A Fermented Wheat Germ Extract Contains Protein Components Active against NSCLC Xenografts In Vivo
Abstract
:1. Introduction
2. Results
2.1. FWGP Has In Vitro Cytotoxicity against Lung Cancer at Low IC50 Values
2.2. FWGP Induces Cell Cycle Arrest in A549 Cells
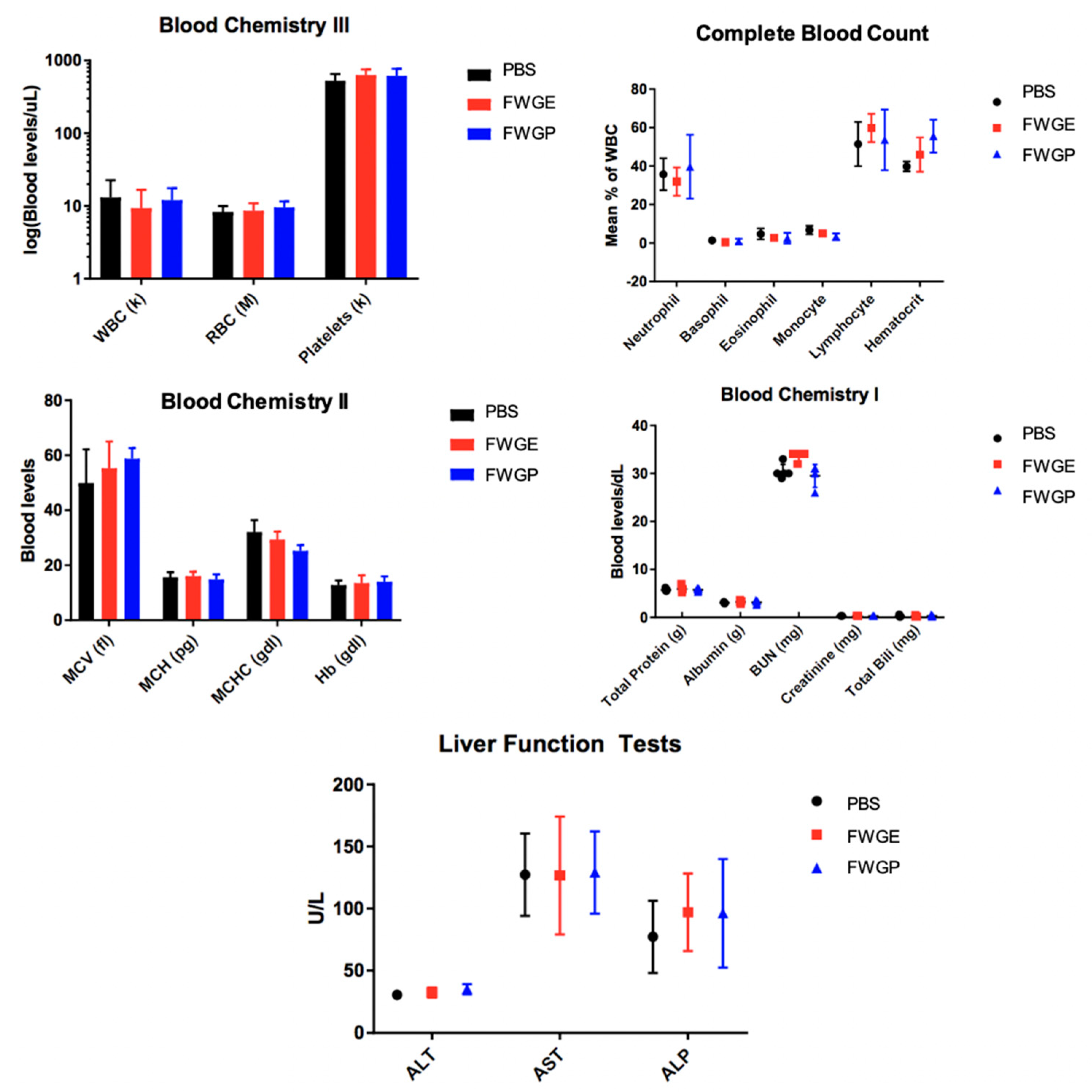
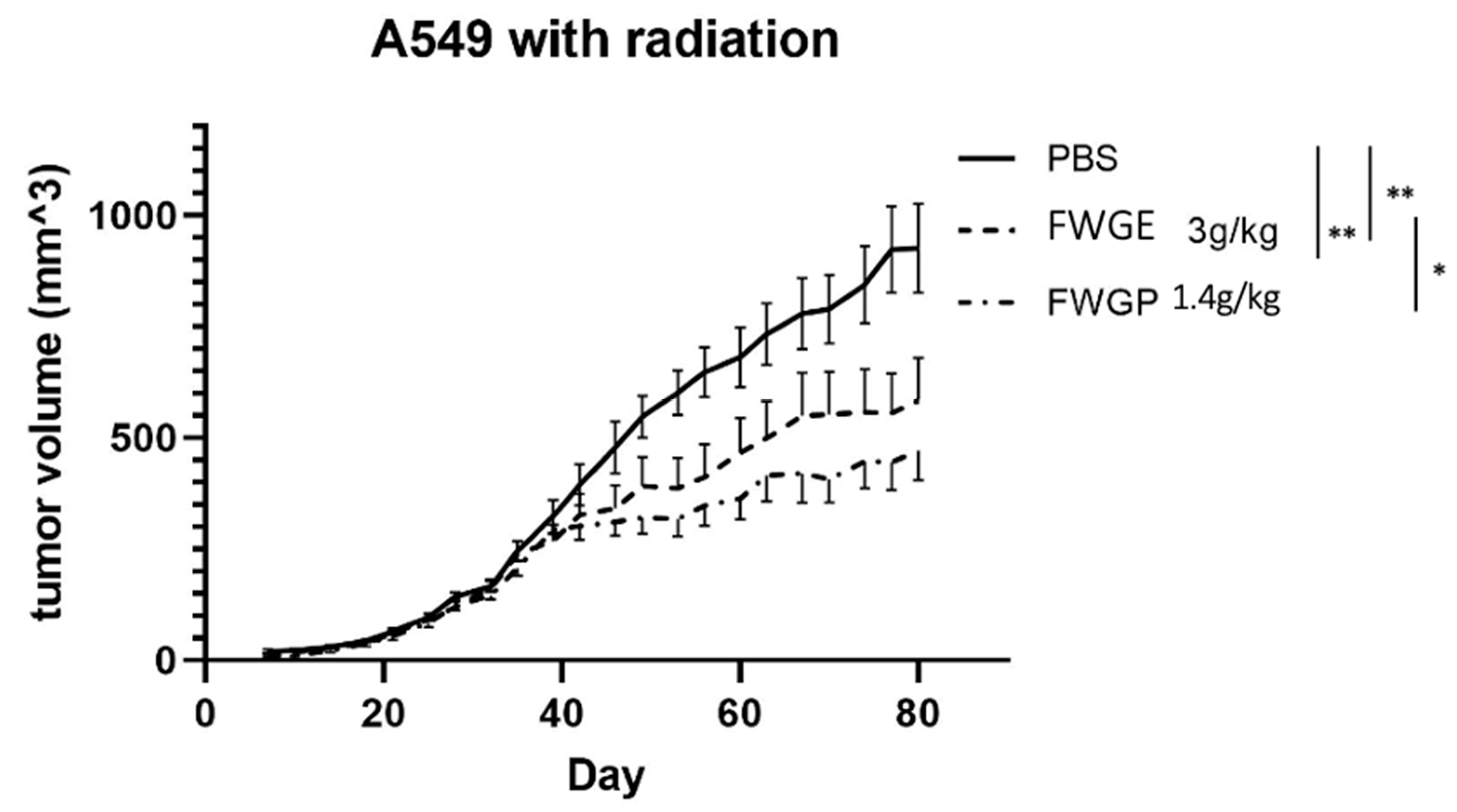
2.3. FWGP Has Tumoricidal Activity with Minimal Toxicity as a Single Agent in Lung Cancer Xenografts
3. Discussion
4. Materials and Methods
4.1. Production of FWGP and FWGE (Avemar)
4.2. Cell lines and Primary Specimens
4.3. Cytotoxicity of FWGP against Lung Cancer Cell Lines In Vitro
4.4. Cell Cycle Arrest Assay with FWGP
4.5. Animals and In Vivo Studies
4.6. Statistical Analyses
4.7. Ethics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Surveillance Epidemiology End Results (SEER) 17: Cancer Stat Facts: Lung and Bronchus Cancer. Available online: https://seer.cancer.gov/statfacts/html/lungb.html (accessed on 2 November 2022).
