Mechanism of Taxanes in the Treatment of Lung Cancer Based on Network Pharmacology and Molecular Docking
Abstract
1. Introduction
2. Materials and Methods
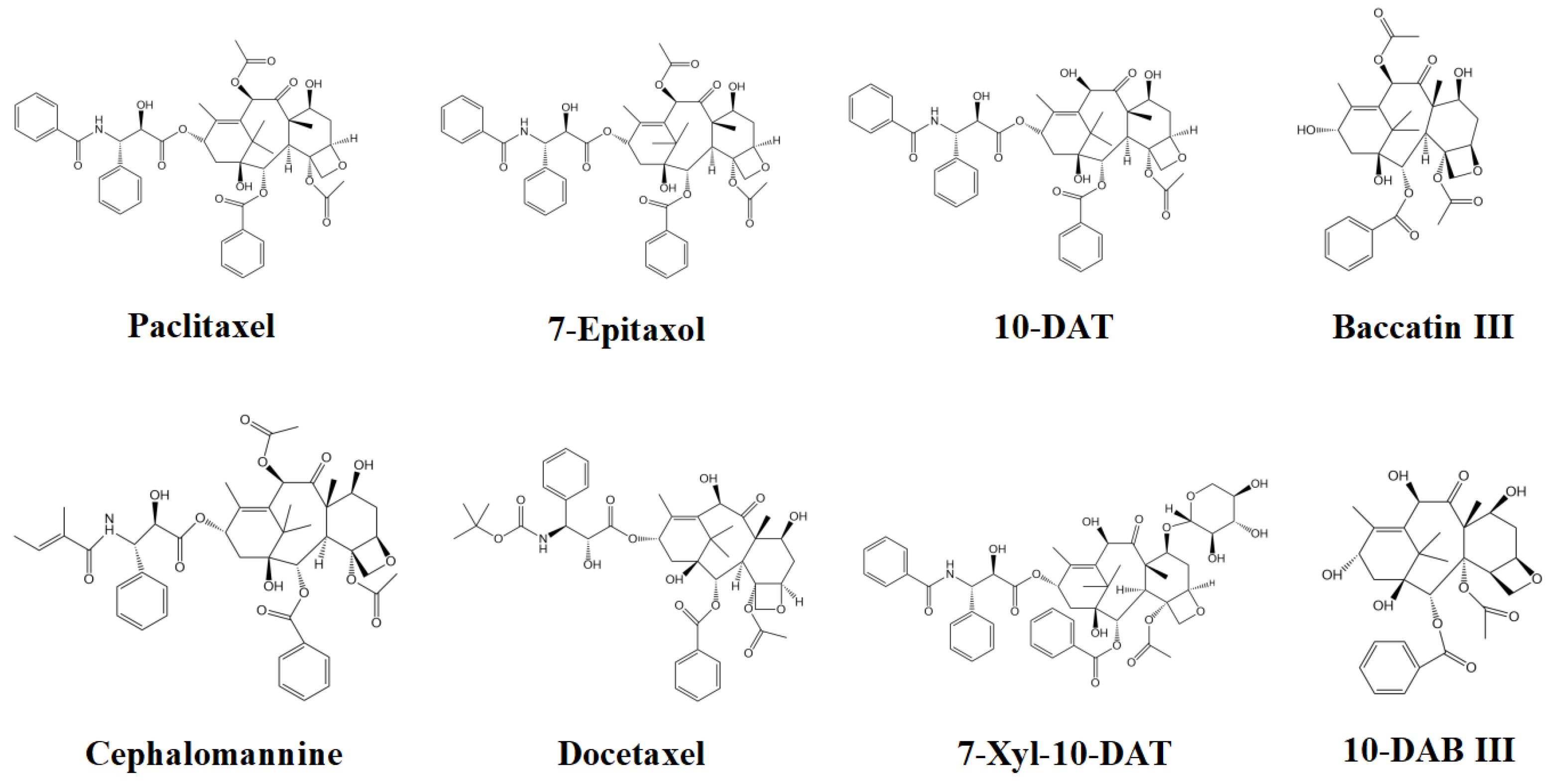
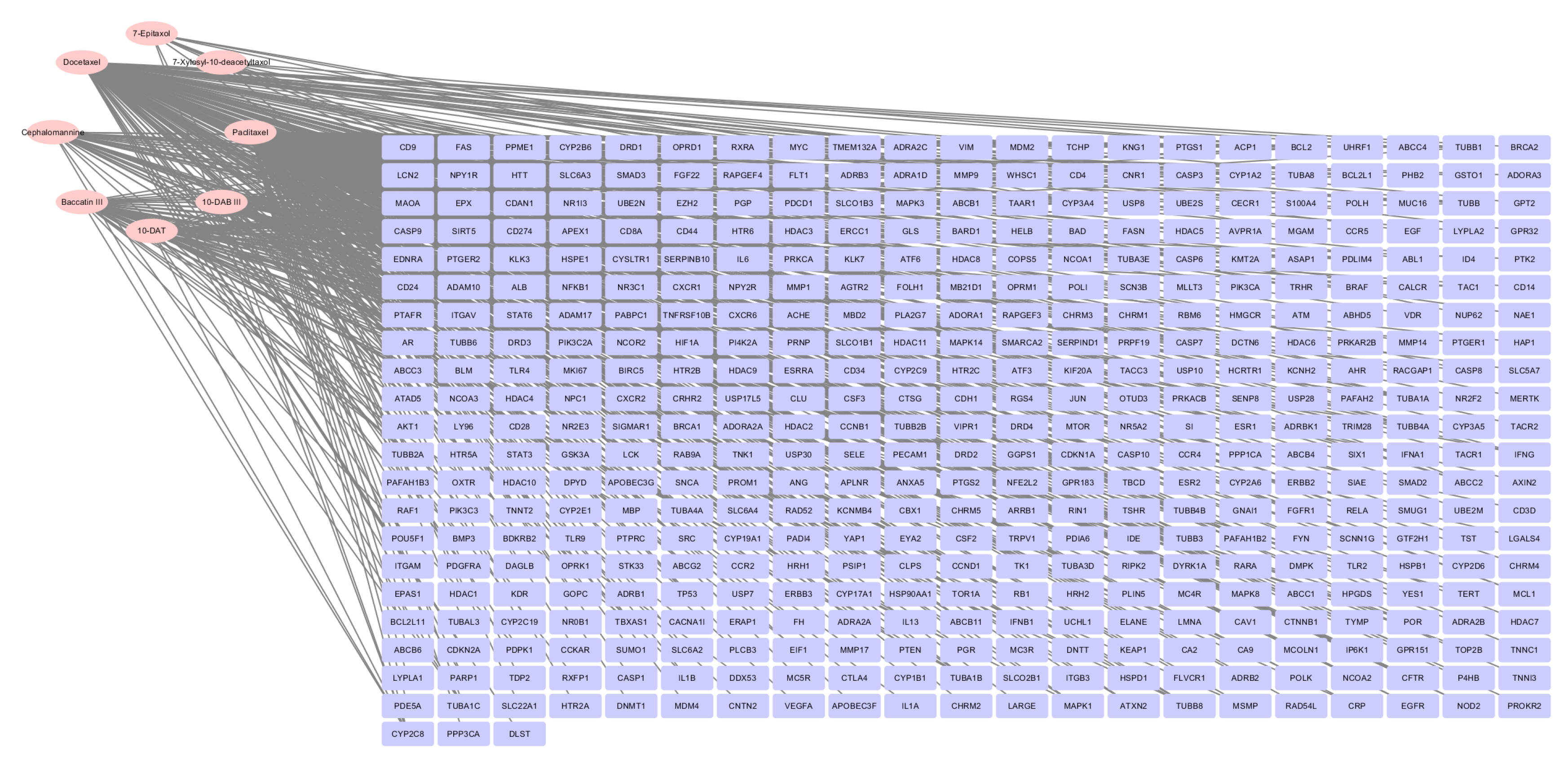
2.1. Screening of Effective Active Compounds of Taxanes and Construction of Compounds-Targets
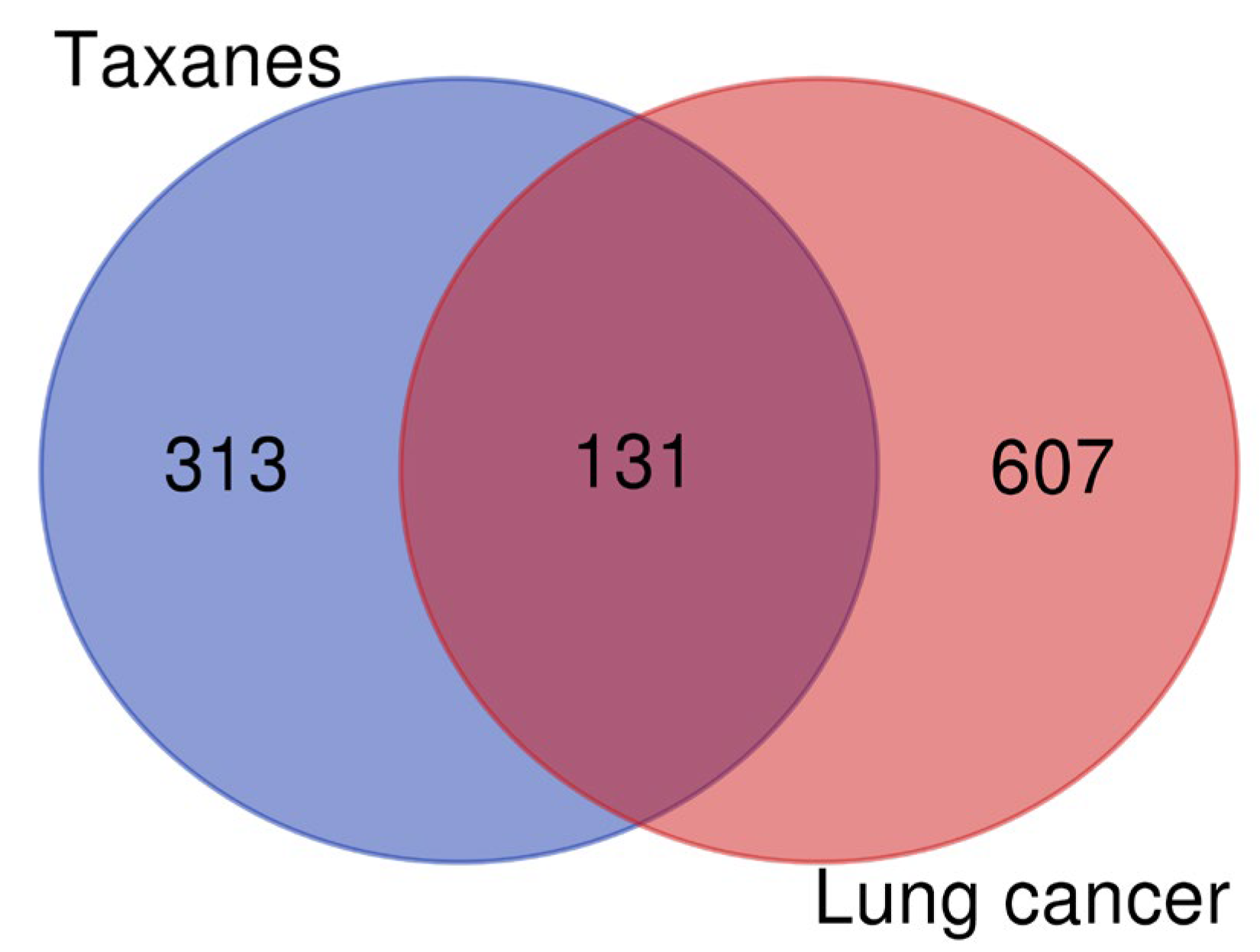
2.2. Screening of Lung Cancer Disease Targets
2.3. Acquisition of Intersection Targets and Construction of PPI Network
2.4. Analysis of GO and KEGG Pathway Enrichment
2.5. Molecular Docking
3. Results
3.1. Construction of Compounds-Targets Network
3.2. Screening of Lung Cancer Disease Targets and Construction of PPI Network of Intersection Targets
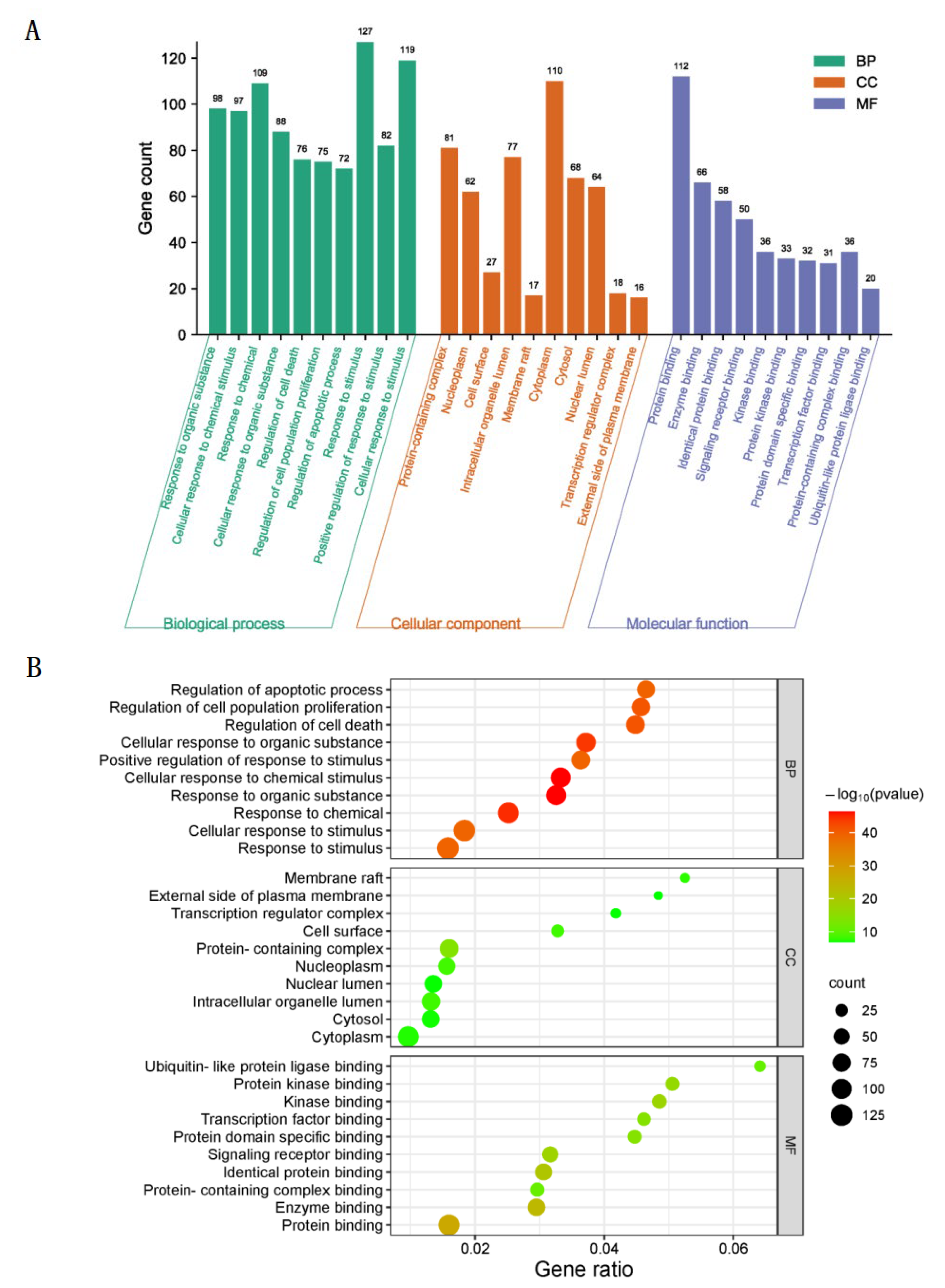
3.3. GO Biological Function Enrichment
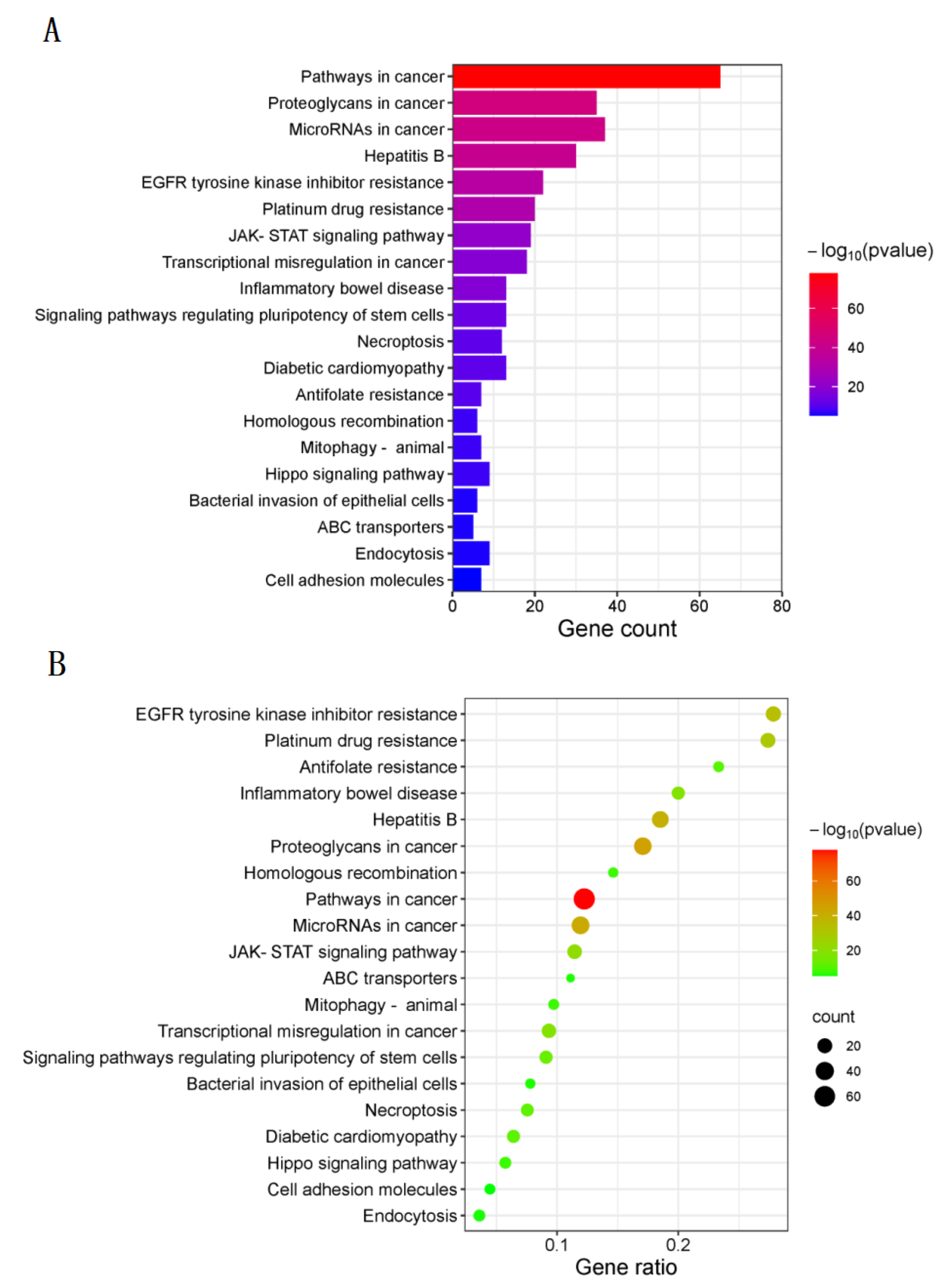
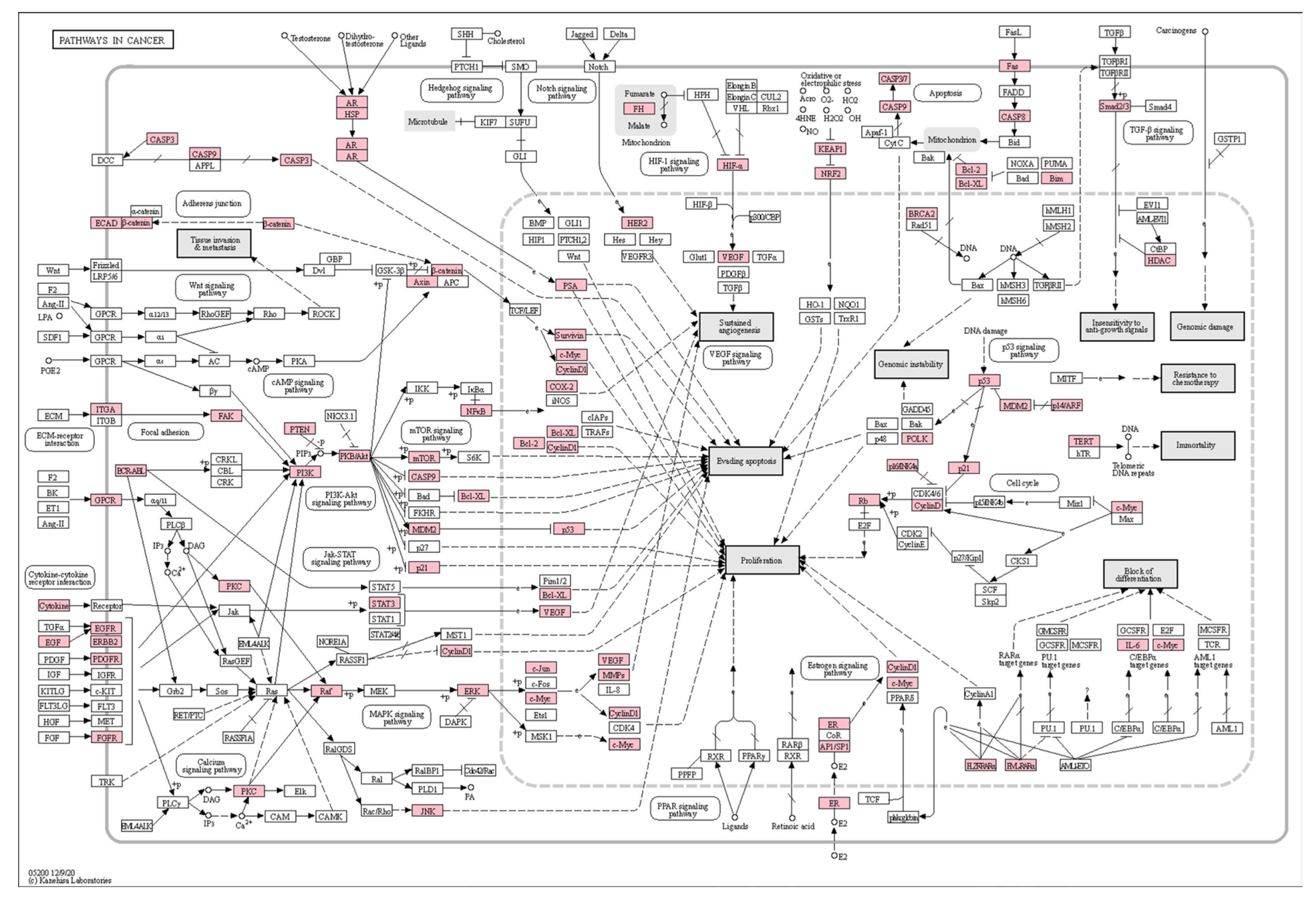
3.4. KEGG Pathway Enrichment
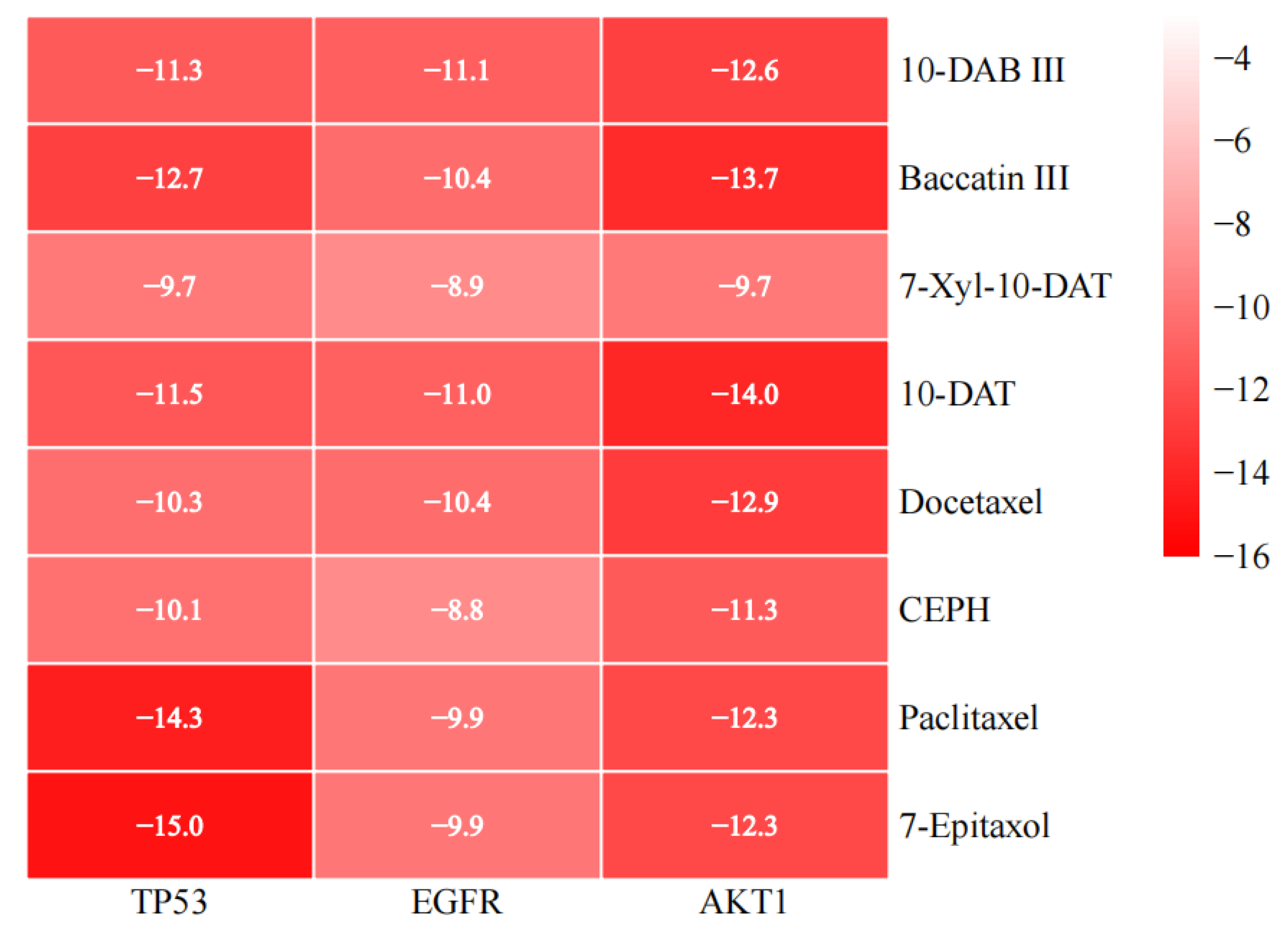
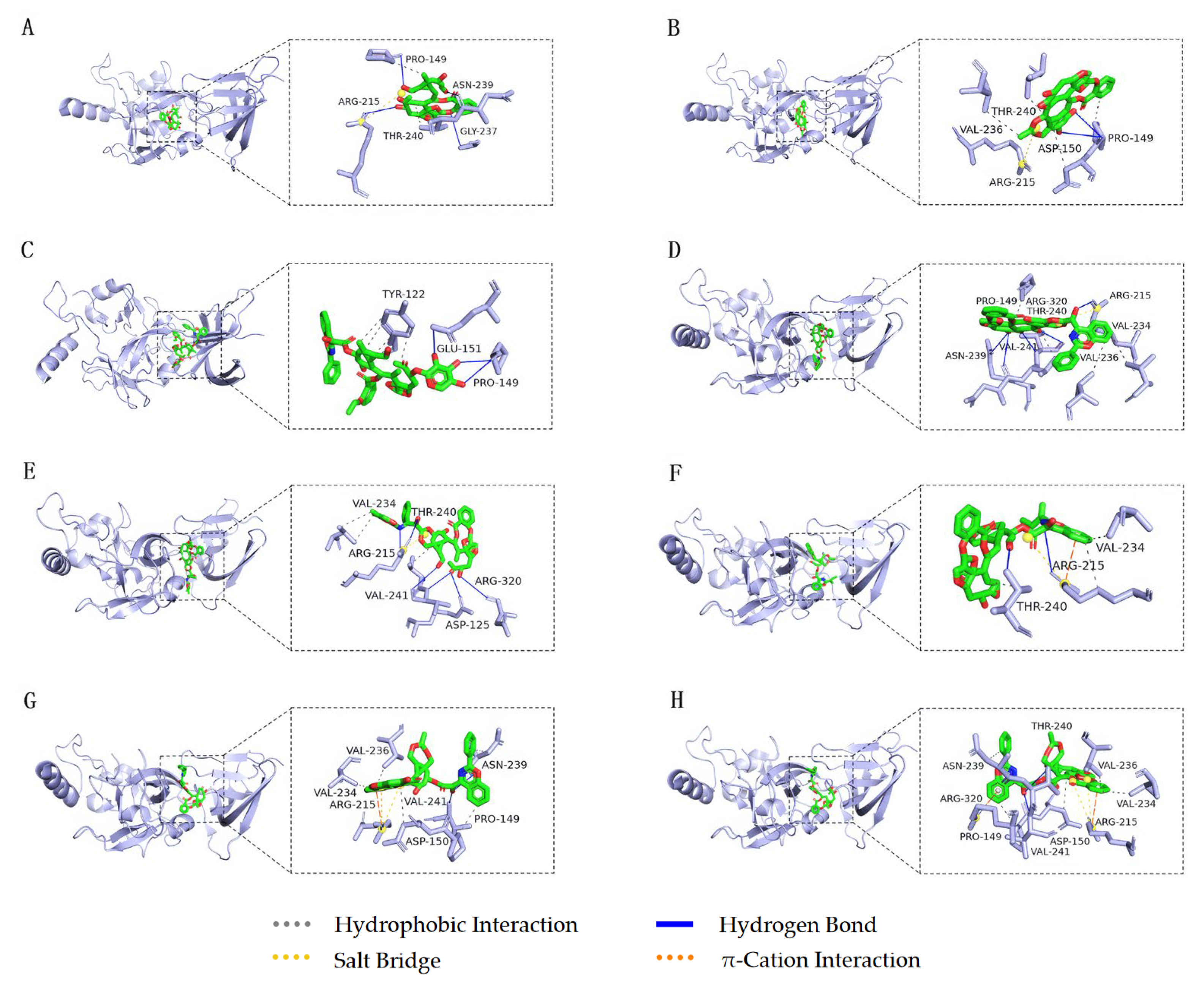
3.5. Molecular Docking
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vrtis, M.C. Advances in Lung Cancer Treatment. Home Healthc. Now 2022, 40, 296–303. [Google Scholar] [CrossRef]
- Kang, D.H.; Choi, S.W.; Sun, P.; Chung, C.; Park, D.; Lee, S.I.; Koh, J.S.; Kim, Y.; Lee, J.E. The rest period between chemotherapy and immunotherapy influences the efficacy of immune checkpoint inhibitors in lung cancer. Thorac. Cancer 2022, 13, 2346–2354. [Google Scholar] [CrossRef] [PubMed]
- Jayarajah, U.; Arulanantham, A.; Fernando, A.; Ilangamge, S.; Seneviratne, S. The incidence and patterns of lung cancers in Sri Lanka from 2001 to 2010: Analysis of national cancer registry data. Eur. J. Cancer Care 2021, 30, e13354. [Google Scholar] [CrossRef] [PubMed]
- Marmor, H.N.; Zorn, J.T.; Deppen, S.A.; Massion, P.P.; Grogan, E.L. Biomarkers in Lung Cancer Screening: A Narrative Review. Curr. Chall. Thorac. Surg. 2023, 5, 5. [Google Scholar] [CrossRef]
- Woodman, C.; Vundu, G.; George, A.; Wilson, C.M. Applications and strategies in nanodiagnosis and nanotherapy in lung cancer. Semin. Cancer Biol. 2021, 69, 349–364. [Google Scholar] [CrossRef]
- Parascandola, M.; Lin, X. Tobacco and the lung cancer epidemic in China. Transl. Lung Cancer Res. 2019, 8, S21–S30. [Google Scholar] [CrossRef]
