X-rays Stimulate Granular Secretions and Activate Protein Kinase C Signaling in Human Platelets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Antibodies and Reagents
2.2. Washed Human Platelets Preparation
2.3. X-rays Treatment
2.4. Flow Cytometric Analysis
2.5. ATP Release Assay
2.6. Platelet Aggregation
2.7. Western Blotting
2.8. Gö 6983 Incubation
2.9. Statistical Analysis
3. Results
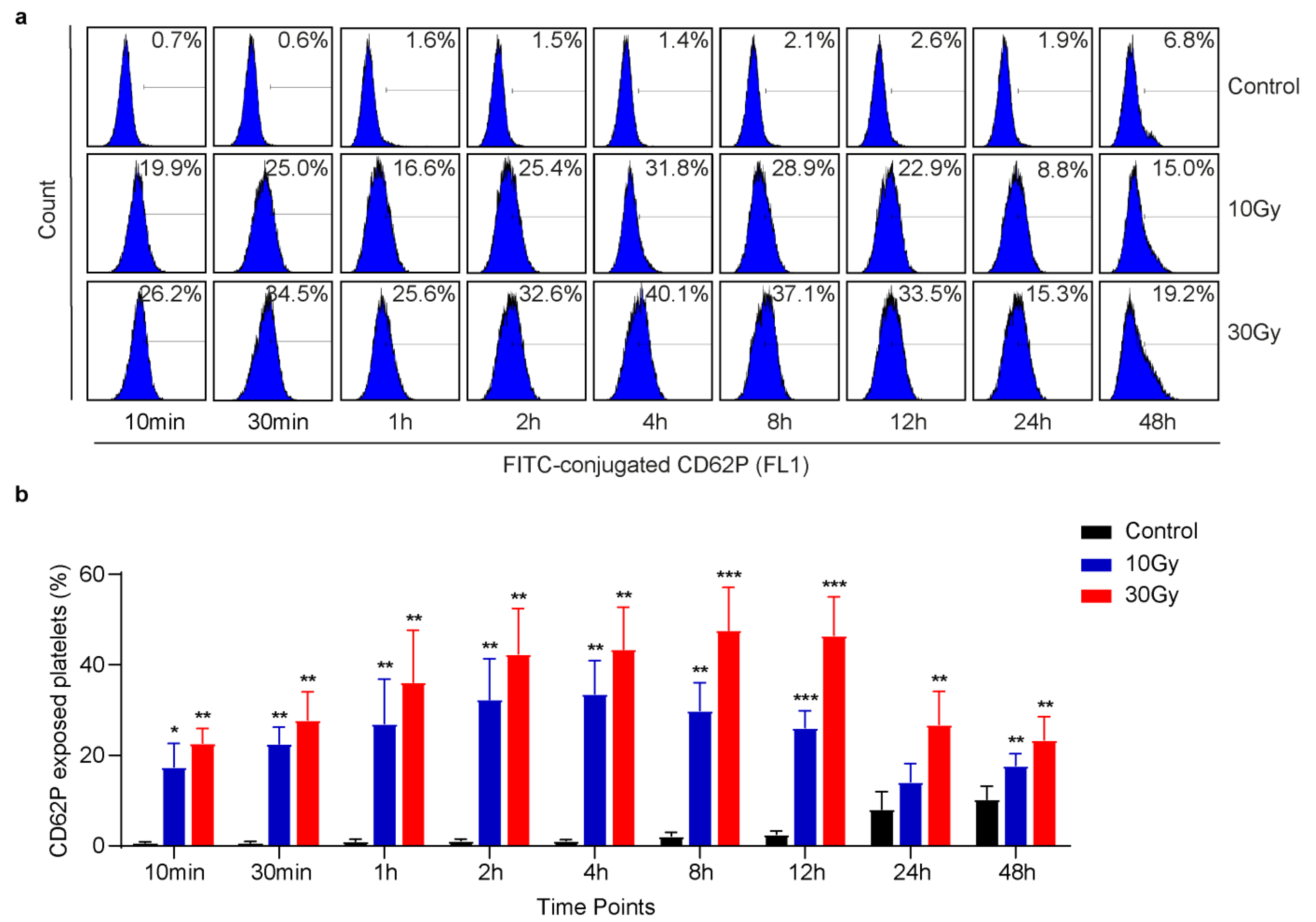
3.1. X-rays Immediately Induce CD62P Exposure
3.2. X-rays Induce ATP Release in Washed Human Platelets
3.3. X-rays Significantly Reduce Platelet Aggregation
3.4. X-rays Induce Apoptosis in Human Platelets in a Time-Dependent Manner
3.5. X-rays Induce Rearrangement of Bcl-2 Family Proteins and Activate PKC Signaling
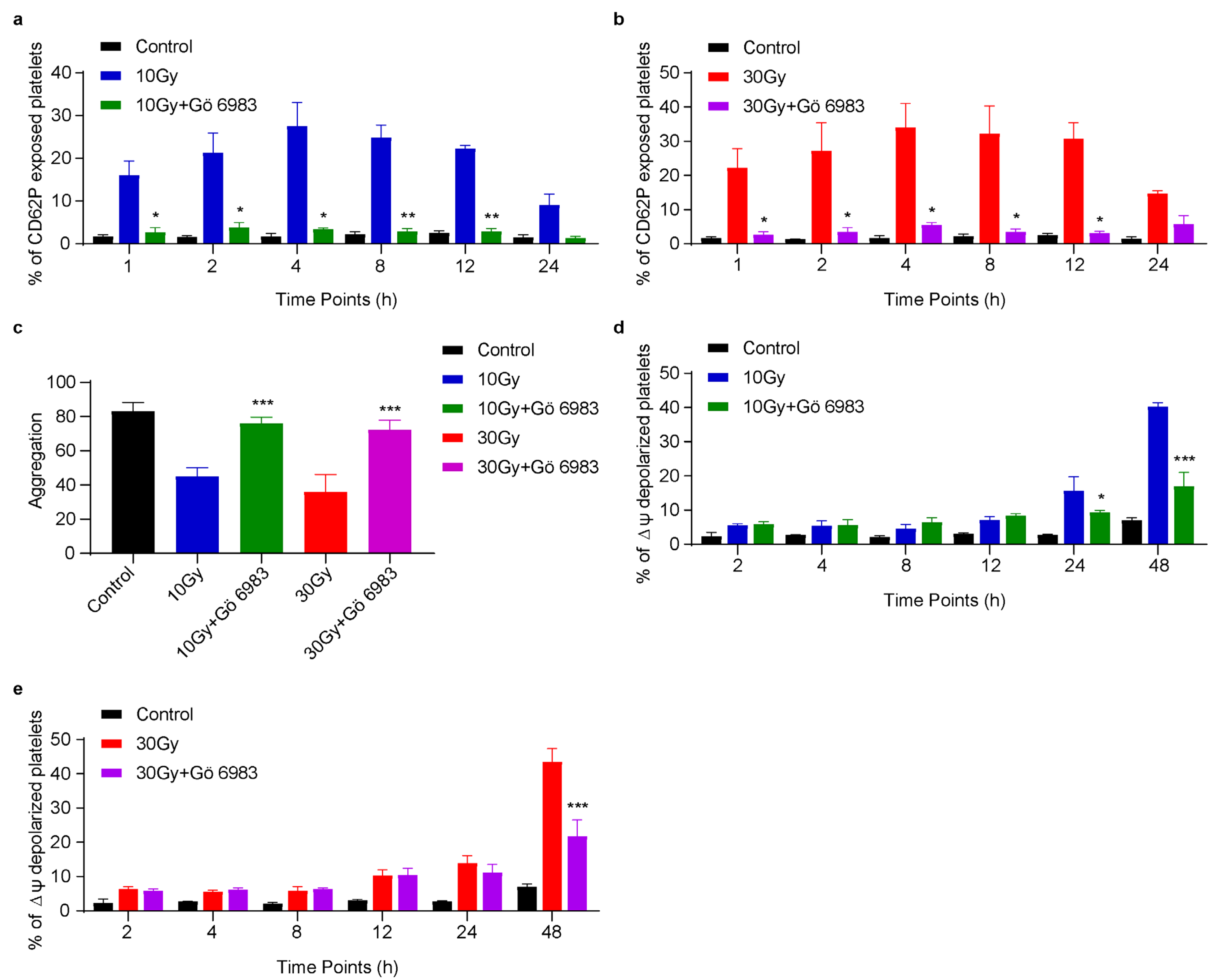
3.6. Gö 6983 Inhibits X-rays Induced CD62P Exposure, ΔΨm Depolarization and Rescues Platelet Aggregation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Panzetta, V.; De Menna, M.; Musella, I.; Pugliese, M.; Quarto, M.; Netti, P.A.; Fusco, S. X-Rays Effects on Cytoskeleton Mechanics of Healthy and Tumor Cells. Cytoskeleton 2017, 74, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Reisz, J.A.; Bansal, N.; Qian, J.; Zhao, W.; Furdui, C.M. Effects of Ionizing Radiation on Biological Molecules—Mechanisms of Damage and Emerging Methods of Detection. Antioxid. Redox Signal. 2014, 21, 260–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houée-Levin, C.; Bobrowski, K. The Use of the Methods of Radiolysis to Explore the Mechanisms of Free Radical Modifications in Proteins. J. Proteom. 2013, 92, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Ljubic, A.; Nikolic, L.; Stefanovic, S.; Popovic, Z.; Bojanic, N.; Anojcic, P.; Markovic, S.; Mijajlovic, M.; Kastratovic, D. Platelets (Thrombocytes)—The Other Recognized Functions. Hosp. Pharmacol.-Int. Multidiscip. J. 2016, 3, 416–421. [Google Scholar] [CrossRef] [Green Version]
- Hosseini, E.; Kianinodeh, F.; Ghasemzadeh, M. Irradiation of Platelets in Transfusion Medicine: Risk and Benefit Judgments. Platelets 2022, 33, 666–678. [Google Scholar] [CrossRef]
- Jacobs, G.P. A Review on the Effects of Ionizing Radiation on Blood and Blood Components. Radiat. Phys. Chem. 1998, 53, 511–523. [Google Scholar] [CrossRef]
