A Metagenome from a Steam Vent in Los Azufres Geothermal Field Shows an Abundance of Thermoplasmatales archaea and Bacteria from the Phyla Actinomycetota and Pseudomonadota
Abstract
:1. Introduction
2. Materials and Methods
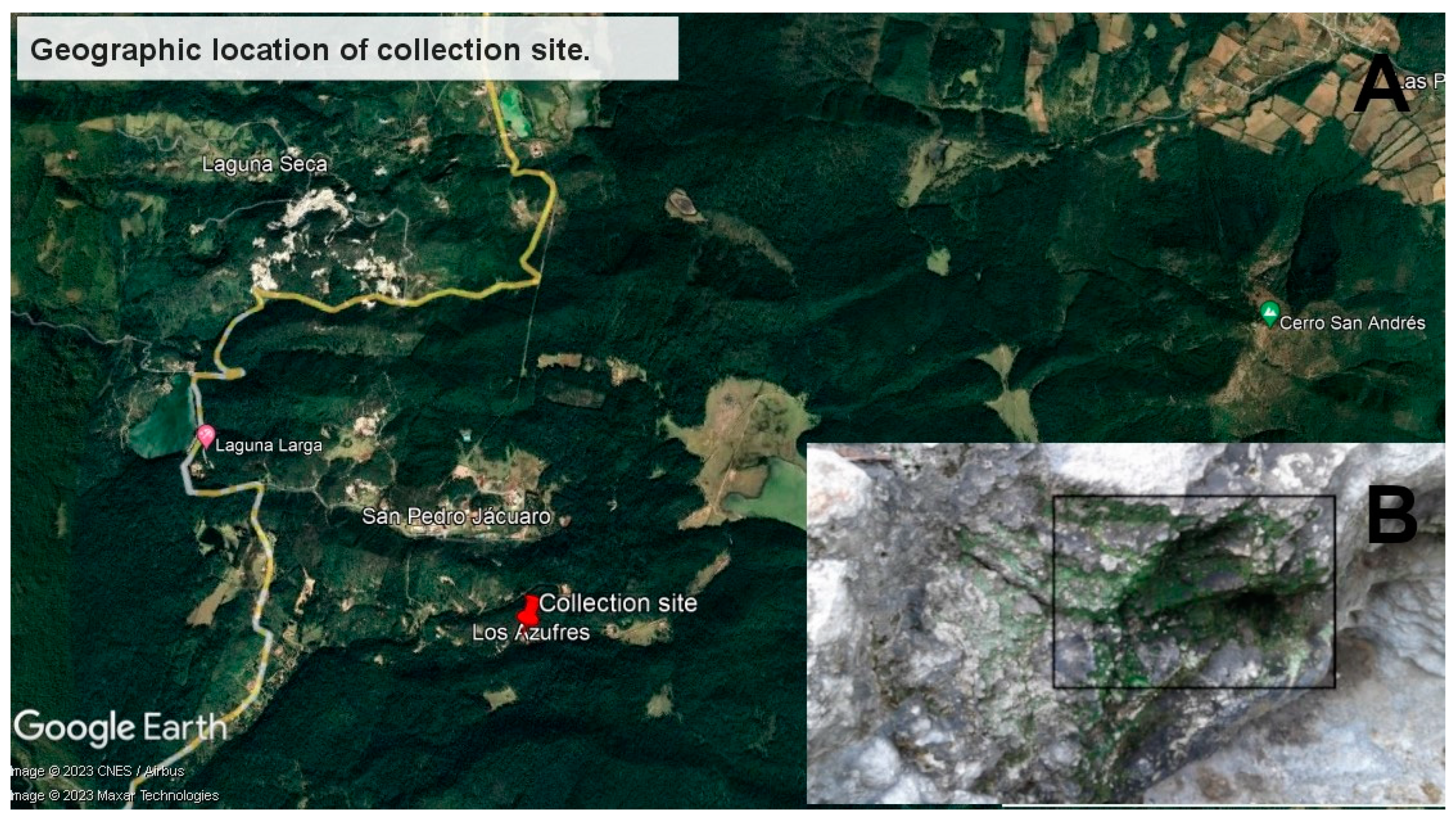
2.1. Sample Collection, DNA Extraction and Sequencing
2.2. Metagenome Assembly and Annotation
2.3. Binning, Taxonomic Classification, and MAG’s Annotation
2.4. Phylogenomic Analysis of Thermoplasmatales archaea and Ferrimicrobium sp. AZ2-2013
2.5. MAG Annotation and Comparative Genomics Analysis
3. Results
3.1. Metagenome Assembly and Annotation
3.2. Metagenome Genes Involved in Metabolic Pathway and Resistances
3.3. Metagenome Viral Sequences
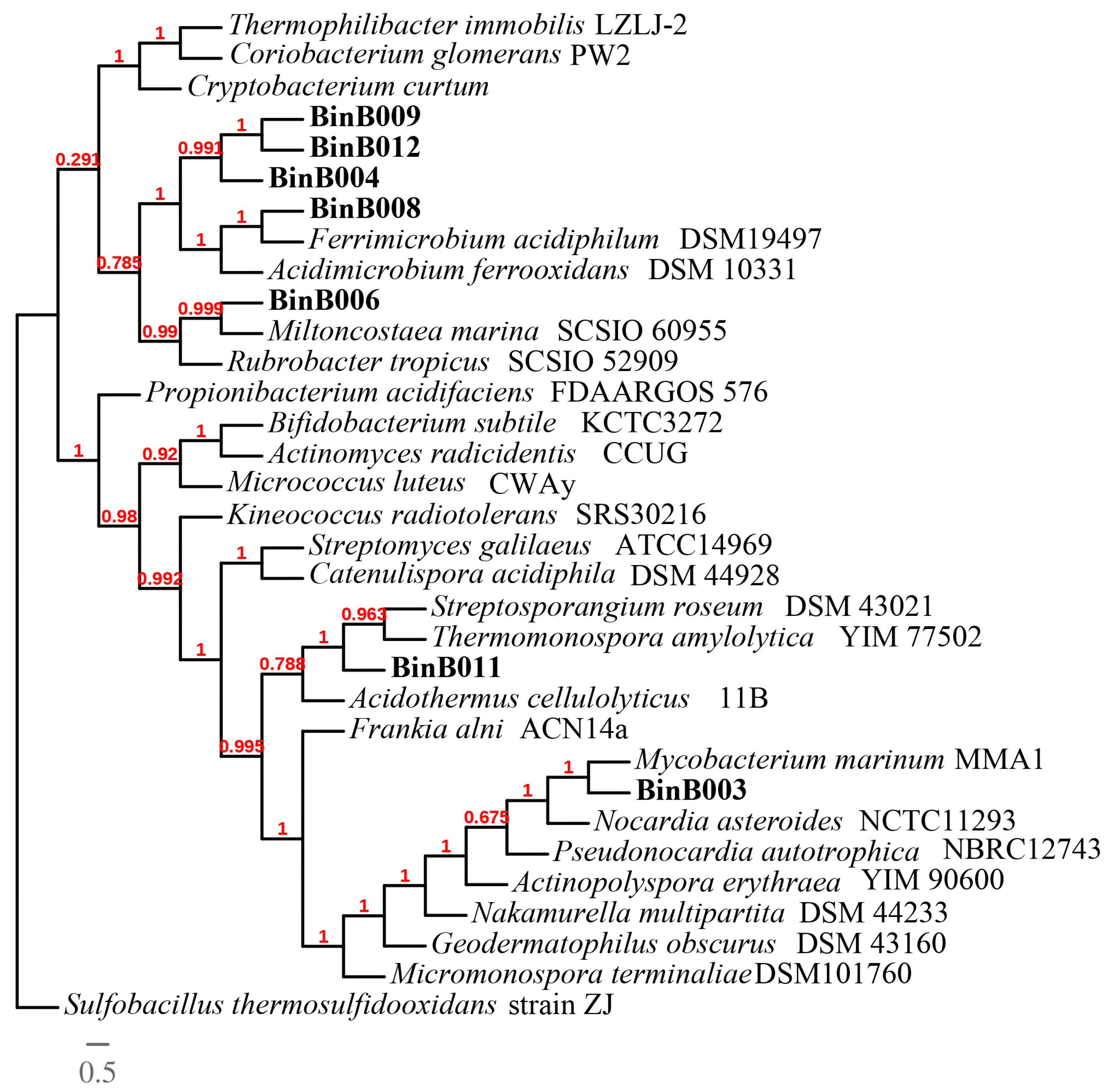
3.4. Binning, Taxonomic Classification, and MAGs Annotation
3.5. Phylogenomic Analysis of Thermoplasmatales archaea and Ferrimicrobium sp. AZ2-2013