- Warkentin, M.T.; Tammemägi, M.C.; Espin-Garcia, O.; Budhathoki, S.; Liu, G.; Hung, R.J. Lung Cancer Absolute Risk Models for Mortality in Asian Population using China Kadoorie Biobank. J. Natl. Cancer Inst. 2022, 114, 1665–1673. [Google Scholar] [CrossRef]
- Takata, Y.; Cai, Q.; Beeghly, F.A.; Li, H.; Shrubsole, M.J.; Ji, B.T.; Gong, Y.; Chow, W.H.; Gao, Y.T.; Wei, Z. Dietary B vitamin and methionine intakes and lung cancer risk among female never smokers in China. Cancer Causes Control 2012, 23, 1965–1975. [Google Scholar] [CrossRef]
- Veeramachaneni, N.K. Commentary: Computed tomography screening for lung cancer at large in China: Early cure or definitive overtreatment? J. Thorac. Cardiovasc. Surg. 2022, 163, 466–467. [Google Scholar] [CrossRef]
- Bridges, C.M.; Smith, E.M.L. What about Alice? Peripheral neuropathy from taxane-containing treatment for advanced nonsmall cell lung cancer. Support. Care Cancer 2014, 22, 2581–2592. [Google Scholar] [CrossRef]
- Jung, J.; Park, S.J.; Chung, H.K.; Kang, H.W.; Lee, S.W.; Min, H.S.; Park, H.J.; Si, Y.S.; Jeong, S.Y.; Choi, E.K. Polymeric Nanoparticles Containing Taxanes Enhance Chemoradiotherapeutic Efficacy in Non-small Cell Lung Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, e77–e83. [Google Scholar] [CrossRef]
- Amir, K.; Azita, H.; Anas, E.A. Establishment of the tandem mass spectrometric fingerprints of taxane-based anticancer compounds. Rapid Commun. Mass Spectrom. RCM 2021, 35, e9107. [Google Scholar] [CrossRef]
- Hao, D.C. Chapter 2—Mining chemodiversity from biodiversity of Taxus plants: Chemistry and chemical biology. In Taxaceae and Cephalotaxaceae; Academic Press: Cambridge, MA, USA, 2021; pp. 53–88. [Google Scholar] [CrossRef]
- Paniagua, N.; Sánchez-Robles, E.M.; Bagues, A.; Martín-Fontelles, M.I.; Girón, R. Behavior and electrophysiology studies of the peripheral neuropathy induced by individual and co-administration of paclitaxel and oxaliplatin in rat. Life Sci. 2021, 277, 119397. [Google Scholar] [CrossRef]