- Tynngård, N.; Studer, M.; Lindahl, T.L.; Trinks, M.; Berlin, G. The Effect of Gamma Irradiation on the Quality of Apheresis Platelets during Storage for 7 Days. Transfusion 2008, 48, 1669–1675. [Google Scholar] [CrossRef]
- Gutmann, C.; Siow, R.; Gwozdz, A.M.; Saha, P.; Smith, A. Reactive Oxygen Species in Venous Thrombosis. Int. J. Mol. Sci. 2020, 21, 1918. [Google Scholar] [CrossRef] [Green Version]
- Verhaar, R.; Dekkers, D.W.C.; De Cuyper, I.M.; Ginsberg, M.H.; De Korte, D.; Verhoeven, A.J. UV-C Irradiation Disrupts Platelet Surface Disulfide Bonds and Activates the Platelet Integrin AlphaIIbbeta3. Blood 2008, 112, 4935–4939. [Google Scholar] [CrossRef] [Green Version]
- Reid, S.; Johnson, L.; Woodland, N.; Marks, D.C. Pathogen Reduction Treatment of Buffy Coat Platelet Concentrates in Additive Solution Induces Proapoptotic Signaling. Transfusion 2012, 52, 2094–2103. [Google Scholar] [CrossRef]
- Gelderman, M.P.; Chi, X.; Zhi, L.; Vostal, J.G. Ultraviolet B Light-Exposed Human Platelets Mediate Acute Lung Injury in a Two-Event Mouse Model of Transfusion. Transfusion 2011, 51, 2343–2357. [Google Scholar] [CrossRef] [PubMed]
- Tsalas, S.; Petrou, E.; Tsantes, A.G.; Sokou, R.; Loukopoulou, E.; Houhoula, D.; Mantzios, P.G.; Kriebardis, A.G.; Tsantes, A.E. Pathogen Reduction Technologies and Their Impact on Metabolic and Functional Properties of Treated Platelet Concentrates: A Systematic Review. Semin. Thromb. Hemost. 2023, 49, 523–541. [Google Scholar] [CrossRef] [PubMed]
- Rola, P.; Doroszko, A.; Szahidewicz-Krupska, E.; Rola, P.; Dobrowolski, P.; Skomro, R.; Szymczyszyn, A.; Mazur, G.; Derkacz, A. Low-Level Laser Irradiation Exerts Antiaggregative Effect on Human Platelets Independently on the Nitric Oxide Metabolism and Release of Platelet Activation Markers. Oxid. Med. Cell. Longev. 2017, 6201797. [Google Scholar] [CrossRef] [PubMed]
- Brill, A.G.; Shenkman, B.; Brill, G.E.; Tamarin, I.; Dardik, R.; Kirichuk, V.F.; Savion, N.; Varon, D. Blood Irradiation by He–Ne Laser Induces a Decrease in Platelet Responses to Physiological Agonists and an Increase in Platelet Cyclic GMP. Platelets 2000, 11, 87–93. [Google Scholar] [CrossRef]
- Johnson, L.; Vekariya, S.; Wood, B.; Costa, M.; Waters, L.; Green, S.; Marks, D.C. The in Vitro Quality of X-Irradiated Platelet Components in PAS-E Is Equivalent to Gamma-Irradiated Components. Transfusion 2021, 61, 3075–3080. [Google Scholar] [CrossRef]
