Physiological and Biochemical Properties of Cotton Seedlings in Response to Cu2+ Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials, Growth Condition, and Treatments
2.2. Determination of Growth Parameters
2.3. Measurement of Photosynthetic Pigment Contents
2.4. Determination of Copper Content
2.5. Detection of H2O2 and MDA Levels
2.6. Measurement of POD Activity and GSH Content
2.7. Determination of Soluble Sugar Content
2.8. Statistical Analysis
3. Results
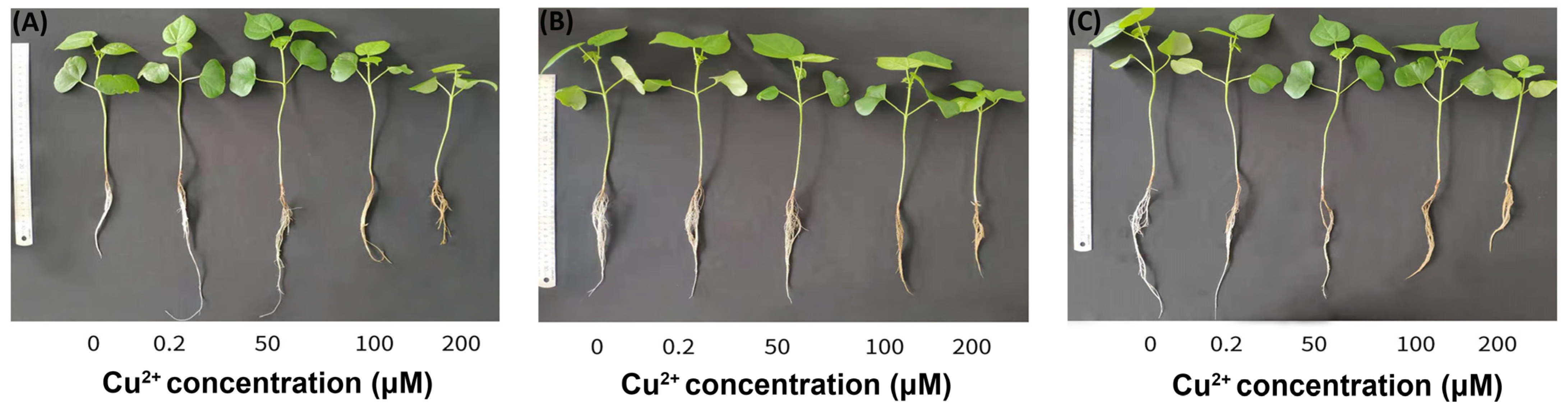
3.1. Plant Growth Conditions under Cu2+ Stress
3.2. Content of Photosynthetic Pigments
3.3. Determination of Cu2+ Content
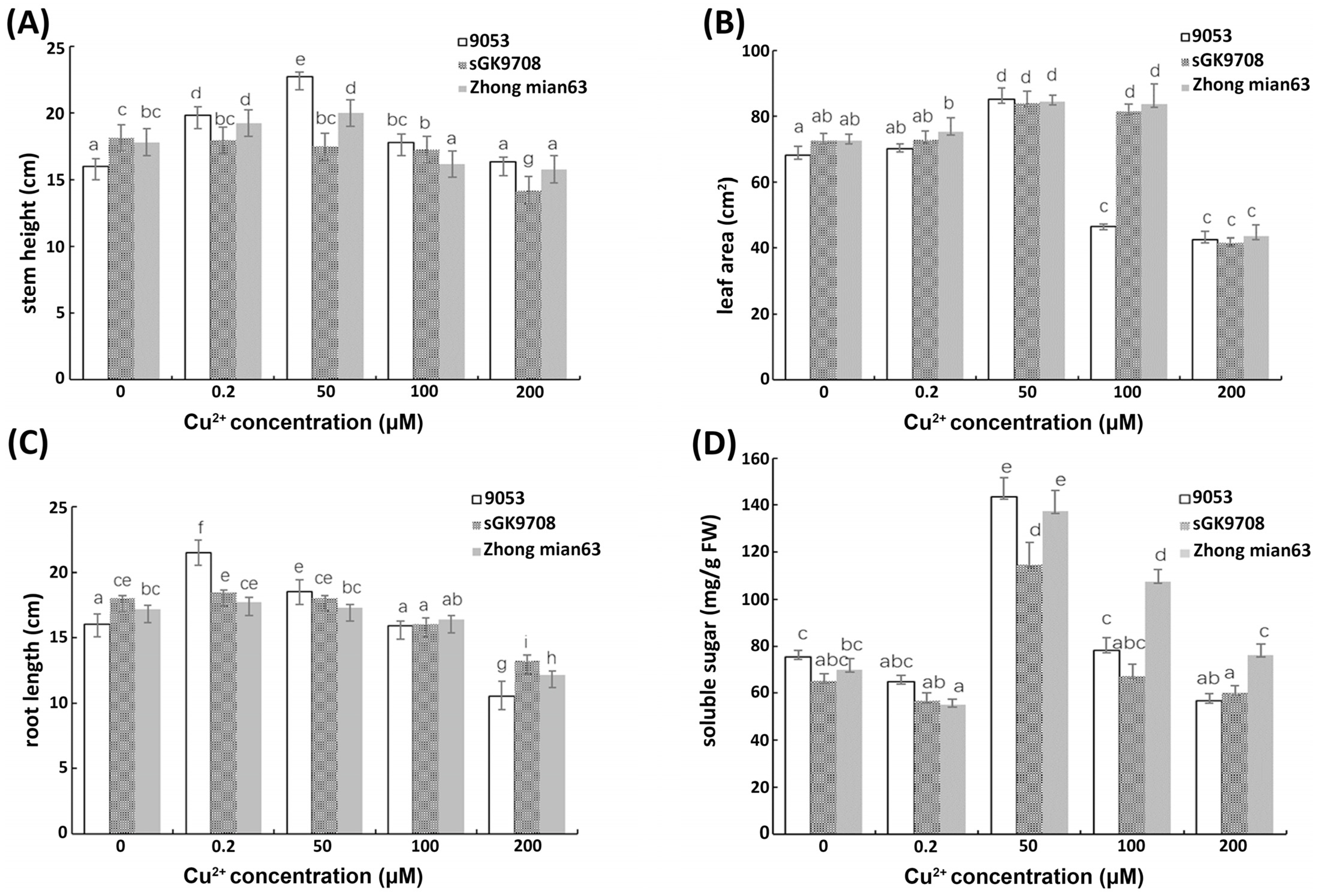
3.4. Determination of Morphological Characters and Soluble Sugar Content
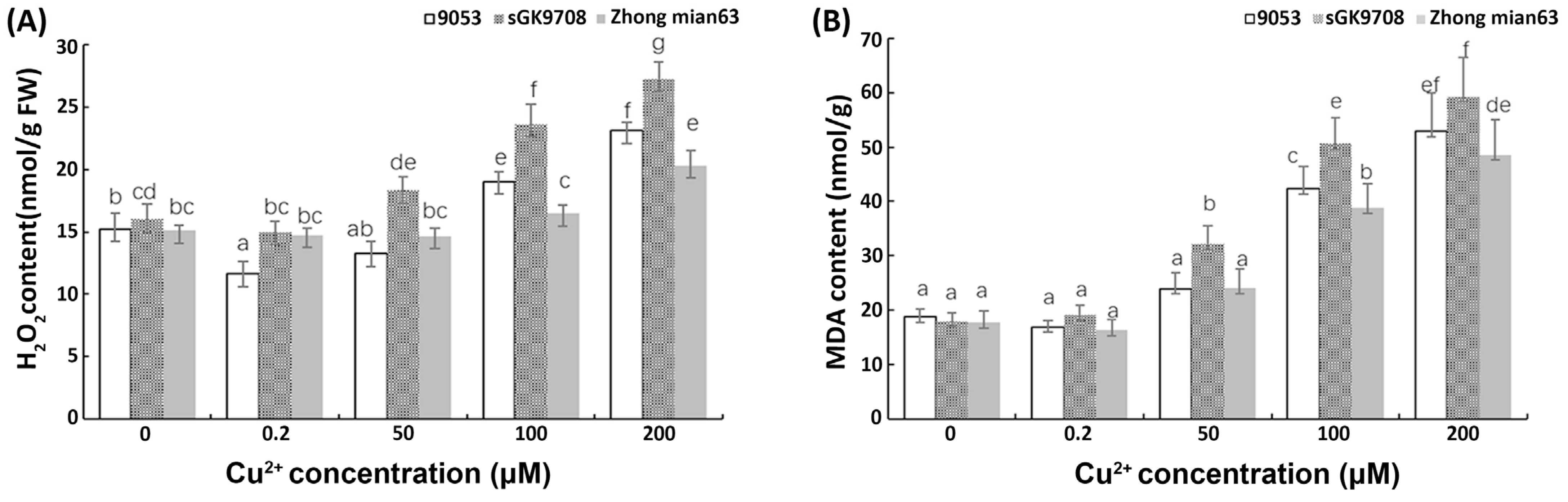
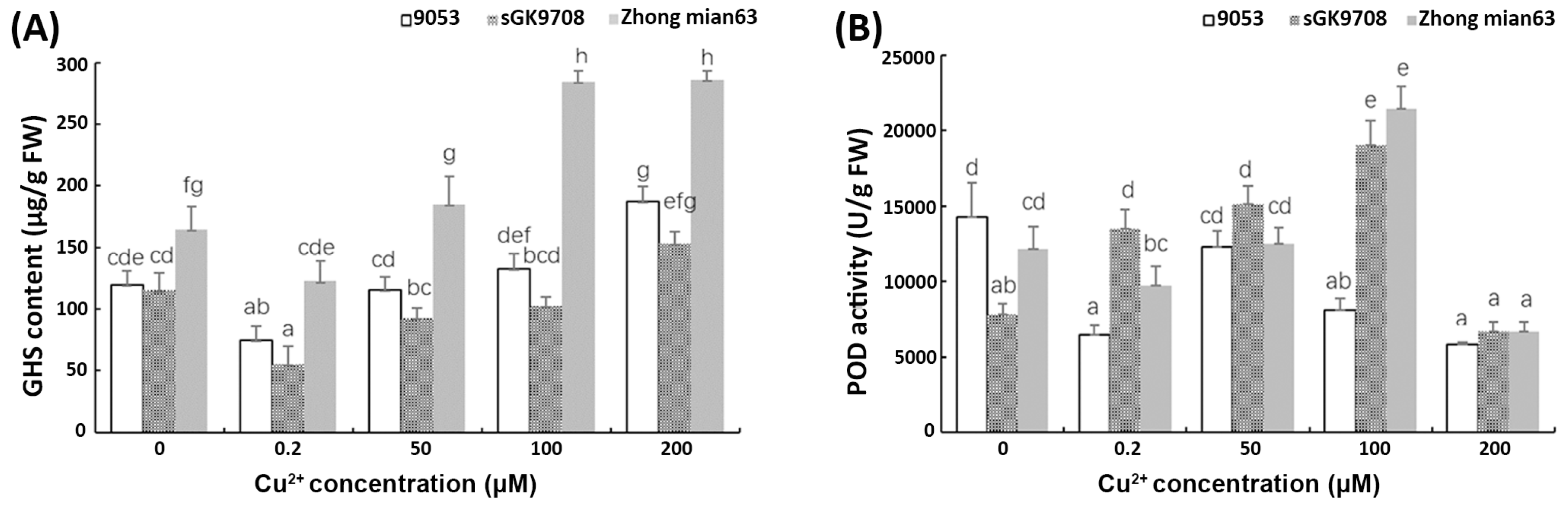
3.5. Determination of H2O2 and MDA Content and Antioxidant Scavenging Ability
4. Discussion
4.1. Morpho-Physiological Changes and Copper Absorption
4.2. ROS Accumulation Levels and Peroxidation
4.3. Antioxidant Alleviation and Soluble Sugar Defensive Mechanisms
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Cu2+ | copper(II) |
| MTs | Metallothioneins |
| PCs | Phytochelatins |
| SOD | superoxide dismutase |
| CAT | catalases |
| POD | Peroxidase |
| GSH | Glutathione |
| AsA | ascorbate |
| ROS | reactive oxygen species |
| MDA | Malondialdehyde |
References
- Chaâbene, Z.; Rorat, A.; Kriaa, W.; Rekik, I.; Mejdoub, H.; Vandenbulcke, F.; Elleuch, A. In-Site and Ex-Site Date Palm Exposure to Heavy Metals Involved Infra-Individual Biomarkers Upregulation. Plants 2021, 10, 137. [Google Scholar] [CrossRef]
- Thounaojam, T.C.; Panda, P.; Mazumdar, P.; Kumar, D.; Sharma, G.D.; Sahoo, L.; Panda, S.K. Excess Copper Induced Oxidative Stress and Response of Antioxidants in Rice. Plant Physiol. Biochem. 2012, 53, 33–39. [Google Scholar] [CrossRef]
- Wuana, R.A.; Okieimen, F.E. Heavy Metals in Contaminated Soils: A Review of Sources, Chemistry, Risks and Best Available Strategies for Remediation. ISRN Ecol. 2011, 2011, 402647. [Google Scholar] [CrossRef]
- Sharma, M.; Kumar, V.; Bhardwaj, R.; Thukral, A.K. Tartaric Acid Mediated Cr Hyperac Cu2+ mulation and Biochemical Alterations in Seedlings of Hordeum vulgare L. J. Plant Growth Regul. 2019, 39, 1–14. [Google Scholar] [CrossRef]
- Yruela, I. Copper in Plants. Braz. J. Plant Physiol. 2005, 17, 145–156. [Google Scholar] [CrossRef]
- Marschner, P. Marschner’s Mineral Nutrition of Higher Plants. Exp. Agric. 2012, 48, 305. [Google Scholar] [CrossRef]
- Shabbir, Z.; Sardar, A.; Shabbir, A.; Abbas, G.; Shamshad, S.; Khalid, S.; Natasha; Murtaza, G.; Dumat, C.; Shahid, M. Copper Uptake, Essentiality, Toxicity, Detoxification and Risk Assessment in Soil-Plant Environment. Chemosphere 2020, 259, 127436. [Google Scholar] [CrossRef]
