Anti-Diabetic Potential of Sargassum horneri and Ulva australis Extracts In Vitro and In Vivo
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of SH and UA Ethanol Extracts
2.2. α-Glucosidase Inhibitory Assay
2.3. Cell Culture and Induction of Insulin Resistance Model
2.4. Cell Viability Assay
2.5. Reactive Oxygen Species (ROS) Detection Assay
2.6. Cellular Glucose Uptake Assay
2.7. Glycogen Content Assay
2.8. Quantitative RT-PCR Analysis
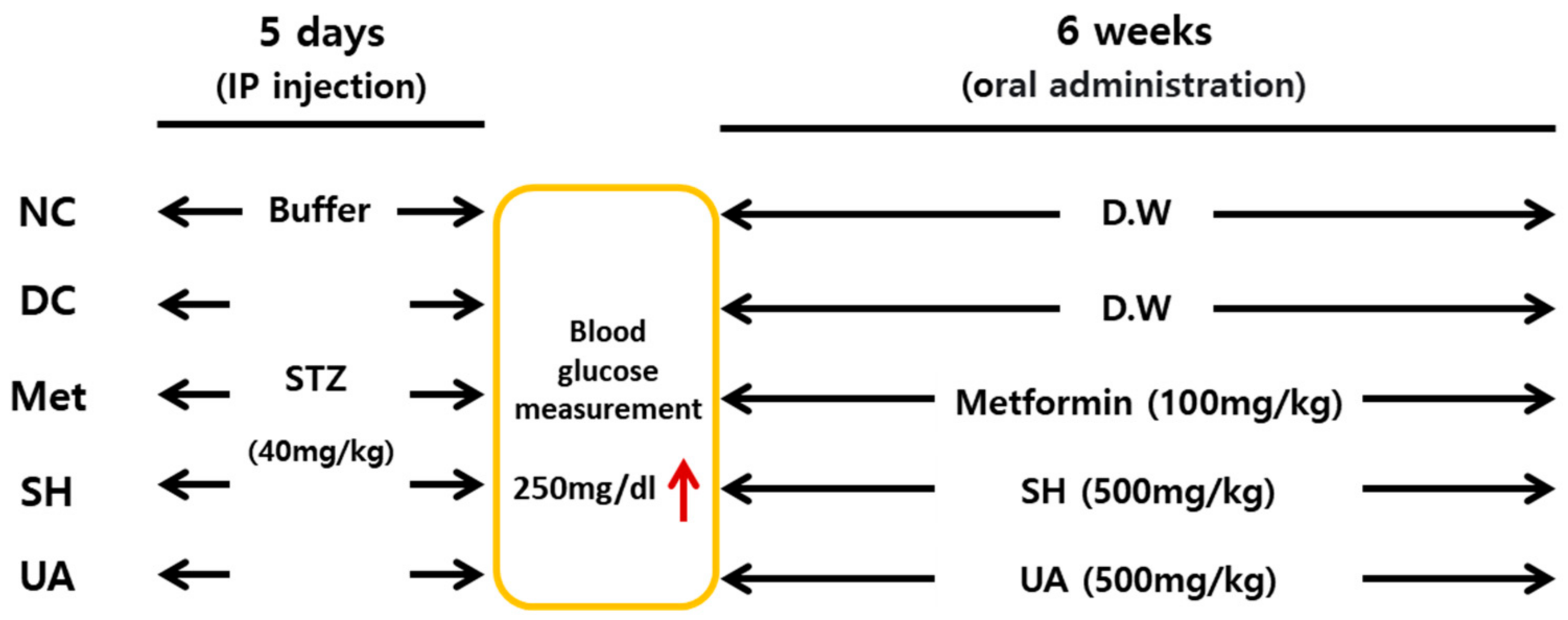
2.9. Animal Experiments
2.10. Weekly Body Weight and Blood Glucose Analysis
2.11. Insulin Tolerance Test and Oral Glucose Tolerance Test
2.12. Serum Biochemical Analysis
2.13. Organ Weight and Histopathological Examination
2.14. Statistical Analysis
3. Results
3.1. α-Glucosidase Inhibitory Activity of SH and UA
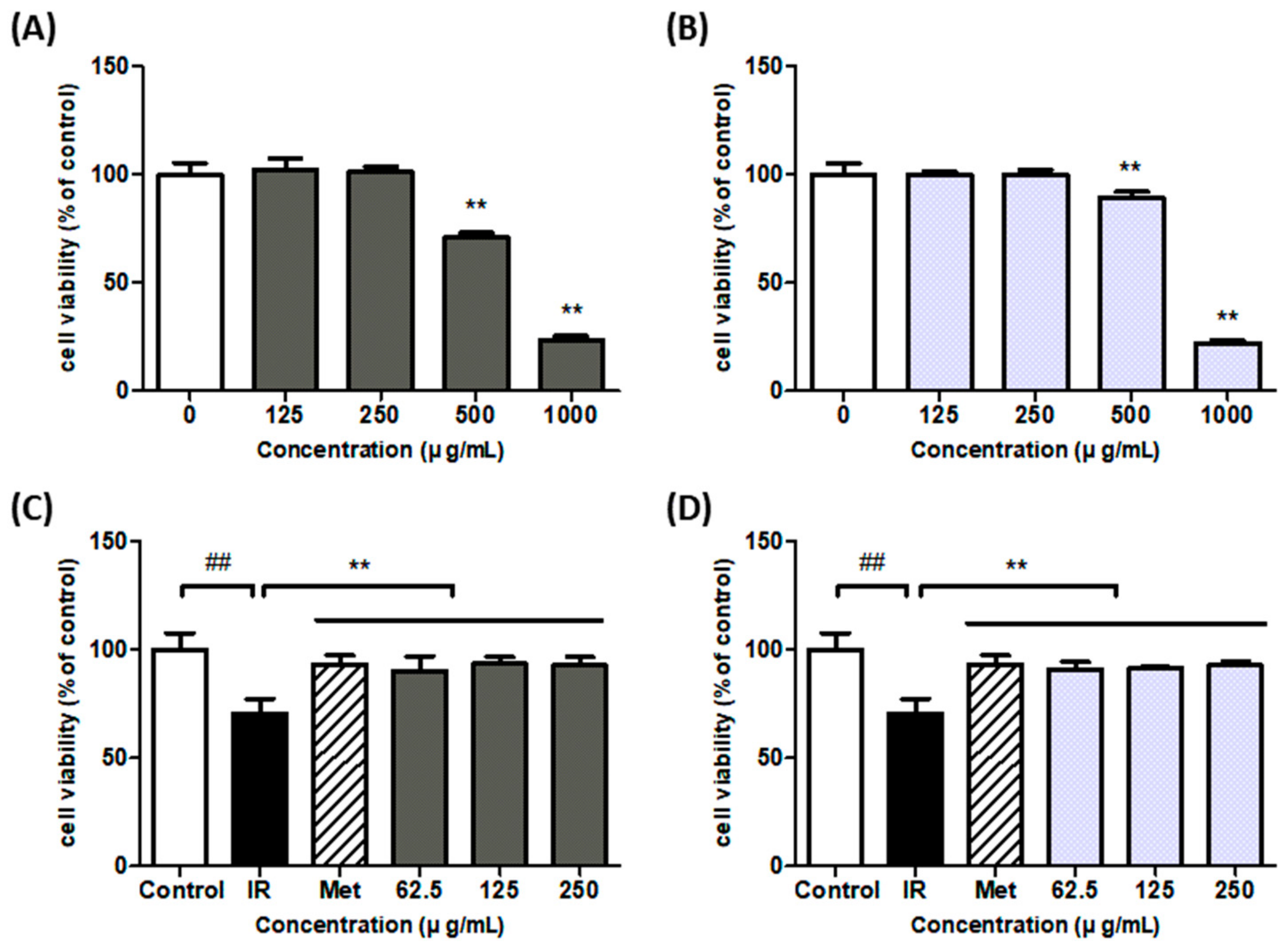
3.2. Cell Viability of HepG2 and IR-HepG2
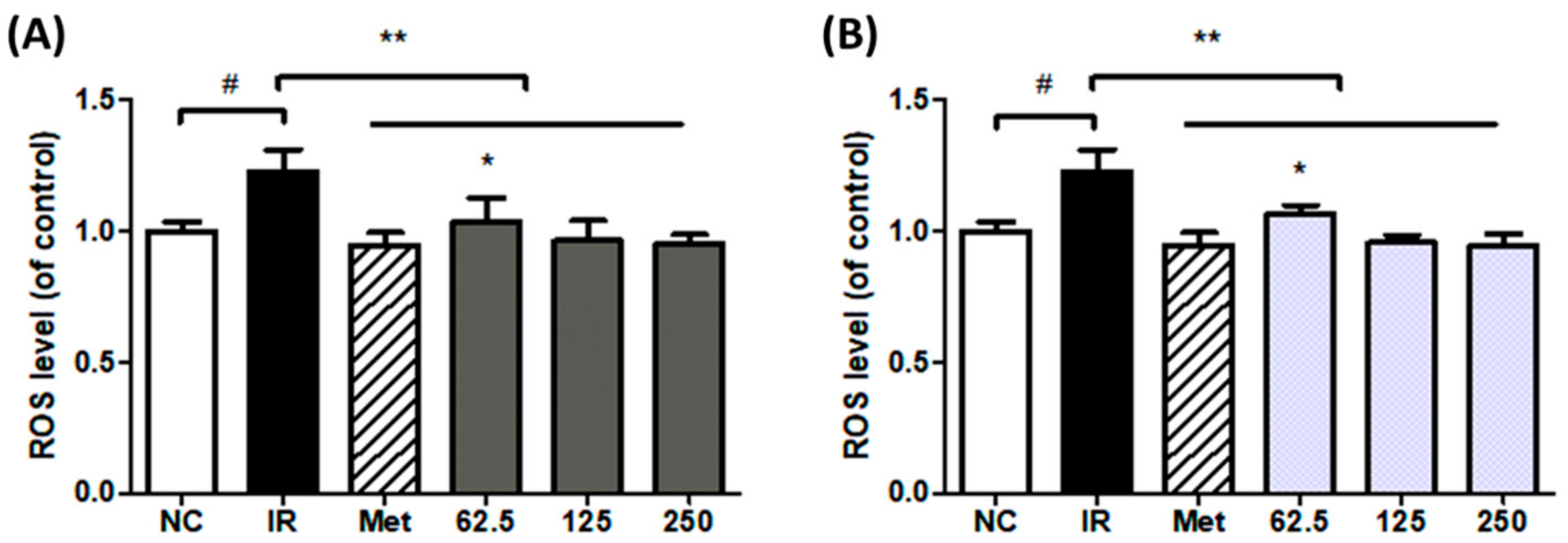
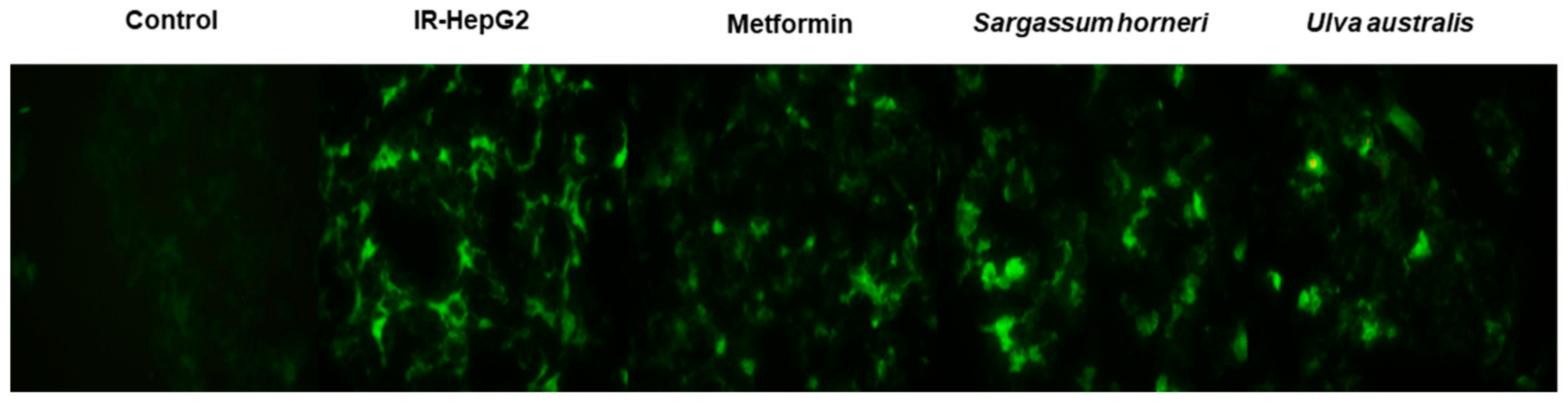
3.3. Effect of Extract Treatment on ROS Content
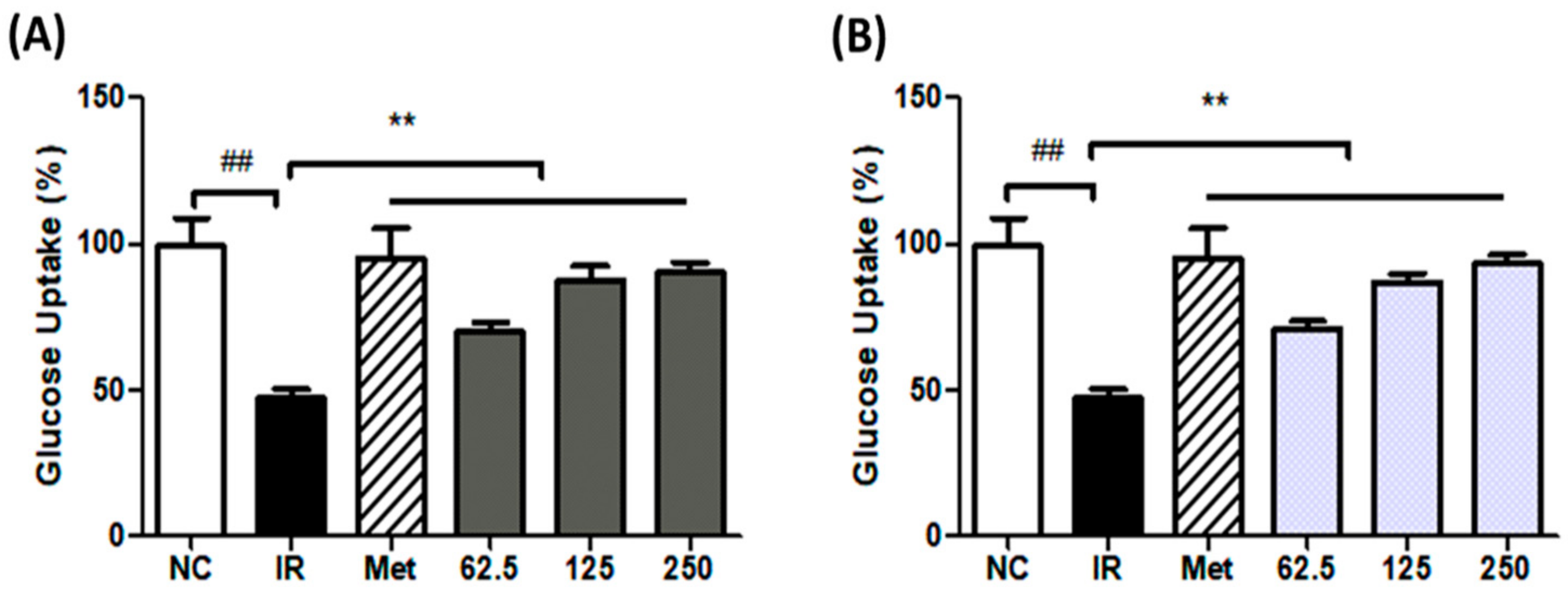
3.4. Effect of Seaweed Extract on Glucose Uptake in IR-HepG2 Cells
3.5. Glycogen Content of IR-HepG2 Cells According to Extract Treatment
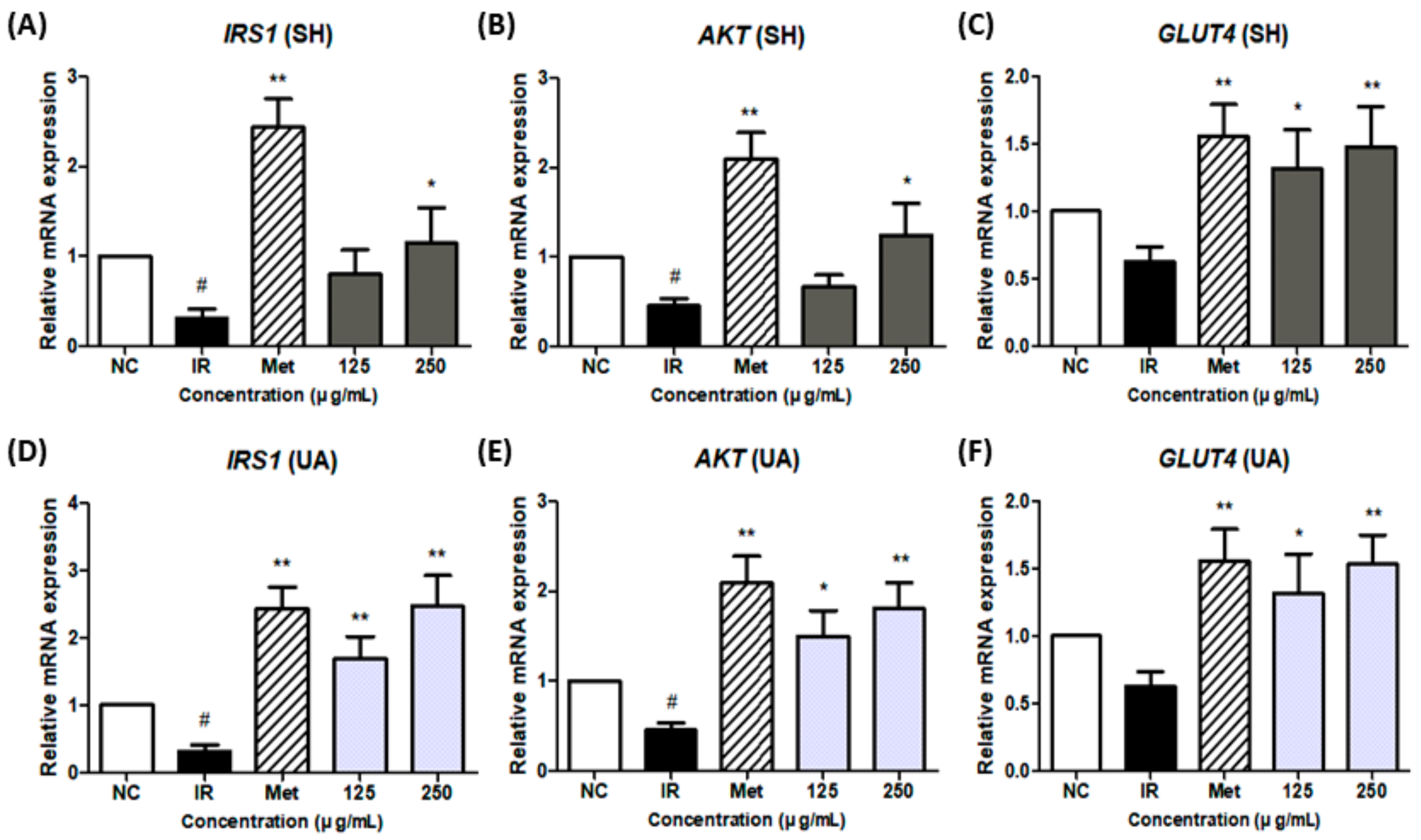
3.6. Effect of Extract Treatment on IRS-1/AKT/GLUT4 Gene Expression
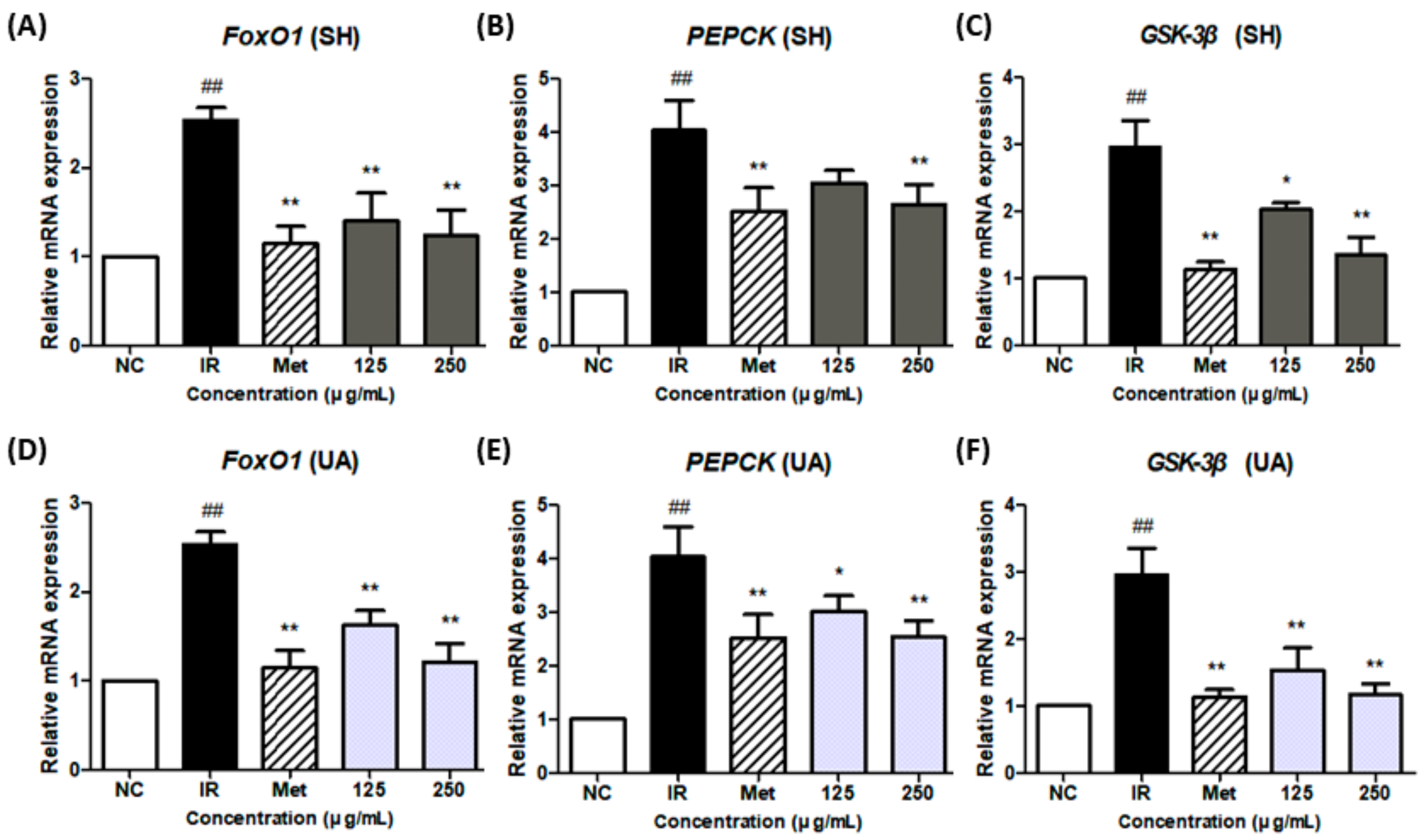
3.7. Effect of Glycogen-Metabolism-Related Gene Expression by Extract Treatment
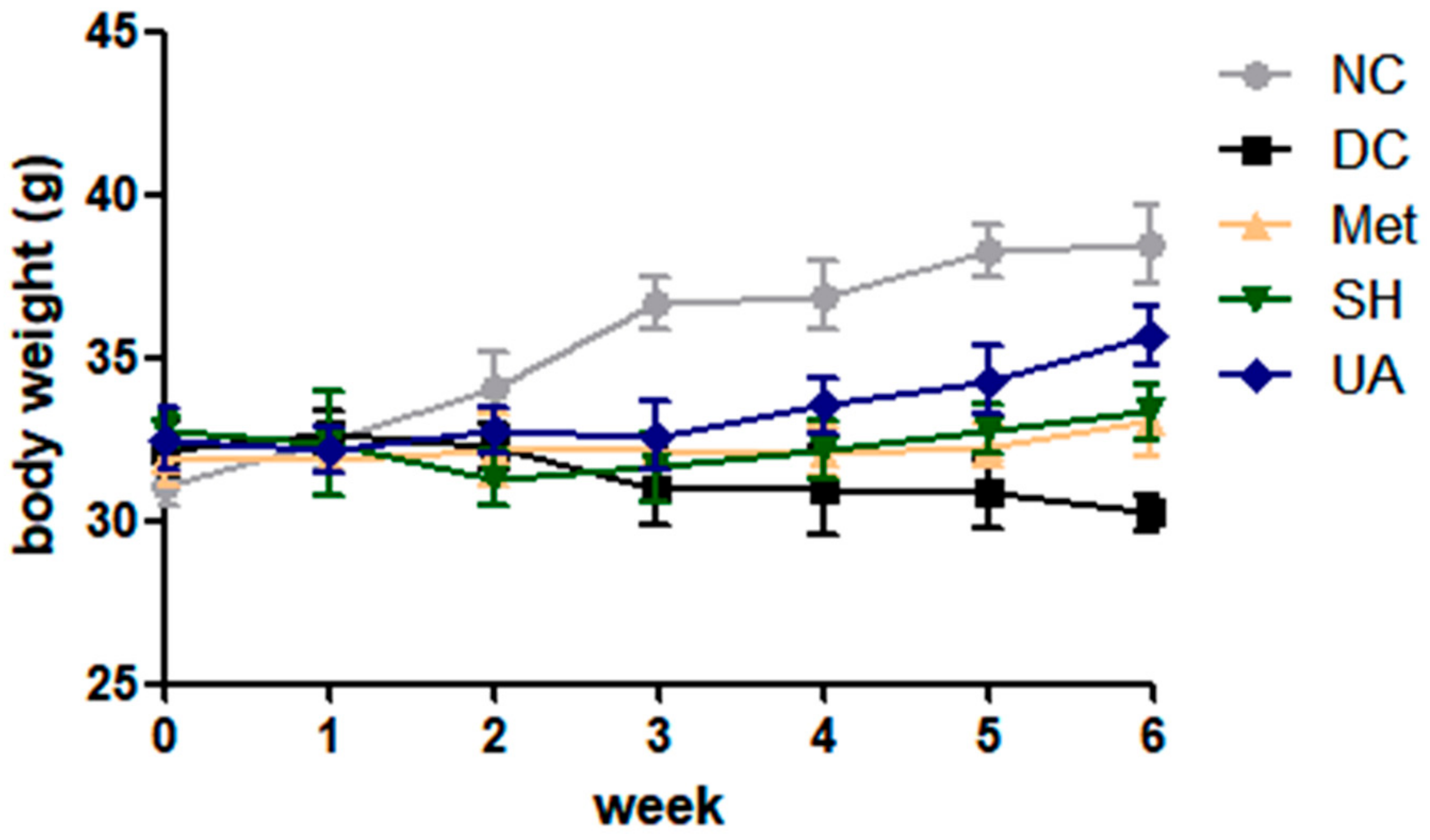
3.8. Changes in Body Weights
3.9. Changes in Blood Glucose at 10-Day Intervals According to the Administration of the Extract
3.10. Analysis of Insulin Tolerance Test and Oral Glucose Tolerance Test
3.11. Serum Biochemical Analysis
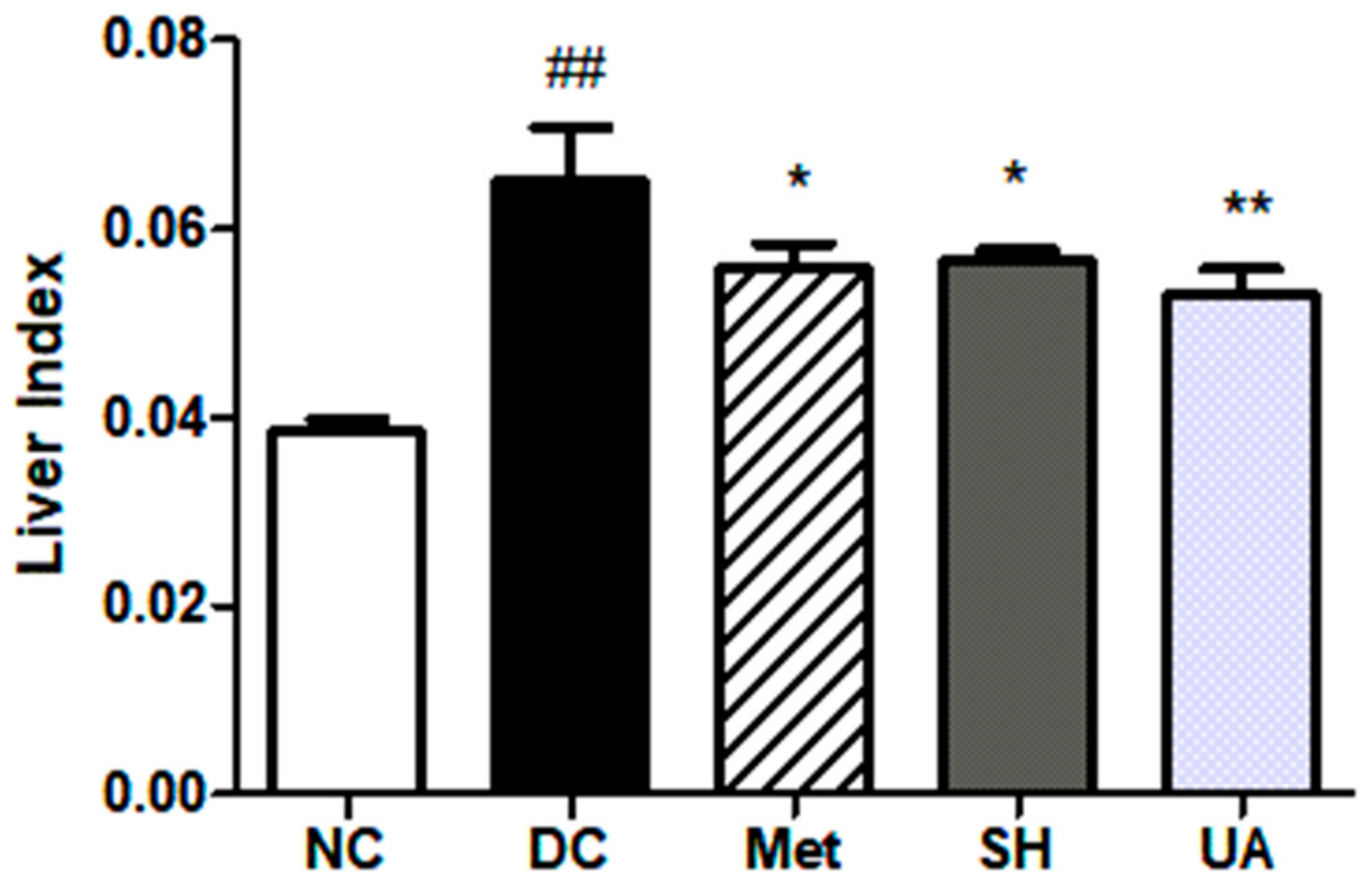
3.12. Tissue Weight and Liver Index Analysis
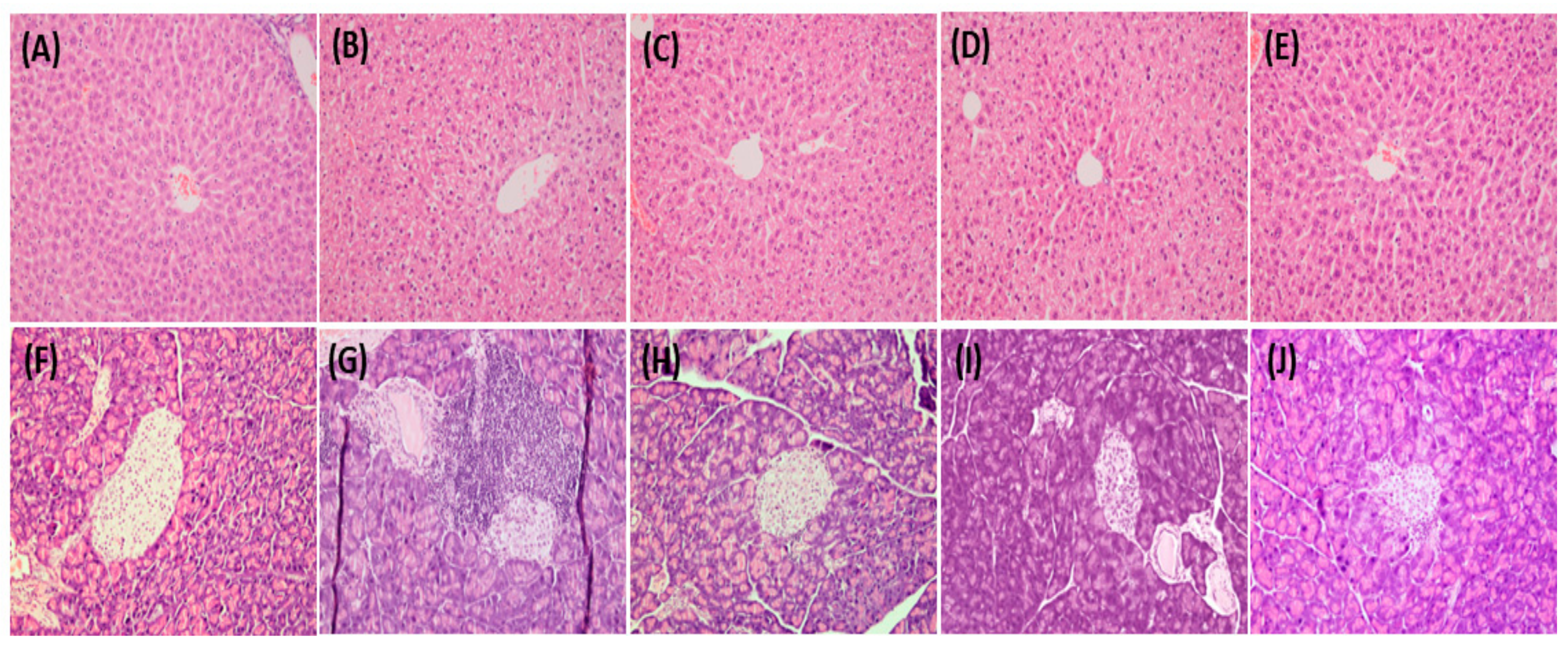
3.13. Histopathological Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DM: | Diabetes mellitus |
| IR: | insulin resistance |
| SH: | Sargassum horneri |
| UA: | Ulva australis |
| ROS: | Reactive oxygen species |
| STZ: | streptozotocin |
| NC: | Normal control |
| DC: | Diabetic control |
| Met: | Metformin |
| AUC: | area under the curve |
| AST: | aspartate aminotransferase |
| ALT: | alanine aminotransferase |
| ALP: | alkaline phosphatase |
| T-P: | total protein |
| TG: | triglycerides |
| CHO: | total cholesterol |
| HDL-C: | high-density lipoprotein cholesterol |
| GLU: | glucose |
| BUN: | blood urea nitrogen |
| CRE: | creatinine |
References
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2011, 34 (Suppl. S1), S62–S69. [Google Scholar] [CrossRef]
- Odeyemi, S.; Dewar, J. In vitro antidiabetic activity affecting glucose uptake in HepG2 cells following their exposure to extracts of Lauridia tetragona (L.f.) R.H. Archer. Processes 2020, 8, 33. [Google Scholar] [CrossRef]
