Selected Technical Aspects of Molecular Allergy Diagnostics
Abstract
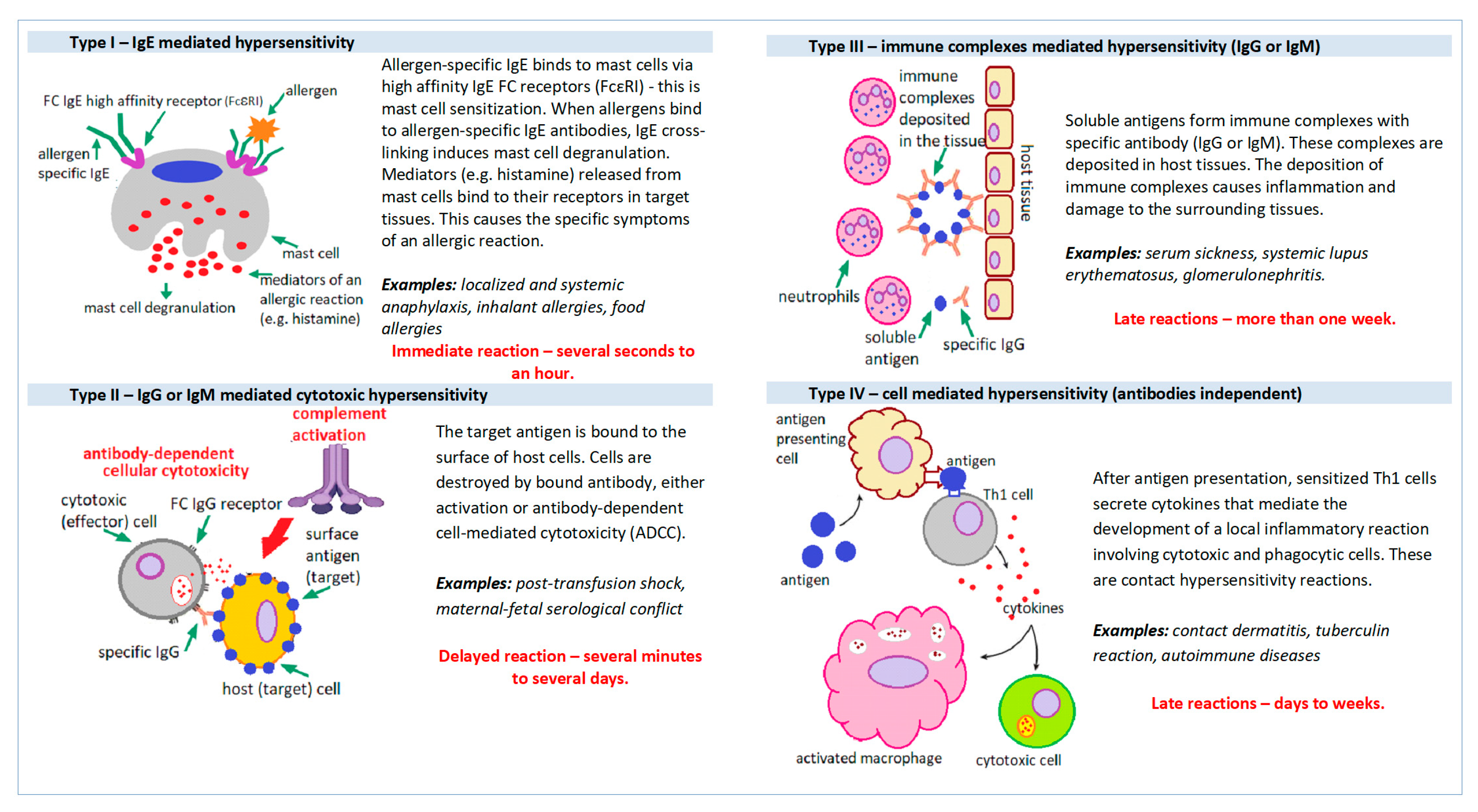
:1. Introduction
2. Allergy Diagnosis Based on the Measurement of Allergen-Specific IgE (sIgE)
Extract-Based and Component (Molecular) Immunological Allergy Diagnostic
3. Molecular Allergy Diagnostic-Technical Aspects and Current Possibilities
3.1. Singleplex (Monocomponent) Molecular Allergy Diagnostic
3.2. Multiplex (Multicomponent) Molecular Allergy Diagnostic
3.2.1. ISAC (Immuno Solid-Phase Allergen Chip; ThermoFisher Scientific, Waltham, MA, USA)
3.2.2. ALEX (ALLERGY XPLORER, MacroArray Diagnostics GmbH (MADx), Vienna, Austria)
4. Summary, Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Tanno, L.K.; Calderon, M.A.; Smith, H.E.; Sanchez-Borges, M.; Sheikh, A.; Demoly, P. Dissemination of definitions and concepts of allergic and hypersensitivity conditions. World Allergy Organ J. 2016, 9, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dispenza, M.C. Classification of hypersensitivity reactions. Allergy Asthma Proc. 2019, 40, 470–473. [Google Scholar] [CrossRef] [PubMed]
- Muraro, A.; Werfel, T.; Hoffmann-Sommergruber, K.; Roberts, G.; Beyer, K.; Bindslev-Jensen, C.; Cardona, V.; Dubois, A.; Dutoit, G.; Eigenmann, P.; et al. EAACI Food Allergy and Anaphylaxis Guidelines Group. EAACI food allergy and anaphylaxis guidelines: Diagnosis and management of food allergy. Allergy 2014, 69, 1008–1025. [Google Scholar] [CrossRef] [PubMed]
- Ansotegui, I.J.; Melioli, G.; Canonica, G.W.; Caraballo, L.; Villa, E.; Ebisawa, M.; Passalacqua, G.; Savi, E.; Ebo, D.; Gómez, R.M.; et al. IgE allergy diagnostics and other relevant tests in allergy, a World Allergy Organization position paper. World Allergy Organ J. 2020, 13, 100080. [Google Scholar] [CrossRef]
