Interactions between the DNA Damage Response and the Telomere Complex in Carcinogenesis: A Hypothesis
Abstract
1. Introduction
2. Review and Hypothesis
2.1. The DNA Damage Response—Telomeres and Cancer
2.2. False Exceptions to the Correlation between Cancer–Telomere Dysfunction: Seckel Syndrome, Cockayne Syndrome, Trichothiodystrophy
2.3. Mutation of the Mre11 Component of the MRN Complex Does Not Result in Telomere Erosion nor Cancer Predisposition
2.4. Chromatin Remodeling Factors Are Concurrently Associated with Telomere Dysfunction and Cancer
2.5. Possible Routes of ATM–Telomere Interaction
2.6. Double-Stranded Breaks versus Chromatin Alteration
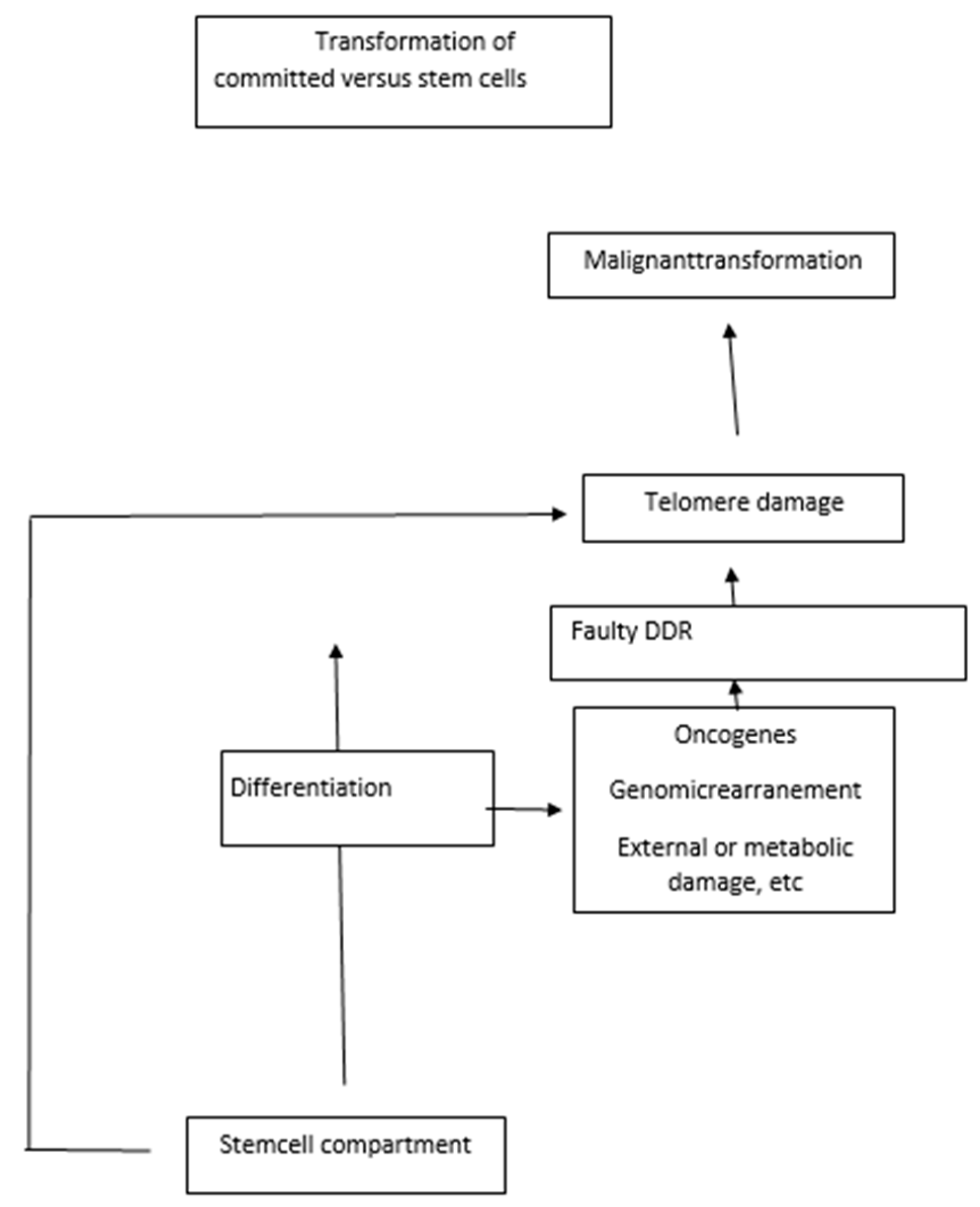
3. Replication Stress Associated with Developmental Blocks Underlies Cancer Transformation
3.1. Replication Stress
3.2. Paradoxical Effects of Chk1
3.3. Other Roles of ATR and the Fanconi Anemia Pathway
4. DNA Damage Associated with Defective Genomic Recombination
4.1. Oncogenesis and Altered Differentiation to Plasma Cells
4.2. Tumorigenesis Associated with Class Switch Recombination and Somatic Hypermutation Induced by Activation-Induced Cytidine Deaminase (AID)
4.3. A DNA Damage Response Is Triggered by Oncogenes
4.4. Disturbed Differentiation and Notch Signaling
5. Telomere Complex Alterations Can Directly Induce Cancer
5.1. Cancer Induced by the Sheltering Protein TRF2
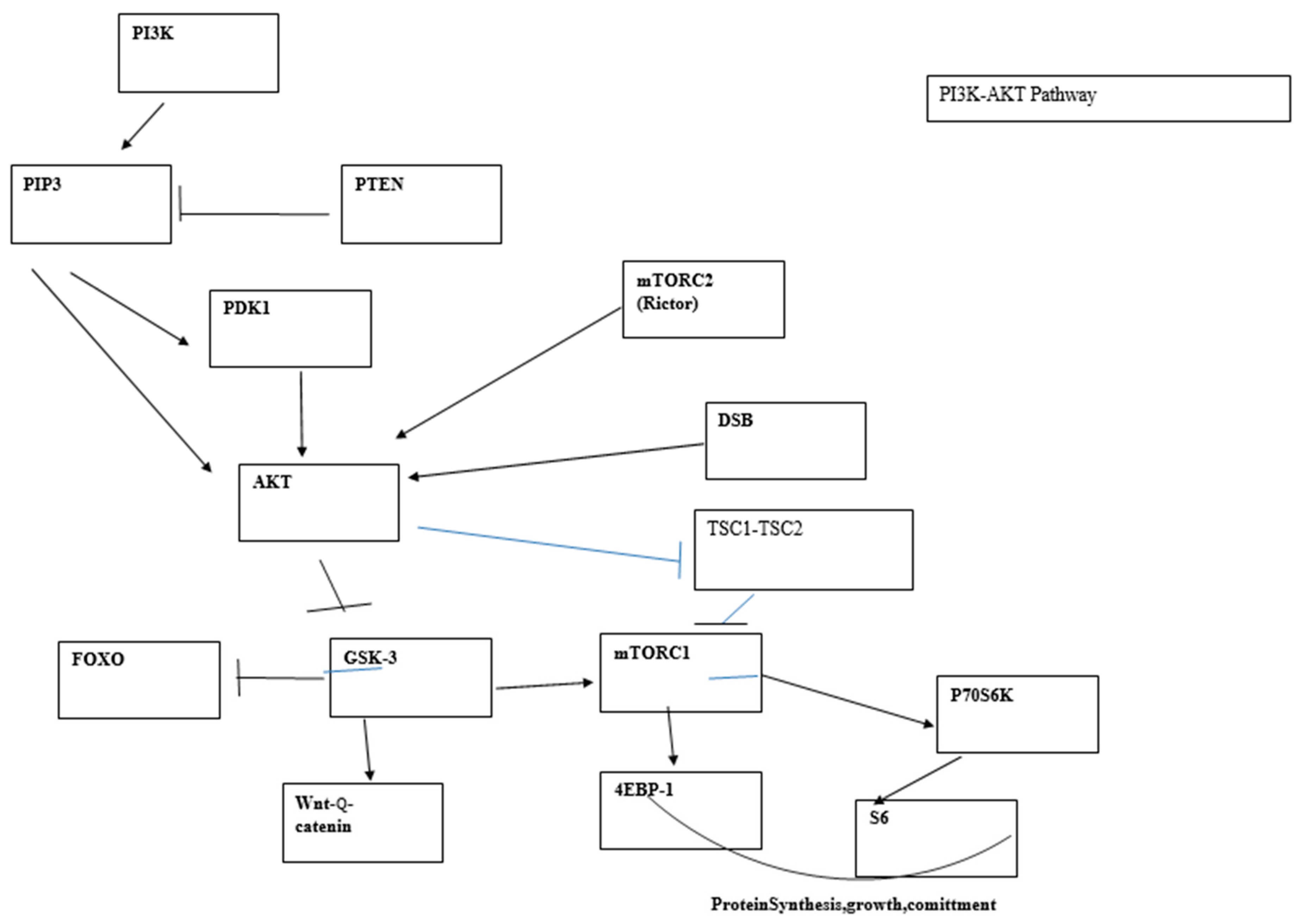
5.2. The PI3K Pathway and DDR
5.3. T-Cell Lymphoma Development Following PI3K/AKT Signaling Is Triggered by V(D)J Recombination Which Coincides with the Tumor Cell Differentiation Stage and Resistance of Neonatal Cells to Leukemia Development
6. Correlation with Clinical Data
7. Further Comments and Conclusions
8. Future Directions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Torres-Montaner, A. The telomere complex and the origin of the cáncer stem cell. Biomark. Res. 2021, 9, 81. [Google Scholar] [CrossRef]
- Chin, K.; Ortiz de Solorzano, C.; Knowles, D.; Jones, A.; Chou, W.; Rodriguez, E.G. In situ analysis of genome instability in breast cancer. Nat. Genet. 2004, 36, 984–988. [Google Scholar] [CrossRef]
- Ferreira, M.S.V.; Crysandt, M.; Ziegler, P.; Hummel, S.; Wilop, S.; Kirschner, M.; Schemionek, M.; Jost, E.; Wagner, W.; Brümmendorf, T.H.; et al. Evidence for a pre-existing telomere deficit in non-clonal hematopoietic stem cells in patients with acute leukemia. Ann. Hematol. 2017, 96, 1457–1461. [Google Scholar] [CrossRef]
- Engelhardt, M.; Mackenzie, K.; Drullinsky, P.; Silver, R.T. Telomerase activity and telomere length in acute and chronic leukemia, pre- and post- ex vivo culture. Cancer Res. 2000, 60, 610–617. [Google Scholar]
- Rufer, N.; Brümmendorf, T.H.; Kolvraa, S.; Bischoff, C.; Christensen, K.; Wadsworth, L.; Schulzer, M.; Lansdorp, P.M. Telomere fluorescence measurements in granulocytes and T lymphocytes subsets point to a high turnover of hematopoietic stem cells and memory T cells in early childhood. J. Exp. Med. 1999, 190, 157–167. [Google Scholar] [CrossRef]
- Torres-Montaner, A. Cancer origin in committed versus stem cells: Hypothetical antineoplastic mechanisms associated with stem cells. Critical Rev. Oncol./Hematol. 2011, 80, 209–224. [Google Scholar] [CrossRef]
- Chen, J.; Tsukamoto, H.; Mushra, L.; Chen, J.; Muñoz, N.M.; Kundra, S. Suppression in human stem cell disorder Beckwith-Wiedemann syndrome find the latest version: TGF-β/β2-spectrin/CTF-regulated tumor suppression in human stem cell disorder Beckwith-Wiedemann syndrome. J. Clin. Investig. 2016, 126, 527–542. [Google Scholar] [CrossRef]
- Passegué, E.; Wagner, E.F.; Weissman, I.L. JunB deficiency leads to a myeloproliferative disorder arising from hematopoietic stem cells. Cell 2004, 119, 431–443. [Google Scholar] [CrossRef]
- Santaguida, M.; Schepers, K.; King, B.; Sabnis, A.J.; Forsberg, E.C.; Attema, J.L.; Braun, B.S.; Passegué, E. JunB protects against myeloid malignancies by limiting hematopoietic stem cell proliferation and differentiation without affecting self-renewal. Cancer Cell 2009, 15, 341–352. [Google Scholar] [CrossRef]
- Nepal, R.M.; Tong Li Kolaj, B.; Edelmann, W.; Marin, A. Msh2-dependent DNA repair mitigates a unique susceptibility of B cell progenitors to c-Myc-induced lymphomas. Proc. Natl. Acad. Sci. USA 2009, 106, 18698–18703. [Google Scholar] [CrossRef]
- Wilson, A.; Murphy, M.J.; Oskarsson, T.; Kaloulis, K.; Bettess, M.D.; Oser, G.M.; Pasche, A.-C.; Knabenhans, C.; MacDonald, H.R.; Trumpp, A. c-Myc controls the balance between stem cell self-renewal and differentiation. Genes. Dev. 2004, 18, 2747–2763. [Google Scholar] [CrossRef]
- Wang, J.; Xie, L.Y.; Allan, S.; Beach, D.; Hannon, G.J. Myc activates telomerase. Genes Dev. 1998, 12, 1769–1774. [Google Scholar] [CrossRef]
- Yamanaka, S. Pluripotent stem cell-based cell therapy-promise and challenges. Cell Stem Cell 2020, 27, 23–531. [Google Scholar] [CrossRef]
- Lieberman, H.B.; Panigtahi, S.K.; Hopkins, K.M. p53 and Rad9, the DNA damage response and regulation of transcription networks. Radiat. Res. 2017, 187, 424–432. [Google Scholar] [CrossRef]