- Liu, Y.; Qiu, Y.; Chen, Q.; Han, X.; Cai, M.; Hao, L. Puerarin suppresses the hepatic gluconeogenesis via activation of PI3K/Akt signaling pathway in diabetic rats and HepG2 cells. Biomed. Pharmacother. 2021, 137, 111325. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.M.; Newton, R.W.; Ruta, D.A.; MacDonald, T.M.; Morris, A.D. Socio-economic status, obesity and prevalence of Type 1 and Type 2 diabetes mellitus. Diabet. Med. 2000, 17, 478–480. [Google Scholar] [CrossRef]
- Lee, J.S.; Kang, Y.H.; Kim, K.K.; Yun, Y.K.; Lim, J.G.; Kim, T.W.; Kim, D.J.; Won, S.Y.; Bae, M.H.; Choi, H.S.; et al. Exploration of optimum conditions for production of saccharogenic mixed grain beverages and assessment of anti-diabetic activity. J. Nutr. Health 2014, 47, 12–22. [Google Scholar] [CrossRef]
- Lei, Y.; Gong, L.; Tan, F.; Liu, Y.; Li, S.; Shen, H.; Zhu, M.; Cai, W.; Xu, F.; Hou, B.; et al. Vaccarin ameliorates insulin resistance and steatosis by activating the AMPK signaling pathway. Eur. J. Pharmacol. 2019, 851, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.Y.; Kim, S.J.; Imm, J.Y. Improvement of blood glucose control in type 2 diabetic db/db mice using Platycodon grandiflorum seed extract. Korean J. Food Sci. Technol. 2020, 52, 81–88. [Google Scholar]
- Zhang, Y.L.; Tan, X.H.; Xiao, M.F.; Li, H.; Mao, Y.Q.; Yang, X.; Tan, H.R. Establishment of liver specific glucokinase gene knockout mice: A new animal model for screening anti-diabetic drugs. Acta Pharmacol. Sin. 2004, 25, 1659–1665. [Google Scholar]
- Moore, M.C.; Coate, K.C.; Winnick, J.J.; An, Z.; Cherrington, A.D. Regulation of hepatic glucose uptake and storage in vivo. Adv. Nutr. 2012, 3, 286–294. [Google Scholar] [CrossRef]
- Kim, D.J.; Kim, J.M.; Kim, T.H.; Baek, J.M.; Kim, H.S.; Choe, M. Anti-diabetic effects of mixed extracts from Lycium chinense, Cordyceps militaris, and Acanthopanax senticosus. Korean J. Plant Res. 2010, 23, 423–429. [Google Scholar]
- Wang, H.; Wang, J.; Zhu, Y.; Yan, H.; Lu, Y. Effects of different intensity exercise on glucose metabolism and hepatic IRS/PI3K/AKT pathway in SD rats exposed with TCDD. Int. J. Environ. Res. Public Health 2021, 18, 13141. [Google Scholar] [CrossRef] [PubMed]
- Raptis, S.A.; Dimitriadis, G.D. Oral hypoglycemic agents: Insulin secretagogues, α-glucosidase inhibitors and insulin sensitizers. Exp. Clin. Endocrinol. Diabetes 2001, 109 (Suppl. S2), S265–S287. [Google Scholar] [CrossRef]
- Sarnobat, D.; Moffett, R.C.; Flatt, P.R.; Tarasov, A.I. Effects of first-line diabetes therapy with biguanides, sulphonylurea and thiazolidinediones on the differentiation, proliferation and apoptosis of islet cell populations. J. Endocrinol. Investig. 2022, 45, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Shannon, E.; Abu-Ghannam, N. Seaweeds as nutraceuticals for health and nutrition. Phycologia 2019, 58, 563–577. [Google Scholar] [CrossRef]