- Eriksson, D.; Stigbrand, T. Radiation-Induced Cell Death Mechanisms. Tumor Biol. 2010, 31, 363–372. [Google Scholar] [CrossRef]
- Leytin, V. Apoptosis in the Anucleate Platelet. Blood Rev. 2012, 26, 51–63. [Google Scholar] [CrossRef]
- Das, J.; Ramani, R.; Suraju, M.O. Polyphenol Compounds and PKC Signaling. Biochim. Biophys. Acta 2016, 1860, 2107–2121. [Google Scholar] [CrossRef] [Green Version]
- Harper, M.T.; Poole, A.W. Diverse Functions of Protein Kinase C Isoforms in Platelet Activation and Thrombus Formation. J. Thromb. Haemost. 2010, 8, 454–462. [Google Scholar] [CrossRef]
- Chen, M.; Yan, R.; Zhou, K.; Li, X.; Zhang, Y.; Liu, C.; Jiang, M.; Ye, H.; Meng, X.; Pang, N.; et al. Akt-Mediated Platelet Apoptosis and Its Therapeutic Implications in Immune Thrombocytopenia. Proc. Natl. Acad. Sci. USA 2018, 115, E10682–E10691. [Google Scholar] [CrossRef] [Green Version]
- Dai, K.; Bodnar, R.; Berndt, M.C.; Du, X. A Critical Role for 14-3-3ζ Protein in Regulating the VWF Binding Function of Platelet Glycoprotein Ib-IX and Its Therapeutic Implications. Blood 2005, 106, 1975–1981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, W.; Zhou, K.; Yang, M.; Sun, C.; Dai, L.; Gu, J.; Yan, R.; Dai, K. Carbamazepine Induces Platelet Apoptosis and Thrombocytopenia Through Protein Kinase A. Front. Pharmacol. 2021, 12, 749930. [Google Scholar] [CrossRef] [PubMed]
- Münzer, P.; Borst, O.; Walker, B.; Schmid, E.; Feijge, M.A.H.; Cosemans, J.M.E.M.; Chatterjee, M.; Schmidt, E.M.; Schmidt, S.; Towhid, S.T.; et al. Acid Sphingomyelinase Regulates Platelet Cell Membrane Scrambling, Secretion, and Thrombus Formation. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 61–71. [Google Scholar] [CrossRef] [Green Version]
- Zhou, K.; Xia, Y.; Yang, M.; Xiao, W.; Zhao, L.; Hu, R.; Shoaib, K.M.; Yan, R.; Dai, K. Actin Polymerization Regulates Glycoprotein Ibα Shedding. Platelets 2022, 33, 381–389. [Google Scholar] [CrossRef]
- Petzold, T.; Ruppert, R.; Pandey, D.; Barocke, V.; Meyer, H.; Lorenz, M.; Zhang, L.; Siess, W.; Massberg, S.; Moser, M. Β1 Integrin-mediated Signals Are Required for Platelet Granule Secretion and Hemostasis in Mouse. Blood 2013, 122, 2723–2731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmermann, R.; Schmidt, S.; Zingsem, J.; Glaser, A.; Weisbach, V.; Ruf, A.; Eckstein, R. Effect