3.6. MAG Annotation and Comparative Genomic Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benson, C.A.; Bizzoco, R.W.; Lipson, D.A.; Kelley, S.T. Microbial Diversity in Nonsulfur, Sulfur and Iron Geothermal Steam Vents. FEMS Microbiol. Ecol. 2011, 76, 74–88. [Google Scholar] [CrossRef]
- Richard, L.; Bizzoco, W.; Kelley, S.T. Volcanic Steam Vents: Life at Low PH and High Temperature. In Extremophiles as Astrobiological Models; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2020; pp. 1–20. ISBN 9781119593096. [Google Scholar]
- Brito, E.M.S.; Romero-Núñez, V.M.; Caretta, C.A.; Bertin, P.; Valerdi-Negreros, J.C.; Guyoneaud, R.; Goñi-Urriza, M. The Bacterial Diversity on Steam Vents from Paricutín and Sapichu Volcanoes. Extremophiles 2019, 23, 249–263. [Google Scholar] [CrossRef] [PubMed]
- Bizzoco, R.L.W.; Kelley, S.T. Geothermal Steam Vents of Hawai’i. Model Ecosyst. Extrem. Environ. 2019, 23–40. [Google Scholar] [CrossRef]
- Ferrari, L. Tectónica y Volcanismo En El Cinturón Volcánico Trans-Mexicano. 2023. Available online: https://www.researchgate.net/publication/260383955 (accessed on 1 May 2023).