- Annic, J.; Babey, H.; Corre, R.; Descourt, R.; Quéré, G.; Renaud, E.; Lambert, M.; Noac’h, P.L.; Dhamelincourt, E.; Nguyen, J.; et al. Real-life second-line epirubicin-paclitaxel regimen as treatment of relapsed small-cell lung cancer: EpiTax study. Cancer Med. 2022, 12, 2658–2665. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Xu, C. Nanoparticle Albumin Bound Paclitaxel in the Third-Line Treatment of Recurrent Small Cell Lung Cancer in Real-World Practice: A Single Center Experience. Technol. Cancer Res. Treat. 2021, 20, 2658–2665. [Google Scholar] [CrossRef]
- Eiff, D.V.; Bozorgmehr, F.; Chung, I.; Bernhardt, D.; Rieken, S.; Liersch, S.; Muley, T.; Kobinger, S.; Thomas, M.; Christopoulos, P.; et al. Paclitaxel for treatment of advanced small cell lung cancer (SCLC): A retrospective study of 185 patients. J. Thorac. Dis. 2020, 12, 782–793. [Google Scholar] [CrossRef]
- Francesco, G.; Kaoru, K.; Federico, C. Future Scenarios for the Treatment of Advanced Non-Small Cell Lung Cancer: Focus on Taxane-Containing Regimens. Oncologist 2010, 15, 1102–1112. [Google Scholar] [CrossRef]
- Liu, X.-X.; Lin, G.-H.; Wang, B.-C. A Bayesian network analysis of immunotherapy and taxane chemotherapy as second- or later-line treatments in non-small cell lung cancer. Medicine 2022, 101, e31751. [Google Scholar] [CrossRef]
- Fanucchi, M.; Khuri, F.R. Taxanes in the treatment of non-small cell lung cancer. Treat. Respir. Med. 2006, 5, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Ghosh, A.K.; Chowdhury, M.; Das, P.K. Folic Acid-Functionalized Carbon Dot-Enabled Starvation Therapy in Synergism with Paclitaxel against Breast Cancer. ACS Appl. Bio Mater. 2022, 5, 2389–2402. [Google Scholar] [CrossRef]
- Yang, Y.H.; Mao, J.W.; Tan, X.L. Research progress on the source, production, and anti-cancer mechanisms of paclitaxel. Chin. J. Nat. Med. 2020, 18, 10–17. [Google Scholar] [CrossRef]
- Hashemi, M.; Zandieh, M.A.; Talebi, Y.; Rahmanian, P.; Shafiee, S.S.; Nejad, M.M.; Babaei, R.; Sadi, F.H.; Rajabi, R.; Abkenar, Z.O.; et al. Paclitaxel and docetaxel resistance in prostate cancer: Molecular mechanisms and possible therapeutic strategies. Biomed. Pharmacother. 2023, 160, 114392. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Aguilar, A.; Ojima, I. Strategies for the drug discovery and development of taxane anticancer therapeutics. Expert Opin. Drug Discov. 2022, 17, 1193–1207. [Google Scholar] [CrossRef]
