Identification of Potential Protein Targets in Extracellular Vesicles Isolated from Chemotherapy-Treated Ovarian Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. EV Extraction and Size Determination
2.3. Proteomic Analysis of EVs
2.4. Gene Ontology and Functional Analysis
2.5. Cancer Treatment Response Gene Signature DataBase Analysis
2.6. Statistical Analysis
3. Results
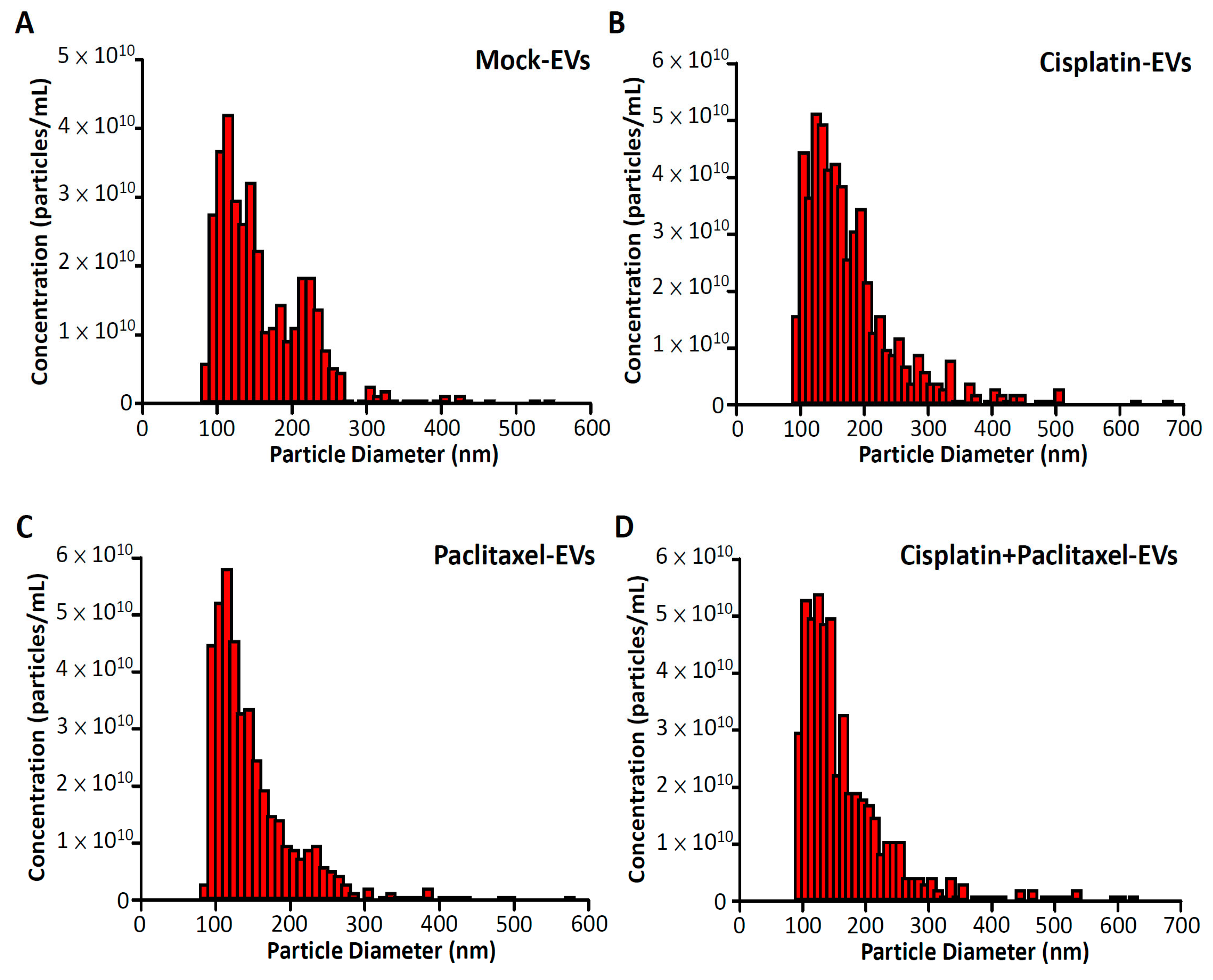
3.1. Sizes of EVs Isolated from Drug-Treated ES2 Cells
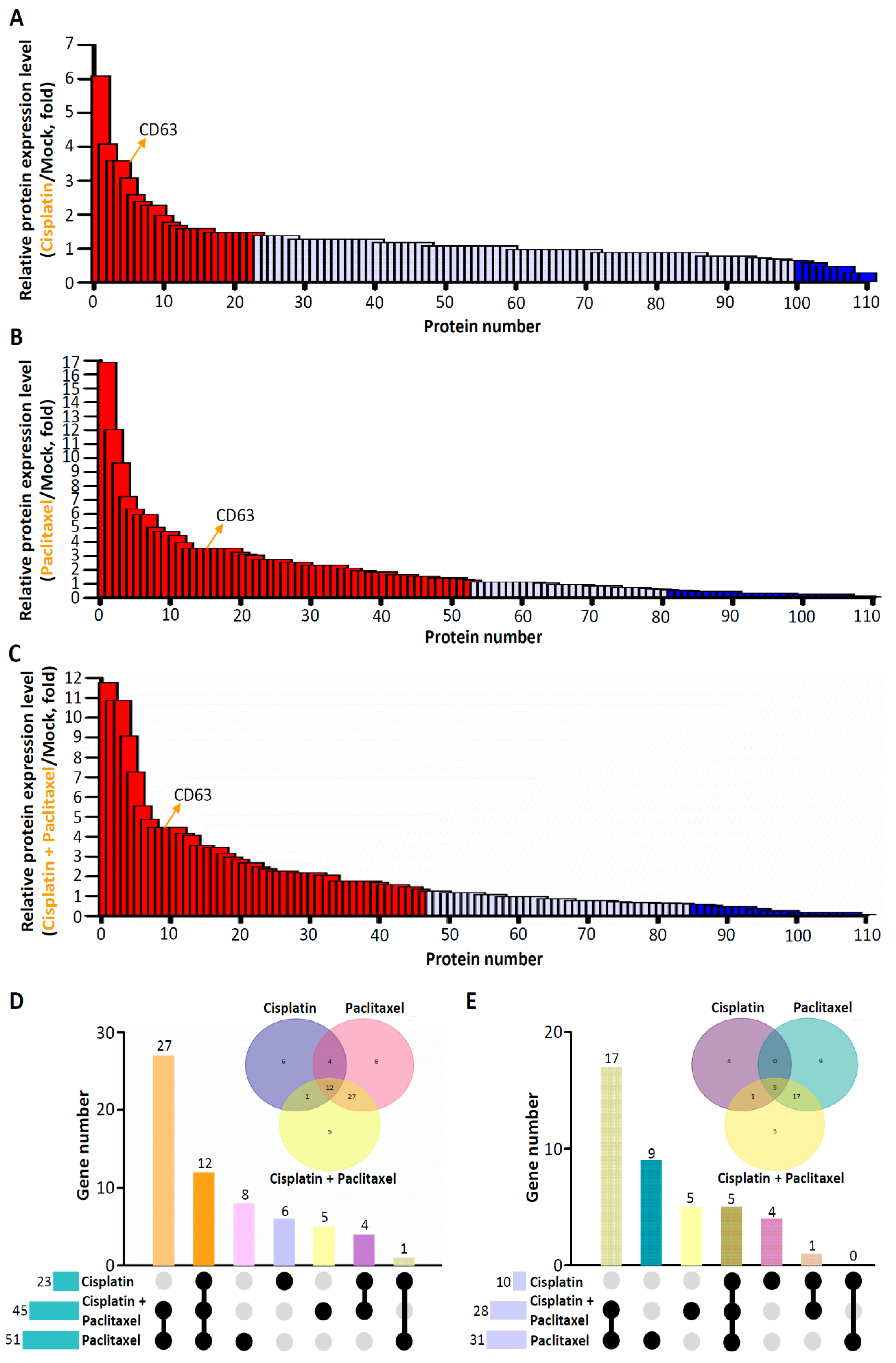
3.2. Differential Expression of EV Proteins in ES2 Cells Treated with Various Chemotherapeutic Drugs
3.3. Results of GO and Functional Enrichment Analyses of Specific Deregulated Proteins in EVs Isolated from ES2 Cells Treated with Both Cisplatin and Paclitaxel
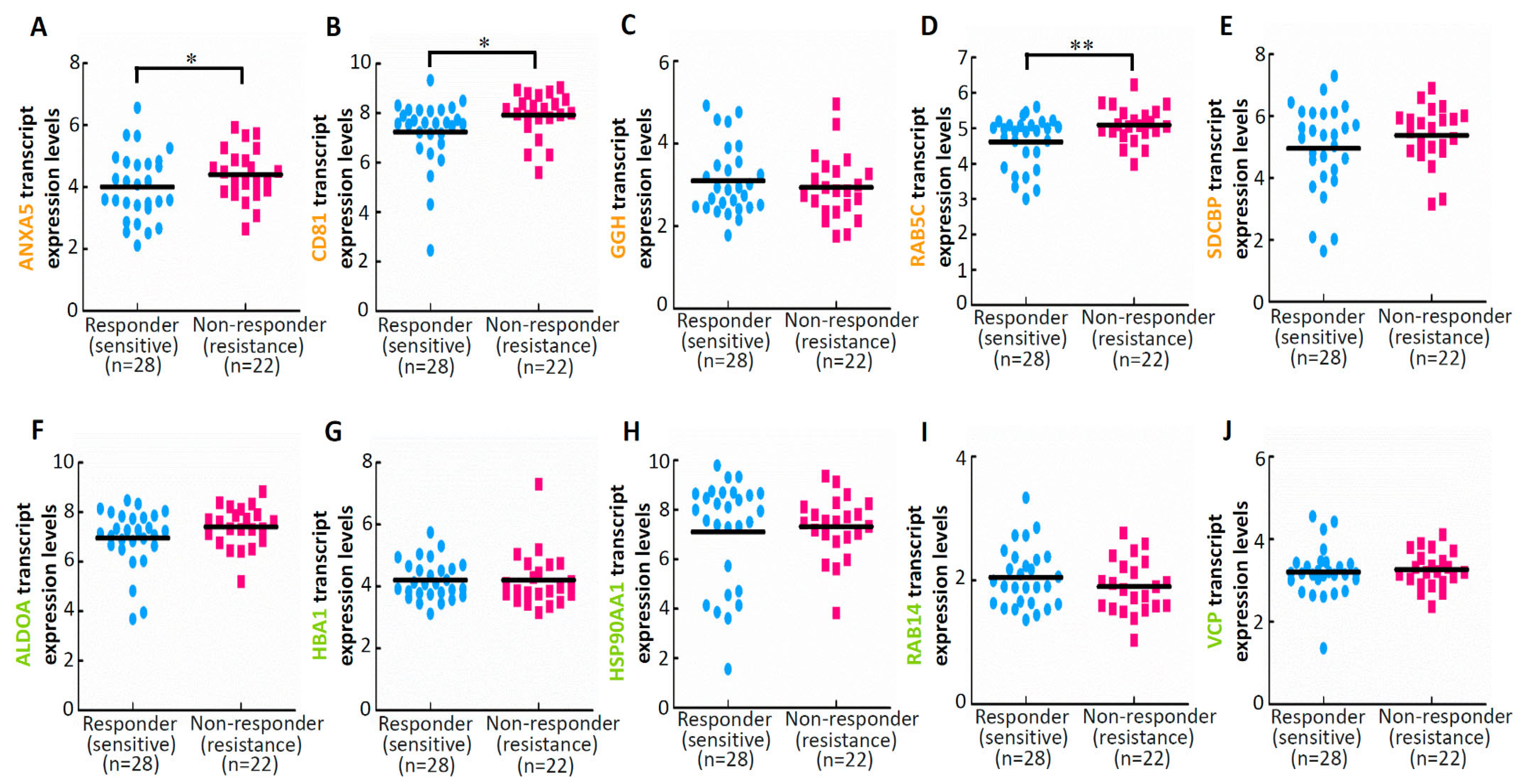
3.4. Results of Drug Sensitivity and Response Analyses of Deregulated Proteins in EVs Isolated from ES2 Cells Treated with Both Cisplatin and Paclitaxel
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Martin, A.; Harter, P.; Leary, A.; Lorusso, D.; Miller, R.E.; Pothuri, B.; Ray-Coquard, I.; Tan, D.S.P.; Bellet, E.; Oaknin, A.; et al. Newly diagnosed and relapsed epithelial ovarian cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. ESMO 2023, 23, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Penninkilampi, R.; Eslick, G.D. Perineal Talc Use and Ovarian Cancer: A Systematic Review and Meta-Analysis. Epidemiology 2018, 29, 41–49. [Google Scholar] [CrossRef]
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef]
- Dilley, J.; Burnell, M.; Gentry-Maharaj, A.; Ryan, A.; Neophytou, C.; Apostolidou, S.; Karpinskyj, C.; Kalsi, J.; Mould, T.; Woolas, R.; et al. Ovarian cancer symptoms, routes to diagnosis and survival—Population cohort study in the ‘no screen’ arm of the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS). Gynecol. Oncol. 2020, 158, 316–322. [Google Scholar] [CrossRef]
- Bast, R.C., Jr.; Hennessy, B.; Mills, G.B. The biology of ovarian cancer: New opportunities for translation. Nat. Rev. Cancer 2009, 9, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Holmes, D. Ovarian cancer: Beyond resistance. Nature 2015, 527, S217. [Google Scholar] [CrossRef]
- Coleridge, S.L.; Bryant, A.; Kehoe, S.; Morrison, J. Chemotherapy versus surgery for initial treatment in advanced ovarian epithelial cancer. Cochrane Database Syst. Rev. 2021, 2, CD005343. [Google Scholar] [CrossRef]
- Elattar, A.; Bryant, A.; Winter-Roach, B.A.; Hatem, M.; Naik, R. Optimal primary surgical treatment for advanced epithelial ovarian cancer. Cochrane Database Syst. Rev. 2011, 2011, CD007565. [Google Scholar] [CrossRef]
