Production of GcMAF with Anti-Inflammatory Properties and Its Effect on Models of Induced Arthritis in Mice and Cystitis in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Experimental Animals
2.3. GcMAF Preparation
2.4. Ex Vivo Activation of PMs with DBP, GcMAF and Pure Enzymes
2.5. Obtaining cDNA
2.6. Real-Time PCR
2.7. Assessment of the Effect of Anti-GcMAF Preparation on the Development of Experimentally Induced Adjuvant Arthritis in Mice
2.8. Assessment of the Effect of Anti-GcMAF on Metabolic Activity of Macrophages in Mice
2.9. Assessment of the Effect of Anti-GcMAF on Hematological Parameters in Mice
2.10. Induction of Chronic Cystitis in Rats
2.11. Method for Studying the Effect of Anti-GcMAF on the Course of Chronic Cystitis
2.12. Statistical Analysis
3. Results
3.1. Experimental Search for the Synthesis Mode of GcMAF Exhibiting Anti-Inflammatory Characteristics and Synthesis of Preparative Amounts of Anti-Inflammatory GcMAF
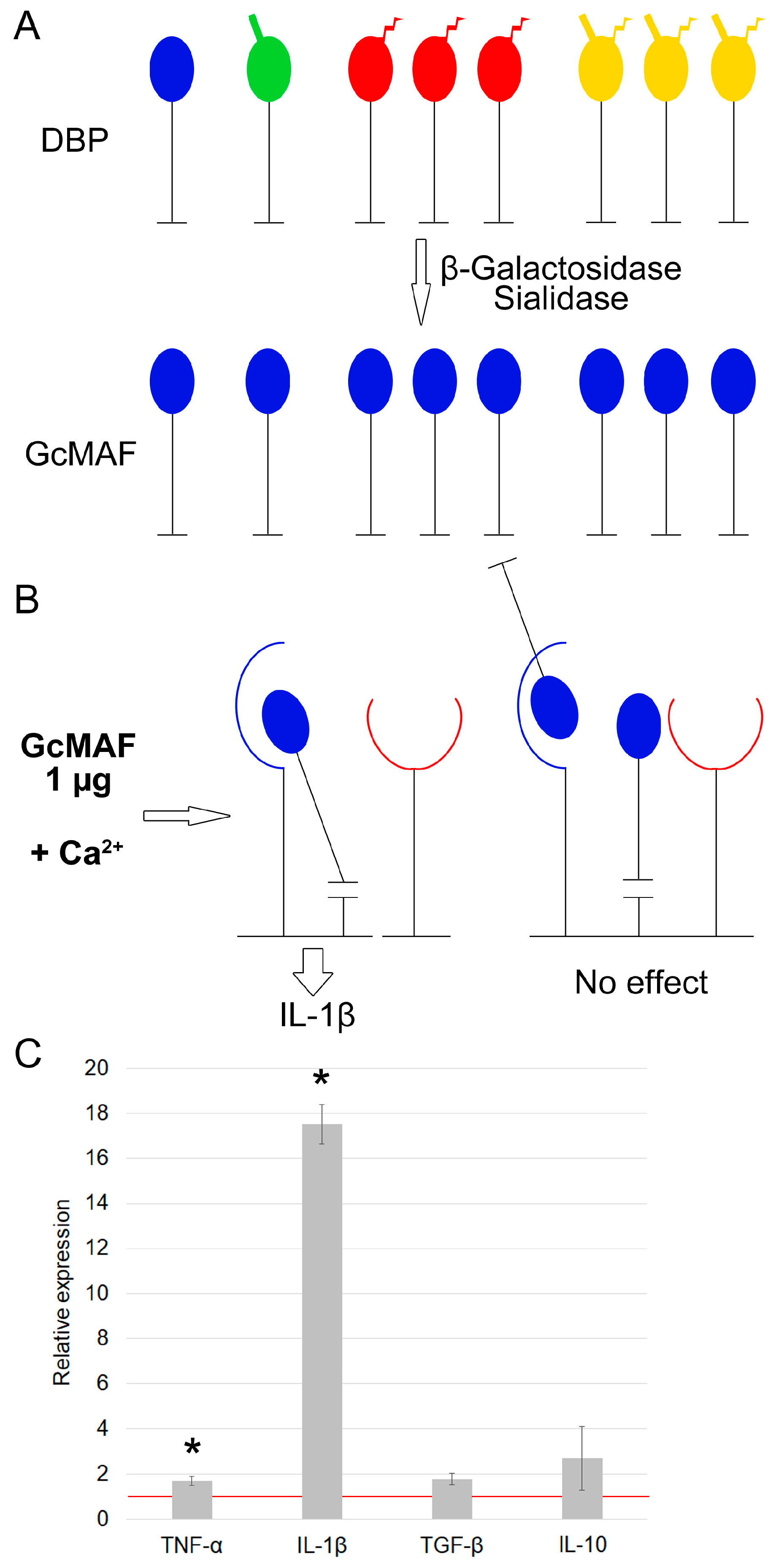
3.1.1. Characterization of mRNA Synthesis by PMs Activated by Exposure to GcMAF Produced by Hydrolysis with Two Enzymes (Sialidase and β–Galactosidase) in the Solution
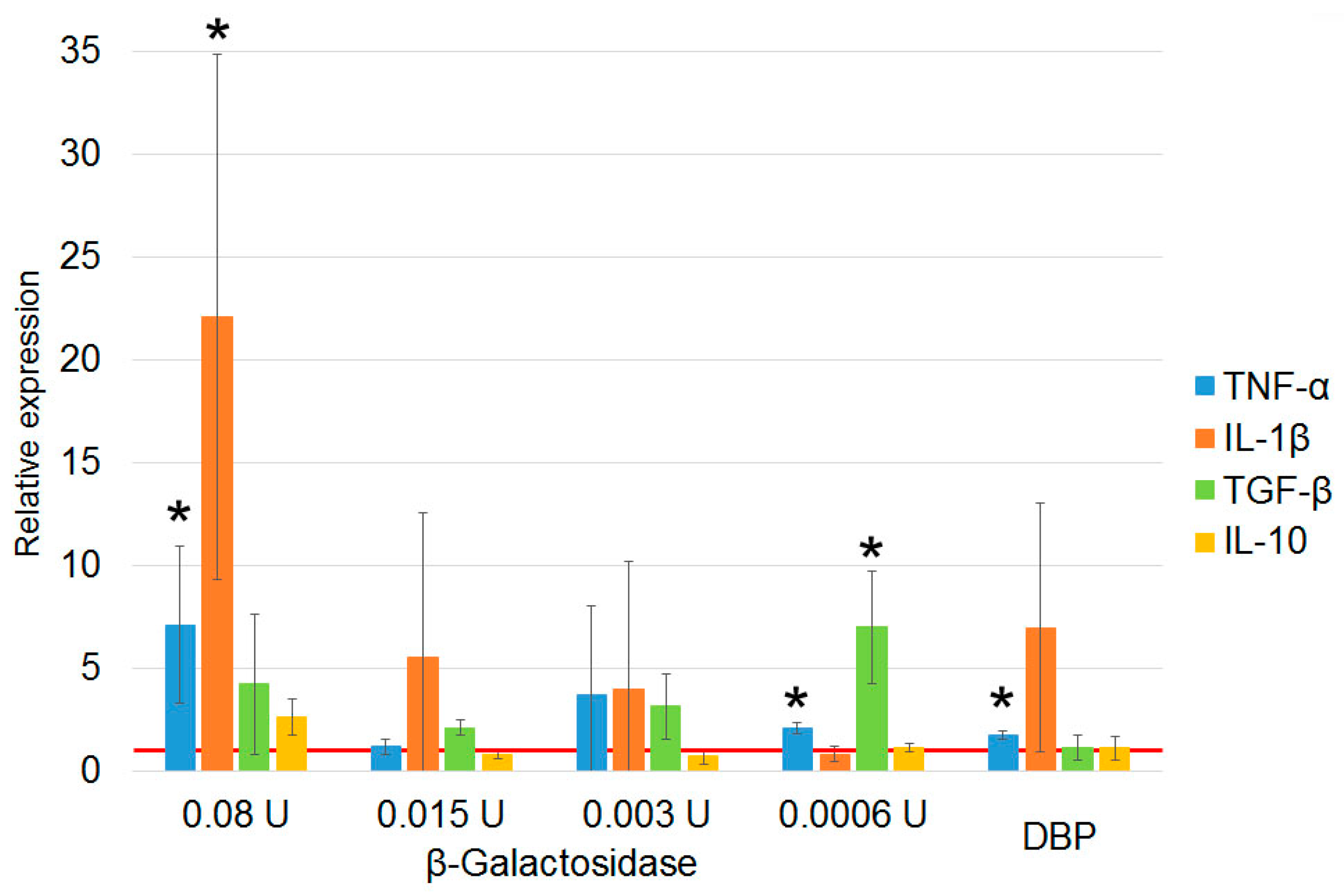
3.1.2. Characterization of Properties of Macrophage-Activating Factor in Different Modes of Deglycosylation with β–Galactosidase Monopreparation in the Solution
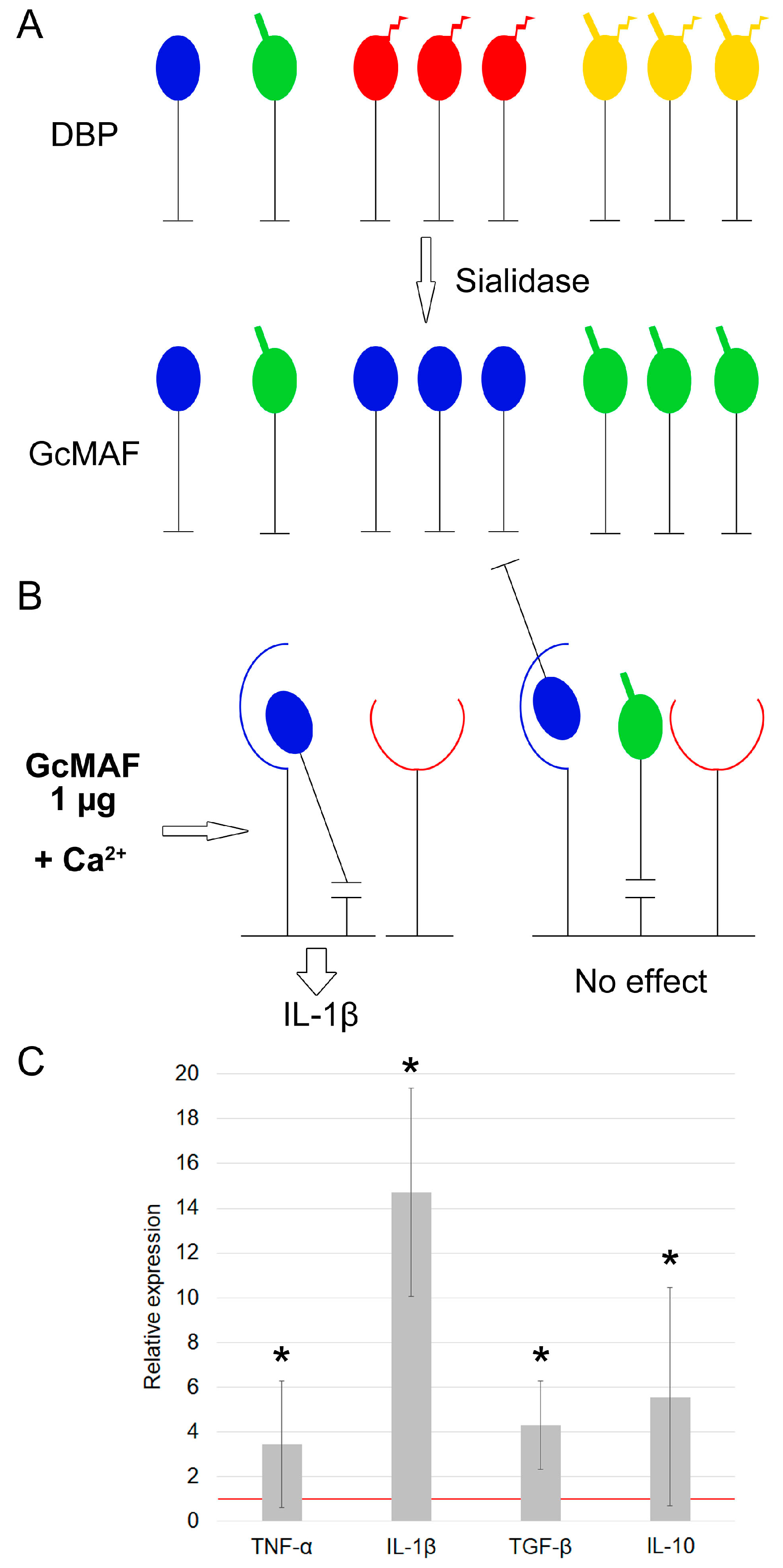
3.1.3. Characterization of Properties of Macrophage-Activating Factor in Different Modes of Glycosylation with Sialidase Monopreparation in the Solution
3.1.4. The Effect of Pure Enzymes on mRNA Synthesis of Analyzed Cytokines by PMs
3.1.5. Quantification of β–Galactosidase Dose upon Treatment of DBP “On Resin” That Is Required for Inducing PMs Toward mRNA Synthesis of Anti-Inflammatory Cytokines
3.2. The Effect of Anti-Inflammatory GcMAF on Clinical Signs of the Inflammatory Response of Arthrosis in Mice and Hemorrhagic Cystitis in Rats
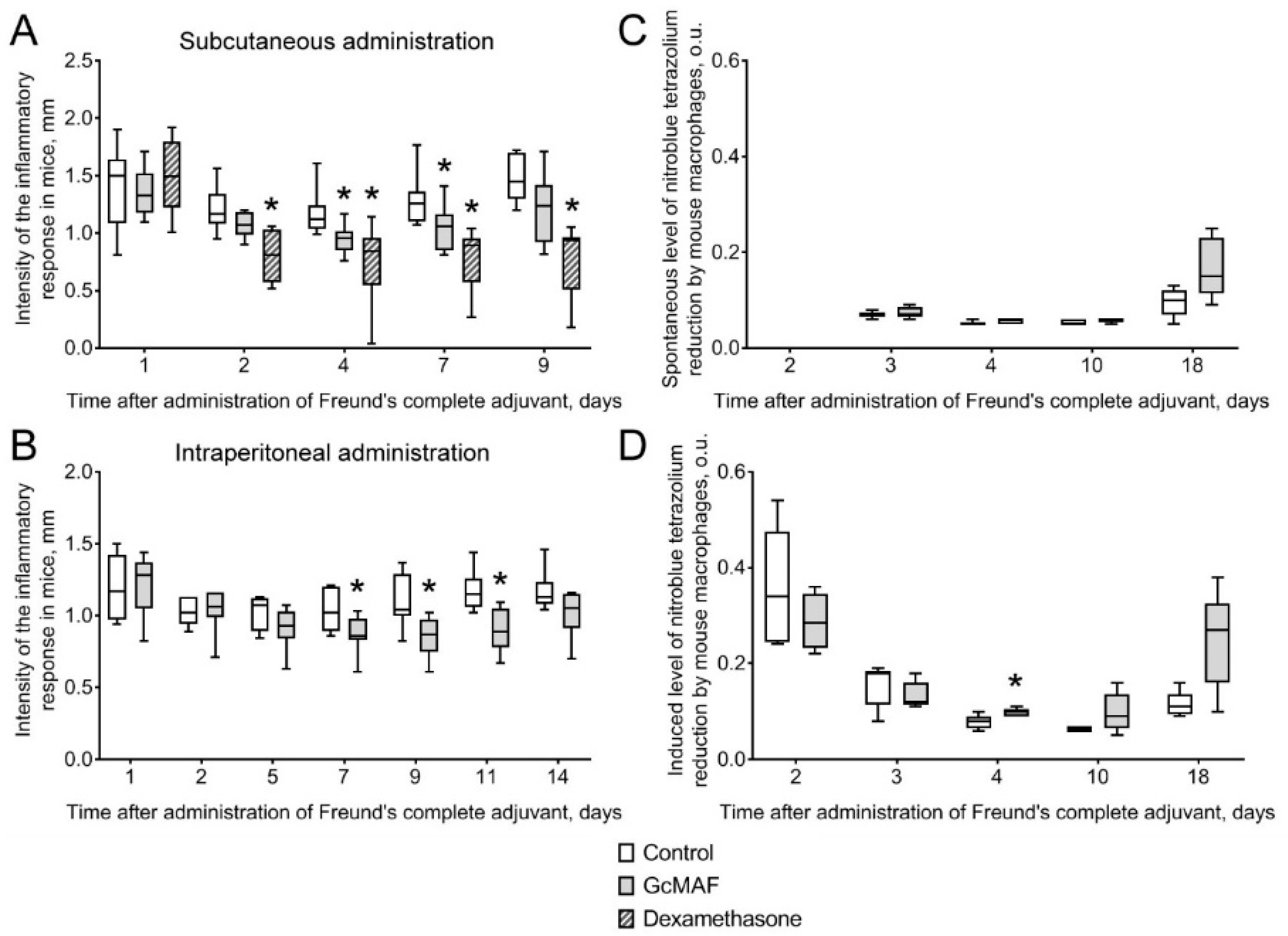
3.2.1. The Effect of Anti-GcMAF on Clinical Signs of the Inflammatory Response in Mice with Adjuvant Arthritis
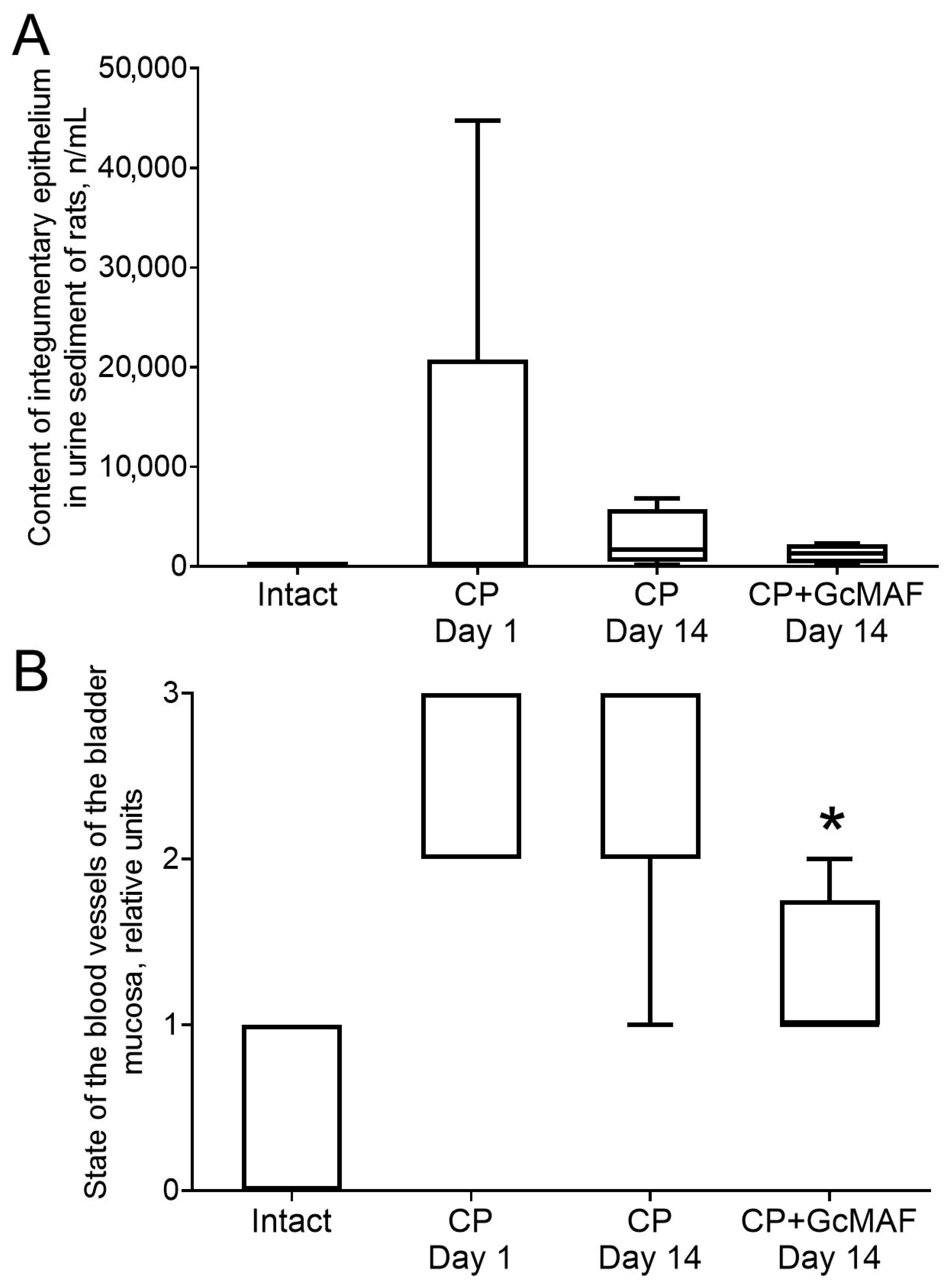
3.2.2. The Effect of Anti-GcMAF on Urine Parameters in Rats with Induced Cystitis
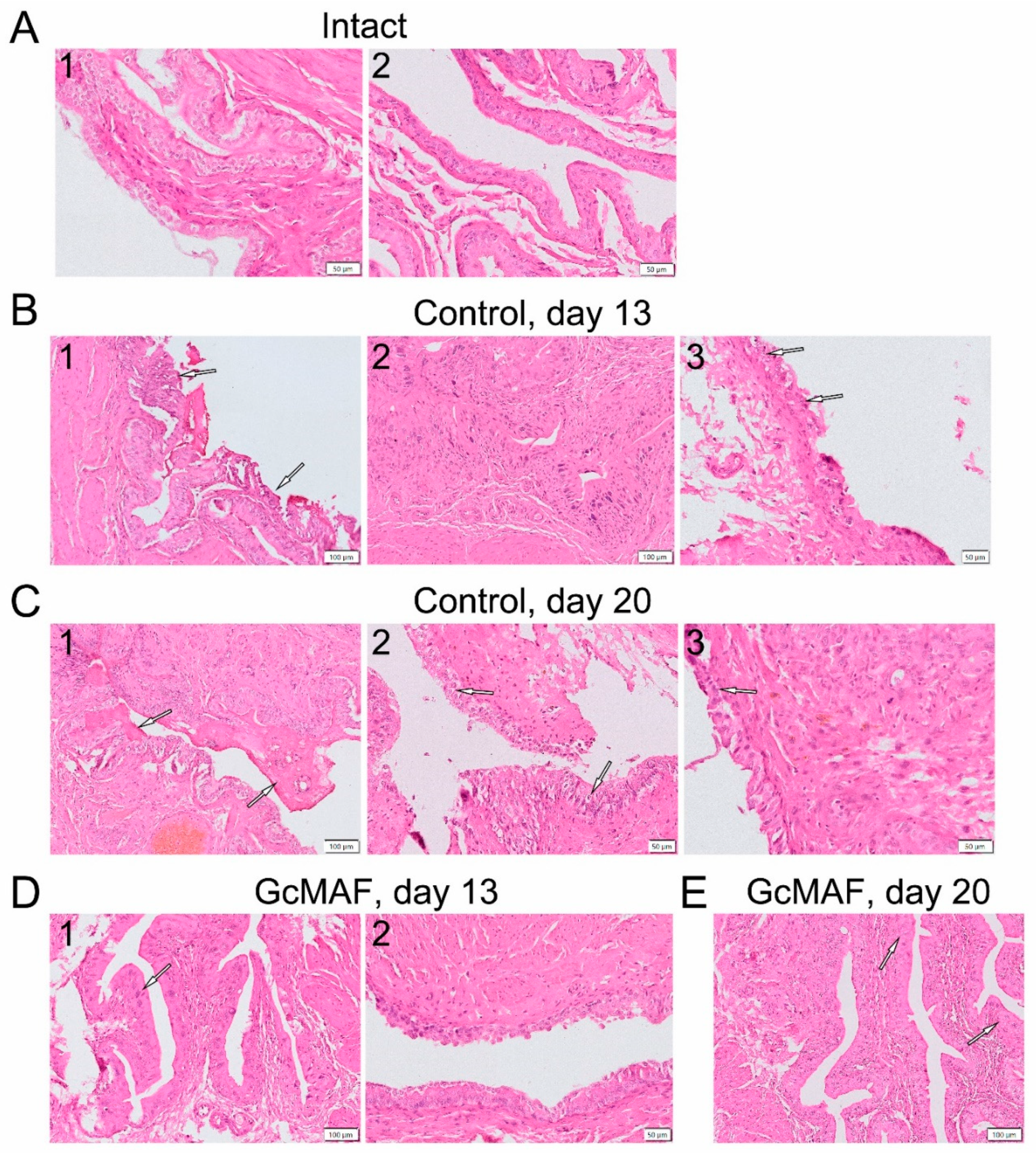