- Hayashi, M.T.; Cesare, A.J.; Fitzpatrick, J.A.J.; Lazzerini-Denchi, E.; Karlseder, J. A telomere dependent DNA damage checkpoint induced by prolonged mitotic arrest. Nat. Struct. Mol. Biol. 2012, 19, 387–394. [Google Scholar] [CrossRef]
- Trkova, M.; Prochazkova, K.; Krutilkova, V. Telomere length in peripheral blood cells of germline TP53 mutation carriers is shorter than that of normal individuals of corresponding age. Cancer 2007, 110, 694–702. [Google Scholar] [CrossRef]
- Tabori, U.; Nauda, S.; Druker, H. Younger age of cancer initiation is associated with shorter telomere length in Li-Fraumeni syndrome. Cancer Res. 2007, 67, 1415–1418. [Google Scholar] [CrossRef]
- Broustas, C.G.; Lieberman, H.B. J Contributions of Rad9 to tumorigenesis. Cell. Biochem. 2012, 113, 742–751. [Google Scholar] [CrossRef][Green Version]
- Barroso-González, J.; García-Expósito, L.; Hoang, S.M.; Roncaioli, J.L.; Ghosh, A. Rad51AP1is an essential mediator of alternative lengthening of telomeres. Mol. Cell 2019, 76, 11–26.e7. [Google Scholar] [CrossRef]
- Bridges, A.E.; Ramachandran, S.; Tamizhmani, K.; Parwal, U.; Lester, A.; Rajpurohit, P.; Morera, D.S.; Hasanali, S.L.; Arjunan, P.; Jedeja, R.N.; et al. Rad51 loss attenuates colon cancer stem cell self-renewal and sensitizes to chemotherapy. Mol. Cancer Res. 2021, 19, 1486–1497. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, H.; Qiao, L. Silencing of Rad51AP1 suppresses epithelial-mesenchymal transition and metastasis in non-small cell lung cancer. Thorac. Cancer 2019, 10, 1748–1763. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.M.; Yadav, T.; Ouyang, J. Alternative lengthening of telomeres through two distinct break induced replication pathways. Cell Rep. 2019, 26, 955–968.c3. [Google Scholar] [CrossRef] [PubMed]
- Hamid, A.B.; Frank, L.E.; Bouley, R.A. Pan-cancer analysis of co-occurring mutations between Rad52 and the BRCA1-BRCA2-PALB axis in human cells. PLoS ONE 2022, 17, e0273736. [Google Scholar] [CrossRef] [PubMed]
- Wahhiby, A.I.; Wong, S.; Slijepcevic, P. Shortened telomeres in murine Scid cells expressing mutant hRad54 coincide with reduction in recombination at telomeres. Mut. Res. 2005, 578, 132–142. [Google Scholar] [CrossRef]
- Li, H.; Zhuang, H.; Gu, T.; Li, G.; Jiang, Y.; Xu, S.; Zhou, Q. Rad54L promotes progression of hepatocellular carcinoma via the homologous recombination repair pathway. Funct. Integr. Genom. 2023, 23, 128. [Google Scholar] [CrossRef]
- Zheng, S.; Yao, L.; Li, F. Homologous recombination repair pathway and Rad54L in early stage lung adenocarcinoma. PeerJ 2021, 9, e10680. [Google Scholar] [CrossRef] [PubMed]
- Burger, K.; Schlackow, M.; Gullerova, M. c-ABL couples RNA polII transcription to DNA double strand breaks. Nucleic Acids Res. 2019, 47, 3467–3484. [Google Scholar] [CrossRef]
- Misri, S.; Pandita, S.; Kumar, R.; Pandita, T. Telomeres, histone code and DNA damage response. Cytogenet. Genome Res. 2008, 122, 297–307. [Google Scholar] [CrossRef]
- Goga, A.; McLaughlin, J.; Pendergast, A.M. Oncogenic activation of c-Abl by mutation within its last exon. Olecular Cell. Biol. 1993, 13, 4967–4975. [Google Scholar]
- Vohodina, J.; Goerhring, L.J.; Liu, B. BRCA1 binds TERRA RNA and suppresses R-loop based telomeric DNA damage. Nat. Commun. 2021, 12, 3542. [Google Scholar] [CrossRef]
- Gunnarsdottir, S.R.; Bjarnason, H.; Thorvaldsdottir, B. BRCA2 haploinsufficiency in telomere maintenance. Genes 2021, 13, 83. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.Y.; Muller, W.J. Oncogenes and tumor suppressor genes. Cold Spring Harb. Perspect. Biol. 2010, 2, a003236. [Google Scholar] [CrossRef] [PubMed]
- Slijecepvic, P. The role of DNA damage response proteins at telomeres-an integrative model. DNA Repair 2006, 5, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- Laspata, N.; Kaur, P.; Mersaoui, S.Y.; Muoio, D.; Liu, Z.S.; Bannister, M.H.; Nguyen, H.D.; Curry, C.; Pascal, J.M.; Poirier, G.G.; et al. PARP1 associates with R-loops to promote their resolution and genome stability. Nucleic Acids Res. 2023, 51, 2215–2237. [Google Scholar] [CrossRef]
- Pazzaglia, S.; Pioli, C. Multifaceted roles of PARP1 in DNA repair and inflammarion: Pathological and therapeutic implications in cancer and non-cancer diseases. Cells 2020, 9, 41. [Google Scholar] [CrossRef]
- Puentes-Pardo, J.D.; Moreno-SanJuan, S.; Casado, J.; Feliu, J.E.; López-Pérez, D.; Sánchez-Uceta, P.; González-Novoa, P.; Galvez, J.; Carazo, Á.; León, J. PARP-1 expression influences cancer stem cell phenotype in colorectal cancer depending on p53. Int. J. Mol. Sci. 2023, 24, 4787. [Google Scholar] [CrossRef]
- Sui, J.; Zhang, S.; Chen, B.P.C. DNA-dependent protein kinase in telomere maintenance and protection. Cell. Mol. Biol. Lett. 2020, 25, 2. [Google Scholar] [CrossRef]
- Hsu, F.M.; Zhang, S.; Chen, B.P.C. Role of DNA-dependent protein kinase catalytic subunit in cancer development and treatment. Transl. Cancer Res. 2012, 1, 22–34. [Google Scholar] [CrossRef]
- Yasaei, H.; Slijepcevic, P. Defective Artemis causes mild telomere dysfunction. Genome Integr. 2010, 1, 3. [Google Scholar] [CrossRef]