- Matulonis, U.A.; Sood, A.K.; Fallowfield, L.; Howitt, B.E.; Sehouli, J.; Karlan, B.Y. Ovarian cancer. Nat. Rev. Dis. Primers 2016, 2, 16061. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.L.; Duska, L.R.; Ramirez, P.T.; Heymach, J.V.; Kamat, A.A.; Modesitt, S.C.; Schmeler, K.M.; Iyer, R.B.; Garcia, M.E.; Miller, D.L.; et al. Phase 1–2 study of docetaxel plus aflibercept in patients with recurrent ovarian, primary peritoneal, or fallopian tube cancer. Lancet Oncol. 2011, 12, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.P.; Hamilton, T.C.; Schilder, R.J. Platinum resistance: The role of DNA repair pathways. Clin. Cancer Res. 2008, 14, 1291–1295. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Cui, M.; Liu, K. Therapeutic strategies to overcome cisplatin resistance in ovarian cancer. Eur. J. Med. Chem. 2022, 232, 114205. [Google Scholar] [CrossRef]
- Cruz, I.N.; Coley, H.M.; Kramer, H.B.; Madhuri, T.K.; Safuwan, N.A.; Angelino, A.R.; Yang, M. Proteomics Analysis of Ovarian Cancer Cell Lines and Tissues Reveals Drug Resistance-associated Proteins. Cancer Genom. Proteom. 2017, 14, 35–51. [Google Scholar] [CrossRef]
- Yang, L.; Xie, H.J.; Li, Y.Y.; Wang, X.; Liu, X.X.; Mai, J. Molecular mechanisms of platinum-based chemotherapy resistance in ovarian cancer (Review). Oncol. Rep. 2022, 47, 82. [Google Scholar] [CrossRef]
- Nowak, M.; Klink, M. The Role of Tumor-Associated Macrophages in the Progression and Chemoresistance of Ovarian Cancer. Cells 2020, 9, 1299. [Google Scholar] [CrossRef]
- Becker, A.; Thakur, B.K.; Weiss, J.M.; Kim, H.S.; Peinado, H.; Lyden, D. Extracellular Vesicles in Cancer: Cell-to-Cell Mediators of Metastasis. Cancer Cell 2016, 30, 836–848. [Google Scholar] [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef]
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Yanez-Mo, M.; Siljander, P.R.; Andreu, Z.; Zavec, A.B.; Borras, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.F.; Rappa, G.; Karbanova, J.; Fontana, S.; Bella, M.A.D.; Pope, M.R.; Parrino, B.; Cascioferro, S.M.; Vistoli, G.; Diana, P.; et al. Itraconazole inhibits nuclear delivery of extracellular vesicle cargo by disrupting the entry of late endosomes into the nucleoplasmic reticulum. J. Extracell. Vesicles 2021, 10, e12132. [Google Scholar] [CrossRef] [PubMed]
- Kogure, A.; Yoshioka, Y.; Ochiya, T. Extracellular Vesicles in Cancer Metastasis: Potential as Therapeutic Targets and Materials. Int. J. Mol. Sci. 2020, 21, 4463. [Google Scholar] [CrossRef]