- Vergote, I.; Macarulla, T.; Hirsch, F.R.; Hagemann, C.; Miller, D.S. Tumor Treating Fields (TTFields) Therapy Concomitant with Taxanes for Cancer Treatment. Cancers 2023, 15, 636. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Zhu, Z.; Wang, H.; Nichols, T.C.; Lui, G.Y.L.; Deng, S.; Rejto, P.A.; VanArsdale, T.; Hardwick, J.S.; Weinrich, S.L.; et al. Combining CDK4/6 inhibition with taxanes enhances anti-tumor efficacy by sustained impairment of pRB-E2F pathways in squamous cell lung cancer. Oncogene 2019, 38, 4125–4141. [Google Scholar] [CrossRef]
- Hammond, E.A.; Pitz, M.; Shay, B. Neuropathic Pain in Taxane-Induced Peripheral Neuropathy: Evidence for Exercise in Treatment. Neurorehabilit. Neural. Repair. 2019, 33, 792–799. [Google Scholar] [CrossRef]
- Hyer, M.L.; Kalev, P.; Fletcher, M.; Chen, C.C.; Aguado-Fraile, E.; Mandley, E.; Newhouse, S.; Lein, M.; Nagaraja, R.; Tuncay, Y.; et al. Abstract 3090: The MAT2A inhibitor, AG-270, combines with both taxanes and gemcitabine to yield enhanced anti-tumor activity in patient-derived xenograft models. Cancer Res. 2020, 80, 3090. [Google Scholar] [CrossRef]
- Khade, P.; Giannakakou, P. 4E-BP1 hyper-phosphorylation senses microtubule damage and plays a critical role in taxane antitumor activity. Cancer Res. 2016, 76, 2896. [Google Scholar] [CrossRef]
- Noor, F.; Tahir ul Qamar, M.; Ashfaq, U.A.; Albutti, A.; Alwashmi, A.S.S.; Aljasir, M.A. Network Pharmacology Approach for Medicinal Plants: Review and Assessment. Pharmaceuticals 2022, 15, 572. [Google Scholar] [CrossRef] [PubMed]
- Alzarea, S.I.; Qasim, S.; Uttra, A.M.; Khan, Y.H.; Aljoufi, F.A.; Ahmed, S.R.; Alanazi, M.; Malhi, T.H. Network Pharmacology and Molecular Docking Based Prediction of Mechanism of Pharmacological Attributes of Glutinol. Processes 2022, 10, 1492. [Google Scholar] [CrossRef]
- Kumar, S.; Abbas, F.; Ali, I.; Gupta, M.K.; Kumar, S.; Garg, M.; Kumar, D. Integrated network pharmacology and in-silico approaches to decipher the pharmacological mechanism of Selaginella tamariscina in the treatment of non-small cell lung cancer. Phytomed. Plus 2023, 3, 100419. [Google Scholar] [CrossRef]