- Jacobs, C.; Huang, Y.; Masud, T. A hypomorphic artemis human disease allele causes aberrant chromosomal rearrangement and tumorigenesis. Hum. Mol. Genet. 2011, 20, 806–819. [Google Scholar] [CrossRef]
- Gan, W.; Liu, P.; Wei, W. Akt promotes tumorigenesis in part through modulating genomic instability via pohosphorylating XLF. Nucleus 2015, 6, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Zahid, S.; El Dahan, M.S.; Iehl, F.; Fernandez-Varela, P. The multifaceted roles of Ku70/Ku80. Int. J. Mol. Sci. 2021, 22, 4134. [Google Scholar] [CrossRef] [PubMed]
- Fell, V.L.; Schild-Poulter, C. The Ku heterodimers: Function in DNA repair and beyond. Mutat. Res. Rev. Mutat. Res. 2015, 763, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.J.; Wang, S.Y.; Lu, H. Ablating putativeKu70 phosphorylation site results in defective DNA damage repair and spontaneous induction of hepatocellular carcinoma. Nucleic Acids Res. 2021, 49, 9836–9850. [Google Scholar] [CrossRef]
- Felgentreff, K.; Baxi, S.N.; Lee, Y.N. Ligase-4 deficiency causes distinctive immune abnormalities in asymptomatic individuals. J. Clin. Immunol. 2016, 36, 341–353. [Google Scholar] [CrossRef]
- Sharma, R.; Lewis, S.; Wlodarski, M.W. DNA repair syndromes and cancer: Insights into genetics and phenotype patterns. Front. Pediatr. 2020, 8, 570084. [Google Scholar] [CrossRef]
- Adelfalk, C.; Lorenz, M.; Serra, V.; von Zglinicki, T.; Hirsch-Kauffmann, M.; Schweiger, M. Accelerated telomere shortening in Fanconi anemia fibroblasts- a longitudinal study. FEBS Lett. 2001, 506, 22–26. [Google Scholar] [CrossRef]
- Stroik, S.; Kurtz, K.; Hendrickson, E.A. CtIP is essential for telomere replication. Nucleic Acids Res. 2019, 47, 8927–8940. [Google Scholar] [CrossRef]
- Mozaffari, N.L.; Pagliarulo, F.; Sartori, A.A. Human CtIP: A double agent in DNA repair and tumorigenesis. Semin. Cell Dev. Biol. 2021, 113, 47–56. [Google Scholar] [CrossRef]
- Paull, T.P.; Lee, J.-H. Rad17, the clamp loader that loads more than clamps. EMBO J. 2014, 33, 783–785. [Google Scholar] [CrossRef][Green Version]
- Sun, J.; Lin, W.; Wang, Q. The cell cycle checkpoint gene, Rad17rs 1045051, is associated with prostate cancer risk. Acta Medica Okoyama 2021, 75, 415–421. [Google Scholar] [CrossRef]
- Yasuda, Y.; Sakai, A. Ito S Genetic polymorphism at codón 546 of the human Rad17 contributes to the risk of esophageal squamous cell carcinoma. Int. J. Mol. Epidemiol. Genet. 2016, 7, 58–66. [Google Scholar] [PubMed]
- Nabetani, A.; Yokoyama, O.; Ishikawa, F. Localization of hRad9, hHus1, hRad1 and hRad17 and caffeine-sensitive DNA replication at the alternative lengthening of telomeres-associated promyelocytic leukemia body. J. Biol. Chem. 2004, 279, 25849–25859. [Google Scholar] [CrossRef] [PubMed]
- Khorrami, A.; Bagheri, M.S.; Tavalleri, M. The functional significance of 14-3-3 proteins in cancer: Focus on lung cancer. Horm. Mol. Biol. Clin. Investig. 2017, 32, 20170032. [Google Scholar] [CrossRef]
- A Stewart, J.; Stewart, J.A.; Wang, Y.; Ackerson, S.M.; Schuck, P.L. Emerging roles of CST in maintaining genome stability and human disease. Front. Biosci. 2018, 23, 1564–1586. [Google Scholar] [CrossRef]
- dos Santos, G.A.; Viana, N.I.; Pimenta, R.; de Camargo, J.A.; Guimaraes, V.R.; Romão, P.; Candido, P.; Ghazarian, V.; Reis, S.T.; Leite, K.R.M.; et al. Pan-cancer analysis reveals that CTC1-STN1-TEN1 (CST) complex may have a key position in oncology. Cancer Genet. 2022, 262–263, 80–90. [Google Scholar] [CrossRef]
- Tummala, H.; Kirwan, M.; Walne, A.J. ERCC6L2 mutations link a distinct bone marrow syndrome to DNA repair and mitochondrial function. Am. J. Hum. Genet. 2014, 94, 246–256. [Google Scholar] [CrossRef]
- Mendez-Bermudez, A.; Royle, N.J. Deficiency in DNA mismatch repair increases the rate of telomere shortening in normal human cells. Hum. Mutat. 2011, 32, 939–946. [Google Scholar] [CrossRef]
- Du, X.; Shen, J.; Kugan, N. Telomere shortening exposes functions for the mouse Werner and Bloom syndrome genes. Mol. Cell. Biol. 2004, 24, 8437–8446. [Google Scholar] [CrossRef]
- Gosh, A.K.; Rossi, M.L.; Singh, K.; Dunn, C.; Ramamoorthy, M.; Croteau, D.L. RECQL4, the protein mutated in Rothmund-Thompson syndrome functions in telomere maintenance. J. Biol. Chem. 2012, 287, 196–209. [Google Scholar] [CrossRef]
- Tanaka, A.; Weinel, S.; Nagy, N.; O’Driscoll, M.; Lai-Cheong, J.E.; Kulp-Shorten, C.L.; Knable, A.; Carpenter, G.; Fisher, S.A.; Hiragun, M.; et al. Germline mutation in ATR in autosomal dominant oropharyngeal cancer syndrome. Am. J. Hum. Genet. 2012, 90, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Flynn, R.L.; Zou, L. ATR: A master conductor of cellular responses to DNA replication stress. Trends Biochem. 2011, 36, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Ruzankina, Y.; Pinzon-Guzman, C.; Asare, A.; Ong, T.; Pontano, L.; Cotsarelis, G.; Zediak, V.P.; Velez, M.; Bhandoola, A.; Brown, E.J. Deletion of the developmentally essential gene ATR in adult mice leads to age-related phenotypes and stem cell loss. Cell Stem Cell 2007, 1, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Epanchintsev, A.; Costanzo, F.; Rauschendorf, M.-A.; Caputo, M.; Ye, T.; Donnio, L.-M.; Proietti-De-Santis, L.; Coin, F.; Laugel, V.; Egly, J.-M. Cockayne’s syndrome A and B proteins regulate transcription arrest after genotoxic stress by promoting ATF3 degradation. Mol. Cell 2017, 68, 1054–1066. [Google Scholar] [CrossRef]