- Wang, W.; Li, M.; Chen, Z.; Xu, L.; Chang, M.; Wang, K.; Deng, C.; Gu, Y.; Zhou, S.; Shen, Y.; et al. Biogenesis and function of extracellular vesicles in pathophysiological processes of skeletal muscle atrophy. Biochem. Pharmacol. 2022, 198, 114954. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Wang, C. A review of the regulatory mechanisms of extracellular vesicles-mediated intercellular communication. Cell Commun. Signal. 2023, 21, 77. [Google Scholar] [CrossRef]
- Tian, T.; Zhu, Y.L.; Zhou, Y.Y.; Liang, G.F.; Wang, Y.Y.; Hu, F.H.; Xiao, Z.D. Exosome uptake through clathrin-mediated endocytosis and macropinocytosis and mediating miR-21 delivery. J. Biol. Chem. 2014, 289, 22258–22267. [Google Scholar] [CrossRef]
- Prada, I.; Meldolesi, J. Binding and Fusion of Extracellular Vesicles to the Plasma Membrane of Their Cell Targets. Int. J. Mol. Sci. 2016, 17, 1296. [Google Scholar] [CrossRef]
- Cocucci, E.; Meldolesi, J. Ectosomes and exosomes: Shedding the confusion between extracellular vesicles. Trends Cell Biol. 2015, 25, 364–372. [Google Scholar] [CrossRef]
- Loyer, X.; Vion, A.C.; Tedgui, A.; Boulanger, C.M. Microvesicles as cell-cell messengers in cardiovascular diseases. Circ. Res. 2014, 114, 345–353. [Google Scholar] [CrossRef]
- Abels, E.R.; Breakefield, X.O. Introduction to Extracellular Vesicles: Biogenesis, RNA Cargo Selection, Content, Release, and Uptake. Cell. Mol. Neurobiol. 2016, 36, 301–312. [Google Scholar] [CrossRef]
- Sheehan, C.; D’Souza-Schorey, C. Tumor-derived extracellular vesicles: Molecular parcels that enable regulation of the immune response in cancer. J. Cell Sci. 2019, 132, jcs235085. [Google Scholar] [CrossRef] [PubMed]
- Clancy, J.W.; D’Souza-Schorey, C. Tumor-Derived Extracellular Vesicles: Multifunctional Entities in the Tumor Microenvironment. Annu. Rev. Pathol. 2023, 18, 205–229. [Google Scholar] [CrossRef] [PubMed]
- Tai, Y.L.; Chen, K.C.; Hsieh, J.T.; Shen, T.L. Exosomes in cancer development and clinical applications. Cancer Sci. 2018, 109, 2364–2374. [Google Scholar] [CrossRef] [PubMed]
- Kreger, B.T.; Johansen, E.R.; Cerione, R.A.; Antonyak, M.A. The Enrichment of Survivin in Exosomes from Breast Cancer Cells Treated with Paclitaxel Promotes Cell Survival and Chemoresistance. Cancers 2016, 8, 111. [Google Scholar] [CrossRef] [PubMed]
- Poggio, M.; Hu, T.; Pai, C.C.; Chu, B.; Belair, C.D.; Chang, A.; Montabana, E.; Lang, U.E.; Fu, Q.; Fong, L.; et al. Suppression of Exosomal PD-L1 Induces Systemic Anti-tumor Immunity and Memory. Cell 2019, 177, 414–427.e413. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Vasaikar, S.; Shi, Z.; Greer, M.; Zhang, B. WebGestalt 2017: A more comprehensive, powerful, flexible and interactive gene set enrichment analysis toolkit. Nucleic Acids Res. 2017, 45, W130–W137. [Google Scholar] [CrossRef]