- Lopez, M.R.T.; Arriaga, M.C.S. Geochemical Evolution of the Los Azufres, Mexico, Geothermal Reservoir. Part I: Water and Salts. In Proceedings of the World Geothermal Congress, Tohoku, Japan, 28 May–10 June 2000; pp. 2257–2262. [Google Scholar]
- Navarrette-Bedolla, M.; Ballesteros-Almanza, L.; Sanchez-Yañez, J.; valdez salas, B.; Hernandez-Duque, G. Biocorrosion in a Geothermal Power Plant. Mater. Perform. 1999, 38, 52–56. [Google Scholar]
- Alfaro-Cuevas-Villanueva, R.; Cortes-Martinez, R.; García-Díaz, J.J.; Galvan-Martinez, R.; Torres-Sanchez, R. Microbiologically Influenced Corrosion of Steels by Thermophilic and Mesophilic Bacteria. Mater. Corros. 2006, 57, 543–548. [Google Scholar] [CrossRef]
- Birkle, P.; Merkel, B. Environmental Impact by Spill of Geothermal Fluids at the Geothermal Field of Los Azufres, Michoacán, Mexico. Water. Air. Soil Pollut. 2000, 124, 371–410. [Google Scholar] [CrossRef]
- Brito, E.M.S.; Villegas-Negrete, N.; Sotelo-González, I.A.; Caretta, C.A.; Goñi-Urriza, M.; Gassie, C.; Hakil, F.; Colin, Y.; Duran, R.; Gutiérrez-Corona, F.; et al. Microbial Diversity in Los Azufres Geothermal Field (Michoacán, Mexico) and Isolation of Representative Sulfate and Sulfur Reducers. Extremophiles 2014, 18, 385–398. [Google Scholar] [CrossRef]
- Torres-Alvarado, I.S. Chemical Equilibrium in Hydrothermal Systems: The Case of Los Azufres Geothermal Field, Mexico. Int. Geol. Rev. 2002, 44, 639–652. [Google Scholar] [CrossRef]
- Lopez, M.R.T.; Arriaga, M.C.S.; Cesar, M. Geochemical Evolution of the Los Azufres, Mexico, Geothermal Reservoir. Part II: Non-Condensible Gases. In Proceedings of the World Geothermal Congress, Tohoku, Japan, 28 May–10 June 2000; pp. 2227–2233. [Google Scholar]
- Valdez Salas, B.; Schorr Wiener, M.; Rioseco de la Peña, L.; Navarrete Bedolla, M. Deterioration of Materials in Geothermal Fields in Mexico. Mater. Corros. 2000, 51, 698–704. [Google Scholar] [CrossRef]
- Servín-Garcidueñas, L.E.; Garrett, R.A.; Amils, R.; Martínez-Romero, E. Genome Sequence of the Acidophilic Bacterium Acidocella Sp. Strain MX-AZ02. Genome Announc. 2013, 1. [Google Scholar] [CrossRef] [Green Version]
- Servín-Garcidueñas, L.E.; Martínez-Romero, E. Draft Genome Sequence of the Sulfolobales Archaeon AZ1, Obtained through Metagenomic Analysis of a Mexican Hot Spring. Genome Announc. 2014, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Servín-Garcidueñas, L.E.; Peng, X.; Garrett, R.A.; Martínez-Romero, E. Genome Sequence of a Novel Archaeal Rudivirus Recovered from a Mexican Hot Spring. Genome Announc. 2013, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Servín-Garcidueñas, L.E.; Peng, X.; Garrett, R.A.; Martínez-Romero, E. Genome Sequence of a Novel Archaeal Fusellovirus Assembled from the Metagenome of a Mexican Hot Spring. Genome Announc. 2013, 1, e0016413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marín-Paredes, R.; Tapia-Torres, Y.; Martínez-Romero, E.; Quesada, M.; Servín-Garcidueñas, L.E. Metagenome Assembly and Metagenome-Assembled Genome of “Candidatus Aramenus Sulfurataquae” from Thermal Sediments from the Los Azufres Volcanic Complex. Microbiol. Resour. Announc. 2021, 10. [Google Scholar] [CrossRef]
- Bolivar-Torres, H.H.; Marín-Paredes, R.; Ramos-Madrigal, C.; Servín-Garcidueñas, L.E. Metagenome-Assembled Genome of Acidibrevibacterium Fodinaquatile FLA01 from Fumarole Sediments from the Los Azufres Geothermal Field. Microbiol. Resour. Announc. 2022, 11. [Google Scholar] [CrossRef]
- Chen, L.-X.; Méndez-García, C.; Dombrowski, N.; Servín-Garcidueñas, L.E.; Eloe-Fadrosh, E.A.; Fang, B.-Z.; Luo, Z.-H.; Tan, S.; Zhi, X.-Y.; Hua, Z.-S.; et al. Metabolic Versatility of Small Archaea Micrarchaeota and Parvarchaeota. ISME J. 2018, 12, 756–775. [Google Scholar] [CrossRef]
- Anders, S. Babraham Bioinformatics—FastQC A Quality Control Tool for High Throughput Sequence Data. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 1 August 2022).