- Labbaci, F.Z.; Boukortt, F.O. Beneficial effects of Algerian green alga Ulva lactuca and its hydroethanolic extract on insulin resistance and cholesterol reverse transport in high-fat/streptozotocin diabetic rats. Prev. Nutr. Food Sci. 2020, 25, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Shikh, E.V.; Makhova, A.A.; Pogozheva, A.V.; Elizarova, E.V. The importance of nuts in the prevention of various diseases. Vopr. Pitan. 2020, 89, 14–21. [Google Scholar] [PubMed]
- Kim, M.S.; Kim, J.Y.; Choi, W.H.; Lee, S.S. Effects of seaweed supplementation on blood glucose concentration, lipid profile, and antioxidant enzyme activities in patients with type 2 diabetes mellitus. Nutr. Res. Pract. 2008, 2, 62–67. [Google Scholar] [CrossRef]
- Sharifuddin, Y.; Chin, Y.X.; Lim, P.E.; Phang, S.M. Potential bioactive compounds from seaweed for diabetes management. Mar. Drugs 2015, 13, 5447–5491. [Google Scholar] [CrossRef]
- Dias, M.K.H.M.; Madusanka, D.M.D.; Han, E.J.; Kim, M.J.; Jeon, Y.J.; Kim, H.S.; Fernando, I.P.S.; Ahn, G. (-)-Loliolide isolated from Sargassum horneri protects against fine dust-induced oxidative stress in human keratinocytes. Antioxidants 2020, 9, 474. [Google Scholar] [CrossRef]
- Lee, Y.H.; Yeo, M.H.; Yoon, S.A.; Hyun, H.B.; Ham, Y.M.; Jung, Y.H.; Chang, K.S. Effects of Sargassum horneri and Ulva australis extracts on body weight and serum glucose levels of Sprague-Dawley rats. Prev. Nutr. Food Sci. 2021, 26, 307–314. [Google Scholar] [CrossRef]
- Kim, H.M.; Jo, J.; Park, C.; Choi, B.J.; Lee, H.G.; Kim, K.Y. Epibionts associated with floating Sargassum horneri in the Korea Strait. Algae 2019, 34, 303–313. [Google Scholar] [CrossRef]
- Kim, J.S.; Kim, B.G.; Kwak, H.S. Simultaneous application of chemicals and temperature for the effective control of trouble seaweed Ulva australis. Weed Turf. Sci. 2018, 7, 35–45. [Google Scholar]
- Gao, G.; Clare, A.S.; Rose, C.; Caldwell, G.S. Eutrophication and warming-driven green tides (Ulva rigida) are predicted to increase under future climate change scenarios. Mar. Pollut. Bull. 2017, 114, 439–447. [Google Scholar] [CrossRef]
- Rasin, A.B.; Silchenko, A.S.; Kusaykin, M.I.; Malyarenko, O.S.; Zueva, A.O.; Kalinovsky, A.I.; Airong, J.; Surits, V.V.; Ermakova, S.P. Enzymatic transformation and anti-tumor activity of Sargassum horneri fucoidan. Carbohydr. Polym. 2020, 246, 116635. [Google Scholar] [CrossRef] [PubMed]
- Fernando, I.P.S.; Jayawardena, T.U.; Sanjeewa, K.K.A.; Wang, L.; Jeon, Y.J.; Lee, W.W. Anti-inflammatory potential of alginic acid from Sargassum horneri against urban aerosol-induced inflammatory responses in keratinocytes and macrophages. Ecotoxicol. Environ. Saf. 2018, 160, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Han, E.J.; Fernando, I.P.S.; Sanjeewa, K.K.A.; Jayawardena, T.U.; Kim, H.J.; Jee, Y.; Kang, S.H.; Jang, J.H.; Jang, J.P.; et al. Anti-allergy effect of mojabanchromanol isolated from Sargassum horneri in bone marrow-derived cultured mast cells. Algal Res. 2020, 48, 101898. [Google Scholar] [CrossRef]