- Kuleshov, M.V.; Jones, M.R.; Rouillard, A.D.; Fernandez, N.F.; Duan, Q.; Wang, Z.; Koplev, S.; Jenkins, S.L.; Jagodnik, K.M.; Lachmann, A.; et al. Enrichr: A comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016, 44, W90–W97. [Google Scholar] [CrossRef]
- Ge, S.X.; Jung, D.; Yao, R. ShinyGO: A graphical gene-set enrichment tool for animals and plants. Bioinformatics 2020, 36, 2628–2629. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, J.; Liu, X.; Wang, X.; Xie, Q.; Zhang, X.; Kong, X.; He, M.; Yang, Y.; Deng, X.; et al. CTR-DB, an omnibus for patient-derived gene expression signatures correlated with cancer drug response. Nucleic Acids Res. 2022, 50, D1184–D1199. [Google Scholar] [CrossRef]
- Wang, J.; Duncan, D.; Shi, Z.; Zhang, B. WEB-based GEne SeT AnaLysis Toolkit (WebGestalt): Update 2013. Nucleic Acids Res. 2013, 41, W77–W83. [Google Scholar] [CrossRef]
- Officer of Nat Rev Cancer. SMART? Time will tell. Nat. Rev. Cancer 2002, 2, 812. [Google Scholar] [CrossRef]
- Arora, T.; Mullangi, S.; Lekkala, M.R. Ovarian Cancer. In StatPearls; Treasure Island (FL) Ineligible Companies: Treasure Island, FL, USA, 2023. [Google Scholar]
- Mikula-Pietrasik, J.; Witucka, A.; Pakula, M.; Uruski, P.; Begier-Krasinska, B.; Niklas, A.; Tykarski, A.; Ksiazek, K. Comprehensive review on how platinum- and taxane-based chemotherapy of ovarian cancer affects biology of normal cells. Cell. Mol. Life Sci. CMLS 2019, 76, 681–697. [Google Scholar] [CrossRef] [PubMed]
- Colombo, N.; Sessa, C.; Bois, A.D.; Ledermann, J.; McCluggage, W.G.; McNeish, I.; Morice, P.; Pignata, S.; Ray-Coquard, I.; Vergote, I.; et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: Pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2019, 29, 728–760. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, R.X.; Chan, K.W.; Hu, J.; Zhang, J.; Wei, L.; Tan, H.; Yang, X.; Liu, H. Exosomal transfer of p-STAT3 promotes acquired 5-FU resistance in colorectal cancer cells. J. Exp. Clin. Cancer Res. 2019, 38, 320. [Google Scholar] [CrossRef]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef]
- Gong, M.; Yu, B.; Wang, J.; Wang, Y.; Liu, M.; Paul, C.; Millard, R.W.; Xiao, D.S.; Ashraf, M.; Xu, M. Mesenchymal stem cells release exosomes that transfer miRNAs to endothelial cells and promote angiogenesis. Oncotarget 2017, 8, 45200–45212. [Google Scholar] [CrossRef]
- Hoshino, A.; Costa-Silva, B.; Shen, T.L.; Rodrigues, G.; Hashimoto, A.; Tesic Mark, M.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015, 527, 329–335. [Google Scholar] [CrossRef]
- Lippert, T.H.; Ruoff, H.J.; Volm, M. Intrinsic and acquired drug resistance in malignant tumors. The main reason for therapeutic failure. Arzneimittelforschung 2008, 58, 261–264. [Google Scholar] [CrossRef]