- Krueger, F. Trim Galore: A Wrapper Tool around Cutadapt and FastQC to Consistently Apply Quality and Adapter Trimming to FastQ Files, with Some Extra Functionality for MspI-Digested RRBS-Type. Available online: https://www.bioinformatics.babraham.ac.uk/projects/trim_galore/ (accessed on 1 August 2022).
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A New Genome Assembly Algorithm and Its Applications to Single-Cell Sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [Green Version]
- Chen, I.M.A.; Chu, K.; Palaniappan, K.; Ratner, A.; Huang, J.; Huntemann, M.; Hajek, P.; Ritter, S.; Varghese, N.; Seshadri, R.; et al. The IMG/M Data Management and Analysis System v.6.0: New Tools and Advanced Capabilities. Nucleic Acids Res. 2021, 49, D751–D763. [Google Scholar] [CrossRef]
- Mukherjee, S.; Stamatis, D.; Li, C.T.; Ovchinnikova, G.; Bertsch, J.; Sundaramurthi, J.C.; Kandimalla, M.; Nicolopoulos, P.A.; Favognano, A.; Chen, I.-M.A.; et al. Twenty-Five Years of Genomes OnLine Database (GOLD): Data Updates and New Features in v.9. Nucleic Acids Res. 2023, 51, D957–D963. [Google Scholar] [CrossRef]
- Menzel, P.; Ng, K.L.; Krogh, A. Fast and Sensitive Taxonomic Classification for Metagenomics with Kaiju. Nat. Commun. 2016, 7, 11257. [Google Scholar] [CrossRef] [Green Version]
- Antipov, D.; Raiko, M.; Lapidus, A.; Pevzner, P.A. Metaviral SPAdes: Assembly of Viruses from Metagenomic Data. Bioinformatics 2020, 36, 4126–4129. [Google Scholar] [CrossRef] [PubMed]
- Seemann, T. Prokka: Rapid Prokaryotic Genome Annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, D.D.; Li, F.; Kirton, E.; Thomas, A.; Egan, R.; An, H.; Wang, Z. MetaBAT 2: An Adaptive Binning Algorithm for Robust and Efficient Genome Reconstruction from Metagenome Assemblies. PeerJ 2019, 2019, e7359. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.W.; Simmons, B.A.; Singer, S.W. MaxBin 2.0: An Automated Binning Algorithm to Recover Genomes from Multiple Metagenomic Datasets. Bioinformatics 2016, 32, 605–607. [Google Scholar] [CrossRef]
- Alneberg, J.; Bjarnason, B.S.; de Bruijn, I.; Schirmer, M.; Quick, J.; Ijaz, U.Z.; Lahti, L.; Loman, N.J.; Andersson, A.F.; Quince, C. Binning Metagenomic Contigs by Coverage and Composition. Nat. Methods 2014, 11, 1144–1146. [Google Scholar] [CrossRef]
- Sieber, C.M.K.; Probst, A.J.; Sharrar, A.; Thomas, B.C.; Hess, M.; Tringe, S.G.; Banfield, J.F. Recovery of Genomes from Metagenomes via a Dereplication, Aggregation and Scoring Strategy. Nat. Microbiol. 2018, 3, 836–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parks, D.H.; Imelfort, M.; Skennerton, C.T.; Hugenholtz, P.; Tyson, G.W. CheckM: Assessing the Quality of Microbial Genomes Recovered from Isolates, Single Cells, and Metagenomes. Genome Res. 2015, 25, 1043–1055. [Google Scholar] [CrossRef] [Green Version]
- Matsen, F.A.; Kodner, R.B.; Armbrust, E.V. Pplacer: Linear Time Maximum-Likelihood and Bayesian Phylogenetic Placement of Sequences onto a Fixed Reference Tree. BMC Bioinform. 2010, 11, 538. [Google Scholar] [CrossRef] [Green Version]
- Hyatt, D.; Chen, G.-L.; LoCascio, P.F.; Land, M.L.; Larimer, F.W.; Hauser, L.J. Prodigal: Prokaryotic Gene Recognition and Translation Initiation Site Identification. BMC Bioinform. 2010, 11, 119. [Google Scholar] [CrossRef] [Green Version]
- von Meijenfeldt, F.A.B.; Arkhipova, K.; Cambuy, D.D.; Coutinho, F.H.; Dutilh, B.E. Robust Taxonomic Classification of Uncharted Microbial Sequences and Bins with CAT and BAT. Genome Biol. 2019, 20, 217. [Google Scholar] [CrossRef] [Green Version]
- Shimoyama, Y. COGclassifier: A Tool for Classifying Prokaryote Protein Sequences into COG Functional Category. GitHub 2022. Available online: https://github.com/moshi4/COGclassifier (accessed on 1 June 2023).