- Dela Cruz, C.S.; Tanoue, L.T.; Matthay, R.A. Lung cancer: Epidemiology, etiology, and prevention. Clin. Chest Med. 2011, 32, 605–644. [Google Scholar] [PubMed]
- Mok, T.S.K.; Wu, Y.-L.; Kudaba, I.; Kowalski, D.M.; Cho, B.C.; Turna, H.Z.; Castro, G., Jr.; Srimuninnimit, V.; Laktionov, K.K.; Bondarenko, I.; et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised, open-label, controlled, phase 3 trial. Lancet 2019, 393, 1819–1830. [Google Scholar] [PubMed]
- Hidvégi, M.; Rásó, E.; Tömösközi-Farkas, R.; Szende, B.; Paku, S.; Prónai, L.; Bocsi, J.; Lapis, K.; Demidov, L.V.; Manziuk, L.V.; et al. MSC, A New Benzoquinone-Containing Natural Product with Antimetastatic Effect. Cancer Biother. Radiopharm. 1999, 14, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Mueller, T.; Jordan, K.; Voigt, W. Promising cytotoxic activity profile of fermented wheat germ extract (Avemar®) in human cancer cell lines. J. Exp. Clin. Cancer Res. 2011, 30, 42. [Google Scholar] [CrossRef]
- Wang, C.W.; Wang, C.K.; Chang, Y.J.; Choong, C.Y.; Lin, C.S.; Tai, C.J.; Tai, C.J. Preclinical evaluation on the tumor suppression efficiency and combination drug effects of fermented wheat germ extract in human ovarian carcinoma cells. Evid.-Based Complement. Altern. Med. 2015, 2015, 570785. [Google Scholar] [CrossRef] [PubMed]
- Tai, C.J.; Wang, W.C.; Wang, C.K.; Wu, C.H.; Yang, M.D.; Chang, Y.J.; Jian, J.Y.; Tai, C.J. Fermented wheat germ extract induced cell death and enhanced cytotoxicity of cisplatin and 5-fluorouracil on human hepatocellular carcinoma cells. Evid.-Based Complement. Altern. Med. 2013, 2013, 121725. [Google Scholar] [CrossRef]
- Yang, M.D.; Chang, W.S.; Tsai, C.W.; Wang, M.F.; Chan, Y.C.; Chan, K.C.; Lu, M.C.; Kao, A.W.; Hsu, C.M.; Bau, D.T. Inhibitory Effects of AVEMAR on Proliferation and Metastasis of Oral Cancer Cells. Nutr. Cancer 2016, 68, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Telekes, A.; Hegedus, M.; Chae, C.H.; Vékey, K. Avemar (wheat germ extract) in cancer prevention and treatment. Nutr. Cancer 2009, 61, 891–899. [Google Scholar] [CrossRef]
- Zalatnai, A.; Lapis, K.; Szende, B.; Rásó, E.; Telekes, A.; Resetár, A.; Hidvégi, M. Wheat germ extract inhibits experimental colon carcinogenesis in F-344 rats. Carcinogenesis 2001, 22, 1649–1652. [Google Scholar] [CrossRef]
- Fajka-Boja, R.; Hidvegi, M.; Shoenfeld, Y.; Ion, G.; Demydenko, D.; Tomoskozi-Farkas, R.; Vizler, C.; Telekes, A.; Resetar, A.; Monostori, E. Fermented wheat germ extract induces apoptosis and downregulation of major histocompatibility complex class I proteins in tumor T and B cell lines. Int. J. Oncol. 2002, 20, 563–570. [Google Scholar] [CrossRef]
- Barisone, G.A.; O’donnell, R.T.; Ma, Y.; Abuhay, M.W.; Lundeberg, K.; Gowda, S.; Tuscano, J.M. A purified, fermented, extract of Triticum aestivum has lymphomacidal activity mediated via natural killer cell activation. PLoS ONE 2018, 13, e0190860. [Google Scholar]
- Zhang, J.Y.; Xiao, X.; Dong, Y.; Wu, J.; Zhou, X.H. Antitumor Activities and Apoptosis-regulated Mechanisms of Fermented Wheat Germ Extract in the Transplantation Tumor Model of Human HT-29 Cells in Nude Mice. Biomed. Environ. Sci. 2015, 28, 718–727. [Google Scholar] [PubMed]