3.2.3. Macroscopic and Histological Examination of Bladder Samples Collected from Rats with Induced Hemorrhagic Cystitis
3.2.4. Hematological and Biochemical Examination of Blood Collected in Rats with Hemorrhagic Cystitis
4. Discussion
4.1. Production of GcMAF with Anti-Inflammatory Properties
- Hence, mRNA expression of pro- or anti-inflammatory cytokine genes is affected by the composition of the population of GcMAF molecules and the content of different variants of glycosylation sites in this population interacting with CLEC10 derivatives.
- In the presence of an excessive amount of GcMAF molecules, their aggregation (or other processes) occurs, and mRNA synthesis of the analyzed cytokines stops.
- The galactose terminal residue does not affect mRNA synthesis. Molecules carrying a terminal galactose residue compete for landing on the “anchor” 29 kDa CLEC10 derivative.
- The GalNAc moiety carrying a terminal sialic acid residue binds to the 63 kDa CLEC10 and induces TGF-β/IL-10 synthesis [28].
- The terminal GalNAc moiety interacts with 65 kDa CLEC10 and induces IL-1β synthesis [28].
4.2. The Effect of GcMAF with Anti-Inflammatory Properties on Models of Induced Arthritis in Mice and Cystitis in Rats
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, F.; Brune, J.L.; Naylor, S.L.; Cupples, R.L.; Naberhaus, K.H.; Bowman, B.H. Human Group-Specific Component (Gc) Is a Member of the Albumin Family. Proc. Natl. Acad. Sci. USA 1985, 82, 7994–7998. [Google Scholar] [CrossRef] [PubMed]
- Morales, E. GcMAF: A Polemic or a Highly Promising Molecule? World Sci. News 2017, 65, 20–36. [Google Scholar]
- Saburi, E.; Saburi, A.; Ghanei, M. Promising Role for Gc-MAF in Cancer Immunotherapy: From Bench to Bedside. Casp. J. Intern. Med. 2017, 8, 228–238. [Google Scholar] [CrossRef]
- Albracht, S.P. Immunotherapy with GcMAF Revisited—A Critical Overview of the Research of Nobuto Yamamoto. Cancer Treat. Res. Commun. 2022, 31, 100537. [Google Scholar] [CrossRef]
- Ruggiero, M.; Ward, E.; Smith, R.; Branca, J.J.V.; Noakes, D.; Morucci, G.; Taubmann, M.; Thyer, L.; Pacini, S. Oleic Acid, Deglycosylated Vitamin D-Binding Protein, Nitric Oxide: A Molecular Triad Made Lethal to Cancer. Anticancer Res. 2014, 34, 3569–3578. [Google Scholar]