- Printz, B.; Lutts, S.; Hausman, J.F.; Sergeant, K. Copper Trafficking in Plants and Its Implication on Cell Wall Dynamics. Front. Plant Sci. 2016, 7, 601. [Google Scholar] [CrossRef]
- Janas, K.M.; Zielińska-Tomaszewska, J.; Rybaczek, D.; Maszewski, J.; Posmyk, M.M.; Amarowicz, R.; Kosińska, A. The Impact of Copper Ions on Growth, Lipid Peroxidation, and Phenolic Compound AcCu2+mulation and Localization in Lentil (Lens culinaris medic.) Seedlings. J. Plant Physiol. 2010, 167, 270–276. [Google Scholar] [CrossRef]
- Lange, B.; van der Ent, A.; Baker, A.J.M.; Echevarria, G.; Mahy, G.; Malaisse, F.; Meerts, P.; Pourret, O.; Verbruggen, N.; Faucon, M.P. Copper and Cobalt AcCu2+mulation in Plants: A Critical Assessment of the Cu2+rrent State of Knowledge. New Phytol. 2017, 213, 537–551. [Google Scholar] [CrossRef]
- Marques, D.M.; Veroneze Júnior, V.; da Silva, A.B.; Mantovani, J.R.; Magalhães, P.C.; de Souza, T.C. Copper Toxicity on Photosynthetic Responses and Root Morphology of Hymenaea courbaril L. (Caesalpinioideae). Water Air Soil Pollut. 2018, 229, 138. [Google Scholar] [CrossRef]
- Jeong-Myeong, K.; Shim, J.K. Toxic Effects of Serpentine Soils on Plant Growth. J. Ecol. Environ. 2008, 31, 327–331. [Google Scholar] [CrossRef]
- Clemens, S. MoleCu2+lar Mechanisms of Plant Metal Tolerance and Homeostasis. Planta 2001, 212, 475–486. [Google Scholar] [CrossRef]
- Zabalza, A.; Gálvez, L.; Marino, D.; Royuela, M.; Arrese-Igor, C.; González, E.M. The Application of Ascorbate or Its Immediate PreCu2+rsor, Galactono-1,4-Lactone, Does Not Affect the Response of Nitrogen-Fixing Pea Nodules to Water Stress. J. Plant Physiol. 2008, 165, 805–812. [Google Scholar] [CrossRef]
- Kang, Y.J.; Eenger, M.D. Effect of Cellular Glutathione Depletion on Cadmium-Induced Cytotoxicity in Human Lung Carcinoma Cells. Cell Biol. Toxicol. 1987, 3, 347–360. [Google Scholar] [CrossRef]
- Jozefczak, M.; Remans, T.; Vangronsveld, J.; Cu2+ypers, A. Glutathione Is a Key Player in Metal-Induced Oxidative Stress Defenses. Int. J. Mol. Sci. 2012, 13, 3145–3175. [Google Scholar] [CrossRef]
- Saleem, M.H.; Kamran, M.; Zhou, Y.; Parveen, A.; Rehman, M.; Ahmar, S.; Malik, Z.; Mustafa, A.; Ahmad Anjum, R.M.; Wang, B.; et al. Appraising Growth, Oxidative Stress and Copper Phytoextraction Potential of Flax (Linum usitatissimum L.) Grown in Soil Differentially Spiked with Copper. J. Environ. Manag. 2020, 257, 109994. [Google Scholar] [CrossRef]
- Sidhu, G.P.S.; Bali, A.S.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Insights into the Tolerance and Phytoremediation Potential of Coronopus didymus L. (Sm) Grown under Zinc Stress. Chemosphere 2020, 244, 125–350. [Google Scholar] [CrossRef]