- Kleine-Tebbe, J.; Jakob, T. Molecular allergy diagnostics using IgE singleplex determinations: Methodological and practical considerations for use in clinical routine: Part 18 of the Series Molecular Allergology. Allergo J. Int. 2015, 24, 185–197. [Google Scholar] [CrossRef]
- Jakob, T.; Forstenlechner, P.; Matricardi, P.; Kleine-Tebbe, J. Molecular allergy diagnostics using multiplex assays: Methodological and practical considerations for use in research and clinical routine: Part 21 of the Series Molecular Allergology. Allergo J. Int. 2015, 24, 320–332. [Google Scholar] [CrossRef] [Green Version]
- Wauthier, L.; Cabo, J.; Eucher, C.; Rosseels, C.; Elsen, M.; Favresse, J. Biotin interference in immunoassays: Water under the bridge? Clin. Chem. Lab. Med. 2023. advance online publication. [Google Scholar] [CrossRef]
- Liu, D.; Gebreab, Y.B.; Hu, J.; Zhou, L.; Zhang, N.; Tong, H.; Chen, B.; Wang, X. Development and Evaluation of an Anti-Biotin Interference Method in Biotin-Streptavidin Immunoassays. Diagnostics 2022, 12, 1729. [Google Scholar] [CrossRef]
- ELISA Handbook Principle, Troubleshooting, Sample Preparation and Assay Protocols. Available online: https://www.bosterbio.com/media/pdf/ELISA_Handbook.pdf (accessed on 3 June 2023).
- Yunginger, J.W.; Ahlstedt, S.; Eggleston, P.A.; Homburger, H.A.; Nelson, H.S.; Ownby, D.R.; Platts-Mills, T.A.; Sampson, H.A.; Sicherer, S.H.; Weinstein, A.M.; et al. Quantitative IgE antibody assays in allergic diseases. J. Allergy Clin. Immunol. 2000, 105 Pt 1, 1077–1084. [Google Scholar] [CrossRef]
- Technical Guide for ELISA. Available online: https://www.seracare.com/globalassets/seracare-resources/tg-protocols-and-troubleshooting.pdf (accessed on 3 June 2023).