- Uto, Y.; Yamamoto, S.; Takeuchi, R.; Nakagawa, Y.; Hirota, K.; Terada, H.; Onizuka, S.; Nakata, E.; Hori, H. Effect of the Gc-Derived Macrophage-Activating Factor Precursor (PreGcMAF) on Phagocytic Activation of Mouse Peritoneal Macrophages. Anticancer Res. 2011, 31, 2489–2492. [Google Scholar]
- Yamamoto, N.; Homma, S. Vitamin D3 Binding Protein (Group-Specific Component) Is a Precursor for the Macrophage-Activating Signal Factor from Lysophosphatidylcholine-Treated Lymphocytes. Proc. Natl. Acad. Sci. USA 1991, 88, 8539–8543. [Google Scholar] [CrossRef]
- Nagasawa, H.; Uto, Y.; Sasaki, H.; Okamura, N.; Murakami, A.; Kubo, S.; Kirk, K.L.; Hori, H. Gc Protein (Vitamin D-Binding Protein): Gc Genotyping and GcMAF Precursor Activity. Anticancer Res. 2005, 25, 3689–3695. [Google Scholar]
- Viau, M.; Constans, J.; Debray, H.; Montreuil, J. Isolation and Characterization of the O-Glycan Chain of the Human Vitamin-D Binding Protein. Biochem. Biophys. Res. Commun. 1983, 117, 324–331. [Google Scholar] [CrossRef]
- Yamamoto, N.; Kumashiro, R. Conversion of Vitamin D3 Binding Protein (Group-Specific Component) to a Macrophage Activating Factor by the Stepwise Action of Beta-Galactosidase of B Cells and Sialidase of T Cells. J. Immunol. 1993, 151, 2794–2802. [Google Scholar] [CrossRef]
- Homma, S.; Yamamoto, M.; Yamamoto, N. Vitamin D-Binding Protein (Group-Specific Component) Is the Sole Serum Protein Required for Macrophage Activation after Treatment of Peritoneal Cells with Lysophosphatidylcholine. Immunol. Cell Biol. 1993, 71, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Naraparaju, V.R.; Yamamoto, N. Roles of β-Galactosidase of B Lymphocytes and Sialidase of T Lymphocytes in Inflammation-Primed Activation of Macrophages. Immunol. Lett. 1994, 43, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Homma, S.; Haddad, J.G.; Kowalski, M.A. Vitamin D3 Binding Protein Required for in Vitro Activation of Macrophages after Alkylglycerol Treatment of Mouse Peritoneal Cells. Immunology 1991, 74, 420–424. [Google Scholar] [PubMed]
- Cooke, N.E.; David, E.V. Serum Vitamin D-Binding Protein Is a Third Member of the Albumin and Alpha Fetoprotein Gene Family. J. Clin. Investig. 1985, 76, 2420–2424. [Google Scholar] [CrossRef]
- Cleve, H.; Constans, J. The Mutants of the Vitamin-D-Binding Protein: More than 120 Variants of the GC/DBP System. Vox Sang. 1988, 54, 215–225. [Google Scholar] [CrossRef]