- Contreras, R.A.; Pizarro, M.; Köhler, H.; Sáez, C.A.; Zúñiga, G.E. Copper Stress Induces Antioxidant Responses and AcCu2+mulation of Sugars and Phytochelatins in Antarctic Colobanthus Quitensis (Kunth) Bartl. Biol. Res. 2018, 51, 48. [Google Scholar] [CrossRef]
- Li, L.; Yan, X.; Li, J.; Tian, Y.; Ren, P. Advances in Cotton Tolerance to Heavy Metal Stress and Applications to Remediate Heavy Metal-Contaminated Farmland Soil. Phyton 2020, 90, 35–50. [Google Scholar] [CrossRef]
- Macdonald, S.; Gale, F.; Hansen, J. Cotton Policy in China. Munich Personal RePEc Archive, U.S. Department of Agriculture, Economic Research 2016. Available online: https://mpra.ub.uni-muenchen.de/id/eprint/70863 (accessed on 27 April 2016).
- Mei, L.; Daud, M.K.; Ullah, N.; Ali, S.; Khan, M.; Malik, Z.; Zhu, S.J. Pretreatment with Salicylic Acid and Ascorbic Acid SPretreatment with Salicylic Acid and Ascorbic Acid Significantly Mitigate Oxidative Stress Induced by Copper in Cotton Genotypes. Environ. Sci. Pollut. Res. 2015, 22, 9922–9931. [Google Scholar] [CrossRef]
- Wang, J.; Moeen-Ud-Din, M.; Yin, R.; Yang, S. ROS Homeostasis Involved in Dose-Dependent Responses of Arabidopsis Seedlings to Copper Toxicity. Genes 2022, 14, 11. [Google Scholar] [CrossRef]
- Elleuch, A.; Chaâbene, Z.; Grubb, D.C.; Drira, N.; Mejdoub, H.; Khemakhem, B. Morphological and Biochemical Behavior of Fenugreek (Trigonella foenum-graecum) under Copper Stress. Ecotoxicol. Environ. Saf. 2013, 98, 46–53. [Google Scholar] [CrossRef]
- Schmitz, D.; Girardi, J.; Jamin, J.; Bundschuh, M.; Geng, B.; Feldmann, R.; Rösch, V.; Riess, K.; Schirmel, J. Copper Uptake and Its Effects on Two Riparian Plant Species, the Native Urtica Dioica, and the Invasive Fallopia Japonica. Plants 2023, 12, 481. [Google Scholar] [CrossRef]
- Sawan, Z.M.; Mahmoud, M.H.; Gregg, B.R. Effect of Foliar Application of Chelated Copper and Manganese on Yield Components and Fibre Properties of Egyptian Cotton (Gossypium barbadense). J. Agric. Sci. 1993, 121, 193–198. [Google Scholar] [CrossRef]
- Lichtenthhaler, H.K.; Wellburn, A.R. Determinations of Total Carotenoids and Chlorophylls a and b of Leaf Extracts in Different Solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Bouazizi, H.; Jouili, H.; Geitmann, A.; El Ferjani, E. Copper Toxicity in Expanding Leaves of Phaseolus vulgaris L.: Antioxidant Enzyme Response and Nutrient Element Uptake. Ecotoxicol. Environ. Saf. 2010, 73, 1304–1308. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Lee, S.; Wen, R. Copper-Caused Oxidative Stress Triggers the Activation of Antioxidant Enzymes via ZmMPK3 in Maize Leaves. PLoS ONE 2018, 13, e0203612. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Lu, H.; Kong, X.; Dai, J.; Li, Z.; Dong, H. Growth, Lint Yield and Changes in Physiological Attributes of Cotton under Temporal Waterlogging. Field Crop. Res. 2016, 194, 83–93. [Google Scholar] [CrossRef]