- Nogales, C.; Mamdouh, Z.M.; List, M.; Kiel, C.; Casas, A.I.; Schmidt, H.H.H.W. Network pharmacology: Curing causal mechanisms instead of treating symptoms. Trends Pharmacol. Sci. 2022, 43, 136–150. [Google Scholar] [CrossRef] [PubMed]
- Pasala, P.K.; Donakonda, M.; Dintakurthi, P.S.N.B.K.; Rudrapal, M.; Gouru, S.A.; Ruksana, K. Investigation of Cardioprotective Activity of Silybin: Network Pharmacology, Molecular Docking, and In Vivo Studies. ChemistrySelect 2023, 8, e202300148. [Google Scholar] [CrossRef]
- Alamri, M.A. Bioinformatics and network pharmacology-based study to elucidate the multi-target pharmacological mechanism of the indigenous plants of Medina valley in treating HCV-related hepatocellular carcinoma. Saudi Pharm. J. 2023, 31, 1125–1138. [Google Scholar] [CrossRef] [PubMed]
- Kamal, I.M.; Chakrabarti, S. MetaDOCK: A Combinatorial Molecular Docking Approach. ACS Omega 2023, 8, 5850–5860. [Google Scholar] [CrossRef]
- Gauri, C.; Dibakar, D. Design and characterizations of pH-responsive drug delivery vehicles using molecular docking. Mater. Technol. 2023, 38, 2196490. [Google Scholar] [CrossRef]
- Rinthong, P.; Pulbutr, P.; Mudjupa, C. Molecular docking studies of Triphala with catalytic portion of HMG-CoA reductase enzyme. J. Herb. Med. Pharmacol. 2023, 12, 262–270. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Azman, A.N.S.S.; Tan, J.J.; Abdullah, M.N.H.; Bahari, H.; Lim, V.; Yong, Y.K. Network Pharmacology and Molecular Docking Analysis of Active Compounds in Tualang Honey against Atherosclerosis. Foods 2023, 12, 1779. [Google Scholar] [CrossRef]
- Voskarides, K.; Giannopoulou, N. The Role of TP53 in Adaptation and Evolution. Cells 2023, 12, 512. [Google Scholar] [CrossRef]
- Yang, D.; Cheng, D.; Tu, Q.; Yang, H.; Sun, B.; Yan, L.; Dai, H.; Luo, J.; Mao, B.; Cao, Y. HUWE1 controls the development of non-small cell lung cancer through down-regulation of p53. Theranostics 2018, 8, 3517–3529. [Google Scholar] [CrossRef]
- Tang, D.; Yue, L.; Yao, R.; Zhou, L.; Yang, Y.; Lu, L.; Gao, W. P53 prevent tumor invasion and metastasis by down-regulating IDO in lung cancer. Oncotarget 2017, 8, 54548–54557. [Google Scholar] [CrossRef]