- Morita, Y.; Wang, R.; Li, X.; Muramatsu, T.; Ueda, M.; Hachimura, S.; Takahashi, S.; Miyakawa, T.; Tanokura, M. Improved Preparation of Group-Specific Component (Gc) Protein to Derive Macrophage Activating Factor. Protein Expr. Purif. 2020, 175, 105714. [Google Scholar] [CrossRef]
- Uto, Y.; Hori, H.; Kubo, K.; Ichihashi, M.; Sakamoto, N.; Mette, M.; Inui, T. GcMAF: Our next-Generation Immunotherapy. Nature 2012, 485, S67–S70. [Google Scholar]
- Borges, C.R.; Jarvis, J.W.; Oran, P.E.; Nelson, R.W. Population Studies of Vitamin D Binding Protein Microheterogeneity by Mass Spectrometry Lead to Characterization of Its Genotype-Dependent O-Glycosylation Patterns. J. Proteome Res. 2008, 7, 4143–4153. [Google Scholar] [CrossRef]
- Ravnsborg, T.; Olsen, D.T.; Thysen, A.H.; Christiansen, M.; Houen, G.; Højrup, P. The Glycosylation and Characterization of the Candidate Gc Macrophage Activating Factor. Biochim. Biophys. Acta-Proteins Proteom. 2010, 1804, 909–917. [Google Scholar] [CrossRef]
- Malik, S.; Fu, L.; Juras, D.J.; Karmali, M.; Wong, B.Y.L.; Gozdzik, A.; Cole, D.E.C. Common Variants of the Vitamin D Binding Protein Gene and Adverse Health Outcomes. Crit. Rev. Clin. Lab. Sci. 2013, 50, 1–22. [Google Scholar] [CrossRef]
- Daiger, S.P.; Schanfield, M.S.; Cavalli Sforza, L.L. Group-Specific Component (Gc) Proteins Bind Vitamin D and 25-Hydroxyvitamin, D. Proc. Natl. Acad. Sci. USA 1975, 72, 2076–2080. [Google Scholar] [CrossRef] [PubMed]
- Borges, C.R.; Rehder, D.S. Glycan Structure of Gc Protein-Derived Macrophage Activating Factor as Revealed by Mass Spectrometry. Arch. Biochem. Biophys. 2016, 606, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Rehder, D.S.; Nelson, R.W.; Borges, C.R. Glycosylation Status of Vitamin D Binding Protein in Cancer Patients. Protein Sci. 2009, 18, 2036–2042. [Google Scholar] [CrossRef] [PubMed]
- Kanie, Y.; Maegawa, Y.; Wei, Y.; Kanie, O. Investigation of the Protective Effect for GcMAF by a Glycosidase Inhibitor and the Glycan Structure of Gc Protein. Molecules 2023, 28, 1570. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y. M1 and M2 Polarization of Macrophages: A Mini-Review. Med. Biol. Sci. Eng. 2019, 2, 1–5. [Google Scholar] [CrossRef]