- Hamilton, R.G.; Matsson, P.N.J.; Chan, S.; Cleve, M.; van Hovanec-Burns, D.; Magnusson, C.; Quicho, R.; Adkinson, N.F. Analytical performance characteristics, quality assurance and clinical utility of immunological assays for human immunoglobulin E (IgE) antibodies of defined allergen specificities. J. Allergy Clin. Immunol. 2015, 135, AB8. Available online: https://clsi.org/media/1414/ila20ed3_sample.pdf (accessed on 3 June 2023). [CrossRef]
- Schmidta, M.; Hoffmanb, D.R. Expression systems for production of recombinant allergens. Int. Arch. Allergy Immunol. 2002, 128, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Tscheppe, A.; Breiteneder, H. Recombinant Allergens in Structural Biology, Diagnosis, and Immunotherapy. Int. Arch. Allergy Immunol. 2017, 172, 187–202. [Google Scholar] [CrossRef] [PubMed]
- Curin, M.; Garib, V.; Valenta, R. Single recombinant and purified major allergens and peptides: How they are made and how they change allergy diagnosis and treatment. Ann. Allergy Asthma. Immunol. 2017, 119, 201–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jutel, M.; Solarewicz-Madejek, K.; Smolinska, S. Recombinant allergens: The present and the future. Hum. Vaccin. Immunother. 2012, 8, 1534–1543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleine-Tebb, J.; Jakob, T. (Eds.) Molecular Allergy Diagnostics Innovation for a Better Patient Management; Springer International Publishing: New York, NY, USA, 2017; ISBN 978-3-319-42498-9. [Google Scholar] [CrossRef]
- Siekierzynska, A.; Piasecka-Kwiatkowska, D.; Litwinczuk, W.; Burzynska, M.; Myszka, A.; Karpinski, P.; Zygala, E.; Piorecki, N.; Springer, E.; Sozanski, T. Molecular and Immunological Identification of Low Allergenic Fruits among Old and New Apple Varieties. Int. J. Mol. Sci. 2021, 22, 3527. [Google Scholar] [CrossRef]
- Ferrari, E.; Breda, D.; Spisni, A.; Burastero, S.E. Component-Resolved Diagnosis Based on a Recombinant Variant of Mus m 1 Lipocalin Allergen. Int. J. Mol. Sci. 2023, 24, 1193. [Google Scholar] [CrossRef]
- Luengo, O.; Labrador-Horrillo, M. Molecular Allergy Diagnosis in Clinical Practice: Frequently Asked Questions. J. Investig. Allergol. Clin. Immunol. 2022, 32, 1–12. [Google Scholar] [CrossRef]
- Barber, D.; Diaz-Perales, A.; Escribese, M.M.; Kleine-Tebbe, J.; Matricardi, P.M.; Ollert, M.; Santos, A.F.; Sastre, J. Molecular allergology and its impact in specific allergy diagnosis and therapy. Allergy 2021, 76, 3642–3658. [Google Scholar] [CrossRef]
- Ansotegui, I.J.; Melioli, G.; Canonica, G.W.; Gomez, R.M.; Jensen-Jarolim, E.; Luengo, O.; Caraballo, L.; Passalacqua, G.; Poulsen, L.K.; Savi, K.; et al. A WAO–ARIA–GA2LEN consensus document on molecular-based allergy diagnosis (PAMD@): Update 2020. World Allergy Organ J. 2020, 13, 100091. [Google Scholar] [CrossRef] [Green Version]
- Neagu, M.; Bostan, M.; Constantin, C. Protein microarray technology: Assisting personalized medicine in oncology (Review). World Acad. Sci. J. 2019, 1, 113–124. [Google Scholar] [CrossRef] [Green Version]
- Westwood, M.; Ramaekers, B.; Lang, S.; Armstrong, N.; Noake, C.; de Kock, S.; Joore, M.; Severens, J.; Kleijnen, J. ImmunoCAP® ISAC and Microtest for multiplet allergen testing in people with difficult to manage allergic disease: A systematic review and cost analysis. Health Technol. Assess. 2016, 20, 1–178. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://www.thermofisher.com/phadia/wo/en/our-solutions/immunocap-allergy-solutions/specific-ige-multiplex.html (accessed on 3 June 2023).