- Pengzhan, Y.; Quanbin, Z.; Ning, L.; Zuhong, X.; Yanmei, W.; Zhi’en, L. Polysaccharides from Ulva pertusa (Chlorophyta) and preliminary studies on their antihyperlipidemia activity. J. Appl. Phycol. 2003, 15, 21–27. [Google Scholar] [CrossRef]
- Chi, Y.; Zhang, M.; Wang, X.; Fu, X.; Guan, H.; Wang, P. Ulvan lyase assisted structural characterization of ulvan from Ulva pertusa and its antiviral activity against vesicular stomatitis virus. Int. J. Biol. Macromol. 2020, 157, 75–82. [Google Scholar] [CrossRef]
- Gao, X.; Qu, H.; Shan, S.; Song, C.; Baranenko, D.; Li, Y.; Lu, W. A novel polysaccharide isolated from Ulva pertusa: Structure and physicochemical property. Carbohydr. Polym. 2020, 233, 115849. [Google Scholar] [CrossRef]
- Lee, Y.H.; Kim, H.R.; Yeo, M.H.; Kim, S.C.; Hyun, H.B.; Ham, Y.M.; Jung, Y.H.; Kim, H.S.; Chang, K.S. Anti-Obesity Potential of Sargassum horneri and Ulva australis Extracts: Study In Vitro and In Vivo. Appl. Sci. 2023, 13, 8951. [Google Scholar] [CrossRef]
- Ding, Y.; Xia, S.; Fang, H.; Niu, B.; Chen, Q. Loureirin B attenuates insulin resistance in HepG2 cells by regulating gluconeogenesis signaling pathway. Eur. J. Pharmacol. 2021, 910, 174481. [Google Scholar] [CrossRef]
- Nie, J.; Chang, Y.; Li, Y.; Zhou, Y.; Qin, J.; Sun, Z.; Li, H. Caffeic acid phenethyl ester (propolis extract) ameliorates insulin resistance by inhibiting JNK and NF-κB inflammatory pathways in diabetic mice and HepG2 cell models. J. Agric. Food Chem. 2017, 65, 9041–9053. [Google Scholar] [CrossRef]
- Shehata, A.M.; Quintanilla-Fend, L.; Bettio, S.; Singh, C.B.; Ammon, H.P.T. Prevention of multiple low-dose streptozotocin (MLD-STZ) diabetes in mice by an extract from gum resin of Boswellia serrata (BE). Phytomedicine 2011, 18, 1037–1044. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Park, S.; Mariadoss, A.V.A.; Sathiyaseelan, A.; Veeraraghavan, V.P.; Kim, S.; Wang, M.H. Chemical composition, antioxidant, and anti-diabetic activities of ethyl acetate fraction of Stachys riederi var. japonica (Miq.) in streptozotocin-induced type 2 diabetic mice. Food Chem. Toxicol. 2021, 155, 112374. [Google Scholar] [CrossRef] [PubMed]
- Safhi, M.M.; Alam, M.F.; Sivakumar, S.M.; Anwer, T. Hepatoprotective potential of Sargassum muticum against STZ-induced diabetic liver damage in wistar rats by inhibiting cytokines and the apoptosis pathway. Anal. Cell. Pathol. Amst. 2019, 2019, 7958701. [Google Scholar] [PubMed]
- Beckman, J.A.; Creager, M.A.; Libby, P. Diabetes and atherosclerosis: Epidemiology, pathophysiology, and management. JAMA 2002, 287, 2570–2581. [Google Scholar] [CrossRef] [PubMed]
- Okayasu, S.; Kitaichi, K.; Hori, A.; Suwa, T.; Horikawa, Y.; Yamamoto, M.; Takeda, J.; Itoh, Y. The evaluation of risk factors associated with adverse drug reactions by metformin in type 2 diabetes mellitus. Biol. Pharm. Bull. 2012, 35, 933–937. [Google Scholar] [CrossRef] [PubMed]
