Inflammation, Autoinflammation and Autoimmunity in Inflammatory Bowel Diseases
Abstract
1. Introduction
2. Inflammation in IBD
2.1. Intestinal Barrier Alterations
2.1.1. The Mucus Layer
2.1.2. Secretory Immunoglobulins
2.1.3. Tight Junctions Alterations
2.1.4. Innate Immune Receptors
2.1.5. Laboratory Testing in IBD: Evaluation of Intestinal Barrier Integrity
2.2. Innate Immune Cells in IBD
2.2.1. Neutrophils and NETs
2.2.2. Dendritic Cells and Macrophages
2.3. Adaptive Immune Cells in IBD
2.3.1. CD4+ T Helper Cells
2.3.2. CD8+ T Cells
2.3.3. CD4+ Treg Cells
2.3.4. B-Lymphocytes
2.4. The Role of Cytokines in IBD
3. Autoinflammation in IBD
3.1. Clinical Studies
3.2. Danger Signals and Receptors
3.3. NOD2 and IBD
3.4. NLRP Inflammasomes and IBD
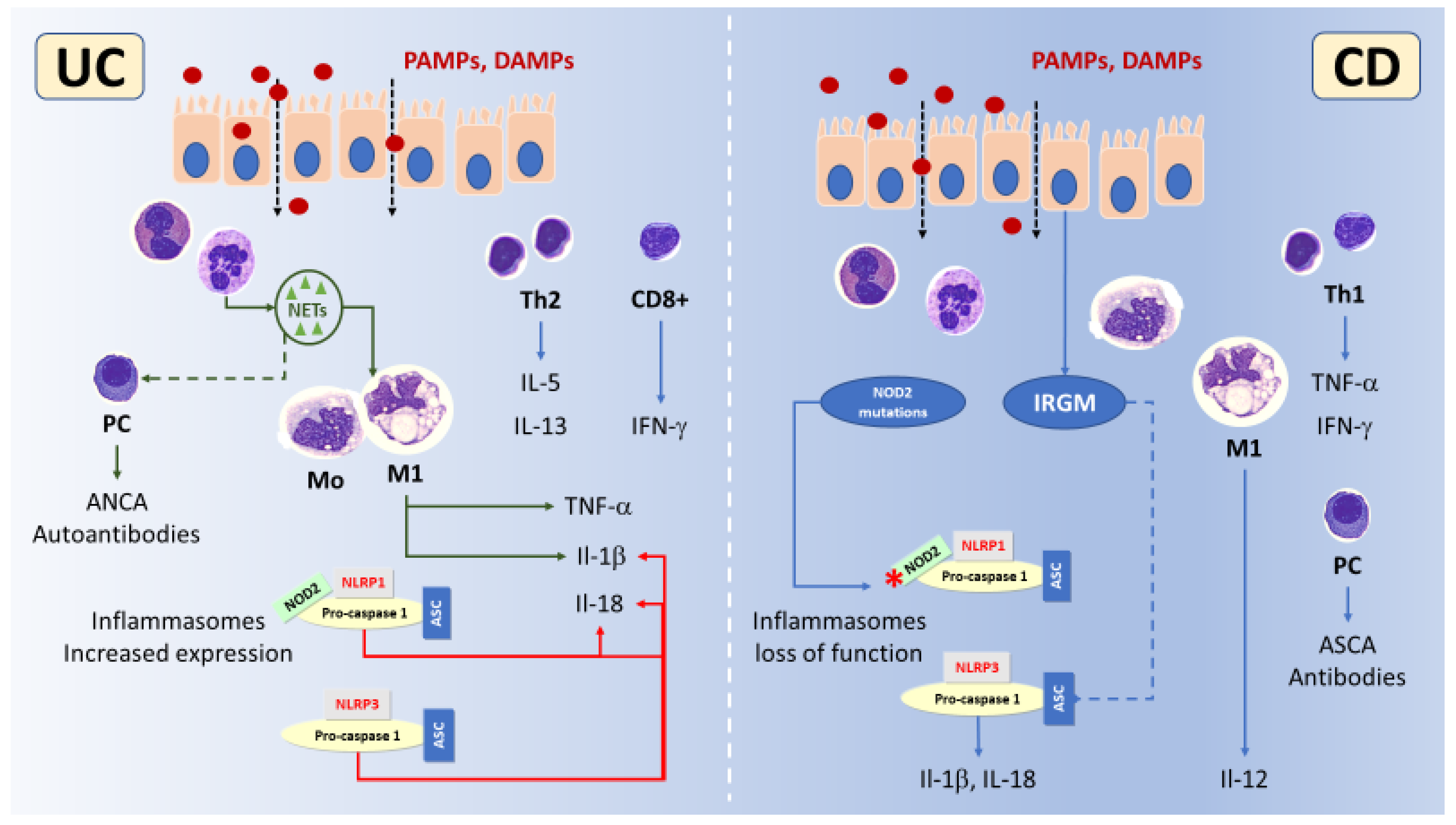
3.5. Inflammasomes Highlights in IBD
3.6. The Role of Laboratory Testing in the Evaluation of Inflammation in IBD
4. Autoimmunity in IBD
Laboratory Testing in IBD: Autoimmunity Markers
5. Concluding Remarks and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Kaplan, G.G.; Ng, S.C. Understanding and Preventing the Global Increase of Inflammatory Bowel Disease. Gastroenterology 2017, 152, 313–321.e2. [Google Scholar] [CrossRef]
- Fiocchi, C. Inflammatory bowel disease pathogenesis: Where are we? J. Gastroenterol. Hepatol. 2015, 30, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Jostins, L.; Ripke, S.; Weersma, R.K.; Duerr, R.H.; McGovern, D.P.; Hui, K.Y.; Lee, J.C.; Philip Schumm, L.; Sharma, Y.; Anderson, C.A.; et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 2012, 491, 119–124. [Google Scholar] [CrossRef]
- Wallace, K.L.; Zheng, L.B.; Kanazawa, Y.; Shih, D.Q. Immunopathology of inflammatory bowel disease. World J. Gastroenterol. 2014, 20, 6–21. [Google Scholar] [CrossRef] [PubMed]
- de Lange, K.M.; Moutsianas, L.; Lee, J.C.; Lamb, C.A.; Luo, Y.; Kennedy, N.A.; Jostins, L.; Rice, D.L.; Gutierrez-Achury, J.; Ji, S.-G.; et al. Genome-wide association study implicates immune activation of multiple integrin genes in inflammatory bowel disease. Nat. Genet. 2017, 49, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Z.; van Sommeren, S.; Huang, H.; Ng, S.C.; Alberts, R.; Takahashi, A.; Ripke, S.; Lee, J.C.; Jostins, L.; Shah, T.; et al. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat. Genet. 2015, 47, 979–986. [Google Scholar] [CrossRef]
- Annunziato, F.; Cosmi, L.; Santarlasci, V.; Maggi, L.; Liotta, F.; Mazzinghi, B.; Parente, E.; Filì, L.; Ferri, S.; Frosali, F.; et al. Phenotypic and functional features of human Th17 cells. J. Exp. Med. 2007, 204, 1849–1861. [Google Scholar] [CrossRef]
- Fuss, I.J.; Neurath, M.; Boirivant, M.; Klein, J.S.; de la Motte, C.; Strong, S.A.; Fiocchi, C.; Strober, W. Disparate CD4+ lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. Crohn’s disease LP cells manifest increased secretion of IFN-gamma, whereas ulcerative colitis LP cells manifest increased secretion of IL-5. J. Immunol. 1996, 157, 1261–1270. [Google Scholar] [CrossRef]
- Baumgart, D.C.; Sandborn, W.J. Crohn’s disease. Lancet 2012, 380, 1590–1605. [Google Scholar] [CrossRef]
- Ordás, I.; Eckmann, L.; Talamini, M.; Baumgart, D.C.; Sandborn, W.J. Ulcerative colitis. Lancet 2012, 380, 1606–1619. [Google Scholar] [CrossRef]
- Esmaily, H.; Sanei, Y.; Abdollahi, M. Autoantibodies and an immune-based rat model of inflammatory bowel disease. World J. Gastroenterol. 2013, 19, 7569–7576. [Google Scholar] [CrossRef] [PubMed]
- Spits, H.; Artis, D.; Colonna, M.; Diefenbach, A.; Di Santo, J.P.; Eberl, G.; Koyasu, S.; Locksley, R.M.; McKenzie, A.N.J.; Mebius, R.E.; et al. Innate lymphoid cells-a proposal for uniform nomenclature. Nat. Rev. Immunol. 2013, 13, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Buonocore, S.; Ahern, P.P.; Uhlig, H.H.; Ivanov, I.I.; Littman, D.R.; Maloy, K.J.; Powrie, F. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature 2010, 464, 1371–1375. [Google Scholar] [CrossRef] [PubMed]
- Songtanin, B.; Peterson, C.J.; Molehin, A.J.; Nugent, K. Biofilms and Benign Colonic Diseases. Int. J. Mol. Sci. 2022, 23, 14259. [Google Scholar] [CrossRef]
- Lu, Q.; Yang, M.F.; Liang, Y.J.; Xu, J.; Xu, H.M.; Nie, Y.Q.; Wang, L.S.; Yao, J.; Li, D.F. Immunology of Inflammatory Bowel Disease: Molecular Mechanisms and Therapeutics. J. Inflamm. Res. 2022, 15, 1825–1844. [Google Scholar] [CrossRef]
- Kofla-Dłubacz, A.; Pytrus, T.; Akutko, K.; Sputa-Grzegrzółka, P.; Piotrowska, A.; Dzięgiel, P. Etiology of IBD-Is It Still a Mystery? Int. J. Mol. Sci. 2022, 23, 12445. [Google Scholar] [CrossRef]
- Alemao, C.A.; Budden, K.F.; Gomez, H.M.; Rehman, S.F.; Marshall, J.E.; Shukla, S.D.; Donovan, C.; Forster, S.C.; Yang, I.A.; Keely, S.; et al. Impact of diet and the bacterial microbiome on the mucous barrier and immune disorders. Allergy 2021, 76, 714–734. [Google Scholar] [CrossRef]
- Basso, D.; Padoan, A.; D’Incà, R.; Arrigoni, G.; Scapellato, M.L.; Contran, N.; Franchin, C.; Lorenzon, G.; Mescoli, C.; Moz, S.; et al. Peptidomic and proteomic analysis of stool for diagnosing IBD and deciphering disease pathogenesis. Clin. Chem. Lab. Med. 2020, 58, 968–979. [Google Scholar] [CrossRef]
- Dharmani, P.; Srivastava, V.; Kissoon-Singh, V.; Chadee, K. Role of intestinal mucins in innate host defense mechanisms against pathogens. J. Innate Immun. 2009, 1, 123–135. [Google Scholar] [CrossRef]
- Kelly, D.; Mulder, I.E. Microbiome and immunological interactions. Nutr. Rev. 2012, 70, S18–S30. [Google Scholar] [CrossRef]
- Miner-Williams, W.M.; Moughan, P.J. Intestinal barrier dysfunction: Implications for chronic inflammatory conditions of the bowel. Nutr. Res. Rev. 2016, 29, 40–59. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.; Shen, J. The roles and functions of Paneth cells in Crohn’s disease: A critical review. Cell Prolif. 2021, 54, e12958. [Google Scholar] [CrossRef] [PubMed]
- Wehkamp, J.; Stange, E.F. An Update Review on the Paneth Cell as Key to Ileal Crohn’s Disease. Front. Immunol. 2020, 11, 646. [Google Scholar] [CrossRef] [PubMed]