of Gamma Radiation on the in Vitro Aggregability of WBC-Reduced Apheresis Platelets. Transfusion 2001, 41, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Kojima, S. Involvement of ATP in Radiation-Induced Bystander Effect as a Signaling Molecule. Yakugaku Zasshi 2014, 134, 743–749. [Google Scholar] [CrossRef] [Green Version]
- Strauss, T.; Sidlik-Muskatel, R.; Kenet, G. Developmental Hemostasis: Primary Hemostasis and Evaluation of Platelet Function in Neonates. Semin. Fetal Neonatal Med. 2011, 16, 301–304. [Google Scholar] [CrossRef]
- Kalovidouris, A.E.; Papayannis, A.G. Effect of Ionizing Radiation on Platelet Function in Vitro. Acta Oncol. 1981, 20, 333–336. [Google Scholar] [CrossRef] [Green Version]
- Kariya, S.; Sawada, K.; Kobayashi, T.; Karashima, T.; Shuin, T.; Nishioka, A.; Ogawa, Y. Combination Treatment of Hydrogen Peroxide and X-Rays Induces Apoptosis in Human Prostate Cancer PC-3 Cells. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 449–454. [Google Scholar] [CrossRef]
- Gyulkhandanyan, A.V.; Allen, D.J.; Mykhaylov, S.; Lyubimov, E.; Ni, H.; Freedman, J.; Leytin, V. Mitochondrial Inner Membrane Depolarization as a Marker of Platelet Apoptosis: Disclosure of Nonapoptotic Membrane Depolarization. Clin. Appl. Thromb. 2017, 23, 139–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crowley, L.C.; Waterhouse, N.J. Detecting Cleaved Caspase-3 in Apoptotic Cells by Flow Cytometry. Cold Spring Harb. Protoc. 2016, 2016, 958–962. [Google Scholar] [CrossRef] [PubMed]
- Leytin, V.; Allen, D.J.; Mutlu, A.; Mykhaylov, S.; Lyubimov, E.; Freedman, J. Platelet Activation and Apoptosis Are Different Phenomena: Evidence from the Sequential Dynamics and the Magnitude of Responses during Platelet Storage. Br. J. Haematol. 2008, 142, 494–497. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, J.; Sun, R.; Zhao, L.; Du, J.; Ruan, C. Calpain Activator Dibucaine Induces Platelet Apoptosis. Int. J. Mol. Sci. 2011, 12, 2125–2137. [Google Scholar] [CrossRef] [Green Version]
- Fernandes-Alnemri, T.; Litwack, G.; Alnemri, E.S. CPP32, a Novel Human Apoptotic Protein with Homology to Caenorhabditis Elegans Cell Death Protein Ced-3 and Mammalian Interleukin-1β-Converting Enzyme. J. Biol. Chem. 1994, 269, 30761–30764. [Google Scholar] [CrossRef]