- Langmead, B.; Salzberg, S.L. Fast Gapped-Read Alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segata, N.; Börnigen, D.; Morgan, X.C.; Huttenhower, C. PhyloPhlAn Is a New Method for Improved Phylogenetic and Taxonomic Placement of Microbes. Nat. Commun. 2013, 4, 2304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-R, L.M.; Konstantinidis, K.T. The Enveomics Collection: A Toolbox for Specialized Analyses of Microbial Genomes and Metagenomes. PeerJ Preprints 2016. [Google Scholar]
- Auch, A.F.; Klenk, H.P.; Göker, M. Standard Operating Procedure for Calculating Genome-to-Genome Distances Based on High-Scoring Segment Pairs. Stand. Genom. Sci. 2010, 2, 142–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korzhenkov, A.A.; Toshchakov, S.V.; Bargiela, R.; Gibbard, H.; Ferrer, M.; Teplyuk, A.V.; Jones, D.L.; Kublanov, I.V.; Golyshin, P.N.; Golyshina, O.V. Archaea Dominate the Microbial Community in an Ecosystem with Low-to-Moderate Temperature and Extreme Acidity. Microbiome 2019, 7, 11. [Google Scholar] [CrossRef] [Green Version]
- Distaso, M.A.; Bargiela, R.; Johnson, B.; McIntosh, O.A.; Williams, G.B.; Jones, D.L.; Golyshin, P.N.; Golyshina, O.V. Microbial Diversity of a Disused Copper Mine Site (Parys Mountain, UK), Dominated by Intensive Eukaryotic Filamentous Growth. Microorganisms 2022, 10, 1694. [Google Scholar] [CrossRef]
- Mesa, V.; Gallego, J.L.R.; González-Gil, R.; Lauga, B.; Sánchez, J.; Méndez-García, C.; Peláez, A.I. Bacterial, Archaeal, and Eukaryotic Diversity across Distinct Microhabitats in an Acid Mine Drainage. Front. Microbiol. 2017, 8, 1756. [Google Scholar] [CrossRef]
- Bond, P.L.; Smriga, S.P.; Banfield, J.F. Phylogeny of Microorganisms Populating a Thick, Subaerial, Predominantly Lithotrophic Biofilm at an Extreme Acid Mine Drainage Site. Appl. Environ. Microbiol. 2000, 66, 3842–3849. [Google Scholar] [CrossRef] [Green Version]
- Dick, G.J.; Andersson, A.F.; Baker, B.J.; Simmons, S.L.; Thomas, B.C.; Yelton, A.P.; Banfield, J.F. Community-Wide Analysis of Microbial Genome Sequence Signatures. Genome Biol. 2009, 10, R85. [Google Scholar] [CrossRef] [Green Version]
- Golyshina, O.V.; Bargiela, R.; Toshchakov, S.V.; Chernyh, N.A.; Ramayah, S.; Korzhenkov, A.A.; Kublanov, I.V.; Golyshin, P.N. Diversity of “Ca. Micrarchaeota” in Two Distinct Types of Acidic Environments and Their Associations with Thermoplasmatales. Genes 2019, 10, 461. [Google Scholar] [CrossRef] [Green Version]
- Arce-Rodríguez, A.; Puente-Sánchez, F.; Avendaño, R.; Martínez-Cruz, M.; de Moor, J.M.; Pieper, D.H.; Chavarría, M. Thermoplasmatales and Sulfur-Oxidizing Bacteria Dominate the Microbial Community at the Surface Water of a CO2-Rich Hydrothermal Spring Located in Tenorio Volcano National Park, Costa Rica. Extremophiles 2019, 23, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Demina, T.A.; Roux, S.; Aiewsakun, P.; Kazlauskas, D.; Simmonds, P.; Prangishvili, D.; Oksanen, H.M.; Krupovic, M. Diversity, Taxonomy, and Evolution of Archaeal Viruses of the Class Caudoviricetes. PLoS Biol. 2021, 19, e3001442. [Google Scholar] [CrossRef] [PubMed]