- Rana, T.; Korolkova, O.Y.; Rachakonda, G.; Williams, A.D.; Hawkins, A.T.; James, S.D.; Sakwe, A.M.; Hui, N.; Wang, L.; Yu, C.; et al. Linking bacterial enterotoxins and alpha defensin 5 expansion in the Crohn’s colitis: A new insight into the etiopathogenetic and differentiation triggers driving colonic inflammatory bowel disease. PLoS ONE 2021, 16, e0246393. [Google Scholar] [CrossRef]
- Eichholz, K.; Tran, T.H.; Chéneau, C.; Tran, T.T.P.; Paris, O.; Pugniere, M.; Kremer, E.J. Adenovirus-α-Defensin Complexes Induce NLRP3-Associated Maturation of Human Phagocytes via Toll-Like Receptor 4 Engagement. J. Virol. 2022, 96, e0185021. [Google Scholar] [CrossRef]
- Hanson, L.; Andersson, B.; Carlsson, B.; Dahlgren, U.; Mellander, L.; Porras, O.; Edén, C.; Söderström, T. The Secretory IgA System. Klin. Pädiatrie 1985, 197, 330–333. [Google Scholar] [CrossRef]
- Alexander, K.L.; Targan, S.R.; Elson, C.O. Microbiota activation and regulation of innate and adaptive immunity. Immunol. Rev. 2014, 260, 206–220. [Google Scholar] [CrossRef]
- Takeuchi, T.; Ohno, H. IgA in human health and diseases: Potential regulator of commensal microbiota. Front. Immunol. 2022, 13, 1024330. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, J.M.; de Zoete, M.R.; Palm, N.W.; Laenen, Y.; Bright, R.; Mallette, M.; Bu, K.; Bielecka, A.A.; Xu, F.; Hurtado-Lorenzo, A.; et al. Immunoglobulin A Targets a Unique Subset of the Microbiota in Inflammatory Bowel Disease. Cell Host Microbe 2021, 29, 83–93.e3. [Google Scholar] [CrossRef]
- Bamias, G.; Kitsou, K.; Rivera-Nieves, J. The Underappreciated Role of Secretory IgA in IBD. Inflamm. Bowel Dis. 2023, 21, izad024. [Google Scholar] [CrossRef]
- Goswami, P.; Das, P.; Verma, A.K.; Prakash, S.; Das, T.K.; Nag, T.C.; Ahuja, V.; Gupta, S.D.; Makharia, G.K. Are alterations of tight junctions at molecular and ultrastructural level different in duodenal biopsies of patients with celiac disease and Crohn’s disease? Virchows Arch. 2014, 465, 521–530. [Google Scholar] [CrossRef]
- Keita, Å.V.; Lindqvist, C.M.; Öst, Å.; Magana, C.D.L.; Schoultz, I.; Halfvarson, J. Gut Barrier Dysfunction-A Primary Defect in Twins with Crohn’s Disease Predominantly Caused by Genetic Predisposition. J. Crohn’s Colitis 2018, 12, 1200–1209. [Google Scholar] [CrossRef]
- Su, L.; Nalle, S.C.; Shen, L.; Turner, E.S.; Singh, G.; Breskin, L.A.; Khramtsova, E.A.; Khramtsova, G.; Tsai, P.Y.; Fu, Y.X.; et al. TNFR2 activates MLCK-dependent tight junction dysregulation to cause apoptosis-mediated barrier loss and experimental colitis. Gastroenterology 2013, 145, 407–415. [Google Scholar] [CrossRef]
- Laudisi, F.; Stolfi, C.; Bevivino, G.; Maresca, C.; Franzè, E.; Troncone, E.; Lolli, E.; Marafini, I.; Pietrucci, D.; Teofani, A.; et al. GATA6 Deficiency Leads to Epithelial Barrier Dysfunction and Enhances Susceptibility to Gut Inflammation. J. Crohn’s Colitis 2022, 16, 301–311. [Google Scholar] [CrossRef]
- Heller, F.; Florian, P.; Bojarski, C.; Richter, J.; Christ, M.; Hillenbrand, B.; Mankertz, J.; Gitter, A.H.; Bürgel, N.; Fromm, M.; et al. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology 2005, 129, 550–564. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Yoshinaga, N.; Tanabe, S. Interleukin-6 (IL-6) regulates claudin-2 expression and tight junction permeability in intestinal epithelium. J. Biol. Chem. 2011, 286, 31263–31271. [Google Scholar] [CrossRef] [PubMed]
- Sugita, K.; Kabashima, K. Tight junctions in the development of asthma, chronic rhinosinusitis, atopic dermatitis, eosinophilic esophagitis, and inflammatory bowel diseases. J. Leukoc. Biol. 2020, 107, 749–762. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Ramos, A.; Viana, G.C.S.; de Macedo Brigido, M.; Almeida, J.F. Neutrophil extracellular traps in inflammatory bowel diseases: Implications in pathogenesis and therapeutic targets. Pharmacol. Res. 2021, 171, 105779. [Google Scholar] [CrossRef]
- Al-Sadi, R.; Engers, J.; Haque, M.; King, S.; Al-Omari, D.; Ma, T.Y. Matrix Metalloproteinase-9 (MMP-9) induced disruption of intestinal epithelial tight junction barrier is mediated by NF-κB activation. PLoS ONE 2021, 16, e0249544. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Lian, H.; Zhong, X.S.; Krishnachaitanya, S.S.; Cong, Y.; Dashwood, R.H.; Savidge, T.C.; Powell, D.W.; Liu, X.; Li, Q. Matrix metalloproteinase 7 contributes to intestinal barrier dysfunction by degrading tight junction protein Claudin-7. Front. Immunol. 2022, 13, 1020902. [Google Scholar] [CrossRef]
- Michielan, A.; D’Incà, R. Intestinal Permeability in Inflammatory Bowel Disease: Pathogenesis, Clinical Evaluation, and Therapy of Leaky Gut. Mediators Inflamm. 2015, 2015, 628157. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Leong, R.W.; Wasinger, V.C.; Ip, M.; Yang, M.; Phan, T.G. Impaired Intestinal Permeability Contributes to Ongoing Bowel Symptoms in Patients With Inflammatory Bowel Disease and Mucosal Healing. Gastroenterology 2017, 153, 723–731.e1. [Google Scholar] [CrossRef] [PubMed]
- Pratt, M.; Forbes, J.D.; Knox, N.C.; Bernstein, C.N.; Van Domselaar, G. Microbiome-Mediated Immune Signaling in Inflammatory Bowel Disease and Colorectal Cancer: Support From Meta-omics Data. Front. Cell Dev. Biol. 2021, 9, 716604. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Wang, K.; Xu, C.; Hao, M.; Li, H.; Ding, L. Intestinal Claudin-7 deficiency impacts the intestinal microbiota in mice with colitis. BMC Gastroenterol. 2022, 22, 24. [Google Scholar] [CrossRef] [PubMed]
- Kordjazy, N.; Haj-Mirzaian, A.; Haj-Mirzaian, A.; Rohani, M.M.; Gelfand, E.W.; Rezaei, N.; Abdolghaffari, A.H. Role of toll-like receptors in inflammatory bowel disease. Pharmacol. Res. 2018, 129, 204–215. [Google Scholar] [CrossRef]
- Lu, Y.; Li, X.; Liu, S.; Zhang, Y.; Zhang, D. Toll-like Receptors and Inflammatory Bowel Disease. Front. Immunol. 2018, 9, 72. [Google Scholar] [CrossRef]
- Science, W. Institute of Genecard. Available online: https://www.genecards.org/ (accessed on 30 January 2022).