- Bundhun, P.K.; Janoo, G.; Teeluck, A.R.; Huang, F. Adverse drug effects observed with vildagliptin versus pioglitazone or rosiglitazone in the treatment of patients with type 2 diabetes mellitus: A systematic review and meta-analysis of randomized controlled trials. BMC Pharmacol. Toxicol. 2017, 18, 66. [Google Scholar] [CrossRef] [PubMed]
- Van Dam, R.M.; Rimm, E.B.; Willett, W.C.; Stampfer, M.J.; Hu, F.B. Dietary patterns and risk for type 2 diabetes mellitus in US men. Ann. Intern. Med. 2002, 136, 201–209. [Google Scholar] [CrossRef]
- Hung, H.Y.; Qian, K.; Morris-Natschke, S.L.; Hsu, C.S.; Lee, K.H. Recent discovery of plant-derived anti-diabetic natural products. Nat. Prod. Rep. 2012, 29, 580–606. [Google Scholar] [CrossRef]
- Burtin, P. Nutritional value of seaweeds. Electron. J. Environ. Agric. Food Chem. 2003, 2, 498–503. [Google Scholar]
- Kadam, S.U.; Prabhasankar, P. Marine foods as functional ingredients in bakery and pasta products. Food Res. Int. 2010, 43, 1975–1980. [Google Scholar] [CrossRef]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Tadera, K.; Minami, Y.; Takamatsu, K.; Matsuoka, T. Inhibition of α-glucosidase and α-amylase by flavonoids. J. Nutr. Sci. Vitaminol. 2006, 52, 149–153. [Google Scholar] [CrossRef]
- Kang, S.H.; Cho, E.K.; Choi, Y.J. α-Glucosidase inhibitory effects for solvent fractions from methanol extracts of Sargassum fulvellum and its antioxidant and alcohol-metabolizing activities. J. Life Sci. 2012, 22, 1420–1427. [Google Scholar] [CrossRef]
- Kellogg, J.; Grace, M.H.; Lila, M.A. Phlorotannins from Alaskan seaweed inhibit carbolytic enzyme activity. Mar. Drugs 2014, 12, 5277–5294. [Google Scholar] [CrossRef]
- Dong, Y.; Sui, L.; Yang, F.; Ren, X.; Xing, Y.; Xiu, Z. Reducing the intestinal side effects of acarbose by baicalein through the regulation of gut microbiota: An in vitro study. Food Chem. 2022, 394, 133561. [Google Scholar] [CrossRef]
- Kim, S.; Kim, S.J.; Oh, J.; Hong, J.H.; Kim, S.Y. Antidiabetic effects of mixed extract from Dendropanax morbiferus, Broussonetia kazinoki, and Cudrania tricuspidata. Herbal Formula Sci. 2019, 27, 223–236. [Google Scholar]
- Aravinthan, A.; Challis, B.; Shannon, N.; Hoare, M.; Heaney, J.; Alexander, G.J.M. Selective insulin resistance in hepatocyte senescence. Exp. Cell Res. 2015, 331, 38–45. [Google Scholar] [CrossRef]
- Rains, J.L.; Jain, S.K. Oxidative stress, insulin signaling, and diabetes. Free Radic. Biol. Med. 2011, 50, 567–575. [Google Scholar] [CrossRef]
- Xu, J.; Li, T.; Xia, X.; Fu, C.; Wang, X.; Zhao, Y. Dietary ginsenoside T19 supplementation regulates glucose and lipid metabolism via AMPK and PI3K pathways and its effect on intestinal microbiota. J. Agric. Food Chem. 2020, 68, 14452–14462. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, T.; Fang, L.; Liu, C.; Liu, X.; Li, H.; Shi, J.; Li, M.; Min, W. Anti-diabetic effect by walnut (Juglans mandshurica Maxim.)-derived peptide LPLLR through inhibiting α-glucosidase and α-amylase, and alleviating insulin resistance of hepatic HepG2 cells. J. Funct. Foods 2020, 69, 103944. [Google Scholar] [CrossRef]