- Ates, B.; Ercal, B.C.; Manda, K.; Abraham, L.; Ercal, N. Determination of Glutathione Disulfide Levels in Biological Samples Using Thiol-Disulfide Exchanging Agent, Dithiothreitol. Biomed. Chromatogr. 2009, 23, 119–123. [Google Scholar] [CrossRef]
- Anjorin, F.B.; Adejumo, S.A.; Agboola, L.; Samuel, Y.D. Proline, Soluble Sugar, Leaf Starch and Relative Water Contents of Four Maize Varieties in Response to Different Watering Regimes. Cercet. Agron. Mold. 2016, 49, 51–62. [Google Scholar] [CrossRef]
- Shahid, M.; Dumat, C.; Khalid, S.; Schreck, E.; Xiong, T.; Niazi, N.K. Foliar Heavy Metal Uptake, Toxicity and Detoxification in Plants: A Comparison of Foliar and Root Metal Uptake. J. Hazard. Mater. 2017, 325, 36–58. [Google Scholar] [CrossRef]
- Wani, W.; Masoodi, K.Z.; Zaid, A.; Wani, S.H.; Shah, F.; Meena, V.S.; Wani, S.A.; Mosa, K.A. Engineering Plants for Heavy Metal Stress Tolerance. Rend. Lincei 2018, 29, 709–723. [Google Scholar] [CrossRef]
- Asgari Lajayer, B.; Ghorbanpour, M.; Nikabadi, S. Heavy Metals in Contaminated Environment: Destiny of Secondary Metabolite Biosynthesis, Oxidative Status and Phytoextraction in Medicinal Plants. Ecotoxicol. Environ. Saf. 2017, 145, 377–390. [Google Scholar] [CrossRef]
- Bardiya-Bhurat, K.; Sharma, S.; Mishra, Y.; Patankar, C. Tagetes Erecta (Marigold), a Phytoremediant for Ni- and Pb-Contaminated Area: A Hydroponic Analysis and Factors Involved. Rend. Lincei 2017, 28, 673–678. [Google Scholar] [CrossRef]
- Bislimi, K.; Sahiti, H.; Halili, J.; Bici, M.; Mazreku, I. Effect of Mining Activity in AcCu2+mulation of Heavy Metals in Soil and Plant (Urtica dioica L). J. Ecol. Eng. 2020, 22, 1–7. [Google Scholar] [CrossRef]
- Brunetto, G.; Bastos de Melo, G.W.; Terzano, R.; Del Buono, D.; Astolfi, S.; Tomasi, N.; Pii, Y.; Mimmo, T.; Cesco, S. Copper AcCu2+mulation in Vineyard Soils: Rhizosphere Processes and Agronomic Practices to Limit Its Toxicity. Chemosphere 2016, 162, 293–307. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, J.; Dou, R.; Gao, X.; Mao, C.; Wang, L. Assessment of the Phytotoxicity of Metal Oxide Nanoparticles on Two Crop Plants, Maize (Zea mays L.) and Rice (Oryza sativa L.). Int. J. Environ. Res. Public Health 2015, 12, 15100–15109. [Google Scholar] [CrossRef]
- Lombardi, L.; Sebastiani, L. Copper Toxicity in Prunus Cerasifera: Growth and Antioxidant Enzymes Responses of in Vitro Grown Plants. Plant Sci. 2005, 168, 797–802. [Google Scholar] [CrossRef]
- Xu, Q.S.; Hu, J.Z.; Xie, K.B.; Yang, H.Y.; Du, K.H.; Shi, G.X. Accumulation and Acute Toxicity of Silver in Potamogeton crispus L. J. Hazard. Mater. 2010, 173, 186–193. [Google Scholar] [CrossRef]