- Frolova, L.; Drastich, P.; Rossmann, P.; Klimesova, K.; Tlaskalova-Hogenova, H. Expression of Toll-like receptor 2 (TLR2), TLR4, and CD14 in biopsy samples of patients with inflammatory bowel diseases: Upregulated expression of TLR2 in terminal ileum of patients with ulcerative colitis. J. Histochem. Cytochem. 2008, 56, 267–274. [Google Scholar] [CrossRef]
- Sánchez-Muñoz, F.; Fonseca-Camarillo, G.; Villeda-Ramírez, M.A.; Miranda-Pérez, E.; Mendivil, E.J.; Barreto-Zúñiga, R.; Uribe, M.; Bojalil, R.; Domínguez-López, A.; Yamamoto-Furusho, J.K. Transcript levels of Toll-Like Receptors 5, 8 and 9 correlate with inflammatory activity in Ulcerative Colitis. BMC Gastroenterol. 2011, 11, 138. [Google Scholar] [CrossRef]
- Østvik, A.E.; Granlund, A.V.; Torp, S.H.; Flatberg, A.; Beisvåg, V.; Waldum, H.L.; Flo, T.H.; Espevik, T.; Damås, J.K.; Sandvik, A.K. Expression of Toll-like receptor-3 is enhanced in active inflammatory bowel disease and mediates the excessive release of lipocalin 2. Clin. Exp. Immunol. 2013, 173, 502–511. [Google Scholar] [CrossRef]
- Zheng, B.; Morgan, M.E.; van de Kant, H.J.; Garssen, J.; Folkerts, G.; Kraneveld, A.D. Transcriptional modulation of pattern recognition receptors in acute colitis in mice. Biochim. Biophys. Acta 2013, 1832, 2162–2172. [Google Scholar] [CrossRef]
- Zheng, B.; Morgan, M.E.; van de Kant, H.J.G.; Garssen, J.; Folkerts, G.; Kraneveld, A.D. Transcriptional modulation of pattern recognition receptors in chronic colitis in mice is accompanied with Th1 and Th17 response. Biochem. Biophys. Rep. 2017, 12, 29–39, Erratum in Biochem. Biophys. Rep. 2018, 13, 147–148. [Google Scholar] [CrossRef] [PubMed]
- Fenton, C.G.; Taman, H.; Florholmen, J.; Sørbye, S.W.; Paulssen, R.H. Transcriptional Signatures That Define Ulcerative Colitis in Remission. Inflamm. Bowel Dis. 2021, 27, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Luo, W.; Tan, B.; Nie, K.; Deng, M.; Wu, S.; Xiao, M.; Wu, X.; Meng, X.; Tong, T.; et al. Roseburia intestinalis stimulates TLR5-dependent intestinal immunity against Crohn’s disease. EBioMedicine 2022, 85, 104285. [Google Scholar] [CrossRef]
- Dastych, M.; Dastych, M.; Novotná, H.; Číhalová, J. Lactulose/mannitol test and specificity, sensitivity, and area under curve of intestinal permeability parameters in patients with liver cirrhosis and Crohn’s disease. Dig. Dis. Sci. 2008, 53, 2789–2792. [Google Scholar] [CrossRef]
- Khoshbin, K.; Khanna, L.; Maselli, D.; Atieh, J.; Breen-Lyles, M.; Arndt, K.; Rhoten, D.; Dyer, R.B.; Singh, R.J.; Nayar, S.; et al. Development and Validation of Test for “Leaky Gut” Small Intestinal and Colonic Permeability Using Sugars in Healthy Adults. Gastroenterology 2021, 161, 463–475.e13. [Google Scholar] [CrossRef]
- Vivier, E.; Malissen, B. Innate and adaptive immunity: Specificities and signaling hierarchies revisited. Nat. Immunol. 2005, 6, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Kałużna, A.; Olczyk, P.; Komosińska-Vassev, K. The Role of Innate and Adaptive Immune Cells in the Pathogenesis and Development of the Inflammatory Response in Ulcerative Colitis. J. Clin. Med. 2022, 11, 400. [Google Scholar] [CrossRef]
- Basso, D.; Zambon, C.F.; Plebani, M. Inflammatory bowel diseases: From pathogenesis to laboratory testing. Clin. Chem. Lab. Med. 2014, 52, 471–481. [Google Scholar] [CrossRef]
- Padoan, A.; D’Incà, R.; Scapellato, M.L.; De Bastiani, R.; Caccaro, R.; Mescoli, C.; Moz, S.; Bozzato, D.; Zambon, C.F.; Lorenzon, G.; et al. Improving IBD diagnosis and monitoring by understanding preanalytical, analytical and biological fecal calprotectin variability. Clin. Chem. Lab. Med. 2018, 56, 1926–1935. [Google Scholar] [CrossRef]
- Dinallo, V.; Marafini, I.; Di Fusco, D.; Laudisi, F.; Franzè, E.; Di Grazia, A.; Figliuzzi, M.M.; Caprioli, F.; Stolfi, C.; Monteleone, I.; et al. Neutrophil Extracellular Traps Sustain Inflammatory Signals in Ulcerative Colitis. J. Crohn’s Colitis 2019, 13, 772–784. [Google Scholar] [CrossRef]
- Abd El Hafez, A.; Mohamed, A.S.; Shehta, A.; Sheta, H.A.E.A.S. Neutrophil extracellular traps-associated protein peptidyl arginine deaminase 4 immunohistochemical expression in ulcerative colitis and its association with the prognostic predictors. Pathol. Res. Pract. 2020, 216, 153102. [Google Scholar] [CrossRef]
- Angelidou, I.; Chrysanthopoulou, A.; Mitsios, A.; Arelaki, S.; Arampatzioglou, A.; Kambas, K.; Ritis, D.; Tsironidou, V.; Moschos, I.; Dalla, V.; et al. REDD1/Autophagy Pathway Is Associated with Neutrophil-Driven IL-1β Inflammatory Response in Active Ulcerative Colitis. J. Immunol. 2018, 200, 3950–3961. [Google Scholar] [CrossRef]
- Cosín-Roger, J.; Ortiz-Masiá, D.; Calatayud, S.; Hernández, C.; Alvarez, A.; Hinojosa, J.; Esplugues, J.V.; Barrachina, M.D. M2 macrophages activate WNT signaling pathway in epithelial cells: Relevance in ulcerative colitis. PLoS ONE 2013, 8, e78128. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Yang, X.; Yue, W.; Xu, X.; Li, B.; Zou, L.; He, R. Chemerin aggravates DSS- induced colitis by suppressing M2 macrophage polarization. Cell. Mol. Immunol. 2014, 11, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Lissner, D.; Schumann, M.; Batra, A.; Kredel, L.I.; Kühl, A.A.; Erben, U.; May, C.; Schulzke, J.D.; Siegmund, B. Monocyte and M1 Macrophage-induced Barrier Defect Contributes to Chronic Intestinal Inflammation in IBD. Inflamm. Bowel Dis. 2015, 21, 1297–1305. [Google Scholar] [CrossRef]
- Cosín-Roger, J.; Ortiz-Masiá, D.; Calatayud, S.; Hernández, C.; Esplugues, J.V.; Barrachina, M.D. The activation of Wnt signaling by a STAT6-dependent macrophage phenotype promotes mucosal repair in murine IBD. Mucosal Immunol. 2016, 9, 986–998. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Jin, Z.; Yu, J.; Liang, J.; Yang, Q.; Li, F.; Shi, X.; Zhu, X.; Zhang, X. Baicalin ameliorates experimental inflammatory bowel disease through polarization of macrophages to an M2 phenotype. Int. Immunopharmacol. 2016, 35, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Tu, L.; Chen, J.; Xu, D.; Xie, Z.; Yu, B.; Tao, Y.; Shi, G.; Duan, L. IL-33-induced alternatively activated macrophage attenuates the development of TNBS-induced colitis. Oncotarget 2017, 8, 27704–27714. [Google Scholar] [CrossRef]
- Jang, S.E.; Min, S.W. Amelioration of colitis in mice by Leuconostoc lactis EJ-1 by M1 to M2 macrophage polarization. Microbiol. Immunol. 2020, 64, 133–142. [Google Scholar] [CrossRef]