- Muralidharan-Chari, V.; Kohan, H.G.; Asimakopoulos, A.G.; Sudha, T.; Sell, S.; Kannan, K.; Boroujerdi, M.; Davis, P.J.; Mousa, S.A. Microvesicle removal of anticancer drugs contributes to drug resistance in human pancreatic cancer cells. Oncotarget 2016, 7, 50365–50379. [Google Scholar] [CrossRef]
- Bebawy, M.; Combes, V.; Lee, E.; Jaiswal, R.; Gong, J.; Bonhoure, A.; Grau, G.E. Membrane microparticles mediate transfer of P-glycoprotein to drug sensitive cancer cells. Leukemia 2009, 23, 1643–1649. [Google Scholar] [CrossRef]
- Zhou, H.L.; Zheng, Y.J.; Cheng, X.Z.; Lv, Y.S.; Gao, R.; Mao, H.P.; Chen, Q. Intercellular transfer of P-glycoprotein from the drug resistant human bladder cancer cell line BIU-87 does not require cell-to-cell contact. J. Urol. 2013, 190, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Li, J.; Chen, W.X.; Cai, Y.Q.; Yu, D.D.; Zhong, S.L.; Zhao, J.H.; Zhou, J.W.; Tang, J.H. Exosomes decrease sensitivity of breast cancer cells to adriamycin by delivering microRNAs. Tumour Biol. J. Int. Soc. Oncodevelopmental Biol. Med. 2016, 37, 5247–5256. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Rodrigues, V.; Di Luca, A.; Sousa, D.; Seca, H.; Meleady, P.; Henry, M.; Lima, R.T.; O’Connor, R.; Vasconcelos, M.H. Multidrug resistant tumour cells shed more microvesicle-like EVs and less exosomes than their drug-sensitive counterpart cells. Biochim. Et Biophys. Acta 2016, 1860, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Campos, A.; Sharma, S.; Obermair, A.; Salomon, C. Extracellular Vesicle-Associated miRNAs and Chemoresistance: A Systematic Review. Cancers 2021, 13, 4608. [Google Scholar] [CrossRef]
- Wang, J.; Hendrix, A.; Hernot, S.; Lemaire, M.; De Bruyne, E.; Van Valckenborgh, E.; Lahoutte, T.; De Wever, O.; Vanderkerken, K.; Menu, E. Bone marrow stromal cell-derived exosomes as communicators in drug resistance in multiple myeloma cells. Blood 2014, 124, 555–566. [Google Scholar] [CrossRef]
- Hu, J.L.; Wang, W.; Lan, X.L.; Zeng, Z.C.; Liang, Y.S.; Yan, Y.R.; Song, F.Y.; Wang, F.F.; Zhu, X.H.; Liao, W.J.; et al. CAFs secreted exosomes promote metastasis and chemotherapy resistance by enhancing cell stemness and epithelial-mesenchymal transition in colorectal cancer. Mol. Cancer 2019, 18, 91. [Google Scholar] [CrossRef]
- Chen, W.X.; Liu, X.M.; Lv, M.M.; Chen, L.; Zhao, J.H.; Zhong, S.L.; Ji, M.H.; Hu, Q.; Luo, Z.; Wu, J.Z.; et al. Exosomes from drug-resistant breast cancer cells transmit chemoresistance by a horizontal transfer of microRNAs. PLoS ONE 2014, 9, e95240. [Google Scholar] [CrossRef]