- Iyama, T.; Wilson, D. III. DNA repair mechanisms in dividing and non-dividing cells. DNA Repair 2013, 12, 620–636. [Google Scholar] [CrossRef]
- Lee, J.-H.; Mand, M.R.; Deshpande, R.A. Ataxia-telangiectasis mutated (ATM) kinase activity is regulated by ATP-driven conformational changes in the Mre11/Rad50/NBs1(MRN) complex. J. Biol. Chem. 2013, 288, 12840–12851. [Google Scholar] [CrossRef]
- Uziel, T.; Lerenthal, Y.; Moyal, L.; Andegeko, Y.; Mittelman, L.; Shiloh, Y. Requirement of the MRN complex for ATM activation by DNA damage. EMBO J. 2003, 22, 5612–5621. [Google Scholar] [CrossRef]
- Matei, I.R.; Guidos, C.J.; Danska, J.S. ATM-dependent DNA damage surveillance in T-cell development and leukemogenesis: The DSB connection. Immunol. Rev. 2006, 209, 142–158. [Google Scholar] [CrossRef]
- Tauchi, H.; Matsuura, S.; Kobayashi, J.; Sakamoto, S.; Komatsu, K. Nijmegen breakage syndrome gene, NSB1, and molecular links to factors for genome stability. Oncogene 2002, 21, 8967–8980. [Google Scholar] [CrossRef]
- Otahalova, B.; Volkova, Z.; Soukupova, J.; Kleiblova, P.; Janatova, M.; Vocka, M.; Macurek, L.; Kleibl, Z. Importance of germ line and somatic alterations in human MRE11, RAS50 and NBN genes coding for MRN complex. Int. J. Mol.Sci. 2023, 24, 5612. [Google Scholar] [CrossRef]
- Williams, B.R.; Mirzoeva, O.K.; Morgan, W.F.; Lin, J.; Dunnick, W.; Petrini, J.H. A murine model of Nijmegen breakage syndrome. Curr. Biol. 2002, 12, 648–653. [Google Scholar] [CrossRef] [PubMed]
- Dumond-Jones, V.; Frapart, P.O.; Tong, W.M.; Sajithal, G.; Hulla, W.; Schmid, G. Non heterozygosity renders mice susceptible totumor formation and ionizing radiation-induced tumorignesis. Cancer Res. 2003, 63, 7263–7269. [Google Scholar]
- Thierfelder, N.; Demuth, I.; Burghardt, N.; Schmelz, K.; Sperling, K.; Chrzanowska, K.H.; Seemanova, E.; Digweed, M. Extreme variation in apoptosis capacity amongst lymphoid cells of Nijmegen breakage syndrome patients. Eur. J. Cell Biol. 2008, 87, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Digweed, M.; Reis, A.; Sperling, K. Nimegen breakage syndrome: Consequences of defective DNA double strand break repair. BioEssays 1999, 21, 649–656. [Google Scholar] [CrossRef]
- Bartkova, J.; Tommiska, J.; Oplustilova, L.; Aaltonen, K.; Tamminen, A.; Heikkinen, T. Aberrations of the Mre11-Rad50-NBS1 DNA damage sensor complex in hman breast cancer: MRE11 as a candidate familial cancer predisposing gene. Mol. Oncol. 2008, 2, 296–316. [Google Scholar] [CrossRef]
- Situ, Y.; Chung, L.; Lee, C.S.; Ho, V. MRN (MRE11-RAD50-NBS1) complex in human cancer and prognosis implications in colorectal cancer. Int. J. Mol. Sci. 2019, 20, 816. [Google Scholar] [CrossRef]
- Gupta, G.P.; Vanness, K.; Barlas, A.; Manova-Todorova, K.O.; Wen, Y.H.; Petrini, J.H. The Mre11 complex suppresses oncogene-driven breast tumorigenesis and metastasis. Mol. Cell 2013, 52, 353–365. [Google Scholar] [CrossRef]
- Spehalski, E.; Capper, K.M.; Smith, C.J.; Morgan, M.J.; Dinkelmann, M.; Buis, J. MRE11 promotes tumorigenesis by facilitating resistance to oncogene-induced replication stress. Cancer Res. 2017, 77, 5327–5338. [Google Scholar] [CrossRef]
- Theusissen, J.-W.F.; Kaplan, M.I.; Hunt, P.A.; Williams, B.R. Checkpoint failure and chromosomal instability without lymphomagenesis in Mre11ATLD1/ATLD1 mice. Mol. Cell 2003, 12, 1511–1523. [Google Scholar] [CrossRef]
- Attwooll, C.L.; Akpmar, M.; Petrini, J.H.J. The Mre11 complex and the response to dysfunctional telomeres. Mol. Cell. Biol. 2009, 29, 5540–5551. [Google Scholar] [CrossRef]
- Ragamin, A.; Yigit, G.; Bousset, K.; Beleggia, F.; Verheijen, F.W.; de Wit, M.Y.; Strom, T.M.; Dörk, T.; Wollnik, B.; Mancini, G.M.S. Human Rad50 deficiency: Confirmation of a distinctive phenotype. Am. J. Med. Genet. Part A 2020, 182, 1378–1386. [Google Scholar] [CrossRef] [PubMed]
- Lombard, D.B.; Chua, K.F.; Mostoslavsky, R.; Franco, S.; Gostissa, M.; Alt, F.W. DNA repair, genome stability and aging. Cell 2005, 120, 497–512. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Liu, Y. R-loops at chromosome ends: From formation, regulation and cellular consequences. Cancers 2023, 15, 2178. [Google Scholar] [CrossRef]
- Bayona-Feliu, A.; Barroso, S.; Muñoz, S. The SWI/SNF chromatin remodeling complex helps resolve R-loop-mediated transcription-replication conflicts. Nat. Genet. 2021, 53, 1050–1063. [Google Scholar] [CrossRef]
- Schaefer, I.-M.; Hornick, J.L. SWI/SNF complex-deficient soft tissue neoplasms: An update. Semin. Diagn. Pathol. 2021, 38, 222–231. [Google Scholar] [CrossRef]
- Goos, J.A.C.; Vogel, W.K.; Mlcochova, H.; Millard, C.J.; Esfandiari, E.; Selman, W.H.; Calpena, E.; Koelling, N.; Carpenter, E.L.; A Swagemakers, S.M.; et al. A de novo substitution in Bcl11b leads to loss of interaction with transcriptional complexes and craniosynostosis. Hum. Mol. Genet. 2019, 28, 2501–2513. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.F.; Sun, A.-A.; Shi, Y.; Li, F.; Pickett, H. A Structural and functional characterization of RBBP4-ZNF827 interaction and its role in NuRD recruitment to telomeres. Biochem. J. 2018, 475, 2667–2679. [Google Scholar] [CrossRef]