- Aulitto, M.; Martinez-Alvarez, L.; Fusco, S.; She, Q.; Bartolucci, S.; Peng, X.; Contursi, P. Genomics, Transcriptomics, and Proteomics of SSV1 and Related Fusellovirus: A Minireview. Viruses 2022, 14, 2082. [Google Scholar] [CrossRef]
- Chan, C.S.; Chan, K.G.; Tay, Y.L.; Chua, Y.H.; Goh, K.M. Diversity of Thermophiles in a Malaysian Hot Spring Determined Using 16S RRNA and Shotgun Metagenome Sequencing. Front. Microbiol. 2015, 6, 177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, D.B.; Bacelar-Nicolau, P.; Okibe, N.; Thomas, A.; Hallberg, K.B. Ferrimicrobium Acidiphilum Gen. Nov., Sp. Nov. and Ferrithrix Thermotolerans Gen. Nov., Sp. Nov.: Heterotrophic, Iron-Oxidizing, Extremely Acidophilic Actinobacteria. Int. J. Syst. Evol. Microbiol. 2009, 59, 1082–1089. [Google Scholar] [CrossRef] [PubMed]
- Eisen, S.; Poehlein, A.; Johnson, D.B.; Daniel, R.; Schlömann, M.; Mühling, M. Genome Sequence of the Acidophilic Iron Oxidizer Ferrimicrobium Acidiphilum Strain T23T. Genome Announc. 2016, 3, e00383-15. [Google Scholar] [CrossRef] [Green Version]
- Urbieta, M.S.; González Toril, E.; Aguilera, A.; Giaveno, M.A.; Donati, E. First Prokaryotic Biodiversity Assessment Using Molecular Techniques of an Acidic River in Neuquén, Argentina. Microb. Ecol. 2012, 64, 91–104. [Google Scholar] [CrossRef]
- Kay, C.M.; Haanela, A.; Johnson, D.B. Microorganisms in Subterranean Acidic Waters within Europe’s Deepest Metal Mine. Res. Microbiol. 2014, 165, 705–712. [Google Scholar] [CrossRef]
- Aytar, P.; Kay, C.M.; Mutlu, M.B.; Çabuk, A.; Johnson, D.B. Diversity of Acidophilic Prokaryotes at Two Acid Mine Drainage Sites in Turkey. Environ. Sci. Pollut. Res. 2015, 22, 5995–6003. [Google Scholar] [CrossRef]
- Kadnikov, V.V.; Gruzdev, E.V.; Ivasenko, D.A.; Beletsky, A.V.; Mardanov, A.V.; Danilova, E.V.; Karnachuk, O.V.; Ravin, N.V. Selection of a Microbial Community in the Course of Formation of Acid Mine Drainage. Microbiology 2019, 88, 292–299. [Google Scholar] [CrossRef]
- Li, J.; Sun, W.; Wang, S.; Sun, Z.; Lin, S.; Peng, X. Bacteria Diversity, Distribution and Insight into Their Role in S and Fe Biogeochemical Cycling during Black Shale Weathering. Environ. Microbiol. 2014, 16, 3533–3547. [Google Scholar] [CrossRef] [PubMed]
- Golyshina, O.V.; Lünsdorf, H.; Kublanov, I.V.; Goldenstein, N.I.; Hinrichs, K.U.; Golyshin, P.N. The Novel Extremely Acidophilic, Cell-Wall-Deficient Archaeon Cuniculiplasma Divulgatum Gen. Nov., Sp. Nov. Represents a New Family, Cuniculiplasmataceae Fam. Nov., of the Order Thermoplasmatales. Int. J. Syst. Evol. Microbiol. 2016, 66, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Golyshina, O.V.; Bargiela, R.; Golyshin, P.N. Cuniculiplasmataceae, Their Ecogenomic and Metabolic Patterns, and Interactions with ‘ARMAN’. Extremophiles 2019, 23, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golyshina, O.V.; Kublanov, I.V.; Tran, H.; Korzhenkov, A.A.; Lünsdorf, H.; Nechitaylo, T.Y.; Gavrilov, S.N.; Toshchakov, S.V.; Golyshin, P.N. Biology of Archaea from a Novel Family Cuniculiplasmataceae (Thermoplasmata) Ubiquitous in Hyperacidic Environments. Sci. Rep. 2016, 6, 39034. [Google Scholar] [CrossRef]

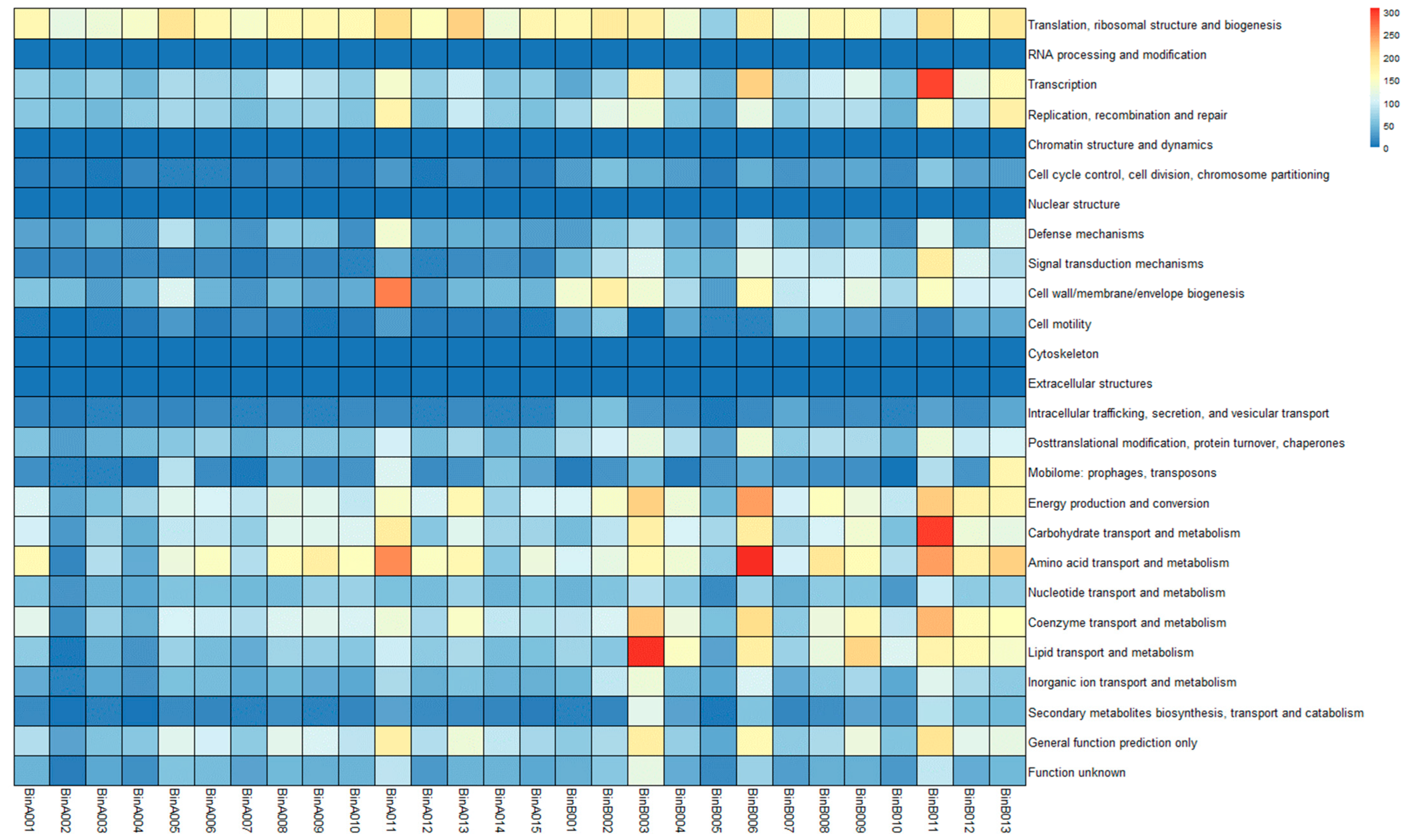