- Ma, X.; Chen, Z.; Hua, D.; He, D.; Wang, L.; Zhang, P.; Wang, J.; Cai, Y.; Gao, C.; Zhang, X.; et al. Essential role for TrpC5-containing extracellular vesicles in breast cancer with chemotherapeutic resistance. Proc. Natl. Acad. Sci. USA 2014, 111, 6389–6394. [Google Scholar] [CrossRef]
- Zhang, F.F.; Zhu, Y.F.; Zhao, Q.N.; Yang, D.T.; Dong, Y.P.; Jiang, L.; Xing, W.X.; Li, X.Y.; Xing, H.; Shi, M.; et al. Microvesicles mediate transfer of P-glycoprotein to paclitaxel-sensitive A2780 human ovarian cancer cells, conferring paclitaxel-resistance. Eur. J. Pharmacol. 2014, 738, 83–90. [Google Scholar] [CrossRef]
- Yang, S.J.; Wang, D.D.; Li, J.; Xu, H.Z.; Shen, H.Y.; Chen, X.; Zhou, S.Y.; Zhong, S.L.; Zhao, J.H.; Tang, J.H. Predictive role of GSTP1-containing exosomes in chemotherapy-resistant breast cancer. Gene 2017, 623, 5–14. [Google Scholar] [CrossRef]
- Coles, B.F.; Kadlubar, F.F. Detoxification of electrophilic compounds by glutathione S-transferase catalysis: Determinants of individual response to chemical carcinogens and chemotherapeutic drugs? Biofactors 2003, 17, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhi, X.; Ji, M.; Wang, Q.; Li, Y.; Xie, J.; Zhao, S. Midkine as a potential diagnostic marker in epithelial ovarian cancer for cisplatin/paclitaxel combination clinical therapy. Am. J. Cancer Res. 2015, 5, 629–638. [Google Scholar] [PubMed]
- Zhang, H.; Zhang, Z.; Guo, T.; Chen, G.; Liu, G.; Song, Q.; Li, G.; Xu, F.; Dong, X.; Yang, F.; et al. Annexin A protein family: Focusing on the occurrence, progression and treatment of cancer. Front. Cell Dev. Biol. 2023, 11, 1141331. [Google Scholar] [CrossRef] [PubMed]
- Virani, N.A.; Thavathiru, E.; McKernan, P.; Moore, K.; Benbrook, D.M.; Harrison, R.G. Anti-CD73 and anti-OX40 immunotherapy coupled with a novel biocompatible enzyme prodrug system for the treatment of recurrent, metastatic ovarian cancer. Cancer Lett. 2018, 425, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Cai, S.; Shen, J.; Peng, H. Tetraspanins: Novel Molecular Regulators of Gastric Cancer. Front. Oncol. 2021, 11, 702510. [Google Scholar] [CrossRef]
- Woodman, P.G. Biogenesis of the sorting endosome: The role of Rab5. Traffic 2000, 1, 695–701. [Google Scholar] [CrossRef]
- Barbera, S.; Nardi, F.; Elia, I.; Realini, G.; Lugano, R.; Santucci, A.; Tosi, G.M.; Dimberg, A.; Galvagni, F.; Orlandini, M. The small GTPase Rab5c is a key regulator of trafficking of the CD93/Multimerin-2/beta1 integrin complex in endothelial cell adhesion and migration. Cell Commun. Signal. 2019, 17, 55. [Google Scholar] [CrossRef]
- Onodera, Y.; Nam, J.M.; Hashimoto, A.; Norman, J.C.; Shirato, H.; Hashimoto, S.; Sabe, H. Rab5c promotes AMAP1-PRKD2 complex formation to enhance beta1 integrin recycling in EGF-induced cancer invasion. J. Cell Biol. 2012, 197, 983–996. [Google Scholar] [CrossRef]