- Conomos, D.; Reddel, R.R.; Picket, H.A. NuRD-ZNF827 recruitment to telomeres creates a molecular scaffold for homologous recombination. Nat. Struct. Mol. Biol. 2014, 21, 760–770. [Google Scholar] [CrossRef] [PubMed]
- Montefiori, L.E.; Mulligan, C.G. Redefining the biological basis of lineage-ambiguous leukemia through genomics: BCL11B deregulation in acute leukemias of ambiguous lineage. Best Pract. Res. Clin. Haematol. 2021, 34, 101329. [Google Scholar] [CrossRef]
- Lennon, M.J.; Jones, S.P.; Lovelace, M.; Guillemin, G.; Brew, B.J. Bcl11b-A critical neurodevelopmental transcription factor- Roles in health and disease. Front. Neurosci. 2017, 11, 89. [Google Scholar] [CrossRef]
- Li, F.; Deng, Z.; Zhang, L.; Wu, C.; Jin, Y.; Hwang, I.; Vladimirova, O.; Xu, L.; Yang, L.; Lu, B.; et al. ATRX loss induces telomere dysfunction and necessitates induction of alternative lengthening of telomeres during human cell immortalization. EMBO J. 2019, 38, e96659. [Google Scholar] [CrossRef] [PubMed]
- Kishi, D.; Lu, K.P. A critical role for Pin2/TRF1 in ATM-dependent regulation. J. Biol. Chem. 2002, 277, 7420–7429. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Lu, K.P. The Pin2/TRF1-interacting protein PinX1 is a potent telomerase inhibitor. Cell 2001, 107, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.Z.; Perrem, K.; Lu, K.P. Role of Pin2/TRF1 in telomere maintenance and cell cycle control. Cell Biochem. 2003, 89, 19–37. [Google Scholar] [CrossRef]
- Huang, W.-J.; Li, M.; Jin, X.-H.; Huang, X.-J.; Zhao, W.; Tian, X.P. Genetic profile and biological implication of Pin2/TRF1-interacting telomerase inhibitor 1 (PinX1) in human cancers: An analysis using the Cancer Genome Atlas. Oncotarget 2017, 8, 67241–67253. [Google Scholar] [CrossRef][Green Version]
- Pierce, A.K.; Humphrey, T.C. Integrating stress-response and cell-cycle checkpoint pathways. Trends Cell Biol. 2001, 11, 426–433. [Google Scholar] [CrossRef]
- Petiniot, L.K.; Weaver, Z.; Barlow, C.; Shen, R.; Eckhaus, M.; Steinberg, S.M.; Ried, T.; Wynshaw-Boris, A.; Hodes, R.J. Recombinase-activating gene (RAG) 2-mediated V(D)J recombination is not essential for tumorigenesis in Atm-deficient mice. Proc. Natl. Acad. Sci. USA 2000, 97, 6664–6669. [Google Scholar] [CrossRef]
- von Boehmer, H. Developmental biology of T cells in T cell-receptor transgenic mice. Annu. Rev. Immunol. 1990, 8, 531–556. [Google Scholar] [CrossRef]
- Nacht, M.; Jacks, T. V (D) J recombination is not required for the development of lymphoma in p53-deficient mice. Cell Growth Differ. 1998, 9, 131–138. [Google Scholar]
- Bester, A.C.; Roniger, M.; Oren, Y.S.; Im, M.M.; Sarni, D.; Chaoat, M.; Bensimon, A.; Zamir, G.; Shewach, D.S.; Kerem, B. Nucleotide deficiency promotes genomic instability in early stages of cancer development. Cell 2011, 145, 435–446. [Google Scholar] [CrossRef]
- Saldivar, J.C.; Miuma, S.; Bene, J.; Hosseini, S.A.; Shibata, H.; Sun, J.; Wheeler, L.J.; Mathews, C.K.; Huebner, K. Initiation of genome instability and preneoplastic processes through loss of Fhit expression. PLoS Genet. 2012, 8, e1003077. [Google Scholar] [CrossRef] [PubMed]
- Lanz, M.C.; Dibitetto, D.; Smolka, M.B. DNA damage kinase signaling: Checkpoint and repair at 30 years. EMBO J. 2019, 38, e101801. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, T.; Said, M.; Naim, V. DNA replication stres and chromosomal instability: Dangerous liaisons. Genes 2020, 11, 642. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, R.; Meuth, M. Chk1 and p21 Cooperate to Prevent Apoptosis during DNA Replication Fork Stress. Mol. Biol. Cell 2006, 17, 402–412. [Google Scholar] [CrossRef]
- López-Contreras, A.J.; Gutierrez-Martinez, P.; Specks, J.; Rodrigo-Perez, S.; Fernandez-Capetillo, O. An extra-allele of Chk1 limits oncogene-induced reoliative stress and promotes transformation. J. Exp. Med. 2012, 209, 455–461. [Google Scholar] [CrossRef]
- García-De-Teresa, B.; Rodríguez, A.; Frias, S. Chromosome instability in Fanconi Anemia:from breaks to phenotypic consequences. Genes 2020, 11, 1528. [Google Scholar] [CrossRef]
- Helbling-Leclerc, A.; Garcin, C.; Rosselli, F. Beyond DNA repair and chromosome instability-Fanconi anaemia as a cellular senescence-associated syndrome. Cell Death Differ. 2021, 28, 1159–1173. [Google Scholar] [CrossRef]
- Collis, S.J.; Ciccia, A.; Deans, A.J.; Horejsi, Z.; Martin, J.S. FANCM and FAPP24 function in ATR-mediated checkpoint signaling independently of the Fanconi anemia core complex. Mol. Cell 2008, 32, 313–324. [Google Scholar] [CrossRef]
- Hořejší, Z.; Collis, S.J.; Boulton, S.J. FANCM-FAAP24 and HCLK2: Roles in ATR signaling and the Fanconi Anemia pathway. Cell Cycle 2009, 8, 1133–1137. [Google Scholar] [CrossRef]
- Runge, K.W.; Zakian, V.A. TEL2, an essential gene required for telomere length regulation and telomere position effect in Saccharmyces cerevisiae. Mol. Cell. Biol. 1996, 6, 3094–3105. [Google Scholar] [CrossRef]