- Demidov, L.V.; Manziuk, L.V.; Kharkevitch, G.Y.; Pirogova, N.A.; Artamonova, E.V. Adjuvant fermented wheat germ extract (Avemar) nutraceutical improves survival of high-risk skin melanoma patients: A randomized, pilot, phase II clinical study with a 7-year follow-up. Cancer Biother. Radiopharm. 2008, 23, 477–482. [Google Scholar] [PubMed]
- Judson, P.L.; Al Sawah, E.; Marchion, D.C.; Xiong, Y.; Bicaku, E.; Zgheib, N.B.; Chon, H.S.; Stickles, X.B.; Hakam, A.; Wenham, R.M.; et al. Characterizing the efficacy of fermented wheat germ extract against ovarian cancer and defining the genomic basis of its activity. Int. J. Gynecol. Cancer 2012, 22, 960–967. [Google Scholar] [CrossRef]
- Szende, B.; Marcsek, Z.; Kocsis, Z.; Tompa, A. Effect of Simultaneous Administration of Avemar® and Cytostatic Drugs on Viability of Cell Cultures, Growth of Experimental Tumors, and Survival of Tumor-Bearing Mice. Cancer Biother. Radiopharm. 2004, 19, 343–349. [Google Scholar] [CrossRef]
- Saiko, P.; Ozsvar-Kozma, M.; Madlener, S.; Bernhaus, A.; Lackner, A.; Grusch, M.; Horvath, Z.; Krupitza, G.; Jaeger, W.; Ammer, K.; et al. Avemar, a nontoxic fermented wheat germ extract, induces apoptosis and inhibits ribonucleotide reductase in human HL-60 promyelocytic leukemia cells. Cancer Lett. 2007, 250, 323–328. [Google Scholar] [CrossRef]
- Boros, L.G.; Nichelatti, M.; Shoenfeld, Y. Fermented wheat germ extract (Avemar) in the treatment of cancer and autoimmune diseases. Ann. N. Y. Acad. Sci. 2005, 1051, 529–542. [Google Scholar] [CrossRef]
- Comín-Anduix, B.; Boros, L.G.G.; Marin, S.; Boren, J.; Callol-Massot, C.; Centelles, J.J.J.; Torres, J.L.L.; Agell, N.; Bassilian, S.; Cascante, M.; et al. Fermented wheat germ extract inhibits glycolysis/pentose cycle enzymes and induces apoptosis through poly(ADP-ribose) polymerase activation in Jurkat T-cell leukemia tumor cells. J. Biol. Chem. 2002, 277, 46408–46414. [Google Scholar] [CrossRef]
- Otto, C.; Hahlbrock, T.; Eich, K.; Karaaslan, F.; Jürgens, C.; Germer, C.-T.; Wiegering, A.; Kämmerer, U. Antiproliferative and antimetabolic effects behind the anticancer property of fermented wheat germ extract. BMC Complement. Altern. Med. 2016, 16, 160. [Google Scholar]
- Bencze, G.; Bencze, S.; Rivera, K.D.; Watson, J.D.; Hidvegi, M.; Orfi, L.; Tonks, N.K.; Pappin, D.J. Mito-oncology agent: Fermented extract suppresses the Warburg effect, restores oxidative mitochondrial activity, and inhibits in vivo tumor growth. Sci. Rep. 2020, 10, 14174. [Google Scholar] [CrossRef]
- Zhang, J.-Y.; Xiao, X.; Dong, Y.; Wu, J.; Yao, F.; Zhou, X.-H. Effect of fermented wheat germ extract with lactobacillus plantarum dy-1 on HT-29 cell proliferation and apoptosis. J. Agric. Food Chem. 2015, 63, 2449–2457. [Google Scholar] [CrossRef] [PubMed]
- Pethig, R.; Gascoyne, P.R.C.; Mclaughlin, J.A.; Szent-Gyorgyi, A. Interaction of the 2,6-dimethoxysemiquinone and ascorbyl free radicals with Ehrlich ascites cells: A probe of cell-surface charge (redox potential/electron-transfer kinetics/cytotoxicity/sulfhydryl groups). Proc. Natl. Acad. Sci. USA 1984, 81, 2088–2091. [Google Scholar] [CrossRef] [PubMed]