| Protein ID | Gene Name | Cisplatin | Paclitaxel | Cisplatin + Paclitaxel |
|---|---|---|---|---|
| P08133 | ANXA6 | ANXA6 | — | — |
| P35613 | BSG | BSG | — | — |
| Q01518 | CAP1 | CAP1 | — | — |
| P68104 | EEF1A1 | EEF1A1 | — | — |
| P06733 | ENO1 | ENO1 | — | — |
| P07195 | LDHB | LDHB | — | — |
| P60709 | ACTB | — | ACTB | — |
| P68032 | ACTC1 | — | ACTC1 | — |
| P04083 | ANXA1 | — | ANXA1 | — |
| P06899 | H2BC11 | — | H2BC11 | — |
| O60814 | H2BC12 | — | H2BC12 | — |
| Q86YZ3 | HRNR | — | HRNR | — |
| P11279 | LAMP1 | — | LAMP1 | — |
| P02788 | LTF | — | LTF | — |
| P08758 | ANXA5 | — | — | ANXA5 |
| P60033 | CD81 | — | — | CD81 |
| Q92820 | GGH | — | — | GGH |
| P51148 | RAB5C | — | — | RAB5C |
| O00560 | SDCBP | — | — | SDCBP |
| P25311 | AZGP1 | AZGP1 | AZGP1 | AZGP1 |
| P08962 | CD63 | CD63 | CD63 | CD63 |
| P01040 | CSTA | CSTA | CSTA | CSTA |
| P20930 | FLG | FLG | FLG | FLG |
| P01859 | IGHG2 | IGHG2 | IGHG2 | IGHG2 |
| P01834 | IGKC | IGKC | IGKC | IGKC |
| P0DOY2 | IGLC2 | IGLC2 | IGLC2 | IGLC2 |
| P13473 | LAMP2 | LAMP2 | LAMP2 | LAMP2 |
| P61626 | LYZ | LYZ | LYZ | LYZ |
| Q06830 | PRDX1 | PRDX1 | PRDX1 | PRDX1 |
| P60900 | PSMA6 | PSMA6 | PSMA6 | PSMA6 |
| P31151 | S100A7 | S100A7 | S100A7 | S100A7 |
| P04075 | ALDOA | ALDOA | ALDOA | — |
| P69905 | HBA1 | HBA1 | HBA1 | — |
| P00338 | LDHA | LDHA | LDHA | — |
| P0CG48 | UBC | UBC | UBC | — |
| P61204 | ARF3 | ARF3 | — | ARF3 |
| P02768 | ALB | — | ALB | ALB |
| P07355 | ANXA2 | — | ANXA2 | ANXA2 |
| P05089 | ARG1 | — | ARG1 | ARG1 |
| P31944 | CASP14 | — | CASP14 | CASP14 |
| P04040 | CAT | — | CAT | CAT |
| P07339 | CTSD | — | CTSD | CTSD |
| P81605 | DCD | — | DCD | DCD |
| Q08554 | DSC1 | — | DSC1 | DSC1 |
| Q02413 | DSG1 | — | DSG1 | DSG1 |
| P15924 | DSP | — | DSP | DSP |
| Q01469 | FABP5 | — | FABP5 | FABP5 |
| Q5D862 | FLG2 | — | FLG2 | FLG2 |
| P04406 | GAPDH | — | GAPDH | GAPDH |
| O75223 | GGCT | — | GGCT | GGCT |
| Q16777 | H2AC20 | — | H2AC20 | H2AC20 |
| P14923 | JUP | — | JUP | JUP |
| P31025 | LCN1 | — | LCN1 | LCN1 |
| P12273 | PIP | — | PIP | PIP |
| P53801 | PTTG1IP | — | PTTG1IP | PTTG1IP |
| P05109 | S100A8 | — | S100A8 | S100A8 |
| P06702 | S100A9 | — | S100A9 | S100A9 |
| O95969 | SCGB1D2 | — | SCGB1D2 | SCGB1D2 |
| Q96P63 | SERPINB12 | — | SERPINB12 | SERPINB12 |
| P29508 | SERPINB3 | — | SERPINB3 | SERPINB3 |
| P22735 | TGM1 | — | TGM1 | TGM1 |
| Q08188 | TGM3 | — | TGM3 | TGM3 |
| P10599 | TXN | — | TXN | TXN |
| Protein ID | Gene Name | Cisplatin | Paclitaxel | Cisplatin + Paclitaxel |
|---|---|---|---|---|
| P04040 | CAT | CAT | — | — |
| Q00610 | CTSD | CTSD | — | — |