| Name | Count | Percent |
|---|---|---|
| Amino acid transport and metabolism | 13,298 | 9.74% |
| Carbohydrate transport and metabolism | 8818 | 6.46% |
| Cell cycle control, cell division, chromosome partitioning | 1649 | 1.21% |
| Cell motility | 1785 | 1.31% |
| Cell wall/membrane/envelope biogenesis | 6417 | 4.70% |
| Chromatin structure and dynamics | 120 | 0.09% |
| Coenzyme transport and metabolism | 7744 | 5.67% |
| Cytoskeleton | 64 | 0.05% |
| Defense mechanisms | 3728 | 2.73% |
| Energy production and conversion | 9578 | 7.02% |
| Extracellular structures | 578 | 0.42% |
| Function unknown | 5598 | 4.10% |
| General function prediction only | 15,042 | 11.02% |
| Inorganic ion transport and metabolism | 7063 | 5.17% |
| Intracellular trafficking, secretion, and vesicular transport | 1799 | 1.32% |
| Lipid transport and metabolism | 7292 | 5.34% |
| Mobilome: prophages, transposons | 3466 | 2.54% |
| Nucleotide transport and metabolism | 3589 | 2.63% |
| Posttranslational modification, protein turnover, chaperones | 5546 | 4.06% |
| RNA processing and modification | 48 | 0.04% |
| Replication, recombination and repair | 6756 | 4.95% |
| Secondary metabolites biosynthesis, transport, and catabolism | 4516 | 3.31% |
| Signal transduction mechanisms | 4455 | 3.26% |
| Transcription | 7452 | 5.46% |
| Translation, ribosomal structure, and biogenesis | 10,130 | 7.42% |
| Bin ID | Completeness | Contamination | Quality of MAGs | Genome Size (bp) | Number of Contigs | N50 (Contigs) | %GC | CAT/BAT Classification |
|---|---|---|---|---|---|---|---|---|
| BinA001 | 98.74 | 0 | High quality | 1,706,574 | 84 | 35,070 | 34.95 | Ferroplasma (G) |
| BinA002 | 69.12 | 1.87 | Low quality | 1,420,676 | 702 | 2148 | 35.95 | Ca. Parvarchaeota (P) |
| BinA003 | 82.07 | 0 | Medium quality | 1,297,165 | 204 | 13,130 | 37.39 | Cuniculiplasma (S) |
| BinA004 | 81.78 | 1.87 | Medium quality | 1,253,227 | 101 | 156,283 | 47.39 | Ca. Micrarchaeota (P) |
| BinA005 | 95.52 | 5.65 | Medium quality | 2,123,351 | 75 | 71,123 | 38.61 | Thermoplasmatales archaeon “E-plasma” (S) |
| BinA006 | 95.39 | 4.84 | High quality | 1,747,204 | 34 | 88,714 | 44.06 | Thermoplasmatales (O) |
| BinA007 | 89.47 | 1.61 | Medium quality | 1,184,382 | 116 | 15,136 | 43.18 | Thermoplasmatales archaeon “A-plasma” (S) |
| BinA008 | 94.72 | 1.61 | High quality | 1,929,173 | 66 | 46,843 | 42.25 | Thermoplasmatales (O) |
| BinA009 | 97.65 | 5.69 | Medium quality | 1,716,143 | 40 | 79,707 | 37.91 | Ferroplasma (G) |
| BinA010 | 94.05 | 3.25 | High quality | 1,556,444 | 61 | 42,667 | 39.16 | Ferroplasma (G) |
| BinA011 | 80.14 | 28.83 | Low quality | 3,842,114 | 370 | 14,845 | 40.9 | Thermoplasmatales (O) |
| BinA012 | 72.92 | 20.33 | Low quality | 1,642,787 | 523 | 4064 | 44.62 | Thermoplasmatales archaeon “A-plasma” (S) |
| BinA013 | 96.37 | 19.35 | Low quality | 2,229,358 | 143 | 64,732 | 44.7 | Thermoplasmatales archaeon “I-plasma” (S) |
| BinA014 | 71.77 | 17.34 | Low quality | 1,586,617 | 201 | 14,693 | 41.52 | Thermoplasmatales (O)) |
| BinA015 | 74.69 | 24.69 | Low quality | 1,450,377 | 162 | 15,441 | 43.6 | Thermoplasmatales (O) |