- Ruggiero, M. Gc Protein-Derived Macrophage Activating Factor (GcMAF) and Autism: Do Clinical Results Require a Novel Interpretation? Am. J. Immunol. 2016, 12, 77–82. [Google Scholar] [CrossRef]
- Dolgova, E.V.; Kirikovich, S.S.; Levites, E.V.; Ruzanova, V.S.; Proskurina, A.S.; Ritter, G.S.; Taranov, O.S.; Varaksin, N.A.; Ryabicheva, T.G.; Leplina, O.Y.; et al. Analysis of the Biological Properties of Blood Plasma Protein with GcMAF Functional Activity. Int. J. Mol. Sci. 2022, 23, 75. [Google Scholar] [CrossRef]
- Kirikovich, S.S.; Levites, E.V.; Proskurina, A.S.; Ritter, G.S.; Peltek, S.E.; Vasilieva, A.R.; Ruzanova, V.S.; Dolgova, E.V.; Oshihmina, S.G.; Sysoev, A.V.; et al. The Molecular Aspects of Functional Activity of Macrophage-Activating Factor GcMAF. Int. J. Mol. Sci. 2023, 24, 17396. [Google Scholar] [CrossRef]
- Ruzanova, V.S.; Kirikovich, S.S.; Levites, E.V.; Proskurina, A.S.; Dolgova, E.V.; Ritter, G.S.; Efremov, Y.R.; Dubatolova, T.D.; Sysoev, A.V.; Koleno, D.I.; et al. The Macrophage Activator GcMAF-RF Enhances the Antitumor Effect of Karanahan Technology through Induction of M2-M1 Macrophage Reprogramming. J. Immunol. Res. 2024, 2024, 7484490. [Google Scholar] [CrossRef]
- Bouillon, R.; Schuit, F.; Antonio, L.; Rastinejad, F. Vitamin D Binding Protein: A Historic Overview. Front. Endocrinol. 2020, 10, 910. [Google Scholar] [CrossRef]
- Chillingworth, N.L.; Donaldson, L.F. Characterisation of a Freund’s Complete Adjuvant-Induced Model of Chronic Arthritis in Mice. J. Neurosci. Methods 2003, 128, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Bogacheva, N.V.; Tuneva, N.A.; Smirnov, A.A.; Galyamova, D.A.; Popescu, L.I. Development of a biological model of immunosuppression using dexamethasone. Vyatka Med. Bull. 2018, 4, 39–43. (In Russian) [Google Scholar]
- Madzhidov, U.V. Suppressor T Cells upon Experimentally Induced Adjuvant Arthritis and Immune Suppression. Zh. Mikrobiol. Epidemiol. Immunobiol. 1984, 1, 65–68. (In Russian) [Google Scholar]
- Novitskii, V.V.; Evtushenko, O.M. Practical Hematology Guidelines; St. Publishing: Tomsk, Russia, 1999. (In Russian) [Google Scholar]