- Khadeja-Tul, K.; Mohammad, S.A.; Mohammad, A.U.; Nektarios, B. P53 versus inflammation: An update. Cell Cycle 2020, 19, 160–162. [Google Scholar] [CrossRef]
- Luca, U.; Paolo, E.C.; Giosia, M.D.; Alberto, D.; Matteo, C. EGFR-Targeted Photodynamic Therapy. Pharmaceutics 2022, 14, 241. [Google Scholar] [CrossRef]
- Li, X.; Zhao, L.; Chen, C.; Nie, J.; Jiao, B. Can EGFR be a therapeutic target in breast cancer? Biochim. Biophys. Acta-Rev. Cancer 2022, 1877, 188789. [Google Scholar] [CrossRef] [PubMed]
- Shariq, O. A novel mechanism for MET and EGFR axis regulation in non-small cell lung cancer involving microRNA-27a and Sprouty2. Thorax 2013, 69, 297. [Google Scholar] [CrossRef]
- Pantazi, P.; Liloglou, T.; Eliopoulos, A. Involvement of long non coding RNAs in EGFR expression regulation in lung cancer. FEBS J. 2015, 282, 389. [Google Scholar] [CrossRef][Green Version]
- Verkoeijen, S.; Ma, Y.-F.; von Roosmalen, W.; Lalai, R.; van Miltenburg, M.H.A.M.; de Graauw, M.; von de Water, B.; Le Dévédec, S.E. Paxillin serine 178 phosphorylation in control of cell migration and metastasis formation through regulation of EGFR expression in breast cancer. J. Cancer Metastasis Treat. 2019, 5, 71–86. [Google Scholar] [CrossRef]
- Siddika, T.; Balasuriya, N.; Frederick, M.I.; Rozik, P.; Heinemann, I.U.; O’Donoghue, P. Delivery of Active AKT1 to Human Cells. Cells 2022, 11, 3834. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.W.; Kim, D.S.; Min, N.Y.; Kim, H.T. Akt1 inhibition by RNA interference sensitizes human non-small cell lung cancer cells to cisplatin. Int. J. Cancer 2008, 122, 2380–2384. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ma, Y.; Hou, X.; Liu, Y.; Li, K.; Xu, S.; Wang, J. miR-185 acts as a tumor suppressor by targeting AKT1 in non-small cell lung cancer cells. Int. J. Clin. Exp. Pathol. 2015, 8, 11854–11862. [Google Scholar] [PubMed]
- Liu, C.; Yang, H.; Xu, Z.; Li, D.; Zhou, M.; Xiao, K.; Shi, Z.; Zhu, L.; Yang, L.; Zhou, R. microRNA-548l is involved in the migration and invasion of non-small cell lung cancer by targeting the AKT1 signaling pathway. J. Cancer Res. Clin. Oncol. 2015, 141, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Broni, E.; Striegel, A.; Ashley, C.; Sakyi, P.O.; Peracha, S.; Velazquez, M.; Bebla, K.; Sodhi, M.; Kwofie, S.K.; Ademokunwa, A.; et al. Molecular Docking and Dynamics Simulation Studies Predict Potential Anti-ADAR2 Inhibitors: Implications for the Treatment of Cancer, Neurological, Immunological and Infectious Diseases. Int. J. Mol. Sci 2023, 24, 6795. [Google Scholar] [CrossRef] [PubMed]