- Melioli, G.; Spenser, C.; Reggiardo, G.; Passalacqua, G.; Compalati, E.; Rogkakou, A.; Riccio, A.M.; Di Leo, E.; Nettis, E.; Canonica, G.W. Allergenius, an expert system for the interpretation of allergen microarray results. World Allergy Organ J. 2014, 7, 15–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bojcukova, J.; Vlas, T.; Forstenlechner, P.; Panzner, P. Comparison of two multiplex arrays in the diagnostics of allergy. Clin. Transl. Allergy 2019, 9, 31–36. [Google Scholar] [CrossRef]
- Platteel, A.C.M.; van der Pol, P.; Murk, J.L.; Verbrugge-Bakker, I.; Hack-Steemers, M.; Roovers, T.H.W.M.; Heron, M. A comprehensive comparison between ISAC and ALEX2 multiplex test systems. Clin. Chem. Lab. Med. 2022, 60, 1046–1052. [Google Scholar] [CrossRef] [PubMed]
- Alessandri, C.; Ferrara, R.; Bernardi, M.L.; Zennaro, D.; Tuppo, L.; Giangrieco, I.; Tamburrini, M.; Mari, A.; Ciardiello, M.A. Diagnosing allergic sensitizations in the third millennium: Why clinicians should know allergen molecule structures. Clin. Transl. Allergy 2017, 7, 21–29. [Google Scholar] [CrossRef]
- De Morais, M.G.; Martins, V.G.; Steffens, D.; Pranke, P.; da Costa, J.A. Biological applications of nanobiotechnology. J. Nanosci. Nanotechnol. 2014, 14, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Salata, O. Applications of nanoparticles in biology and medicine. J. Nanotechnol. 2004, 2, 3. [Google Scholar] [CrossRef] [Green Version]
- Reagen, S.; Zhao, J.X. Analysis of Nanomaterials on Biological and Environmental Systems and New Analytical Methods for Improved Detection. Int. J. Mol. Sci. 2022, 23, 6331. [Google Scholar] [CrossRef]
- Popescu, F.D.; Vieru, M. Precision medicine allergy immunoassay methods for assessing immunoglobulin E sensitization to aeroallergen molecules. World J. Methodol. 2018, 8, 17–36. [Google Scholar] [CrossRef]
- Heffler, E.; Puggioni, F.; Peveri, S.; Montagni, M.; Canonica, G.W.; Melioli, G. Extended IgE profile based on an allergen macroarray: A novel tool for precision medicine in allergy diagnosis. World Allergy Organ J. 2018, 11, 7–14. [Google Scholar] [CrossRef] [Green Version]
- Kochuyt, A.M.; Van Hoeyveld, E.M.; Stevens, E.A. Prevalence and clinical relevance of specific immunoglobulin E to pollen caused by sting-induced specific immunoglobulin E to cross-reacting carbohydrate determinants in Hymenoptera venoms. Clin. Exp. Allergy 2005, 35, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Mari, A. IgE to cross-reactive carbohydrate determinants: Analysis of the distribution and appraisal of the in vivo and in vitro reactivity. Int. Arch. Allergy Immunol. 2002, 129, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Altmann, F. Coping with cross-reactive carbohydrate determinants in allergy diagnosis. Allergo J. Int. 2016, 25, 98–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Jiang, Q.; Yang, Y.; Zhang, W.; Yang, L.; Zhu, R. Cross-Reacting Carbohydrate Determinants Inhibitor Can Improve the Diagnostic Accuracy in Pollen and Food Allergy. J. Asthma Allergy 2022, 15, 713–725. [Google Scholar] [CrossRef]
- Sinson, E.; Ocampo, C.; Liao, C.; Nguyen, S.; Dinh, L.; Rodems, K.; Whitters, E.; Hamilton, R.G. Cross-reactive carbohydrate determinant interference in cellulose-based IgE allergy tests utilizing recombinant allergen components. PLoS ONE 2020, 15, e0231344. [Google Scholar] [CrossRef] [Green Version]
- Luo, W.; Huang, H.; Zheng, P.; Zheng, J.; Sun, B. CCD Inhibition Test Can Improve the Accuracy of the Detection of Pollen and Seed Food Allergen-Specific IgE in Southern China. J. Asthma Allergy 2021, 14, 439–447. [Google Scholar] [CrossRef]
- Holzweber, F.; Svehla, E.; Fellner, W.; Dalik, T.; Stubler, S.; Hemmer, W.; Altmann, F. Inhibition of IgE binding to cross-reactive carbohydrate determinants enhances diagnostic selectivity. Allergy 2013, 68, 1269–1277. [Google Scholar] [CrossRef] [Green Version]
- Available online: www.proglycan.com (accessed on 7 May 2023).