- Guo, Q.; Chen, Z.; Santhanam, R.K.; Xu, L.; Gao, X.; Ma, Q.; Xue, Z.; Chen, H. Hypoglycemic effects of polysaccharides from corn silk (Maydis stigma) and their beneficial roles via regulating the PI3K/Akt signaling pathway in L6 skeletal muscle myotubes. Int. J. Biol. Macromol. 2019, 121, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.H.; Lee, J.Y.; Kang, S.B.; Park, J.S.; Lee, B.W.; Kang, E.S.; Ahn, C.W.; Lee, H.C.; Cha, B.S. Dietary monounsaturated fatty acids but not saturated fatty acids preserve the insulin signaling pathway via IRS-1/PI3K in rat skeletal muscle. Lipids 2010, 45, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Kousteni, S. FoxO1, the transcriptional chief of staff of energy metabolism. Bone 2012, 50, 437–443. [Google Scholar] [CrossRef]
- Webb, A.E.; Brunet, A. FOXO transcription factors: Key regulators of cellular quality control. Trends Biochem. Sci. 2014, 39, 159–169. [Google Scholar] [CrossRef]
- Huang, X.; Liu, G.; Guo, J.; Su, Z. The PI3K/AKT pathway in obesity and type 2 diabetes. Int. J. Biol. Sci. 2018, 14, 1483–1496. [Google Scholar] [CrossRef]
- Adisa, R.A.; Choudhary, M.I.; Olorunsogo, O.O. Hypoglycemic activity of Buchholzia coriacea (Capparaceae) seeds in streptozotocin-induced diabetic rats and mice. Exp. Toxicol. Pathol. 2011, 63, 619–625. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, X.T.; Zhang, H.Y.; Yao, H.Y.; Zhang, H. Effect of Vaccinium bracteatum Thunb. leaves extract on blood glucose and plasma lipid levels in streptozotocin-induced diabetic mice. J. Ethnopharmacol. 2010, 130, 465–469. [Google Scholar] [CrossRef]
- Qudus, B.; Aroyehun, A.; Abdul Razak, S.; Palaniveloo, K.; Nagappan, T.; Suraiza Nabila Rahmah, N.; Wee Jin, G.; Chellappan, D.K.; Chellian, J.; Kunnath, A.P. Bioprospecting cultivated tropical green algae, Caulerpa racemosa (Forsskal) J. Agardh: A perspective on nutritional properties, antioxidative capacity and anti-diabetic potential. Foods 2020, 9, 1313. [Google Scholar] [CrossRef]
- Renitta, R.E.; Narayanan, R.; Samrot, A.V. Antidiabetic potential of methanolic extracts of Sargassum wightii in streptozotocin induced diabetic mice. Biocatal. Agric. Biotechnol. 2020, 28, 101763. [Google Scholar] [CrossRef]
- Boye, A.; Acheampong, D.O.; Gyamerah, E.O.; Asiamah, E.A.; Addo, J.K.; Mensah, D.A.; Brah, A.S.; Ayiku, P.J. Glucose lowering and pancreato-protective effects of Abrus precatorius (L.) leaf extract in normoglycemic and STZ/Nicotinamide-Induced diabetic rats. J. Ethnopharmacol. 2020, 258, 112918. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.W.; Han, J.S. Hypoglycemic effect of Sargassum ringgoldianum extract in STZ-induced diabetic mice. Prev. Nutr. Food Sci. 2012, 17, 8–13. [Google Scholar] [CrossRef] [PubMed]