- Clarke, T.C.; Black, L.I.; Stussman, B.J.; Nahin, R.L. Trends in the Use of Complementary Health Approaches Among Adults: United States, 2002–2012. Natl. Health Stat. Rep. 2002, 79, 1–16. [Google Scholar]
- Imir, N.G.; Aydemir, E.; Şimşek, E. Mechanism of the anti-angiogenic effect of avemar on tumor cells. Oncol. Lett. 2018, 15, 2673–2678. [Google Scholar] [CrossRef] [PubMed]
- Saiko, P.; Ozsvar-Kozma, M.; Graser, G.; Lackner, A.; Grusch, M.; Madlener, S.; Krupitza, G.; Jaeger, W.; Hidvegi, M.; Agarwal, R.P.; et al. Avemar, a nontoxic fermented wheat germ extract, attenuates the growth of sensitive and 5-FdUrd/Ara-C cross-resistant H9 human lymphoma cells through induction of apoptosis. Oncol. Rep. 2009, 21, 787–791. [Google Scholar]
- Silobricic, V.; Zietman, A.L.; Ramsay, J.R.; Suit, H.D.; Sedlacek, R.S. Residual immunity of athymic NCr/sed nude mice and the xenotransplantation of human tumors. Int. J. Cancer 1990, 45, 325–333. [Google Scholar] [CrossRef]
- Jakab, F.; Shoenfeld, Y.; Balogh, Á; Nichelatti, M.; Hoffmann, A.; Kahán, Z.; Lapis, K.; Mayer,, Á; Sápy, P.; Szentpétery, F.; et al. A medical nutriment has supportive value in the treatment of colorectal cancer. Br. J. Cancer 2003, 89, 465–469. [Google Scholar] [CrossRef]
- Heimbach, J.T.; Sebestyen, G.; Semjen, G.; Kennepohl, E. Safety studies regarding a standardized extract of fermented wheat germ. Int. J. Toxicol. 2007, 26, 253–259. [Google Scholar] [CrossRef]
- Barisone, G.A.; Ngo, T.; Tran, M.; Cortes, D.; Shahi, M.H.; Nguyen, T.-V.; Perez-Lanza, D.; Matayasuwan, W.; Díaz, E. Role of MXD3 in proliferation of DAOY human medulloblastoma cells. PLoS ONE 2012, 7, e38508. [Google Scholar]

| Cell Line | Tumor Type | IC50 (μg/mL) (SD) |
|---|---|---|
| A549 | Non-small cell lung cancer (NSCLC) | 71.74 (±13) |
| H1650 | Bronchoalveolar carcinoma | 22.52 (±5) |
| Calu-1 | NSCLC | 20.99 (±8) |
| H1975 | NSCLC | 12.88 (±3) |
| HCC827 | Lung adenocarcinoma | 17.32 (±6) |
| H460 | Large cell lung cancer | 62.82 (±29) |
| Time Point/Treatment | %G1 | %S | %G2 |
|---|---|---|---|
| Untreated | 33.1 | 46.6 | 11.3 |
| FWGP (20 μg/mL), 24 h | 33.6 | 48.1 | 10.3 |
| FWGP (20 μg/mL), 48 h | 57.4 | 29.6 | 12.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Levis, D.J.; Meckler, J.F.; O’Donnell, R.T.; Tuscano, J.M. A Fermented Wheat Germ Extract Contains Protein Components Active against NSCLC Xenografts In Vivo. Curr. Issues Mol. Biol. 2023, 45, 7087-7096. https://doi.org/10.3390/cimb45090448
Levis DJ, Meckler JF, O’Donnell RT, Tuscano JM. A Fermented Wheat Germ Extract Contains Protein Components Active against NSCLC Xenografts In Vivo. Current Issues in Molecular Biology. 2023; 45(9):7087-7096. https://doi.org/10.3390/cimb45090448
Chicago/Turabian StyleLevis, Daniel J., Joshua F. Meckler, Robert T. O’Donnell, and Joseph M. Tuscano. 2023. "A Fermented Wheat Germ Extract Contains Protein Components Active against NSCLC Xenografts In Vivo" Current Issues in Molecular Biology 45, no. 9: 7087-7096. https://doi.org/10.3390/cimb45090448