- Zhuang, H.; Lv, Q.; Zhong, C.; Cui, Y.; He, L.; Zhang, C.; Yu, J. Tiliroside Ameliorates Ulcerative Colitis by Restoring the M1/M2 Macrophage Balance via the HIF-1α/glycolysis Pathway. Front. Immunol. 2021, 12, 649463. [Google Scholar] [CrossRef]
- Zhu, H.; Tong, S.; Yan, C.; Zhou, A.; Wang, M.; Li, C. Triptolide attenuates LPS-induced activation of RAW 264.7 macrophages by inducing M1-to-M2 repolarization via the mTOR/STAT3 signaling. Immunopharmacol. Immunotoxicol. 2022, 44, 894–901. [Google Scholar] [CrossRef]
- Song, W.J.; Li, Q.; Ryu, M.O.; Ahn, J.O.; Ha Bhang, D.; Chan Jung, Y.; Youn, H.Y. TSG-6 Secreted by Human Adipose Tissue-derived Mesenchymal Stem Cells Ameliorates DSS- induced colitis by Inducing M2 Macrophage Polarization in Mice. Sci. Rep. 2017, 7, 5187. [Google Scholar] [CrossRef]
- Song, J.Y.; Kang, H.J.; Hong, J.S.; Kim, C.J.; Shim, J.Y.; Lee, C.W.; Choi, J. Umbilical cord-derived mesenchymal stem cell extracts reduce colitis in mice by re-polarizing intestinal macrophages. Sci. Rep. 2017, 7, 9412. [Google Scholar] [CrossRef]
- Kawata, Y.; Tsuchiya, A.; Seino, S.; Watanabe, Y.; Kojima, Y.; Ikarashi, S.; Tominaga, K.; Yokoyama, J.; Yamagiwa, S.; Terai, S. Early injection of human adipose tissue-derived mesenchymal stem cell after inflammation ameliorates dextran sulfate sodium- induced colitis in mice through the induction of M2 macrophages and regulatory T cells. Cell Tissue Res. 2019, 376, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Xu, H.; Wang, G.; Liu, M.; Tian, D.; Yuan, Z. Extracellular vesicles derived from bone marrow mesenchymal stem cells attenuate dextran sodium sulfate-induced ulcerative colitis by promoting M2 macrophage polarization. Int. Immunopharmacol. 2019, 72, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liang, Z.; Wang, F.; Zhou, C.; Zheng, X.; Hu, T.; He, X.; Wu, X.; Lan, P. Exosomes from mesenchymal stromal cells reduce murine colonic inflammation via a macrophage-dependent mechanism. JCI Insight. 2019, 4, e131273. [Google Scholar] [CrossRef]
- Cao, X.; Duan, L.; Hou, H.; Liu, Y.; Chen, S.; Zhang, S.; Liu, Y.; Wang, C.; Qi, X.; Liu, N.; et al. IGF-1C hydrogel improves the therapeutic effects of MSCs on colitis in mice through PGE2-mediated M2 macrophage polarization. Theranostics 2020, 10, 7697–7709. [Google Scholar] [CrossRef]
- Gómez-Ferrer, M.; Amaro-Prellezo, E.; Dorronsoro, A.; Sánchez-Sánchez, R.; Vicente, Á.; Cosín-Roger, J.; Barrachina, M.D.; Baquero, M.C.; Valencia, J.; Sepúlveda, P. HIF-Overexpression and Pro-Inflammatory Priming in Human Mesenchymal Stromal Cells Improves the Healing Properties of Extracellular Vesicles in Experimental Crohn’s Disease. Int. J. Mol. Sci. 2021, 22, 11269. [Google Scholar] [CrossRef] [PubMed]
- Altemus, J.; Dadgar, N.; Li, Y.; Lightner, A.L. Adipose tissue-derived mesenchymal stem cells’ acellular product extracellular vesicles as a potential therapy for Crohn’s disease. J. Cell. Physiol. 2022, 237, 3001–3011. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Chen, Q.; Li, Y.; Wan, J.; Li, J.; Tang, S. HIF-1α-Overexpressing Mesenchymal Stem Cells Attenuate Colitis by Regulating M1-like Macrophages Polarization toward M2-like Macrophages. Biomedicines 2023, 11, 825. [Google Scholar] [CrossRef]
- Qian, W.; Huang, L.; Xu, Y.; Lu, W.; Wen, W.; Guo, Z.; Zhu, W.; Li, Y. Hypoxic ASCs-derived Exosomes Attenuate Colitis by Regulating Macrophage Polarization via miR-216a-5p/HMGB1 Axis. Inflamm. Bowel Dis. 2023, 29, 602–619. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Liu, W.; Chen, H.; Zuo, T.; Wu, X. Immune Cell Landscaping Reveals Distinct Immune Signatures of Inflammatory Bowel Disease. Front. Immunol. 2022, 13, 861790. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, T.K.; Brown, E.M.; Plichta, D.R.; Johansen, J.; Twardus, S.W.; Delorey, T.M.; Lau, H.; Vlamakis, H.; Moon, J.J.; Xavier, R.J.; et al. The CD4+ T cell response to a commensal-derived epitope transitions from a tolerant to an inflammatory state in Crohn’s disease. Immunity 2022, 55, 1909–1923.e6. [Google Scholar] [CrossRef] [PubMed]
- Bharti, S.; Bharti, M. The Business of T Cell Subsets and Cytokines in the Immunopathogenesis of Inflammatory Bowel Disease. Cureus 2022, 14, 27290. [Google Scholar] [CrossRef]
- Globig, A.M.; Mayer, L.S.; Heeg, M.; Andrieux, G.; Ku, M.; Otto-Mora, P.; Hipp, A.V.; Zoldan, K.; Pattekar, A.; Rana, N.; et al. Exhaustion of CD39-Expressing CD8+ T Cells in Crohn’s Disease Is Linked to Clinical Outcome. Gastroenterology 2022, 163, 965–981.e31. [Google Scholar] [CrossRef]
- Lee, J.C.; Lyons, P.A.; McKinney, E.F.; Sowerby, J.M.; Carr, E.J.; Bredin, F.; Rickman, H.M.; Ratlamwala, H.; Hatton, A.; Rayner, T.F.; et al. Gene expression profiling of CD8+ T cells predicts prognosis in patients with Crohn disease and ulcerative colitis. J. Clin. Investig. 2011, 121, 4170–4179. [Google Scholar] [CrossRef]
- Marafini, I.; Sedda, S.; Dinallo, V.; Monteleone, G. Inflammatory cytokines: From discoveries to therapies in IBD. Expert Opin. Biol. Ther. 2019, 19, 1207–1217. [Google Scholar] [CrossRef]
- Pickard, J.M.; Zeng, M.Y.; Caruso, R.; Núñez, G. Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunol. Rev. 2017, 279, 70–89. [Google Scholar] [CrossRef]
- Ashwood, P.; Harvey, R.; Verjee, T.; Wolstencroft, R.; Thompson, R.P.H.; Powell, J.J. Functional interactions between mucosal IL-1, IL-ra and TGF-beta 1 in ulcerative colitis. Inflamm. Res. 2004, 53, 53–59. [Google Scholar] [CrossRef]
- Neurath, M.F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014, 14, 329–342. [Google Scholar] [CrossRef]
- Abraham, C.; Abreu, M.T.; Turner, J.R. Pattern Recognition Receptor Signaling and Cytokine Networks in Microbial Defenses and Regulation of Intestinal Barriers: Implications for Inflammatory Bowel Disease. Gastroenterology 2022, 162, 1602–1616.e6. [Google Scholar] [CrossRef] [PubMed]
- Fujino, S.; Andoh, A.; Bamba, S.; Ogawa, A.; Hata, K.; Araki, Y.; Bamba, T.; Fujiyama, Y. Increased expression of interleukin 17 in inflammatory bowel disease. Gut 2003, 52, 65–70. [Google Scholar] [CrossRef]
- Moldoveanu, A.C.; Diculescu, M.; Braticevici, C.F. Cytokines in inflammatory bowel disease. Rom. J. Intern. Med. 2015, 53, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Giuffrida, P.; Caprioli, F.; Facciotti, F.; Di Sabatino, A. The role of interleukin-13 in chronic inflammatory intestinal disorders. Autoimmun. Rev. 2019, 18, 549–555. [Google Scholar] [CrossRef]