- Chen, J.L.; Zhou, X.; Ding, H.L.; Zhan, H.L.; Yang, F.; Li, W.B.; Xie, J.C.; Liu, X.G.; Xu, Y.C.; Su, M.Z.; et al. Neuregulin-1-ErbB Signaling Promotes Microglia Activation Contributing to Mechanical Allodynia of Cyclophosphamide-Induced Cystitis. Neurourol. Urodyn. 2019, 38, 1250–1260. [Google Scholar] [CrossRef]
- Ding, H.; Chen, J.; Su, M.; Lin, Z.; Zhan, H.; Yang, F.; Li, W.; Xie, J.; Huang, Y.; Liu, X.; et al. BDNF Promotes Activation of Astrocytes and Microglia Contributing to Neuroinflammation and Mechanical Allodynia in Cyclophosphamide-Induced Cystitis. J. Neuroinflammation 2020, 17, 19. [Google Scholar] [CrossRef]
- Augé, C.; Gamé, X.; Vergnolle, N.; Lluel, P.; Chabot, S. Characterization and Validation of a Chronic Model of Cyclophosphamide-Induced Interstitial Cystitis/Bladder Pain Syndrome in Rats. Front. Pharmacol. 2020, 11, 572871. [Google Scholar] [CrossRef]
- Gray, K.J.; Engelmann, U.H.; Johnson, E.H.; Fishman, I.J. Evaluation of Misoprostol Cytoprotection of the Bladder with Cyclophosphamide (Cytoxan) Therapy. J. Urol. 1986, 136, 497–500. [Google Scholar] [CrossRef]
- Varki, A.; Cummings, R.D.; Esko, J.D.; Freeze, H.H.; Stanley, P.; Bertozzi, C.R.; Hart, G.W.; Etzler, M.E. Essentials of Glycobiology. Cold Spring Harb. 2009, 39, 2015–2017. [Google Scholar]
- Lima, M.; Baynes, J.W. Glycation. In Encyclopedia of Biological Chemistry, 2nd ed.; Academic Press: Cambridge, MA, USA, 2013; pp. 405–411. [Google Scholar] [CrossRef]
- Takeuchi, H.; Haltiwanger, R.S. Significance of Glycosylation in Notch Signaling. Biochem. Biophys. Res. Commun. 2014, 453, 235. [Google Scholar] [CrossRef]
- Hoober, K.J. Asgr1 and Its Enigmatic Relative, CLEC10A. Int. J. Mol. Sci. 2020, 21, 4818. [Google Scholar] [CrossRef]
- Klimp, A.H.; De Vries, E.G.E.; Scherphof, G.L.; Daemen, T. A Potential Role of Macrophage Activation in the Treatment of Cancer. Crit. Rev. Oncol. Hematol. 2002, 44, 143–161. [Google Scholar] [CrossRef] [PubMed]
- Zaal, A.; Li, R.J.E.; Lübbers, J.; Bruijns, S.C.M.; Kalay, H.; van Kooyk, Y.; van Vliet, S.J. Activation of the C-Type Lectin MGL by Terminal GalNAc Ligands Reduces the Glycolytic Activity of Human Dendritic Cells. Front. Immunol. 2020, 11, 305. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.K.; Huang, S.C.C.; Sergushichev, A.; Lampropoulou, V.; Ivanova, Y.; Loginicheva, E.; Chmielewski, K.; Stewart, K.M.; Ashall, J.; Everts, B.; et al. Network Integration of Parallel Metabolic and Transcriptional Data Reveals Metabolic Modules That Regulate Macrophage Polarization. Immunity 2015, 42, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Geeraerts, X.; Bolli, E.; Fendt, S.M.; Van Ginderachter, J.A. Macrophage Metabolism as Therapeutic Target for Cancer, Atherosclerosis, and Obesity. Front. Immunol. 2017, 8, 250637. [Google Scholar] [CrossRef] [PubMed]
- van Vliet, S.J.; Gringhuis, S.I.; Geijtenbeek, T.B.H.; van Kooyk, Y. Regulation of Effector T Cells by Antigen-Presenting Cells via Interaction of the C-Type Lectin MGL with CD45. Nat. Immunol. 2006, 7, 1200–1208. [Google Scholar] [CrossRef]
- Napoletano, C.; Zizzari, I.G.; Rughetti, A.; Rahimi, H.; Irimura, T.; Clausen, H.; Wandall, H.H.; Belleudi, F.; Bellati, F.; Pierelli, L.; et al. Targeting of Macrophage Galactose-Type C-Type Lectin (MGL) Induces DC Signaling and Activation. Eur. J. Immunol. 2012, 42, 936–945. [Google Scholar] [CrossRef]
- van Vliet, S.J.; Bay, S.; Vuist, I.M.; Kalay, H.; García-Vallejo, J.J.; Leclerc, C.; van Kooyk, Y. MGL Signaling Augments TLR2-Mediated Responses for Enhanced IL-10 and TNF-α Secretion. J. Leukoc. Biol. 2013, 94, 315–323. [Google Scholar] [CrossRef]
- Heger, L.; Balk, S.; Lühr, J.J.; Heidkamp, G.F.; Lehmann, C.H.K.; Hatscher, L.; Purbojo, A.; Hartmann, A.; Garcia-Martin, F.; Nishimura, S.I.; et al. CLEC10A Is a Specific Marker for Human CD1c+ Dendritic Cells and Enhances Their Toll-like Receptor 7/8-Induced Cytokine Secretion. Front. Immunol. 2018, 9, 347880. [Google Scholar] [CrossRef]
- Mortezai, N.; Behnken, H.N.; Kurze, A.K.; Ludewig, P.; Buck, F.; Meyer, B.; Wagener, C. Tumor-Associated Neu5Ac-Tn and Neu5Gc-Tn Antigens Bind to C-Type Lectin CLEC10A (CD301, MGL). Glycobiology 2013, 23, 844–852. [Google Scholar] [CrossRef]
- Diniz, A.; Coelho, H.; Dias, J.S.; van Vliet, S.J.; Jiménez-Barbero, J.; Corzana, F.; Cabrita, E.J.; Marcelo, F. The Plasticity of the Carbohydrate Recognition Domain Dictates the Exquisite Mechanism of Binding of Human Macrophage Galactose-Type Lectin. Chem.-A Eur. J. 2019, 25, 13945–13955. [Google Scholar] [CrossRef]