- González-Mendoza, D.; Espadas y Gil, F.; Escoboza-Garcia, F.; Santamaría, J.M.; Zapata-Perez, O. Copper Stress on Photosynthesis of Black Mangle (Avicennia germinans). An. Acad. Bras. Cienc. 2013, 85, 665–670. [Google Scholar] [CrossRef]
- Rehman, M.; Liu, L.; Wang, Q.; Saleem, M.H.; Bashir, S.; Ullah, S.; Peng, D. Copper Environmental Toxicology, Recent Advances, and Future Outlook: A Review. Environ. Sci. Pollut. Res. 2019, 26, 18003–18016. [Google Scholar] [CrossRef]
- Hindarti, D.; Larasati, A.W. Copper (Cu2+) and Cadmium (Cd) Toxicity on Growth, Chlorophyll-a and Carotenoid Content of Phytoplankton Nitzschia Sp. IOP Conf. Ser. Earth Environ. Sci. 2019, 236, 12053. [Google Scholar] [CrossRef]
- Wang, L.; Yang, L.; Yang, F.; Li, X.; Song, Y.; Wang, X.; Hu, X. Involvements of H2O2 and Metallothionein in NO-Mediated Tomato Tolerance to Copper Toxicity. J. Plant Physiol. 2010, 167, 1298–1306. [Google Scholar] [CrossRef]
- Tang, S.; Zhang, H.; Li, L.; Liu, X.; Chen, L.; Chen, W.; Ding, Y. Exogenous Spermidine Enhances the Photosynthetic and Antioxidant Capacity of Rice under Heat Stress during Early Grain-Filling Period. Funct. Plant Biol. 2018, 45, 911–921. [Google Scholar] [CrossRef]
- Hossain, M.S.; Abdelrahman, M.; Tran, C.D.; Nguyen, K.H.; Chu, H.D.; Watanabe, Y.; Hasanuzzaman, M.; Mohsin, S.M.; Fujita, M.; Tran, L.S.P. Insights into Acetate-Mediated Copper Homeostasis and Antioxidant Defense in Lentil under Excessive Copper Stress. Environ. Pollut. 2020, 258, 113544. [Google Scholar] [CrossRef]
- Chakilam, S. Metal Effect on Carotenoid Content of Cyanobacteria. Int. J. Bot. 2012, 8, 192–197. [Google Scholar] [CrossRef]
- Woldetsadik, D.; Drechsel, P.; Keraita, B.; Itanna, F.; Gebrekidan, H. Heavy Metal AcCu2+mulation and Health Risk Assessment in Wastewater-Irrigated Urban Vegetable Farming Sites of Addis Ababa, Ethiopia. Int. J. Food Contam. 2017, 4, 1–13. [Google Scholar] [CrossRef]
- Adnan, M.; Xiao, B.; Xiao, P.; Zhao, P.; Li, R.; Bibi, S. Research Progress on Heavy Metals Pollution in the Soil of Smelting Sites in China. Toxics 2022, 10, 231. [Google Scholar] [CrossRef]
- Mir, A.R.; Pichtel, J.; Hayat, S. Copper: Uptake, Toxicity and Tolerance in Plants and Management of Cu2+-Contaminated Soil. BioMetals 2021, 34, 737–759. [Google Scholar] [CrossRef]
- Shah, J.; Kachroo, P.; Nandi, A.; Klessig, D.F. A Recessive Mutation in the Arabidopsis SSI2 Gene Confers SA- and NPR1-Independent Expression of PR Genes and Resistance against Bacterial and Oomycete Pathogens. Plant J. 2001, 25, 563–574. [Google Scholar] [CrossRef]
- Zehra, A.; Choudhary, S.; Wani, K.I.; Naeem, M.; Khan, M.M.A.; Aftab, T. Exogenous Abscisic Acid Mediates ROS Homeostasis and Maintains Glandular Trichome to Enhance Artemisinin Biosynthesis in Artemisia Annua under Copper Toxicity. Plant Physiol. Biochem. 2020, 156, 125–134. [Google Scholar] [CrossRef]