- Bamias, G.; Zampeli, E.; Domènech, E. Targeting neutrophils in inflammatory bowel disease: Revisiting the role of adsorptive granulocyte and monocyte apheresis. Expert Rev. Gastroenterol. Hepatol. 2022, 16, 721–735. [Google Scholar] [CrossRef]
- Chen, G.Y.; Nez, G. Inflammasomes in intestinal inflammation and cancer. Gastroenterology 2011, 141, 1986–1999. [Google Scholar] [CrossRef] [PubMed]
- Opipari, A.; Franchi, L. Role of inflammasomes in intestinal inflammation and Crohn’s disease. Inflamm. Bowel Dis. 2015, 21, 173–181. [Google Scholar] [CrossRef]
- de Jesus, A.A.; Goldbach-Mansky, R. Monogenic autoinflammatory diseases: Concept and clinical manifestations. Clin. Immunol. 2013, 147, 155–174. [Google Scholar] [CrossRef] [PubMed]
- Bernot, A.; Clepet, C.; Dasilva, C.; Devaud, C.; Petit, J.L.; Caloustian, C.; Cruaud, C.; Samson, D.; Pulcini, F.; Weissenbach, J.; et al. A candidate gene for familial Mediterranean fever. Nat. Genet. 1997, 17, 25–31. [Google Scholar] [CrossRef]
- Aksentijevich, I.; Centola, M.; Deng, Z.; Sood, R.; Balow, J.; Wood, G.; Zaks, N.; Mansfield, E.; Chen, X.; Eisenberg, S.; et al. Ancient missense mutations in a new member of the RoRet gene family are likely to cause familial Mediterranean fever. The International FMF Consortium. Cell 1997, 90, 797–807. [Google Scholar] [CrossRef]
- Gattorno, M.; Hofer, M.; Federici, S.; Vanoni, F.; Bovis, F.; Aksentijevich, I.; Anton, J.; Arostegui, J.I.; Barron, K.; Ben-Cherit, E.; et al. Classification criteria for autoinflammatory recurrent fevers. Ann. Rheum. Dis. 2019, 78, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Georgin-Lavialle, S.; Fayand, A.; Rodrigues, F.; Bachmeyer, C.; Savey, L.; Grateau, G. Autoinflammatory diseases: State of the art. Presse Med. 2019, 48, e25–e48. [Google Scholar] [CrossRef] [PubMed]
- Tyler, P.M.; Bucklin, M.L.; Zhao, M.; Maher, T.J.; Rice, A.J.; Ji, W.; Warner, N.; Pan, J.; Morotti, R.; McCarthy, P.; et al. Human autoinflammatory disease reveals ELF4 as a transcriptional regulator of inflammation. Nat. Immunol. 2021, 22, 1118–1126. [Google Scholar] [CrossRef] [PubMed]
- Goldbach-Mansky, R. Immunology in clinic review series; focus on autoinflammatory diseases: Update on monogenic autoinflammatory diseases: The role of interleukin (IL)-1 and an emerging role for cytokines beyond IL-1. Clin. Exp. Immunol. 2012, 167, 391–404. [Google Scholar] [CrossRef]
- Shen, Y.; Qian, L.; Luo, H.; Li, X.; Ruan, Y.; Fan, R.; Si, Z.; Chen, Y.; Li, L.; Liu, Y. The Significance of NLRP Inflammasome in Neuropsychiatric Disorders. Brain Sci. 2022, 12, 1057. [Google Scholar] [CrossRef]
- Rosenstiel, P.; Till, A.; Schreiber, S. NOD-like receptors and human diseases. Microbes Infect. 2007, 9, 648–657. [Google Scholar] [CrossRef]
- Borzutzky, A.; Fried, A.; Chou, J.; Bonilla, F.A.; Kim, S.; Dedeoglu, F. NOD2-associated diseases: Bridging innate immunity and autoinflammation. Clin. Immunol. 2010, 134, 251–261. [Google Scholar] [CrossRef]
- Xu, Q.; Zhou, X.; Strober, W.; Mao, L. Inflammasome Regulation: Therapeutic Potential for Inflammatory Bowel Disease. Molecules 2021, 26, 1725. [Google Scholar] [CrossRef]
- Girardin, S.E.; Boneca, I.G.; Viala, J.; Chamaillard, M.; Labigne, A.; Thomas, G.; Philpott, D.J.; Sansonetti, P.J. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J. Biol. Chem. 2003, 278, 8869–8872. [Google Scholar] [CrossRef]
- Honjo, H.; Watanabe, T.; Kamata, K.; Minaga, K.; Kudo, M. RIPK2 as a New Therapeutic Target in Inflammatory Bowel Diseases. Front. Pharmacol. 2021, 12, 650403. [Google Scholar] [CrossRef]
- Topal, Y.; Gyrd-Hansen, M. RIPK2 NODs to XIAP and IBD. Semin. Cell Dev. Biol. 2021, 109, 144–150. [Google Scholar] [CrossRef] [PubMed]
- de Iudicibus, S.; Franca, R.; Martelossi, S.; Ventura, A.; Decorti, G. Molecular mechanism of glucocorticoid resistance in inflammatory bowel disease. World J. Gastroenterol. 2011, 17, 1095–1108. [Google Scholar] [CrossRef] [PubMed]
- Tye, H.; Yu, C.H.; Simms, L.A.; de Zoete, M.R.; Kim, M.L.; Zakrzewski, M.; Penington, J.S.; Harapas, C.R.; Souza-Fonseca-Guimaraes, F.; Wockner, L.F.; et al. NLRP1 restricts butyrate producing commensals to exacerbate inflammatory bowel disease. Nat. Commun. 2018, 9, 3728. [Google Scholar] [CrossRef] [PubMed]
- Villani, A.C.; Lemire, M.; Fortin, G.; Louis, E.; Silverberg, M.S.; Collette, C.; Baba, N.; Libioulle, C.; Belaiche, J.; Bitton, A.; et al. Common variants in the NLRP3 region contribute to Crohn’s disease susceptibility. Nat. Genet. 2009, 41, 71–76. [Google Scholar] [CrossRef]
- Georges, M. The long and winding road from correlation to causation. Nat. Genet. 2011, 43, 180–181. [Google Scholar] [CrossRef]
- Brest, P.; Lapaquette, P.; Souidi, M.; Lebrigand, K.; Cesaro, A.; Vouret-Craviari, V.; Mari, B.; Barbry, P.; Mosnier, J.F.; Hébuterne, X.; et al. A synonymous variant in IRGM alters a binding site for miR-196 and causes deregulation of IRGM-dependent xenophagy in Crohn’s disease. Nat. Genet. 2011, 43, 242–245. [Google Scholar] [CrossRef]
- Mehto, S.; Jena, K.K.; Nath, P.; Chauhan, S.; Kolapalli, S.P.; Das, S.K.; Sahoo, P.K.; Jain, A.; Taylor, G.A.; Chauhan, S. The Crohn’s Disease Risk Factor IRGM Limits NLRP3 Inflammasome Activation by Impeding Its Assembly and by Mediating Its Selective Autophagy. Mol. Cell 2019, 73, 429–445.e7. [Google Scholar] [CrossRef]
- Gonçalves, P.; Di Santo, J.P. An Intestinal Inflammasome—The ILC3-Cytokine Tango. Trends Mol. Med. 2016, 22, 269–271. [Google Scholar] [CrossRef]
- Steiner, A.; Reygaerts, T.; Pontillo, A.; Ceccherini, I.; Moecking, J.; Moghaddas, F.; Davidson, S.; Caroli, F.; Grossi, A.; Castro, F.F.M.; et al. Recessive NLRC4-Autoinflammatory Disease Reveals an Ulcerative Colitis Locus. J. Clin. Immunol. 2022, 42, 325–335. [Google Scholar] [CrossRef]
- Romberg, N.; Al Moussawi, K.; Nelson-Williams, C.; Stiegler, A.L.; Loring, E.; Choi, M.; Overton, J.; Meffre, E.; Khokha, M.K.; Huttner, A.J.; et al. Mutation of NLRC4 causes a syndrome of enterocolitis and autoinflammation. Nat. Genet. 2014, 46, 1135–1139. [Google Scholar] [CrossRef]