- Available online: https://www.macroarraydx.com/products/alex (accessed on 3 June 2023).
- Villalta, D.; Tonutti, E.; Bizzaro, N.; Brusca, I.; Sargentini, V.; Asero, R.; Bilo, M.B.; Manzotti, G.; Murzilli, F.; Cecchi, L.; et al. Recommendations for the use of molecular diagnostics in the diagnosis of allergic diseases. Eur. Ann. Allergy Clin. Immunol. 2018, 50, 51–58. [Google Scholar] [CrossRef]
- Peveri, S.; Pattini, S.; Costantino, M.T.; Incorvaia, C.; Montagni, M.; Roncallo, C.; Villalta, D.; Savi, E. Molecular diagnostics improves diagnosis and treatment of respiratory allergy and food allergy with economic optimization and cost saving. Allergol. Immunopathol. 2019, 47, 64–72. [Google Scholar] [CrossRef]
- Diem, L.; Neuher, B.; Rohrhofer, J.; Koidl, L.; Asero, R.; Brockow, K.; Diaz Perales, A.; Faber, M.; Gebhardt, J.; Torres, M.J.; et al. Real-life evaluation of molecular multiplex IgE test methods in the diagnosis of pollen associated food allergy. Allergy 2022, 77, 3028–3040. [Google Scholar] [CrossRef]
- Santos, A.F.; Kulis, M.D.; Sampson, H.A. Bringing the Next Generation of Food Allergy Diagnostics into the Clinic. J. Allergy Clin. Immunol. Pract. 2022, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Suprun, M.; Getts, R.; Raghunathan, R.; Grishina, G.; Witmer, M.; Gimenez, G.; Sampson, H.A.; Suárez-Fariñas, M. Novel Bead-Based Epitope Assay is a sensitive and reliable tool for profiling epitope-specific antibody repertoire in food allergy. Sci. Rep. 2019, 9, 18425. [Google Scholar] [CrossRef] [PubMed] [Green Version]


