernández-García, L.; López, M.; Bleriot, I.; Bou, G.; García-Contreras, R.; Wood, T.K.; et al. Relationship between Quorum Sensing and Secretion Systems. Front. Microbiol. 2019, 10, 1100. [Google Scholar] [CrossRef]
- Unno, M.; Cho, O.; Sugita, T. Inhibition of Propionibacterium acnes Lipase Activity by the Antifungal Agent Ketoconazole. Microbiol. Immunol. 2017, 61, 42–44. [Google Scholar] [CrossRef]
- Nakase, K.; Tashiro, A.; Yamada, T.; Ikoshi, H.; Noguchi, N. Shiunko and Chuoko, topical Kampo medicines, inhibit the expression of gehA encoding the extracellular lipase in Cutibacterium acnes. J. Dermatol. 2019, 46, 308–313. [Google Scholar] [CrossRef]
- Wunnoo, S.; Saising, J.; Voravuthikunchai, S.P. Rhodomyrtone inhibits lipase production, biofilm formation, and disorganizes established biofilm in Propionibacterium acnes. Anaerobe 2017, 43, 61–68. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cañellas-Santos, M.; Rosell-Vives, E.; Montell, L.; Bilbao, A.; Goñi-de-Cerio, F.; Fernandez-Campos, F. Anti-Inflammatory and Anti-Quorum Sensing Effect of Camellia sinensis Callus Lysate for Treatment of Acne. Curr. Issues Mol. Biol. 2023, 45, 3997-4016. https://doi.org/10.3390/cimb45050255
Cañellas-Santos M, Rosell-Vives E, Montell L, Bilbao A, Goñi-de-Cerio F, Fernandez-Campos F. Anti-Inflammatory and Anti-Quorum Sensing Effect of Camellia sinensis Callus Lysate for Treatment of Acne. Current Issues in Molecular Biology. 2023; 45(5):3997-4016. https://doi.org/10.3390/cimb45050255
Chicago/Turabian StyleCañellas-Santos, Mariona, Elisabet Rosell-Vives, Laia Montell, Ainhoa Bilbao, Felipe Goñi-de-Cerio, and Francisco Fernandez-Campos. 2023. "Anti-Inflammatory and Anti-Quorum Sensing Effect of Camellia sinensis Callus Lysate for Treatment of Acne" Current Issues in Molecular Biology 45, no. 5: 3997-4016. https://doi.org/10.3390/cimb45050255
APA StyleCañellas-Santos, M., Rosell-Vives, E., Montell, L., Bilbao, A., Goñi-de-Cerio, F., & Fernandez-Campos, F. (2023). Anti-Inflammatory and Anti-Quorum Sensing Effect of Camellia sinensis Callus Lysate for Treatment of Acne. Current Issues in Molecular Biology, 45(5), 3997-4016. https://doi.org/10.3390/cimb45050255