- Pal, D.; Outram, S.P.; Basu, A. Upregulation of PKCη by PKCε and PDK1 Involves Two Distinct Mechanisms and Promotes Breast Cancer Cell Survival. Biochim. Biophys. Acta 2013, 1830, 4040–4045. [Google Scholar] [CrossRef] [Green Version]
- Mallhi, R.S.; Biswas, A.K.; Philip, J.; Chatterjee, T. To Study the Effects of Gamma Irradiation on Single Donor Apheresis Platelet Units by Measurement of Cellular Counts, Functional Indicators and a Panel of Biochemical Parameters, in Order to Assess Pre-Transfusion Platelet Quantity and Quality during the shelf life of the product. Med. J. Armed Forces India 2016, 72, 19–26. [Google Scholar] [CrossRef] [Green Version]
- Zeddies, S.; De Cuyper, I.M.; Van Der Meer, P.F.; Daal, B.B.; De Korte, D.; Gutiérrez, L.; Thijssen-Timmer, D.C. Pathogen Reduction Treatment Using Riboflavin and Ultraviolet Light Impairs Platelet Reactivity toward Specific Agonists in Vitro. Transfusion 2014, 54, 2292–2300. [Google Scholar] [CrossRef]
- Salunkhe, V.; De Cuyper, I.M.; Papadopoulos, P.; van der Meer, P.F.; Daal, B.B.; Villa-Fajardo, M.; de Korte, D.; van den Berg, T.K.; Gutiérrez, L. A Comprehensive Proteomics Study on Platelet Concentrates: Platelet Proteome, Storage Time and Mirasol Pathogen Reduction Technology. Platelets 2019, 30, 368–379. [Google Scholar] [CrossRef]
- Ay, C.; Jungbauer, L.V.; Sailer, T.; Tengler, T.; Koder, S.; Kaider, A.; Panzer, S.; Quehenberger, P.; Pabinger, I.; Mannhalter, C. High Concentrations of Soluble P-Selectin Are Associated with Risk of Venous Thromboembolism and the P-Selectin Thr715 Variant. Clin. Chem. 2007, 53, 1235–1243. [Google Scholar] [CrossRef] [Green Version]
- Cerletti, C.; Tamburrelli, C.; Izzi, B.; Gianfagna, F.; De Gaetano, G. Platelet-Leukocyte Interactions in Thrombosis. Thromb. Res. 2012, 129, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Etulain, J.; Martinod, K.; Wong, S.L.; Cifuni, S.M.; Schattner, M.; Wagner, D.D. P-Selectin Promotes Neutrophil Extracellular Trap Formation in Mice. Blood 2015, 126, 242–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehrpoori, M.; Hosseini, E.; Amini Kafi-Abad, S.; Ghasemzadeh, M. The Effect of Pre-Storage Leukoreduction on the Levels of Expression and Shedding of the pro-Inflammatory Molecule P-Sel in Random PRP Platelets. Sci. J. Iran. Blood Transfus. Organ. 2015, 12, 153–162. [Google Scholar]