- Fan, H.-C.; Chang, F.-W.; Tsai, J.-D.; Lin, K.-M.; Chen, C.-M.; Lin, S.-Z.; Liu, C.-A.; Harn, H.-J. Telomeres and cancer. Life 2021, 11, 1405. [Google Scholar] [CrossRef] [PubMed]
- Sherman, M.H.; Kuraishy, A.I.; Deshpande, C.; Hong, J.S.; Cacalano, N.A.; Gatti, R.A.; Manis, J.P.; Damore, M.A.; Pellegrini, M.; Teitell, M.A. AID-induced genotoxic stress promotes B cell differentiation in the germinal center via ATM and LKB1 signaling. Mol. Cell 2010, 39, 873–885. [Google Scholar] [CrossRef] [PubMed]
- Çakan, E.; Gunaydin, G. Activation induced cytidine deaminase: An old friend with new faces. Front. Immunol. 2022, 13, 965312. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, I.-M.; Hiai, H.; Kakazu, N.; Yamada, S.; Muramatsu, M.; Kinoshita, K.; Honjo, T. Constitutive expression of AID leads to tumorigenesis. J. Exp. Med. 2003, 197, 1173–1181. [Google Scholar] [CrossRef]
- Pusapati, R.V.; Rounbehler, R.J.; Hong, S.; Powers, J.T.; Yan, M.; Kiguchi, K.; McArthur, M.J.; Wong, P.K.; Johnson, D.G. ATM promotes apoptosis and suppresses tumorigenesis in response to Myc. Proc. Natl. Acad. Sci. USA 2006, 103, 1446–1451. [Google Scholar] [CrossRef]
- Allman DKarnell, F.G.; Punt, J.A.; Bakkour, S. Separation of Notch1 promoted lineage commitment and expansion/transformation in developing T cells. J. Exp. Med. 2001, 194, 99–106. [Google Scholar] [CrossRef]
- Ciofani, M.; Schmitt, T.M.; Ciofani, A.; Michie, A.M.; Çuburu, N.; Aublin, A.; Maryanski, J.L.; Zúñiga-Pflücker, J. C Obligatory role for cooperative signaling by pre-TCR and Notch during thymocyte differentiation. J. Immunol. 2004, 172, 5230–5239. [Google Scholar] [CrossRef]
- Campese, A.F.; Garbe, A.I.; Zhang, F.; Grassi, F.; Screpanti, I.; von Boehmer, H. Notch1-dependent lymphomagenesis is assisted by but does not essentially require pre-TCR signaling. Blood 2006, 108, 305–310. [Google Scholar] [CrossRef]
- Cieslak, A.; Payet-Bornet, D.; Asnafi, V. RUNX1 as a recombinase cofactor. Oncotarget 2015, 6, 21793–21794. [Google Scholar] [CrossRef]
- Blanco, R.; Muñoz, P.; Flores, J.M.; Klatt, P.; Blasco, M.A. Telomerase abrogation dramatically accelerates TRF2-induced epithelial carcinogenesis. Genes Dev. 2007, 21, 206–220. [Google Scholar] [CrossRef]
- Muñoz RBlanco, M.A. Blasco Role of the TRF2 telomeric protein in cancer and aging. Cell Cycle 2006, 5, 718–721. [Google Scholar] [CrossRef]
- Imran, S.A.M.; Yazid, M.D.; Cui, W.; Lokanathan, Y. The intra and extratelomeric role of TRF2 in the DNA damage response. Int. J. Mol. Sci. 2021, 22, 9900. [Google Scholar] [CrossRef]
- Bozulic, L.; Surucu, B.; Hynx, D.; Hemmings, B.A. PKBα/Akt1 acts downstream of DNA-PK in the DNA double strand break reponse and promotes survival. Mol. Cell 2008, 30, 203–213. [Google Scholar] [CrossRef]
- Bassi, C.; Fortin, J.; Snow, B.E.; Wakeham, A.; Ho, J.; Haight, J.; You-Ten, A.; Cianci, E.; Buckler, L.; Gorrini, C.; et al. The PTEN and ATM axis controls the G1/S cell cycle checkpoint and tumorigenesis in HER2-positive breast cancer. Cell Deth Differ. 2021, 28, 3036–3051. [Google Scholar] [CrossRef]
- Fruman, D.A.; Rommel, C. PI3K and cancer: Lessons, challenges and opportunities. Nat. Rev. Drug Discov. 2014, 13, 140–156. [Google Scholar] [CrossRef]
- Shen, W.H.; Balajee, A.S.; Wang, J.; Wu, H.; Eng, C.; Pandolfi, P.P.; Yin, Y. Essential role for nuclear Pten in maintaining chromosomal integrity. Cell 2007, 128, 157–170. [Google Scholar] [CrossRef]
- Planchon, S.M.; Waite, K.A.; Eng, C. The nuclear affairs of Ptem. J. Cell Sci. 2008, 121, 249–253. [Google Scholar] [CrossRef]
- Skeen, J.E.; Bhaskar, P.T.; Chen, C.-C.; Chen, W.C. AKT deficiency impairs normal cell proliferation and suppresses oncogenesis in a p53-independent and TORC1-dependent manner. Cancer Cell 2006, 10, 269–280. [Google Scholar] [CrossRef]
- Hannan, K.M.; Brandengurger, Y.; Jenkins, A.; Sharkey, K. mTOR-dependent regulation of ribosomal gene transcription requires S6K1 and is mediated by phosphorylation of the carboxy-terminal activation domain of the nucleolar transcription factor UBF. Mol. Cell. Biol. 2003, 23, 8862–8877. [Google Scholar] [CrossRef]
- Torres-Montaner, A.; Bolivar, J.; Astola, A.; Gimenez-Mas, J.A. Immunohistochemical detection of ribosomal transcription factor UBF and AgNOR staining identify apoptotic events in neoplastic cells od Hodgkin’s disease and other lymphoid cells. J. Histochem. Cytochem. 2000, 48, 1521–1530. [Google Scholar] [CrossRef]
- Cheng, C.; Liu, Y.; Liu, R.; Ikenoue, T.; Guan, K.-L.; Liu, Y.; Zheng, P. TSC-mTOR maintains quiescence and function of hematopoietic stem cells by repressing mitochondrial biogenesis and reactive oxygen species. J. Exp. Med. 2008, 205, 2397–2408. [Google Scholar] [CrossRef]
- Lee, J.Y.; Nakada, D.; Yilmaz, O.H.; Tothova, Z.; Joseph, N.M.; Lim, M.S.; Gilliland, D.G.; Morrison, S.J. mTOR activation induces tumor suppressors that inhibit leukemogenesis and deplete hematopoietic stem cells after Pten deletion. Cell Stem Cell 2010, 7, 593–605. [Google Scholar] [CrossRef]