- Bang, S.W.; Choi, S.; Jin, X.; Jung, S.E.; Choi, J.W.; Seo, J.S.; Kim, J.K. Transcriptional Activation of Rice CINNAMOYL-CoA REDUCTASE 10 by OsNAC5, Contributes to Drought Tolerance by Modulating Lignin AcCu2+mulation in Roots. Plant Biotechnol. J. 2022, 20, 736–747. [Google Scholar] [CrossRef] [PubMed]
- Sami, A.; Shah, F.A.; Abdullah, M.; Zhou, X.; Yan, Y.; Zhu, Z.; Zhou, K. Melatonin Mitigates Cadmium and Aluminium Toxicity through Modulation of Antioxidant Potential in Brassica napus L. Plant Biol. 2020, 22, 679–690. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Y.Q.; Yan, X.W.; Wei, G.H.; Zhang, J.H.; Fang, L.C. Rhizobium InoCu2+lation Enhances Copper Tolerance by Affecting Copper Uptake and Regulating the Ascorbate-Glutathione Cycle and Phytochelatin Biosynthesis-Related Gene Expression in Medicago Sativa Seedlings. Ecotoxicol. Environ. Saf. 2018, 162, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Pardo, B.; Fernández-PasCual, M.; Zornoza, P. Copper Microlocalisation, Ultrastructural Alterations and Antioxidant Responses in the Nodules of White Lupin and Soybean Plants Grown under Conditions of Copper Excess. Environ. Exp. Bot. 2012, 84, 52–60. [Google Scholar] [CrossRef]
- Dat, J.; Vandenabeele, S.; Vranová, E.; Van Montagu, M.; Inzé, D.; Van Breusegem, F. Dual Action of the Active Oxygen Species during Plant Stress Responses. Cell. Mol. Life Sci. 2000, 57, 779–795. [Google Scholar] [CrossRef]
- Noctor, G.; Foyer, C.H. Ascorbate and Glutathione: Keeping Active Oxygen under Control. Annu. Rev. Plant Biol. 1998, 49, 249–279. [Google Scholar] [CrossRef]
- Chen, Z.-H.; Wang, Y.; Aroca, R.; Wani, O.A.; Lone, J.K.; Manhas, S.; Kour, N.; Alam, P.; Ahmad, A.; Ahmad, P. Reactive Oxygen Species in Plants: From Source to Sink. Antioxidants 2022, 11, 225. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, J.Z.; Chow, W.S.; Sun, L.L.; Chen, J.W.; Chen, Y.J.; Peng, C.L. The Influence of Low Temperature on Photosynthesis and Antioxidant Enzymes in Sensitive Banana and Tolerant Plantain (Musa Sp.) Cu2+ltivars. Photosynthetica 2011, 49, 201–208. [Google Scholar] [CrossRef]
- Ferreira, P.A.A.; Tiecher, T.; Tiecher, T.L.; de Melo Rangel, W.; Soares, C.R.F.S.; Deuner, S.; Tarouco, C.P.; Giachini, A.J.; Nicoloso, F.T.; Brunetto, G.; et al. Effects of Rhizophagus Clarus and P Availability in the Tolerance and Physiological Response of MuCu2+na Cinereum to Copper. Plant Physiol. Biochem. 2018, 122, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.Q.; Wang, Y.S.; Lou, Z.P.; De Dong, J. Effect of Heavy Metal Stress on Antioxidative Enzymes and Lipid Peroxidation in Leaves and Roots of Two Mangrove Plant Seedlings (Kandelia candel and Bruguiera gymnorrhiza). Chemosphere 2007, 67, 44–50. [Google Scholar] [CrossRef]