- Elinav, E.; Strowig, T.; Kau, A.L.; Henao-Mejia, J.; Thaiss, C.A.; Booth, C.J.; Peaper, D.R.; Bertin, J.; Eisenbarth, S.C.; Gordon, J.I.; et al. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell 2011, 145, 745–757. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wilson, J.E.; Koenigsknecht, M.J.; Chou, W.C.; Montgomery, S.A.; Truax, A.D.; Brickey, W.J.; Packey, C.D.; Maharshak, N.; Matsushima, G.K.; et al. NLRP12 attenuates colon inflammation by maintaining colonic microbial diversity and promoting protective commensal bacterial growth. Nat. Immunol. 2017, 18, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Tal, Y.; Ribak, Y.; Khalaila, A.; Shamriz, O.; Marcus, N.; Zinger, A.; Meiner, V.; Schuster, R.; Lewis, E.C.; Nahum, A. Toll-like receptor 3 (TLR3) variant and NLRP12 mutation confer susceptibility to a complex clinical presentation. Clin. Immunol. 2020, 212, 108249. [Google Scholar] [CrossRef]
- Bernstein, C.N.; Eliakim, A.; Fedail, S.; Fried, M.; Gearry, R.; Goh, K.L.; Hamid, S.; Khan, A.G.; Khalif, I.; Ng, S.C.; et al. World Gastroenterology Organisation Global Guidelines Inflammatory Bowel Disease: Update August 2015. J. Clin. Gastroenterol. 2016, 50, 803–818. [Google Scholar] [CrossRef] [PubMed]
- Cappello, M.; Morreale, G.C. The Role of Laboratory Tests in Crohn’s Disease. Clin. Med. Insights Gastroenterol. 2016, 9, 51–62. [Google Scholar] [CrossRef]
- Li, T.; Qian, Y.; Bai, T.; Li, J. Prediction of complications in inflammatory bowel disease using routine blood parameters at diagnosis. Ann. Transl. Med. 2022, 10, 185. [Google Scholar] [CrossRef] [PubMed]
- Alper, A.; Zhang, L.; Pashankar, D.S. Correlation of Erythrocyte Sedimentation Rate and C-Reactive Protein With Pediatric Inflammatory Bowel Disease Activity. J. Pediatr. Gastroenterol. Nutr. 2017, 65, e25–e27. [Google Scholar] [CrossRef]
- Ruffolo, C.; Scarpa, M.; Faggian, D.; Basso, D.; D’Incà, R.; Plebani, M.; Sturniolo, G.C.; Bassi, N.; Angriman, I. Subclinical intestinal inflammation in patients with Crohn’s disease following bowel resection: A smoldering fire. J. Gastrointest. Surg. 2010, 14, 24–31. [Google Scholar] [CrossRef]
- Scarpa, M.; D’Incà, R.; Basso, D.; Ruffolo, C.; Polese, L.; Bertin, E.; Luise, A.; Frego, M.; Plebani, M.; Sturniolo, G.C.; et al. Fecal lactoferrin and calprotectin after ileocolonic resection for Crohn’s disease. Dis. Colon Rectum 2007, 50, 861–869. [Google Scholar] [CrossRef]
- Ardizzone, S.; Sarzi Puttini, P.; Cassinotti, A.; Bianchi Porro, G. Extraintestinal Manifestations of Inflammatory Bowel Disease in Children. Dig. Liver Dis. 2008, 40, S253–S259. [Google Scholar] [CrossRef]
- Cassinotti, A.; Sarzi-Puttini, P.; Fichera, M.; Shoenfeld, Y.; de Franchis, R.; Ardizzone, S. Immunity, autoimmunity and inflammatory bowel disease. Autoimmun. Rev. 2014, 13, 1–2. [Google Scholar] [CrossRef]
- Fiorino, G.; Danese, S.; Pariente, B.; Allez, M. Paradoxical immune-mediated inflammation in inflammatory bowel disease patients receiving anti-TNF-α agents. Autoimmun. Rev. 2014, 13, 15–19. [Google Scholar] [CrossRef]
- Su, C.G.; Judge, T.A.; Lichtenstein, G.R. Extraintestinal manifestations of inflammatory bowel disease. Gastroenterol. Clin. North Am. 2002, 31, 307–327. [Google Scholar] [CrossRef]
- Das, K.M. Relationship of extraintestinal involvements in inflammatory bowel disease: New insights into autoimmune pathogenesis. Dig. Dis. Sci. 1999, 44, 1–13. [Google Scholar] [CrossRef]
- Halling, M.L.; Kjeldsen, J.; Knudsen, T.; Nielsen, J.; Hansen, L.K. Patients with inflammatory bowel disease have increased risk of autoimmune and inflammatory diseases. World J. Gastroenterol. 2017, 23, 6137–6146. [Google Scholar] [CrossRef] [PubMed]
- Prideaux, L.; De Cruz, P.; Ng, S.C.; Kamm, M.A. Serological antibodies in inflammatory bowel disease: A systematic review. Inflamm. Bowel Dis. 2012, 18, 1340–1355. [Google Scholar] [CrossRef] [PubMed]
- Bossuyt, X. Serologic markers in inflammatory bowel disease. Clin. Chem. 2006, 52, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Broberger, O.; Perlmann, P. Autoantibodies in human ulcerative colitis. J. Exp. Med. 1959, 110, 657–674. [Google Scholar] [CrossRef] [PubMed]
- Ramponi, G.; Folci, M.; De Santis, M.; Damoiseaux, J.G.M.C.; Selmi, C.; Brunetta, E. The biology, pathogenetic role, clinical implications, and open issues of serum anti-neutrophil cytoplasmic antibodies. Autoimmun. Rev. 2021, 20, 102759. [Google Scholar] [CrossRef]
- Moiseev, S.; Cohen Tervaert, J.W.; Arimura, Y.; Bogdanos, D.P.; Csernok, E.; Damoiseaux, J.; Ferrante, M.; Flores-Suárez, L.F.; Fritzler, M.J.; Invernizzi, P.; et al. 2020 international consensus on ANCA testing beyond systemic vasculitis. Autoimmun. Rev. 2020, 19, 102618. [Google Scholar] [CrossRef] [PubMed]
- van Beers, J.J.B.C.; Vanderlocht, J.; Roozendaal, C.; Damoiseaux, J. Detection of anti-neutrophil cytoplasmic antibodies (ANCA) by indirect immunofluorescence. Methods Mol. Biol. 2019, 1901, 47–62. [Google Scholar] [CrossRef]
- Savige, J.; Gillis, D.; Benson, E.; Davies, D.; Esnault, V.; Falk, R.J.; Hagen, E.C.; Jayne, D.; Jennette, J.C.; Paspaliaris, B.; et al. International consensus statement on testing and reporting of antineutrophil cytoplasmic antibodies (ANCA). Am. J. Clin. Pathol. 1999, 111, 507–513. [Google Scholar] [CrossRef]
- Folci, M.; Ramponi, G.; Solitano, V.; Brunetta, E. Serum ANCA as Disease Biomarkers: Clinical Implications Beyond Vasculitis. Clin. Rev. Allergy Immunol. 2022, 63, 107–123. [Google Scholar] [CrossRef] [PubMed]
- Calabresi, P.; Thayer, W.R.; Spiro, H.M. Demonstration of Circulating Antinuclear Globulins in Ulcerative Colitis. J. Clin. Invest. 1961, 40, 2126–2133. [Google Scholar] [CrossRef] [PubMed]
- Mahler, M.; Damoiseaux, J.; Ballet, V.; Dillaerts, D.; Bentow, C.; Tervaert, J.C.; Blockmans, D.; Boeckxstaens, G.; Aguilera-lizarraga, J. PR3-anti-neutrophil cytoplasmic antibodies (ANCA) in ulcerative colitis. Clin. Chem. Lab. Med. 2018, 56, 27–30. [Google Scholar]
- Main, J.; McKenzie, H.; Yeaman, G.R.; Kerr, M.A.; Robson, D.; Pennington, C.R.; Parratt, D. Antibody to Saccharomyces cerevisiae (bakers’ yeast) in Crohn’s disease. Br. Med. J. 1988, 297, 1105–1106. [Google Scholar] [CrossRef]
- Quinton, J.F.; Sendid, B.; Reumaux, D.; Duthilleul, P.; Cortot, A.; Grandbastien, B.; Charrier, G.; Targan, S.R.; Colombel, J.F.; Poulain, D. Anti-Saccharomyces cerevisiae manna antibodies combined with antineutrophil cytoplasmic autoantibodies in inflammatory bowel disease: Prevalence and diagnostic role. Gut 1998, 42, 788–791. [Google Scholar] [CrossRef] [PubMed]