- Kim, K.; Li, J.; Tseng, A.; Andrews, R.K.; Cho, J. NOX2 Is Critical for Heterotypic Neutrophil-Platelet Interactions during Vascular Inflammation. Blood 2015, 126, 1952–1964. [Google Scholar] [CrossRef] [Green Version]
- Lu, Q.; Malinauskas, R.A. Comparison of Two Platelet Activation Markers Using Flow Cytometry After In Vitro Shear Stress Exposure of Whole Human Blood. Artif. Organs 2011, 35, 137–144. [Google Scholar] [CrossRef]
- Cho, J.H.; Wool, G.D.; Tjota, M.Y.; Gutierrez, J.; Mikrut, K.; Miller, J.L. Functional Assessment of Platelet Dense Granule ATP Release. Am. J. Clin. Pathol. 2021, 155, 863–872. [Google Scholar] [CrossRef]
- Aibibula, M.; Naseem, K.M.; Sturmey, R.G. Glucose Metabolism and Metabolic Flexibility in Blood Platelets. J. Thromb. Haemost. 2018, 16, 2300–2314. [Google Scholar] [CrossRef] [Green Version]
- Birk, A.V.; Broekman, M.J.; Gladek, E.M.; Robertson, H.D.; Drosopoulos, J.H.F.; Marcus, A.J.; Szeto, H.H. Role of Extracellular ATP Metabolism in Regulation of Platelet Reactivity. J. Lab. Clin. Med. 2002, 140, 166–175. [Google Scholar] [CrossRef]
- Fullard, J. The Role of the Platelet Glycoprotein IIb/IIIa in Thrombosis and Haemostasis. Curr. Pharm. Des. 2004, 10, 1567–1576. [Google Scholar] [CrossRef]
- Shakir, E.A.; Rasheed Naji, N.A. In Vitro Impact of Laser Irradiation on Platelet Aggregation. Lasers Med. Sci. 2018, 33, 1717–1721. [Google Scholar] [CrossRef]
- Nodeh, F.K.; Hosseini, E.; Ghasemzadeh, M. The Effect of Gamma Irradiation on Platelet Redox State during Storage. Transfusion 2021, 61, 579–593. [Google Scholar] [CrossRef] [PubMed]
- Leytin, V.; Gyulkhandanyan, A.V.; Freedman, J. Platelet Apoptosis Can Be Triggered Bypassing the Death Receptors. Clin. Appl. Thromb. 2019, 25, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Stivala, S.; Gobbato, S.; Infanti, L.; Reiner, M.F.; Bonetti, N.; Meyer, S.C.; Camici, G.G.; Lüscher, T.F.; Buser, A.; Beer, J.H. Amotosalen/Ultraviolet A Pathogen Inactivation Technology Reduces Platelet Activatability, Induces Apoptosis and Accelerates Clearance. Haematologica 2017, 102, 1650–1660. [Google Scholar] [CrossRef]