- BelHadj, S.; Hentati, O.; Elfeki, A.; Hamden, K. Inhibitory activities of Ulva lactuca polysaccharides on digestive enzymes related to diabetes and obesity. Arch. Physiol. Biochem. 2013, 119, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, H.; Hu, X.; Shi, J.; Shao, D.; Jin, M. Antidiabetic effects of different polysaccharide fractions from Artemisia sphaerocephala Krasch seeds in db/db mice. Food Hydrocoll. 2019, 91, 1–9. [Google Scholar] [CrossRef]
- Silawat, N.; Gupta, V.B. Chebulic acid attenuates ischemia reperfusion induced biochemical alteration in diabetic rats. Pharm. Biol. 2013, 51, 23–29. [Google Scholar] [CrossRef]
- Elangovan, A.; Subramanian, A.; Durairaj, S.; Ramachandran, J.; Lakshmanan, D.K.; Ravichandran, G.; Nambirajan, G.; Thilagar, S. Antidiabetic and hypolipidemic efficacy of skin and seed extracts of Momordica cymbalaria on alloxan induced diabetic model in rats. J. Ethnopharmacol. 2019, 241, 111989. [Google Scholar] [CrossRef]
- Fedor, D.; Kelley, D.S. Prevention of insulin resistance by n-3 polyunsaturated fatty acids. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 138–146. [Google Scholar] [CrossRef]
- Park, J.; Song, D.H.; Park, J.S.; Nam, J.Y.; Kim, C.S.; Kim, D.M.; Ahn, C.W.; Cha, B.S.; Lim, S.K.; Kim, K.R.; et al. A case of hepatomegaly due to diabetic glycogenosis reversed by glycemic control. J. Korean Endocr. Soc. 2004, 19, 223–228. [Google Scholar]




| Gene | GenBank Accession | Forward Primer (5′ to 3′) | Reverse Primer (5′ to 3′) | Amplicon (bp) |
|---|---|---|---|---|
| IRS-1 1 | NM_005544.3 | GAG TCC CAG CAC CAA CAG AA | CCT TGC CAC CCA TGC AGA TA | 374 |
| AKT 2 | NM_005163.2 | GGA CAA GGA CGG GCA CAT TA | CGA CCG CAC ATC ATC TCG TA | 192 |
| GLUT4 3 | NM_001042.3 | GCT GAA GGA TGA GAA GCG GA | TGT CTC GAA GAT GCT GGT CG | 172 |
| GSK-3β 4 | NM_002093 | CGA GAC ACA CCT GCA CTC TT | TCT GTC CAC GGT CTC CAG TA | 163 |
| FoxO1 5 | NM_002015 | GTG GAT GGT CAA GAG CGT GC | TGC CAC CCT CTG GAT TGA GC | 170 |
| PEPCK 6 | NM-002591.3 | AAG AGA CAC AGT GCC CAT CC | ACG TAG GGT GAA TCC GTC AG | 201 |
| β-actin 7 | NM_001101.5 | ATG GAT GAT GAT ATC GCC GCG | TCT CCA TGT CGT CCC AGT TG | 250 |
| Groups | Initial Weight (g) | Final Weight (g) | Body Weight Gain (g) |
|---|---|---|---|
| NC | 31.03 ± 0.61 | 38.44 ± 1.22 | 6.93 ± 1.22 |
| DC | 31.96 ± 0.78 | 30.24 ± 1.06 | −0.86 ± 0.57 ## |
| Met | 31.88 ± 0.79 | 33.07 ± 1.08 | 1.28 ± 0.45 * |
| SH | 32.54 ± 0.47 | 33.33 ± 0.88 | 1.12 ± 0.36 * |
| UA | 32.49 ± 0.95 | 35.65 ± 0.93 | 2.54 ± 0.43 ** |
| Groups | Date of the Blood Glucose Measurement | ||||
|---|---|---|---|---|---|
| 0 | 10 | 20 | 30 | 40 | |
| NC | 160.00 ± 12.01 | 157.50 ± 13.83 | 146.25 ± 9.86 | 128.50 ± 11.61 | 124.00 ± 12.71 |
| DC | 440.50 ± 48.74 ## | 516.75 ± 43.27 ## | 521.75 ± 33.93 ## | 531.25 ± 55.43 ## | 547.75 ± 36.62 ## |
| Met | 437.00 ± 36.40 | 422.40 ± 43.63 * | 414.25 ± 23.16 ** | 418.00 ± 43.47 * | 452.25 ± 47.27 * |