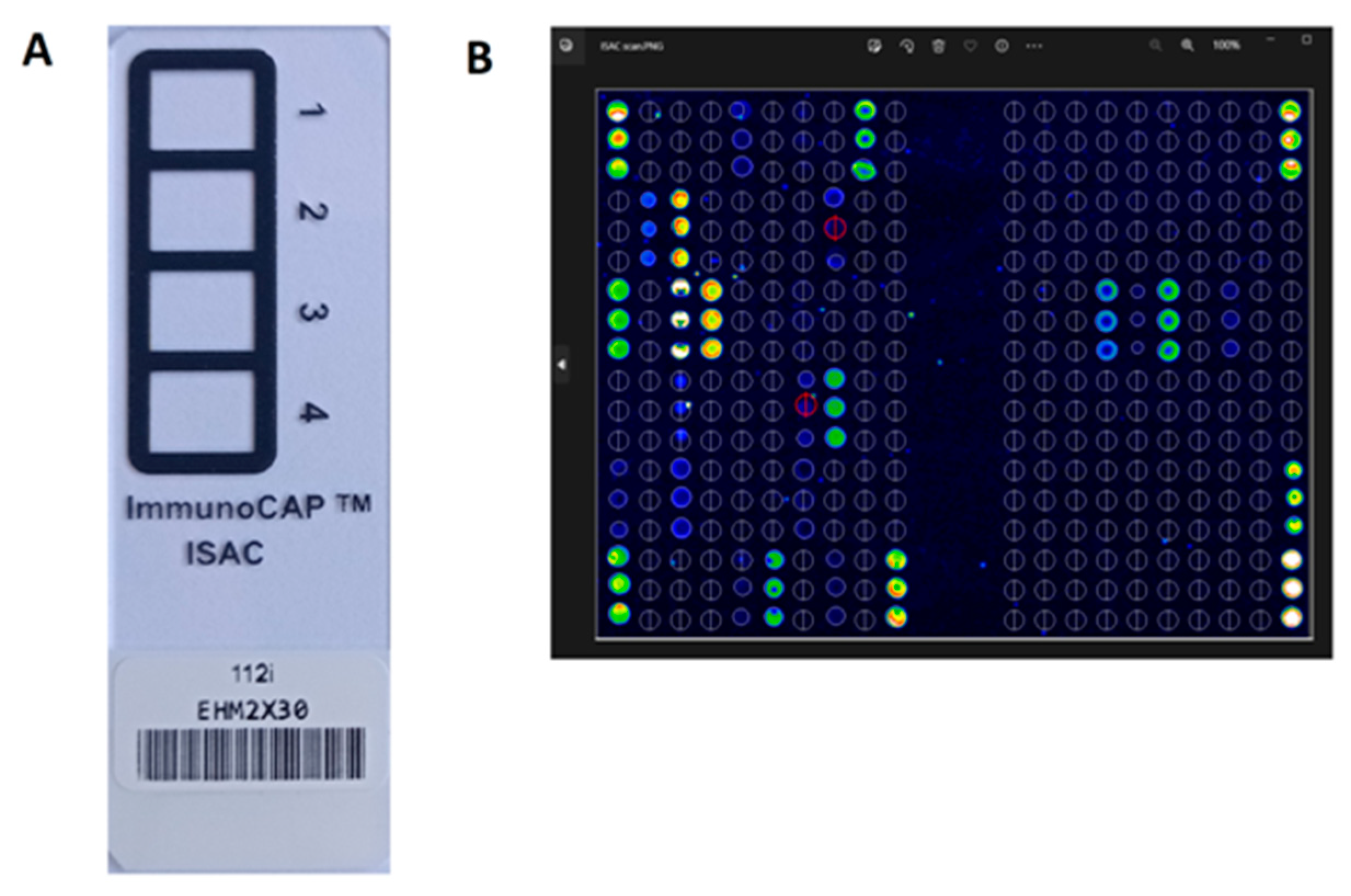

| ISACE112i Immuno Solid-Phase Allergen Chip | ALEX2 Allergy Xplorer | |
|---|---|---|
| The number of allergens on the test matrix | 112 | 296 |
| The number of allergen molecules | 112 | 177 |
| Number of allergen extracts | 0 | 119 |
| Number of recombinant molecules | 74 | 125 |
| Number of native molecules | 38 | 52 |
| Method of sIgE determination | Semi-quantitative | Quantitative |
| test result reporting unit | ISU-E | kU/L |
| Total IgE determination | No | 1–2500 kU/L |
| CCD blocking | No | yes |
| CCD detection | Yes | yes |
| Serum volume needed for testing | 30 µL | 100 µL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lis, K.; Bartuzi, Z. Selected Technical Aspects of Molecular Allergy Diagnostics. Curr. Issues Mol. Biol. 2023, 45, 5481-5493. https://doi.org/10.3390/cimb45070347
Lis K, Bartuzi Z. Selected Technical Aspects of Molecular Allergy Diagnostics. Current Issues in Molecular Biology. 2023; 45(7):5481-5493. https://doi.org/10.3390/cimb45070347
Chicago/Turabian StyleLis, Kinga, and Zbigniew Bartuzi. 2023. "Selected Technical Aspects of Molecular Allergy Diagnostics" Current Issues in Molecular Biology 45, no. 7: 5481-5493. https://doi.org/10.3390/cimb45070347
APA StyleLis, K., & Bartuzi, Z. (2023). Selected Technical Aspects of Molecular Allergy Diagnostics. Current Issues in Molecular Biology, 45(7), 5481-5493. https://doi.org/10.3390/cimb45070347