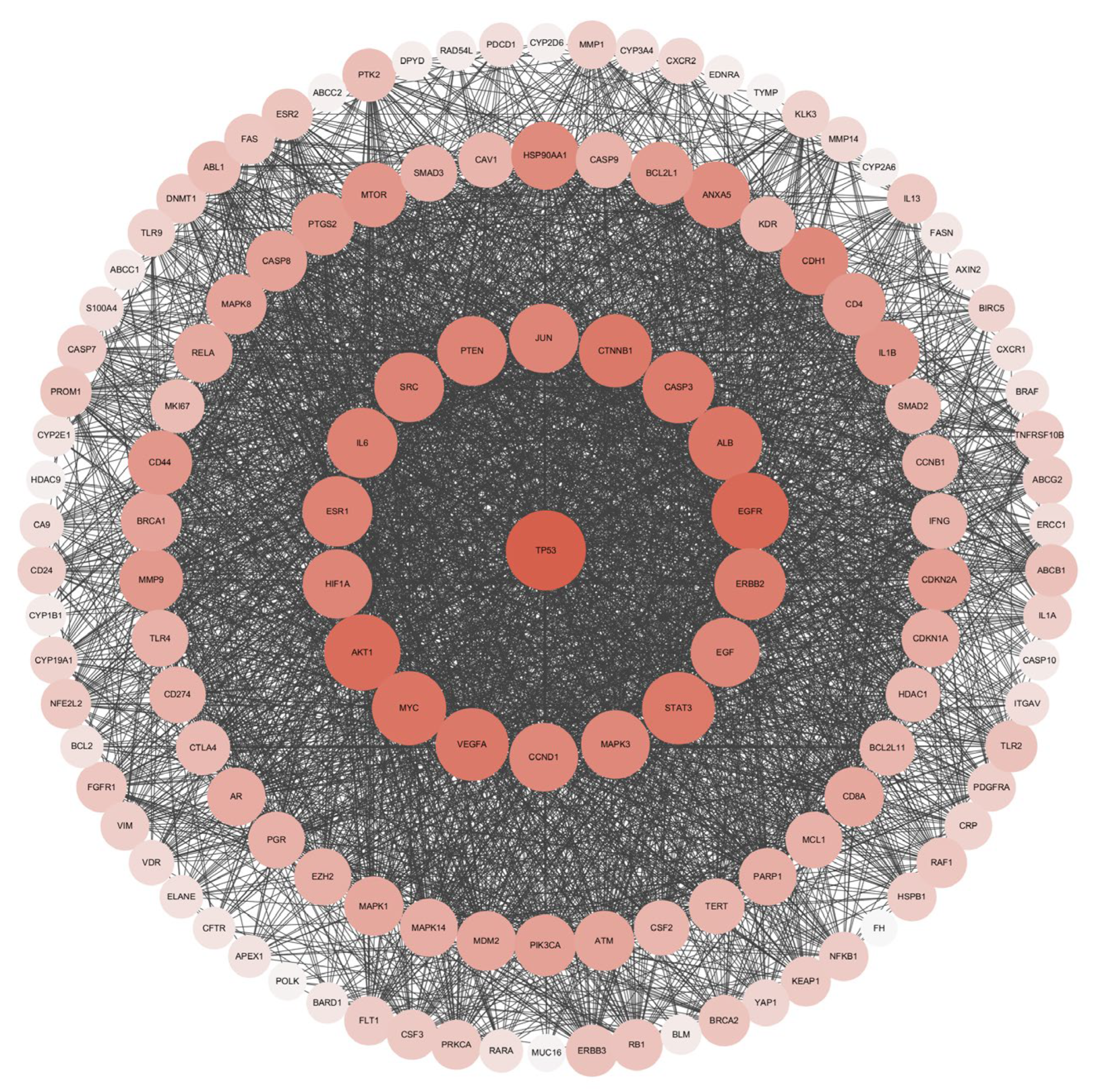


| No. | Gene | Protein Target | Betweenness Centrality | Closeness Centrality | Clustering Coefficient | Degree |
|---|---|---|---|---|---|---|
| 1 | TP53 | Tumor protein p53 | 0.0651 | 0.9353 | 0.4120 | 121 |
| 2 | EGFR | Epidermal growth factor receptor | 0.0335 | 0.8904 | 0.4571 | 114 |
| 3 | AKT1 | RAC-alpha serine/threonine-protein kinase | 0.0271 | 0.8725 | 0.4704 | 111 |
| 4 | MYC | MYC proto-oncogene, BHLH transcription factor | 0.0241 | 0.8387 | 0.4996 | 105 |
| 5 | ALB | Albumin | 0.0337 | 0.8333 | 0.4832 | 104 |
| 6 | CTNNB1 | Catenin beta 1 | 0.0193 | 0.8228 | 0.5218 | 102 |
| 7 | VEGFA | Vascular endothelial growth factor A | 0.0187 | 0.8228 | 0.5205 | 102 |
| 8 | STAT3 | Signal transducer and activator of transcription 3 | 0.0139 | 0.8176 | 0.5430 | 101 |
| 9 | CASP3 | Caspase 3 | 0.0161 | 0.8075 | 0.5448 | 99 |
| 10 | ERBB2 | Erb-B2 receptor tyrosine kinase 2 | 0.0211 | 0.8025 | 0.5092 | 98 |
| 11 | IL6 | Interleukin 6 | 0.0150 | 0.7879 | 0.5330 | 95 |
| 12 | ESR1 | Estrogen receptor 1 | 0.0190 | 0.7831 | 0.5427 | 94 |
| 13 | PTEN | Phosphatase and tensin homolog | 0.0117 | 0.7784 | 0.5676 | 93 |
| 14 | SRC | SRC proto-oncogene, non-receptor tyrosine kinase | 0.0185 | 0.7784 | 0.5640 | 93 |
| 15 | JUN | Jun proto-oncogene, AP-1 transcription factor subunit | 0.0132 | 0.7738 | 0.5707 | 92 |
| 16 | HIF1A | Hypoxia inducible factor 1 subunit alpha | 0.0136 | 0.7738 | 0.5791 | 92 |
| 17 | EGF | Epidermal growth factor | 0.0101 | 0.7692 | 0.5819 | 91 |
| 18 | CCND1 | Cyclin D1 | 0.0090 | 0.7647 | 0.5900 | 90 |
| 19 | MAPK3 | Mitogen-activated protein kinase 3 | 0.0102 | 0.7647 | 0.5760 | 90 |
| PubChem CID | Compounds | Targets | PDB ID | Uniprot ID | Binding Energy (kcal/mol) |
|---|---|---|---|---|---|
| 154272 | 10-DAB III | TP53 | 6MXY | P04637 | −11.3 |
| 154272 | 10-DAB III | EGFR | 3POZ | P00533 | −11.1 |
| 154272 | 10-DAB III | AKT1 | 3OS5 | P31749 | −12.6 |