- Cobbett, C.; Goldsbrough, P. Phytochelatins and Metallothioneins: Roles in Heavy Metal Detoxification and Homeostasis. Annu. Rev. Plant Biol. 2002, 53, 159–182. [Google Scholar] [CrossRef] [PubMed]
- Couée, I.; Sulmon, C.; Gouesbet, G.; El Amrani, A. Involvement of Soluble Sugars in Reactive Oxygen Species Balance and Responses to Oxidative Stress in Plants. J. Exp. Bot. 2006, 57, 449–459. [Google Scholar] [CrossRef]
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.Q. Heavy Metals and Pesticides Toxicity in AgriCu2+ltural Soil and Plants: Ecological Risks and Human Health Implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Tóth, G.; Hermann, T.; Da Silva, M.R.; Montanarella, L. Heavy Metals in AgriCu2+ltural Soils of the European Union with Implications for Food Safety. Environ. Int. 2016, 88, 299–309. [Google Scholar] [CrossRef]
- Reckova, S.; Tuma, J.; Dobrev, P.; Vankova, R. Influence of Copper on Hormone Content and Selected Morphological, Physiological and Biochemical Parameters of Hydroponically Grown Zea Mays Plants. Plant Growth Regul. 2019, 89, 191–201. [Google Scholar] [CrossRef]
- Zahid, K.R.; Ali, F.; Shah, F.; Younas, M.; Shah, T.; Shahwar, D.; Hassan, W.; Ahmad, Z.; Qi, C.; Lu, Y.; et al. Response and Tolerance Mechanism of Cotton Gossypium Hirsutum L. To Elevated Temperature Stress: A Review. Front. Plant Sci. 2016, 7, 937. [Google Scholar] [CrossRef]
- Kumar, V.; Pandita, S.; Singh Sidhu, G.P.; Sharma, A.; Khanna, K.; Kaur, P.; Bali, A.S.; Setia, R. Copper Bioavailability, Uptake, Toxicity and Tolerance in Plants: A Comprehensive Review. Chemosphere 2021, 262, 127810. [Google Scholar] [CrossRef]
| Cotton Variety | Cu2+ Concentration (μM) | Chlorophyll a (mg/g FW) | Chlorophyll b (mg/g FW) | Chlorophyll (a + b) (mg/g FW) | Chlorophyll a/b | Carotenoid (mg/g FW) |
|---|---|---|---|---|---|---|
| 9053 | 0 | 0.28 ± 0.01 bcd | 0.08 ± 0.01 b | 0.36 ± 0.02 bcd | 3.36 ± 0.14 c | 0.051 ± 0.003 bc |
| 0.2 | 0.33 ± 0.05 cd | 0.09 ± 0.02 bc | 0.43 ± 0.06 cd | 3.52 ± 0.09 c | 0.048 ± 0.006 ab | |
| 50 | 0.30 ± 0.04 cd | 0.09 ± 0.02 bc | 0.39 ± 0.06 cd | 3.12 ± 0.09 b | 0.045 ± 0.004 ab | |
| 100 | 0.27 ± 0.03 bcd | 0.08 ± 0.01 b | 0.35 ± 0.04 bcd | 3.26 ± 0.06 bc | 0.049 ± 0.007 abc | |
| 200 | 0.11 ± 0.01 a | 0.04 ± 0.01 a | 0.16 ± 0.01 a | 2.64 ± 0.21 a | 0.049 ± 0.004 abc | |
| Zhongmian 63 | 0 | 0.24 ± 0.02 bcd | 0.08 ± 0.01 b | 0.31 ± 0.02 bcd | 3.06 ± 0.20 c | 0.044 ± 0.003 ab |
| 0.2 | 0.25 ± 0.04 bc | 0.08 ± 0.02 b | 0.32 ± 0.05 bc | 3.06 ± 0.11 b | 0.038 ± 0.004 a | |
| 50 | 0.35 ± 0.06 d | 0.11 ± 0.02 c | 0.46 ± 0.08 d | 3.15 ± 0.03 b | 0.050 ± 0.005 bc | |
| 100 | 0.28 ± 0.10 cd | 0.09 ± 0.03 bc | 0.37 ± 0.13 bcd | 3.11 ± 0.10 b | 0.048 ± 0.016 abc | |