- Kharas, M.G.; Okabe, R.; Ganis, J.J.; Gozo, M.; Khandan, T.; Paktinat, M.; Gilliland, D.G.; Gritsman, K. Constitutively active AKT depletes hematopoietic stem cells and induces leukemia in mice. Blood 2010, 115, 1406–1415. [Google Scholar] [CrossRef]
- Huang, J.; Nguyen-McCarty, M.; O Hexner, E.; Danet-Desnoyers, G.; Klein, P.S. Maintenance of hematopoietic stem cells through regulation of Wnt and mTOR pathways. Nat. Med. 2012, 18, 1778–1785. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, Y.; Bersenev, A.; O’brien, W.T.; Tong, W.; Emerson, S.G.; Klein, P.S. Pivotal role for glycogen synthase kinase-3 in hematopoietic stem cell homeostasis in mice. J. Clin. Investig. 2009, 119, 3519–3529. [Google Scholar] [CrossRef]
- Murtaza, G.; Khan, A.K.; Rashid, R.; Muneer, S.; Hasan, S.M.F.; Chen, J. Foxo transciptional factors and long-term living. Oxidative Med. Cell. Longev. 2017, 2017, 1–8. [Google Scholar] [CrossRef]
- Murtaza, G.; Khan, A.K.; Rashid, R.; Muneer, S.; Hasan, S.M.F.; Chen, J. Ablation in mice of the mTORC components raptor, rictor or mLST8 reveals that mTORC2 is required for signaling to AKT_FOXO and PKC alpha but not S6K1. Dev. Cell 2006, 11, 859–871. [Google Scholar] [CrossRef]
- Miyamoto, K.; Araki, K.Y.; Naka, K.; Arai, F. Foxo3a is essential for the maintenance of the hematopoietic stem cell pool. Cell Stem Cell 2007, 1, 101–112. [Google Scholar] [CrossRef]
- Miyamoto, K.; Araki, K.Y.; Naka, K.; Arai, F.; Takubo, K.; Yamazaki, S.; Matsuoka, S.; Miyamoto, T.; Ito, K.; Ohmura, M.; et al. Phosphoinositide-dependent kinase 1 controls migration and malignant transformation but not cell growth and proliferation in Pten-null lymphocytes. J. Exp. Med. 2009, 206, 2441–2454. [Google Scholar]
- Guertin, D.A.; Stevens, D.M.; Saitoh, M.; Kinkel, S.; Crosby, K.; Sheen, J.-H.; Mullholland, D.J.; Magnuson, M.A.; Wu, H.; Sabatini, D.M. The mTOR complex 2 is required for the development of prostate cancer induced by Pten loss in mice. Cancer Cell 2009, 15, 148–159. [Google Scholar] [CrossRef]
- Magee, J.A.; Ikenoue, T.; Nakada, D.; Lee, J.Y.; Guan, K.-L.; Morrison, S.J. Temporal changes in Pten and mTORC2 regulation of hematopoietic stem cell self-renewal and leukemia suppression. Cell Stem Cell 2012, 11, 415–428. [Google Scholar] [CrossRef]
- Duronio, V. The life of a cell: Apoptosis regulation by the PI3K/PKB pathway. Biochem. J. 2008, 415, 333–344. [Google Scholar] [CrossRef]
- Sykes, S.M.; Lane, S.W.; Bullinger, L.; Kalaitzidis, D.; Yusuf, R.; Saez, B.; Ferraro, F.; Mercier, F.; Singh, H.; Brumme, K.M.; et al. AKT/FOXO signaling enforces reversible differentiation blockade in myeloid leukemia. Cell 2011, 146, 697–708. [Google Scholar] [CrossRef]
- Hu, M.C.-T.; Lee, D.-F.; Xia, W.; Golfman, L.S.; Ou-Yang, F.; Yang, J.-Y.; Zou, Y.; Bao, S.; Hanada, N.; Saso, H.; et al. IκB kinase promotes tumorigenesis through inhibition of Forkhead FOXO3a. Cell 2004, 117, 225–237. [Google Scholar] [CrossRef]
- Cai, Y.; Kandula, V.; Ye, X.; Irwin, M.G.; Xia, Z. Decoding telomere protein Rap1: Its telomeric and nontelomeric functions and potential implications in diabetic cardiomyopathy. Cell Cycle 2017, 16, 1765–1773. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Banin, S.; Ouyang, H.; Li, G.C.; Courtois, G.; Shiloh, Y.; Karin, M.; Rotman, G. ATM is required for IκB kinase (IKK) activation in response to DNA double strand breaks. J. Biol. Chem. 2001, 270, 8898–8903. [Google Scholar] [CrossRef] [PubMed]
- Shats, I.; Gatza, M.L.; Liu, B.; Angus, S.P.; You, L.; Nevins, J.R. FOXO transcription factors control E2F1 transcriptional specificity and apoptotic function. Cancer Res. 2013, 73, 6056–6067. [Google Scholar] [CrossRef] [PubMed]
- Ryo, A.; Liou, Y.-C.; Wulf, G.; Nakamura, M.; Lee, S.W.; Lu, K.P. Pin1 is an E2F target gene essential for Neu/Ras-induced transformation of mammary epithelial cells. Mol. Cell. Biol. 2002, 22, 5281–5295. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Nolla, H.; Suzuki, A.; Mak, T.W.; Winoto, A. Normal development is an integral parto f tumorigenesis in T-cell specific PTen-deficient mice. Prac. Natl. Acad. Sci. USA 2008, 105, 2022–2027. [Google Scholar] [CrossRef] [PubMed]
- Puc, J.; Keniry, M.; Li, H.S.; Pandita, T.K.; Choudhury, A.D.; Memeo, L.; Mansukhani, M.; Murty, V.V.; Gaciong, Z.; Meek, S.E.; et al. Lack of Pten sequesters CHK1 and initiates genetic instability. Cancer Cell 2005, 7, 193–204. [Google Scholar] [CrossRef]
- Campbell, P.J.; Getz, G.; Korbel, G.; Stuart, J.M.; Jennings, J.L.; Stein, L.D.; Perry, M.D.; Nahal-Bose, H.; Kuellett, B.F.; Francis, L.; et al. Pan-cancer analysis of whole genomes. Nature 2020, 578, 82–93. [Google Scholar] [CrossRef]
- Rheinbay, E.; Muhling Nielsen, M.; Abascal, F.; Wala Jeremiah, A.; Shapira, O.; Tiao, G.; Hornshej, H.; Hess Julian, M.; Juul, R.I.; Lin, Z.; et al. Analysis of non-coding somatic drivers in 2658 cancer whole genomes. Nature 2020, 578, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Fasching, C.L.; Bower, K.; Reddel, R.R. Telomerase-independent telomere length maintenance in the absence of alternative llengthening of telomeres-associated promyelocytic leukemia bodies. Cancer Res. 2005, 65, 2722–2729. [Google Scholar] [CrossRef] [PubMed]