| 65366 | Baccatin III | TP53 | 6MXY | P04637 | −12.7 |
| 65366 | Baccatin III | EGFR | 3POZ | P00533 | −10.4 |
| 65366 | Baccatin III | AKT1 | 3OS5 | P31749 | −13.7 |
| 46783796 | 7-Xyl-10-DAT | TP53 | 6MXY | P04637 | −9.7 |
| 46783796 | 7-Xyl-10-DAT | EGFR | 3POZ | P00533 | −8.9 |
| 46783796 | 7-Xyl-10-DAT | AKT1 | 3OS5 | P31749 | −9.7 |
| 155831 | 10-DAT | TP53 | 6MXY | P04637 | −11.5 |
| 155831 | 10-DAT | EGFR | 3POZ | P00533 | −11.0 |
| 155831 | 10-DAT | AKT1 | 3OS5 | P31749 | −14.0 |
| 148124 | Docetaxel | TP53 | 6MXY | P04637 | −10.3 |
| 148124 | Docetaxel | EGFR | 3POZ | P00533 | −10.4 |
| 148124 | Docetaxel | AKT1 | 3OS5 | P31749 | −12.9 |
| 6436208 | CEPH | TP53 | 6MXY | P04637 | −10.1 |
| 6436208 | CEPH | EGFR | 3POZ | P00533 | −8.8 |
| 6436208 | CEPH | AKT1 | 3OS5 | P31749 | −11.3 |
| 36314 | Paclitaxel | TP53 | 6MXY | P04637 | −14.3 |
| 36314 | Paclitaxel | EGFR | 3POZ | P00533 | −9.9 |
| 36314 | Paclitaxel | AKT1 | 3OS5 | P31749 | −12.3 |
| 184492 | 7-Epitaxol | TP53 | 6MXY | P04637 | −15.0 |
| 184492 | 7-Epitaxol | EGFR | 3POZ | P00533 | −9.9 |
| 184492 | 7-Epitaxol | AKT1 | 3OS5 | P31749 | −12.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhao, Z.; Li, W.; Tang, Y.; Wang, S. Mechanism of Taxanes in the Treatment of Lung Cancer Based on Network Pharmacology and Molecular Docking. Curr. Issues Mol. Biol. 2023, 45, 6564-6582. https://doi.org/10.3390/cimb45080414
Zhang Y, Zhao Z, Li W, Tang Y, Wang S. Mechanism of Taxanes in the Treatment of Lung Cancer Based on Network Pharmacology and Molecular Docking. Current Issues in Molecular Biology. 2023; 45(8):6564-6582. https://doi.org/10.3390/cimb45080414
Chicago/Turabian StyleZhang, Yajing, Zirui Zhao, Wenlong Li, Yuanhu Tang, and Shujie Wang. 2023. "Mechanism of Taxanes in the Treatment of Lung Cancer Based on Network Pharmacology and Molecular Docking" Current Issues in Molecular Biology 45, no. 8: 6564-6582. https://doi.org/10.3390/cimb45080414
APA StyleZhang, Y., Zhao, Z., Li, W., Tang, Y., & Wang, S. (2023). Mechanism of Taxanes in the Treatment of Lung Cancer Based on Network Pharmacology and Molecular Docking. Current Issues in Molecular Biology, 45(8), 6564-6582. https://doi.org/10.3390/cimb45080414