| 200 | 0.19 ± 0.02 ab | 0.07 ± 0.01 b | 0.26 ± 0.03 ab | 2.67 ± 0.07 a | 0.055 ± 0.006 bc | |
| sGK9708 | ||||||
| 0 | 0.30 ± 0.03 cd | 0.10 ± 0.02 bc | 0.40 ± 0.04 cd | 3.09 ± 0.17 bc | 0.050 ± 0.003 bc | |
| 0.2 | 0.32 ± 0.08 cd | 0.09 ± 0.03 bc | 0.42 ± 0.11 cd | 3.37 ± 0.03 bc | 0.044 ± 0.005 ab | |
| 50 | 0.34 ± 0.06 cd | 0.10 ± 0.02 bc | 0.43 ± 0.08 cd | 3.39 ± 0.13 bc | 0.068 ± 0.004 abc | |
| 100 | 0.29 ± 0.03 cd | 0.09 ± 0.01 bc | 0.38 ± 0.04 bcd | 3.13 ± 0.09 b | 0.049 ± 0.003 abc | |
| 200 | 0.17 ± 0.08 a | 0.07 ± 0.02 b | 0.24 ± 0.1 ab | 2.34 ± 0.4 a | 0.058 ± 0.007 c |
| Cotton Variety | Cu2+ Concentration (μM) | Leaf (μg/g DW) | Stem (μg/g DW) | Root (μg/g DW) |
|---|---|---|---|---|
| 9053 | 0 | 8.67 ± 0.85 a | 4.43 ± 0.70 a° | 20.27 ± 1.46 a |
| 0.2 | 10.40 ± 1.20 a | 5.33 ± 0.35 a | 22.20 ± 2.00 a | |
| 50 | 28.95 ± 3.13 b | 8.70 ± 1.45 bc | 121.67 ± 5.8 b | |
| 100 | 46.27 ± 4.76 c | 15.87 ± 2.06 e | 262.87 ± 23.1 de | |
| 200 | 75.54 ± 11.40 e | 25.37 ± 2.80 g | 386.30 ± 31.96 f | |
| Zhongmian 63 | 0 | 7.60 ± 0.36 a | 4.47 ± 0.70 a | 20.47 ± 1.74 a |
| 0.2 | 8.83 ± 0.81 a | 5.43 ± 0.59 a | 24.77 ± 3.32 a | |
| 50 | 23.79 ± 2.36 b | 9.03 ± 0.83 c | 158.63 ± 10.92 bc | |
| 100 | 39.43 ± 5.80 c | 13.60 ± 1.55 de | 286.20 ± 17.76 de | |
| 200 | 65.07 ± 4.73 d | 20.57 ± 2.41 f | 489.20 ± 32.03 g | |
| sGK9708 | 0 | 8.50 ± 0.70 a | 5.63 ± 0.45 ab | 18.57 ± 1.12 a |
| 0.2 | 9.47 ± 1.20 a | 6.30 ± 0.50 abc | 20.07 ± 2.14 a | |
| 50 | 30.27 ± 1.99 b | 12.40 ± 2.05 d | 139.83 ± 10.63 bc | |
| 100 | 43.53 ± 3.56 c | 20.27 ± 1.96 f | 253.00 ± 10.50 d | |
| 200 | 76.97 ± 8.06 e | 33.90 ± 3.60 h | 475.73 ± 24.83 g |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, H.; Zhou, K.-H.; Zhao, G.; Wang, P.-P.; Yang, D.-G.; Ma, X.-F.; Gao, J.-S. Physiological and Biochemical Properties of Cotton Seedlings in Response to Cu2+ Stress. Curr. Issues Mol. Biol. 2023, 45, 4050-4062. https://doi.org/10.3390/cimb45050258
Zhou H, Zhou K-H, Zhao G, Wang P-P, Yang D-G, Ma X-F, Gao J-S. Physiological and Biochemical Properties of Cotton Seedlings in Response to Cu2+ Stress. Current Issues in Molecular Biology. 2023; 45(5):4050-4062. https://doi.org/10.3390/cimb45050258
Chicago/Turabian StyleZhou, Hao, Ke-Hai Zhou, Gang Zhao, Pei-Pei Wang, Dai-Gang Yang, Xiong-Feng Ma, and Jun-Shan Gao. 2023. "Physiological and Biochemical Properties of Cotton Seedlings in Response to Cu2+ Stress" Current Issues in Molecular Biology 45, no. 5: 4050-4062. https://doi.org/10.3390/cimb45050258
APA StyleZhou, H., Zhou, K.-H., Zhao, G., Wang, P.-P., Yang, D.-G., Ma, X.-F., & Gao, J.-S. (2023). Physiological and Biochemical Properties of Cotton Seedlings in Response to Cu2+ Stress. Current Issues in Molecular Biology, 45(5), 4050-4062. https://doi.org/10.3390/cimb45050258