| P02788 | LTF | LTF | — | — |
| P22735 | TGM1 | TGM1 | — | — |
| P60033 | CD81 | — | CD81 | — |
| Q99829 | CPNE1 | — | CPNE1 | — |
| O75131 | CPNE3 | — | CPNE3 | — |
| P0DMV8 | HSPA1A | — | HSPA1A | — |
| P05556 | ITGB1 | — | ITGB1 | — |
| P00558 | PGK1 | — | PGK1 | — |
| P51148 | RAB5C | — | RAB5C | — |
| O00560 | SDCBP | — | SDCBP | — |
| P02786 | TFRC | — | TFRC | — |
| P04075 | ALDOA | — | — | ALDOA |
| P69905 | HBA1 | — | — | HBA1 |
| P07900 | HSP90AA1 | — | — | HSP90AA1 |
| P61106 | RAB14 | — | — | RAB14 |
| P55072 | VCP | — | — | VCP |
| P54709 | ATP1B3 | ATP1B3 | ATP1B3 | ATP1B3 |
| Q00610 | CLTC | CLTC | CLTC | CLTC |
| P60842 | EIF4A1 | EIF4A1 | EIF4A1 | EIF4A1 |
| Q08380 | LGALS3BP | LGALS3BP | LGALS3BP | LGALS3BP |
| Q9NZM1 | MYOF | MYOF | MYOF | MYOF |
| Q86YZ3 | HRNR | HRNR | — | HRNR |
| O43707 | ACTN4 | — | ACTN4 | ACTN4 |
| P05023 | ATP1A1 | — | ATP1A1 | ATP1A1 |
| P35613 | BSG | — | BSG | BSG |
| P62879 | GNB2 | — | GNB2 | GNB2 |
| P04439 | HLA-A | — | HLA-A | HLA-A |
| P26006 | ITGA3 | — | ITGA3 | ITGA3 |
| P26038 | MME | — | MME | MME |
| Q9NZM1 | MSN | — | MSN | MSN |
| O75340 | PDCD6 | — | PDCD6 | PDCD6 |
| Q8WUM4 | PDCD6IP | — | PDCD6IP | PDCD6IP |
| P62937 | PPIA | — | PPIA | PPIA |
| P26022 | PTX3 | — | PTX3 | PTX3 |
| P61586 | RHOA | — | RHOA | RHOA |
| P27105 | STOM | — | STOM | STOM |
| P68363 | TUBA1B | — | TUBA1B | TUBA1B |
| P62258 | YWHAE | — | YWHAE | YWHAE |
| P63104 | YWHAZ | — | YWHAZ | YWHAZ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, C.-Y.; Ni, Y.-C.; Nguyen, H.D.; Wu, Y.-F.; Lee, K.-H. Identification of Potential Protein Targets in Extracellular Vesicles Isolated from Chemotherapy-Treated Ovarian Cancer Cells. Curr. Issues Mol. Biol. 2023, 45, 7417-7431. https://doi.org/10.3390/cimb45090469
Chan C-Y, Ni Y-C, Nguyen HD, Wu Y-F, Lee K-H. Identification of Potential Protein Targets in Extracellular Vesicles Isolated from Chemotherapy-Treated Ovarian Cancer Cells. Current Issues in Molecular Biology. 2023; 45(9):7417-7431. https://doi.org/10.3390/cimb45090469
Chicago/Turabian StyleChan, Chia-Yi, Yi-Chun Ni, Hieu Duc Nguyen, Yung-Fu Wu, and Kuen-Haur Lee. 2023. "Identification of Potential Protein Targets in Extracellular Vesicles Isolated from Chemotherapy-Treated Ovarian Cancer Cells" Current Issues in Molecular Biology 45, no. 9: 7417-7431. https://doi.org/10.3390/cimb45090469
APA StyleChan, C.-Y., Ni, Y.-C., Nguyen, H. D., Wu, Y.-F., & Lee, K.-H. (2023). Identification of Potential Protein Targets in Extracellular Vesicles Isolated from Chemotherapy-Treated Ovarian Cancer Cells. Current Issues in Molecular Biology, 45(9), 7417-7431. https://doi.org/10.3390/cimb45090469