- Levine, A.J.; Oren, M. The First 30 Years of P53: Growing Ever More Complex. Nat. Rev. Cancer 2009, 9, 749–758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Orazi, G.; Cecchinelli, B.; Bruno, T.; Manni, I.; Higashimoto, Y.; Saito, S.; Gostissa, M.; Coen, S.; Marchetti, A.; Del Sal, G.; et al. Homeodomain-Interacting Protein Kinase-2 Phosphorylates P53 at Ser 46 and Mediates Apoptosis. Nat. Cell Biol. 2002, 4, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Kong, C.; Zhang, Z.; Zhu, Y.; Zhang, Y.; Chen, X. Reduction of Protein Kinase C α (PKC-α) Promote Apoptosis via down-Regulation of Dicer in Bladder Cancer. J. Cell. Mol. Med. 2015, 19, 1085–1093. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Xia, L.; Chen, G.Q. Protein Kinase Cδ in Apoptosis: A Brief Overview. Arch. Immunol. Ther. Exp. 2012, 60, 361–372. [Google Scholar] [CrossRef]
- Mizuno, K.; Noda, K.; Araki, T.; Imaoka, T.; Kobayashi, Y.; Akita, Y.; Shimonaka, M.; Kishi, S.; Ohno, S. The Proteolytic Cleavage of Protein Kinase C Isotypes, Which Generates Kinase and Regulatory Fragments, Correlates with Fas-Mediated and 12-O-Tetradecanoyl-Phorbol-13-Acetate-Induced Apoptosis. Eur. J. Biochem. 1997, 250, 7–18. [Google Scholar] [CrossRef]
- Ghayur, T.; Hugunin, M.; Talanian, R.V.; Ratnofsky, S.; Quinlan, C.; Emoto, Y.; Pandey, P.; Datta, R.; Huang, Y.; Kharbanda, S.; et al. Proteolytic Activation of Protein Kinase C Delta by an ICE/CED 3-like Protease Induces Characteristics of Apoptosis. J. Exp. Med. 1996, 184, 2399–2404. [Google Scholar] [CrossRef]
- Kajimoto, T.; Ohmori, S.; Shirai, Y.; Sakai, N.; Saito, N. Subtype-Specific Translocation of the Delta Subtype of Protein Kinase C and Its Activation by Tyrosine Phosphorylation Induced by Ceramide in HeLa Cells. Mol. Cell. Biol. 2001, 21, 1769–1783. [Google Scholar] [CrossRef] [Green Version]
- Kajimoto, T.; Shirai, Y.; Sakai, N.; Yamamoto, T.; Matsuzaki, H.; Kikkawa, U.; Saito, N. Ceramide-Induced Apoptosis by Translocation, Phosphorylation, and Activation of Protein Kinase Cdelta in the Golgi Complex. J. Biol. Chem. 2004, 279, 12668–12676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hara, T.; Namba, H.; Yang, T.T.; Nagayama, Y.; Fukata, S.; Kuma, K.; Ishikawa, N.; Ito, K.; Yamashita, S. Ionizing Radiation Activates C-Jun NH2-Terminal Kinase (JNK/SAPK) via a PKC-Dependent Pathway in Human Thyroid Cells. Biochem. Biophys. Res. Commun. 1998, 244, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Rolfe, M.; Proud, C.G. Ca2+-Independent Protein Kinase C Activity Is Required for A1-Adrenergic-Receptor-Mediated Regulation of Ribosomal Protein S6 Kinases in Adult Cardiomyocytes. Biochem. J. 2003, 373, 603–611. [Google Scholar] [CrossRef] [Green Version]