| BinB001 | 93.97 | 0 | High quality | 1,754,364 | 20 | 114,024 | 66.67 | Gammaproteobacteria (C) |
| BinB002 | 99.38 | 0 | High quality | 2,395,071 | 53 | 80,710 | 62.41 | Pseudomonadota (P) |
| BinB003 | 99.55 | 0.45 | High quality | 4,091,439 | 176 | 47,256 | 66.49 | Actinomycetales (O) |
| BinB004 | 92.74 | 0.85 | High quality | 2,246,041 | 483 | 6521 | 58.95 | Actinomycetota (P) |
| BinB005 | 39.56 | 0.85 | Low quality | 1,299,422 | 822 | 1563 | 66.42 | Bacteria |
| BinB006 | 97.86 | 1.14 | High quality | 3,765,924 | 74 | 128,327 | 70.35 | Actinomycetota(P) |
| BinB007 | 62.65 | 1.3 | Low quality | 1,844,347 | 418 | 4609 | 65.59 | Gammaproteobacteria (C) |
| BinB008 | 96.58 | 1.38 | High quality | 2,544,086 | 60 | 60,560 | 57.97 | Ferrimicrobium (S) |
| BinB009 | 94.79 | 2.91 | High quality | 2,895,140 | 349 | 10,670 | 73.1 | Actinomycetota (P) |
| BinB010 | 51.91 | 3.23 | Low quality | 1,990,973 | 1119 | 1860 | 73.16 | Bacteria |
| BinB011 | 97.59 | 3.48 | High quality | 5,183,776 | 320 | 24,927 | 71.89 | Actinomycetales (O) |
| BinB012 | 94.87 | 4.7 | High quality | 2,730,528 | 96 | 50,090 | 73.93 | Actinomycetota (P) |
| BinB013 | 94.02 | 8.93 | Medium quality | 3,691,086 | 559 | 8930 | 68.55 | Bacteria |
| BinE001 | NA | NA | NA | 162,145 | 15 | 89,086 | 37.92 | Cyanidiaceae (F) |
| BinE002 | NA | NA | NA | 11,989,646 | 192 | 113,036 | 53.45 | Cyanidiaceae (F) |
| BinE003 | NA | NA | NA | 113,433 | 6 | 78,601 | 28.14 | Cyanidiaceae (F) |
| BinE004 | NA | NA | NA | 142,617 | 5 | 72,671 | 28.44 | Cyanidiaceae (F) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marín-Paredes, R.; Bolívar-Torres, H.H.; Coronel-Gaytán, A.; Martínez-Romero, E.; Servín-Garcidueñas, L.E. A Metagenome from a Steam Vent in Los Azufres Geothermal Field Shows an Abundance of Thermoplasmatales archaea and Bacteria from the Phyla Actinomycetota and Pseudomonadota. Curr. Issues Mol. Biol. 2023, 45, 5849-5864. https://doi.org/10.3390/cimb45070370
Marín-Paredes R, Bolívar-Torres HH, Coronel-Gaytán A, Martínez-Romero E, Servín-Garcidueñas LE. A Metagenome from a Steam Vent in Los Azufres Geothermal Field Shows an Abundance of Thermoplasmatales archaea and Bacteria from the Phyla Actinomycetota and Pseudomonadota. Current Issues in Molecular Biology. 2023; 45(7):5849-5864. https://doi.org/10.3390/cimb45070370
Chicago/Turabian StyleMarín-Paredes, Roberto, Hermes H. Bolívar-Torres, Alberto Coronel-Gaytán, Esperanza Martínez-Romero, and Luis E. Servín-Garcidueñas. 2023. "A Metagenome from a Steam Vent in Los Azufres Geothermal Field Shows an Abundance of Thermoplasmatales archaea and Bacteria from the Phyla Actinomycetota and Pseudomonadota" Current Issues in Molecular Biology 45, no. 7: 5849-5864. https://doi.org/10.3390/cimb45070370
APA StyleMarín-Paredes, R., Bolívar-Torres, H. H., Coronel-Gaytán, A., Martínez-Romero, E., & Servín-Garcidueñas, L. E. (2023). A Metagenome from a Steam Vent in Los Azufres Geothermal Field Shows an Abundance of Thermoplasmatales archaea and Bacteria from the Phyla Actinomycetota and Pseudomonadota. Current Issues in Molecular Biology, 45(7), 5849-5864. https://doi.org/10.3390/cimb45070370