- Saibeni, S.; Folli, C.; de Franchis, R.; Borsi, G.; Vecchi, M. Diagnostic role and clinical correlates of anti-Saccharomyces cerevisiae antibodies (ASCA) and anti-neutrophil cytoplasmic antibodies (p-ANCA) in Italian patients with inflammatory bowel diseases. Dig. Liver Dis. 2003, 35, 862–868. [Google Scholar] [CrossRef]
- Vermeire, S.; Joossens, S.; Peeters, M.; Monsuur, F.; Marien, G.; Bossuyt, X.; Groenen, P.; Vlietinck, R.; Rutgeerts, P. Comparative study of ASCA (Anti-Saccharomyces cerevisiae antibody) assays in inflammatory bowel disease. Gastroenterology 2001, 120, 827–833. [Google Scholar] [CrossRef]
- Torres, J.; Petralia, F.; Sato, T.; Wang, P.; Telesco, S.E.; Choung, R.S.; Strauss, R.; Li, X.J.; Laird, R.M.; Gutierrez, R.L.; et al. Serum Biomarkers Identify Patients Who Will Develop Inflammatory Bowel Diseases Up to 5 Years Before Diagnosis. Gastroenterology 2020, 159, 96–104. [Google Scholar] [CrossRef]
- Schwarz, M.; Spector, L.; Gargir, A.; Shtevi, A.; Gortler, M.; Altstock, R.T.; Dukler, A.A.; Dotan, N. A new kind of carbohydrate array, its use for profiling antiglycan antibodies, and the discovery of a novel human cellulose-binding antibody. Glycobiology 2003, 13, 749–754. [Google Scholar] [CrossRef]
- Seow, C.H.; Stempak, J.M.; Xu, W.; Lan, H.; Griffiths, A.M.; Greenberg, G.R.; Steinhart, A.H.; Dotan, N.; Silverberg, M.S. Novel anti-glycan antibodies related to inflammatory bowel disease diagnosis and phenotype. Am. J. Gastroenterol. 2009, 104, 1426–1434. [Google Scholar] [CrossRef]
- Rojas, A.; Schneider, I.; Lindner, C.; Gonzàlez, I.; Morales, M.A. Receptor for advanced glycation end-products axis and coronavirus disease 2019 in inflammatory bowel diseases: A dangerous liaison? World J. Gastroenterol. 2021, 27, 2270–2280. [Google Scholar] [CrossRef] [PubMed]
- Leibovitzh, H.; Lee, S.H.; Raygoza Garay, J.A.; Espin-Garcia, O.; Xue, M.; Neustaeter, A.; Goethel, A.; Huynh, H.Q.; Griffiths, A.M.; Turner, D.; et al. Immune response and barrier dysfunction-related proteomic signatures in preclinical phase of Crohn’s disease highlight earliest events of pathogenesis. Gut 2023. [Google Scholar] [CrossRef] [PubMed]
- Vich Vila, A.; Hu, S.; Andreu-Sánchez, S.; Collij, V.; Jansen, B.H.; Augustijn, H.E.; Bolte, L.A.; Ruigrok, R.A.A.A.; Abu-Ali, G.; Giallourakis, C.; et al. Faecal metabolome and its determinants in inflammatory bowel disease. Gut 2023, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, K.G.; Desai, D.C.; Ashavaid, T.F.; Dherai, A.J. Microbiome and metabolome in inflammatory bowel disease. J. Gastroenterol. Hepatol. 2023, 38, 34–43. [Google Scholar] [CrossRef]
- Kriger-Sharabit, O.A.; Kopylov, U. Harnessing the Power of Precision Medicine and Novel Biomarkers to Treat Crohn’s Disease. J. Clin. Med. 2023, 12, 2696. [Google Scholar] [CrossRef]
- Gubatan, J.; Levitte, S.; Patel, A.; Balabanis, T.; Wei, M.T.; Sinha, S.R. Artificial intelligence applications in inflammatory bowel disease: Emerging technologies and future directions. World J. Gastroenterol. 2021, 27, 1920–1935. [Google Scholar] [CrossRef]
- Sahoo, D.; Swanson, L.; Sayed, I.M.; Katkar, G.D.; Ibeawuchi, S.R.; Mittal, Y.; Pranadinata, R.F.; Tindle, C.; Fuller, M.; Stec, D.L.; et al. Artificial intelligence guided discovery of a barrier-protective therapy in inflammatory bowel disease. Nat. Commun. 2021, 12, 4246. [Google Scholar] [CrossRef]
- Pernencar, C.; Saboia, I.; Dias, J.C. How Far Can Conversational Agents Contribute to IBD Patient Health Care—A Review of the Literature. Front. Public Health 2022, 10, 862432. [Google Scholar] [CrossRef]
- Zand, A.; Sharma, A.; Stokes, Z.; Reynolds, C.; Montilla, A.; Sauk, J.; Hommes, D. An Exploration Into the Use of a Chatbot for Patients with Inflammatory Bowel Diseases: Retrospective Cohort Study. J. Med. Internet Res. 2020, 22, e15589. [Google Scholar] [CrossRef] [PubMed]
| TLR | Adapter | Compartments | Ligands | Cell Types | Main Alterations in IBD |
|---|---|---|---|---|---|
| TLR1 | MyD88/TRIAP | CS | Di- and tri-acylated lipopeptides | Mø, B, MCs | No variation in IBD |
| TLR2 | MyD88/TRIAP | CS | Bacterial lipoproteins or lipopeptides | Mø, B, MCs | Increased in active UC |
| TLR3 | TRIF | IC | Double-stranded RNA (viral infection) | Mø, B, MCs, N, Myeloid DCs, IECs | Increased in active UC and CD |
| TLR4 | MyD88/TRIAP, TRIF/TRAM | CS | LPS, free fatty acids | Mø, B, MCs, N, Myeloid DCs, IECs | Increased in UC and CD |
| TLR5 | MyD88 | CS | Bacterial flagellins | Mø, Myeloid DCs, IECs | Decreased in CD |
| TLR6 | MyD88/TRAF6 and NF-κB pathway | CS | Di- and tri-acylated lipopeptides | Mø, B, MCs | Increased in UC |
| TLR7 | MyD88 | IC/Endosomal | Single stranded RNA (viral inflammation) | Mø | Increased in UC |
| TLR8 | MyD88 | IC/endosomal | RNA degradation products specific to microorganism (GU-rich single stranded RNA) | Mø, DCs | Increased in UC |
| TLR9 | MyD88/TRAF6 | IC | Nucleid acid | Mø, B, plasmacytoid DCs | Increased in UC |
| Cytokine | Source of Secretion | Potential Function in Pathogenesis of Chronic Intestinal Inflammation in IBD |
|---|---|---|
| IFN alpha and IFN beta | DCs | Promote epithelial generation and induce IL-10 producing cells |
| IFN gamma | T cells and ILCs | Activate macrophages, augment antigen processing and induce epithelial cell death |
| TNF-alpha | Macrophages, DC and T cells | Pro-inflammatory action, pro-inflammatory cytokine production and angiogenesis, induce epithelial cell death, mediate T cell resistance against apoptosis and induce cachexia |
| IL-1 | Neutrophils and macrophages | Pro-inflammatory actions: augment neutrophil recruitment, stimulate IL-6 production by macrophages, activate ILCs and promote tumor development. Significantly increased in UC patients |
| IL-6 | Macrophages, fibroblasts and T cells | Perform pro-inflammatory action by means of IL-6 soluble receptor. Activate T cells and prevent apoptosis (via STAT3), induce macrophage activation, recruit immune cells, activate acute-phase proteins, induce epithelial cell proliferation |
| IL-10 | T cells | Exert anti-inflammatory effects that inhibit both antigen presentation and subsequent release of pro-inflammatory cytokines, and induce STAT3 signaling in regulatory T cells |