| SH | 435.50 ± 51.50 | 424.00 ± 37.30 * | 391.50 ± 42.80 ** | 392.30 ± 46.80 ** | 446.30 ± 33.00 * |
| UA | 434.17 ± 57.22 | 423.00 ± 41.32 * | 359.75 ± 32.96 ** | 336.25 ± 29.79 ** | 387.00 ± 42.07 ** |
| NC | DC | Met | SH | UA | |
|---|---|---|---|---|---|
| AST (U/L) | 131.3 ± 21.6 | 249.6 ± 27.0 ## | 247.8 ± 19.8 | 236.5 ± 25.5 | 238.7 ± 22.1 |
| ALT (U/L) | 23.5 ± 3.3 | 64.5 ± 8.7 ## | 62.5 ± 9.4 | 54.5 ± 7.8 | 36.8 ± 7.5 ** |
| ALP (U/L) | 93.1 ± 9.9 | 130.5 ± 28.0 | 128.3 ± 15.0 | 110.2 ± 16.3 | 111.5 ± 16.0 |
| T-P (g/dL) | 5.0 ± 0.3 | 4.9 ± 0.5 | 4.6 ± 0.5 | 5.0 ± 0.6 | 5.0 ± 0.4 |
| TG (mg/dL) | 16.1 ± 3.0 | 258.4 ± 26.0 ## | 78.4 ± 17.3 ** | 78.9 ± 16.5 ** | 79.1 ± 12.3 ** |
| CHO (mg/dL) | 90.0 ± 8.6 | 125.5 ± 12.7 ## | 93.5 ± 9.3 * | 96.3 ± 8.7 * | 97.0 ± 8.4 * |
| HDL-C (mg/dL) | 112.5 ± 6.9 | 83.7 ± 7.0 # | 117.4 ± 14.5 * | 92.6 ± 7.3 | 116.0 ± 12.9 * |
| GLU (mg/dL) | 98.0 ± 11.2 | 748.0 ± 38.8 ## | 624.0 ± 38.5 ** | 658.0 ± 38.1 ** | 602.0 ± 34.8 ** |
| BUN (mg/dL) | 30.5 ± 2.6 | 39.4 ± 8.5 | 28.7 ± 3.0 | 28.4 ± 3.4 | 28.2 ± 3.7 |
| CRE (mg/dL) | 0.4 ± 0.1 | 0.7 ± 0.2 | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.5 ± 0.1 |
| NC | DC | Met | SH | UA | |
|---|---|---|---|---|---|
| Liver | 1.34 ± 0.08 | 2.04 ± 0.24 ## | 1.69 ± 0.09 * | 1.72 ± 0.08 | 1.68 ± 0.09 * |
| Kidney | 0.59 ± 0.05 | 0.58 ± 0.09 | 0.58 ± 0.06 | 0.55 ± 0.03 | 0.56 ± 0.03 |
| Pancreas | 0.18 ± 0.02 | 0.21 ± 0.05 | 0.20 ± 0.02 | 0.18 ± 0.01 | 0.17 ± 0.02 |
| Spleen | 0.09 ± 0.01 | 0.11 ± 0.02 | 0.08 ± 0.02 | 0.11 ± 0.04 | 0.09 ± 0.02 |
| Heart | 0.18 ± 0.02 | 0.16 ± 0.01 | 0.15 ± 0.01 | 0.16 ± 0.01 | 0.17 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-H.; Kim, H.-R.; Yeo, M.-H.; Kim, S.-C.; Hyun, H.-B.; Ham, Y.-M.; Jung, Y.-H.; Kim, H.-S.; Chang, K.-S. Anti-Diabetic Potential of Sargassum horneri and Ulva australis Extracts In Vitro and In Vivo. Curr. Issues Mol. Biol. 2023, 45, 7492-7512. https://doi.org/10.3390/cimb45090473
Lee Y-H, Kim H-R, Yeo M-H, Kim S-C, Hyun H-B, Ham Y-M, Jung Y-H, Kim H-S, Chang K-S. Anti-Diabetic Potential of Sargassum horneri and Ulva australis Extracts In Vitro and In Vivo. Current Issues in Molecular Biology. 2023; 45(9):7492-7512. https://doi.org/10.3390/cimb45090473
Chicago/Turabian StyleLee, Young-Hyeon, Hye-Ran Kim, Min-Ho Yeo, Sung-Chun Kim, Ho-Bong Hyun, Young-Min Ham, Yong-Hwan Jung, Hye-Sook Kim, and Kyung-Soo Chang. 2023. "Anti-Diabetic Potential of Sargassum horneri and Ulva australis Extracts In Vitro and In Vivo" Current Issues in Molecular Biology 45, no. 9: 7492-7512. https://doi.org/10.3390/cimb45090473
APA StyleLee, Y. -H., Kim, H. -R., Yeo, M. -H., Kim, S. -C., Hyun, H. -B., Ham, Y. -M., Jung, Y. -H., Kim, H. -S., & Chang, K. -S. (2023). Anti-Diabetic Potential of Sargassum horneri and Ulva australis Extracts In Vitro and In Vivo. Current Issues in Molecular Biology, 45(9), 7492-7512. https://doi.org/10.3390/cimb45090473