- Geserick, C.; Tejera, A.; González-Suárez, E.; Klatt, P.; A Blasco, M. Expression of mTert in primary murine cells links the growth-promoting effects of telomerase to transforming growth factor-β signaling. Oncogene 2006, 25, 4310–4319. [Google Scholar] [CrossRef][Green Version]
- Sprung, C.N.; Reynolds, G.E.; Jasin, M.; Murnane, J.P. Chromosome healing in mouse embryonic stem cells. Proc. Natl. Acad. Sci. USA 1999, 96, 6781–6786. [Google Scholar] [CrossRef]
- Sprung, C.N.; Reynolds, G.E.; Jasin, M.; Murnane, J.P. Tcl1 functions as a transcriptional regulator and is directly involved in the pathogenesis of CLL. Proc. Natl. Acad. Sci. USA 2008, 105, 19643–19648. [Google Scholar]
- Cabezas-Wallscheid, N.; Buettner, F.; Sommerkamp, P.; Klimmeck, D. Vitamin A-Retinoic acid signaling regulates hematopoietic stem cell dormancy. Cell 2017, 169, 807–823. [Google Scholar] [CrossRef]
- Liao, X.-H.; Zhang, A.L.; Zheng, M.; Li, M.-Q.; Chen, C.P.; Xu, H.; Chu, Q.-S.; Yang, D.; Lu, W.; Tsai, T.-F.; et al. Chemical or genetic Pin1 inhibition exerts potent anticancer activity against hepatocellular carcinoma by blocking multiple cancer-driving pathways. Sci. Rep. 2017, 7, srep43639. [Google Scholar] [CrossRef]
- Liou, Y.-C.; Ryo, A.; Huang, H.-K.; Lu, P.-J.; Bronson, R.; Fujimori, F.; Uchida, T.; Hunter, T.; Lu, K.P. Loss of Pin1 function in the mouse causes phenotypes resembling cyclin D1-null phenotypes. Proc. Natl. Acad. Sci. USA 2002, 99, 1335–1340. [Google Scholar] [CrossRef]
- Shen, M.; Stukenberg, P.T.; Kirschner, M.W.; Lu, K.P. The essential mitotic peptidyl-prolyl isomerase Pin1 binds and regulates mitosis-specific phosphoproteins. Genes. Dev. 1998, 12, 706–720. [Google Scholar] [CrossRef]
- Bao, L.; Kimzey, A.; Sauter, G.; Sowadski, J.M.; Lu, K.P.; Wang, D.-G. Prevalent overexpression of prolyl isomerase Pin1 in human cancers. Am. J. Pathol. 2004, 164, 1727–1737. [Google Scholar] [CrossRef]
| Telomere Dysfunction | Cancer Predisposition | |
|---|---|---|
| * P53 | Yes [16] | Yes [17] |
| Rad 9 | Yes [18] | Yes [18] |
| Rad 51 | Yes [19,20] | Yes [21] |
| Rad 52 | Yes [22] | Yes [22,23] |
| Rad 54 | Yes [24] | Yes [25,26] |
| c-Abl | Yes [27,28] | Yes [29] |
| BRCA1-BRCA2 | Yes [30,31] | Yes [32] |
| ** PARP-1 | Yes [33,34,35] | Yes [36] |
| *** DNA-PKcs | Yes [28,37] | Yes [38] |
| Artemis | Yes [28,39] | Yes [40] |
| Cerunnos | Yes [41] | Yes [41] |
| Ku70-Ku80 | Yes [42,43] | Yes [43,44] |
| Ligase IV | Yes [45,46] | Yes [46] |
| Fanconi Anemia | Yes [47] | Yes [46] |
| ^ CtIP | Yes [48] | Yes [48,49] |
| Rad17 | Yes [50] | Yes [51,52] |
| 9-1-1 complex | Yes [53] | Yes [53] |
| 14-3-3 complex | Yes [28] | Yes [54] |
| CTC complex | Yes [55,56] | Yes [55,56] |
| Telomere Dysfunction | Cancer Predisposition | |
|---|---|---|
| (See comment) ERCC6like2 (ERCC6L2) | Yes [57] | Yes [46] |
| CMMRD-Xeroderma pigmentosum | Yes [58] | Yes [33,46] |
| Bloom syndrome | Yes [59] | Yes [46,59] |
| Werner syndrome | Yes [59] | Yes [46,59] |
| Rothmund–Thomson syndrome | Yes [60] | Yes [46] |
| ATM-Mutated | Mer11 (AT-LD) | NBN (Nijmegen Breakage Syndrome) (NBS) |
|---|---|---|
| Deficient initial DNA repair. | Deficient initial DNA repair. | Deficient initial DNA repair. |
| Hypersensitivity to IR-induced chromosome breakage, increased translocations (chromosomal instability). | Hypersensitivity to IR-induced chromosome breakage, increased translocations (chromosomal instability). | Hypersensitivity to IR-induced chromosome breakage, increased translocations (chromosomal instability). |
| IR-induced thymocyte apoptosis relative to wild-type: 58% versus 90%. Severe intra-S and G2/M checkpoint defects. Telomere shortening. Cancer predisposition. | IR-induced thymocyte apoptosis relative to wild-type: Sightly reduced (81% versus 90%. Intra-S and G2/M checkpoints less severe than in ATM deficiency and comparable to Nsb1 null. No telomere shortening. No cancer predisposition (or mild). | IR-induced thymocyte apoptosis: Extreme variations in apoptotic capacity (in neural tissue similar to wild-type). Intra-S and G2/M checkpoints less severe than in ATM deficiency and comparable to Mre11 hypomorphic. Telomere shortening. Cancer predisposition. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Montaner, A. Interactions between the DNA Damage Response and the Telomere Complex in Carcinogenesis: A Hypothesis. Curr. Issues Mol. Biol. 2023, 45, 7582-7616. https://doi.org/10.3390/cimb45090478
Torres-Montaner A. Interactions between the DNA Damage Response and the Telomere Complex in Carcinogenesis: A Hypothesis. Current Issues in Molecular Biology. 2023; 45(9):7582-7616. https://doi.org/10.3390/cimb45090478
Chicago/Turabian StyleTorres-Montaner, Antonio. 2023. "Interactions between the DNA Damage Response and the Telomere Complex in Carcinogenesis: A Hypothesis" Current Issues in Molecular Biology 45, no. 9: 7582-7616. https://doi.org/10.3390/cimb45090478
APA StyleTorres-Montaner, A. (2023). Interactions between the DNA Damage Response and the Telomere Complex in Carcinogenesis: A Hypothesis. Current Issues in Molecular Biology, 45(9), 7582-7616. https://doi.org/10.3390/cimb45090478