- Murriel, C.L.; Churchill, E.; Inagaki, K.; Szweda, L.I.; Mochly-Rosen, D. Protein Kinase Cdelta Activation Induces Apoptosis in Response to Cardiac Ischemia and Reperfusion Damage: A Mechanism Involving BAD and the Mitochondria. J. Biol. Chem. 2004, 279, 47985–47991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fei, Y.D.; Li, W.; Hou, J.W.; Guo, K.; Chen, X.M.; Chen, Y.H.; Wang, Q.; Xu, X.L.; Wang, Y.P.; Li, Y.G. Oxidative Stress-Induced Afterdepolarizations and Protein Kinase C Signaling. Int. J. Mol. Sci. 2017, 18, 688. [Google Scholar] [CrossRef] [PubMed]
- Young, L.H.; Balin, B.J.; Weis, M.T. Gö 6983: A Fast Acting Protein Kinase C Inhibitor That Attenuates Myocardial Ischemia/Reperfusion Injury. Cardiovasc. Drug Rev. 2005, 23, 255–272. [Google Scholar] [CrossRef]
- Zhao, L.L.; Chen, M.X.; Zhang, M.Y.; Dai, K.S. Protein Kinase C Activation Induces Platelet Apoptosis. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2013, 21, 1207–1210. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.S.; Liu, C.; Meng, F.; Yang, M.; Zhou, K.; Hu, R.; Wang, X.; Dai, K. X-rays Stimulate Granular Secretions and Activate Protein Kinase C Signaling in Human Platelets. Curr. Issues Mol. Biol. 2023, 45, 6024-6039. https://doi.org/10.3390/cimb45070380
Khan MS, Liu C, Meng F, Yang M, Zhou K, Hu R, Wang X, Dai K. X-rays Stimulate Granular Secretions and Activate Protein Kinase C Signaling in Human Platelets. Current Issues in Molecular Biology. 2023; 45(7):6024-6039. https://doi.org/10.3390/cimb45070380
Chicago/Turabian StyleKhan, Muhammad Shoaib, Chunliang Liu, Fanbi Meng, Mengnan Yang, Kangxi Zhou, Renping Hu, Xuexiang Wang, and Kesheng Dai. 2023. "X-rays Stimulate Granular Secretions and Activate Protein Kinase C Signaling in Human Platelets" Current Issues in Molecular Biology 45, no. 7: 6024-6039. https://doi.org/10.3390/cimb45070380
APA StyleKhan, M. S., Liu, C., Meng, F., Yang, M., Zhou, K., Hu, R., Wang, X., & Dai, K. (2023). X-rays Stimulate Granular Secretions and Activate Protein Kinase C Signaling in Human Platelets. Current Issues in Molecular Biology, 45(7), 6024-6039. https://doi.org/10.3390/cimb45070380