| IL-12 | Macrophages and DC | Induce Th1 cell differentiation via STAT4 activation in T cells, stimulate Th1-type cytokine production and activate ILCs; a link between innate and adaptive resistance |
| IL-13 | T cells, mast cells, basophil and eosinophil and NKT cells | Induce intestinal epithelial cell alterations and barrier function; induce fibrosis |
| IL-17 | Th17 cells and ILCs | Induce pro-inflammatory factors (including TNF-α, IL-6 and IL-1β) and anti-inflammatory effects in the mucosa; IL-17A exerts pro-fibrotic functions |
| IL-18 | IECs | Act in synergy with IL-12 to promote the production of INF-g, causing severe intestinal inflammation |
| IL-21 | Th1 cells | Induce production of TNF-α, IL-1, IL-6 and IL-8 in the mucosa, recruit neutrophils, induce secretion of matrix metalloproteinases by fibroblasts and favor tumor development |
| IL-22 | T cells, ILC, neutrophils and DC | Exert a pro-inflammatory effect; increased in both CD and UC. Activate production of antimicrobial peptides by epithelial cells, induce proliferation of epithelial cells and favor tumor development via STAT3 activation |
| IL-23 | Macrophages and DCs | Activate mucosal immune cells (e.g., T cells and macrophages) cells, augment TNF-α production and stabilize effector Th17 cell phenotype |
| IL-27 | Macrophages | Exert pro-inflammatory effects by inducing T cell activation and Th1-type cytokine production and exert anti-inflammatory effects by blocking T cell expansion and inhibiting cytokine production by neutrophils |
| IL-33 | Epithelial cells and myofibroblasts | Suppress Th1-type cytokine production and induce neutrophil influx |
| Family | Protein | Gene | Mutation-Related Diseases | IBD-Associated | Function | Complex |
|---|---|---|---|---|---|---|
| NLR | MHC class II transactivator | CIITA | Bare lymphocyte syndrome RA | Unknown | Positive regulator of class II MHC | |
| NLRB | NRL family apoptosis inhibitory protein | NAIP | Spinal muscular atrophy | Unknown | Anti-apoptotic (inhibits CASP3, CASP7 and CASP9) | Sensor component of NLRC4 that recognizes and binds CprI from pathogenic bacteria C. violaceum |
| NLRC | NOD1 | NOD1 | IBD, Asthma, Behcet’s disease and sarcoidosis | Yes | Innate and adaptive immune responses and cellular homeostasis. Binds bacterial peptidoglycans, single-stranded RNA (ssRNA) from viruses and the metabolite sphingosine-1-phosphate | Interacts with RIPK2 activating NF-kB and MAPK signaling pathways |
| NLRC | NOD2 | NOD2 | Crohn’s disease and Blau syndrome | Yes | Innate and adaptive immune responses and cellular homeostasis. Binds LPS by recognizing the muramyl dipeptide (MDP), single-stranded RNA (ssRNA) from viruses and the metabolite sphingosine-1-phosphate | Interacts with RIPK2 activating NF-kB and MAPK signaling pathways. Interacts with NLRP1 leading to IL-1 release. Interacts with ATG16L1 leading to autophagy |
| NLRC | NOD-like receptor caspase recruitment domain containing proteins 3–5 | NLRC3 | Yes | Negative regulator of the innate immune response (negative regulation of NF-kB and type I interferon signaling pathways) | Prevents NLRP3 inflammasome formation and may affect NOD1- or NOD2-mediated NF-kB activation | |
| NRLC4 | FCAS 4 Autoinflammation with infantile enterocolitis | Yes | Innate immune response. Promotes caspase-1 activation, cytokine production and macrophage pyroptosis | Homo-oligomerizes in the NLRC4 inflammasome and enters the NRLP3 inflammasome | ||
| NRLC5 | Pityriasis rubra pilaris Bare lymphocytic syndrome type I FMF | Unknown | Negative regulator of the innate immune response (negative regulation of NF-kB and type I interferon signaling pathways) | |||
| NLRP | NACHT, LRR, and PRD containing proteins 1–14 | NLRP1-14 | NRLP1: VAMAS1 MSPC JRRP | Yes | NLRP1: Innate immunity and inflammation. Cytokines IL-1, IL-18 and gasdermin-D (GSDMD), leading to pyroptosis, an inflammatory form of programmed cell death | NRLP1 inflammasome response to various pathogen-associated signals, recruits pro-caspase-1 (proCASP1) and promotes caspase-1 (CASP1) activation; may be activated by MDP in a NOD2-dependent manner |
| NRLP3: FCAS1 AIADK MWS CINCA syndrome | Yes | NLRP3: regulation of inflammation, immune response, and apoptosis. Stimulated by extracellular ATP, reactive oxygen species, K(+) efflux, crystals of monosodium urate or cholesterol, amyloid-beta fibers, environmental or industrial particles and nanoparticles, cytosolic dsRNA | NRLP3 inflammasome upstream activator of NF-kappaB signaling | |||
| NLRP12: FCAS2 | Yes | NLRP12 potent mitigator of inflammation Primarily expressed in dendritic cells and macrophages, inhibits both canonical and non-canonical NF-kB and ERK activation pathways Functions as a negative regulator of NOD2 by targeting it to degradation via the proteasome pathway. Promotes bacterial tolerance | ||||
| NLRX | NOD-like receptor with “unknown” domain | NLRX1 | Histiocytic sarcoma Combined oxidative phosphorylation deficiency 4 Mooren Ulcer Mitochondrial Complex V Nuclear deficiency Type 3 | Unknown | Regulator of mitochondrial antivirus responses. Promotes autophagy Enhances NF-kB and JUN N-terminal kinase dependent signaling through the production of reactive oxygen species. Regulates energy metabolism in a sex-dependent manner | Regulates NLRP3 inflammasome activation to attenuate apoptosis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padoan, A.; Musso, G.; Contran, N.; Basso, D. Inflammation, Autoinflammation and Autoimmunity in Inflammatory Bowel Diseases. Curr. Issues Mol. Biol. 2023, 45, 5534-5557. https://doi.org/10.3390/cimb45070350
Padoan A, Musso G, Contran N, Basso D. Inflammation, Autoinflammation and Autoimmunity in Inflammatory Bowel Diseases. Current Issues in Molecular Biology. 2023; 45(7):5534-5557. https://doi.org/10.3390/cimb45070350
Chicago/Turabian StylePadoan, Andrea, Giulia Musso, Nicole Contran, and Daniela Basso. 2023. "Inflammation, Autoinflammation and Autoimmunity in Inflammatory Bowel Diseases" Current Issues in Molecular Biology 45, no. 7: 5534-5557. https://doi.org/10.3390/cimb45070350
APA StylePadoan, A., Musso, G., Contran, N., & Basso, D. (2023). Inflammation, Autoinflammation and Autoimmunity in Inflammatory Bowel Diseases. Current Issues in Molecular Biology, 45(7), 5534-5557. https://doi.org/10.3390/cimb45070350