- Marcelo, F.; Supekar, N.; Corzana, F.; Van Der Horst, J.C.; Vuist, I.M.; Live, D.; Boons, G.J.P.H.; Smith, D.F.; Van Vliet, S.J. Identification of a Secondary Binding Site in Human Macrophage Galactose-Type Lectin by Microarray Studies: Implications for the Molecular Recognition of Its Ligands. J. Biol. Chem. 2019, 294, 1300–1311. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, W.; Cao, W.; Liu, K.; Su, S.; Ma, J.; Li, X. Selective Synthesis of α- and β-Glycosides of N-Acetyl Galactosamine Using Rare Earth Metal Triflates. Front. Chem. 2022, 10, 1029911. [Google Scholar] [CrossRef] [PubMed]
- Munkley, J.; Elliott, D.J.; Munkley, J.; Elliott, D.J. Hallmarks of Glycosylation in Cancer. Oncotarget 2016, 7, 35478–35489. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Wang, L.; Zurawski, S.; Oh, S. Signaling Cascade through DC-ASGPR Induces Transcriptionally Active CREB for IL-10 Induction and Immune Regulation. J. Immunol. 2019, 203, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Udalova, I.A.; Mantovani, A.; Feldmann, M. Macrophage Heterogeneity in the Context of Rheumatoid Arthritis. Nat. Publ. Gr. 2016, 12, 472–485. [Google Scholar] [CrossRef]
- Homma, Y.; Ueda, T.; Tomoe, H.; Lin, A.T.L.; Kuo, H.C.; Lee, M.H.; Oh, S.J.; Kim, J.C.; Lee, K.S. Clinical Guidelines for Interstitial Cystitis and Hypersensitive Bladder Updated in 2015. Int. J. Urol. 2016, 23, 542–549. [Google Scholar] [CrossRef]
- Birder, L.A. Pathophysiology of Interstitial Cystitis. Int. J. Urol. 2019, 26, 12–15. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirikovich, S.S.; Levites, E.V.; Proskurina, A.S.; Ritter, G.S.; Dolgova, E.V.; Ruzanova, V.S.; Oshihmina, S.G.; Snegireva, J.S.; Gamaley, S.G.; Sysoeva, G.M.; et al. Production of GcMAF with Anti-Inflammatory Properties and Its Effect on Models of Induced Arthritis in Mice and Cystitis in Rats. Curr. Issues Mol. Biol. 2024, 46, 10934-10959. https://doi.org/10.3390/cimb46100650
Kirikovich SS, Levites EV, Proskurina AS, Ritter GS, Dolgova EV, Ruzanova VS, Oshihmina SG, Snegireva JS, Gamaley SG, Sysoeva GM, et al. Production of GcMAF with Anti-Inflammatory Properties and Its Effect on Models of Induced Arthritis in Mice and Cystitis in Rats. Current Issues in Molecular Biology. 2024; 46(10):10934-10959. https://doi.org/10.3390/cimb46100650
Chicago/Turabian StyleKirikovich, Svetlana S., Evgeniy V. Levites, Anastasia S. Proskurina, Genrikh S. Ritter, Evgeniya V. Dolgova, Vera S. Ruzanova, Sofya G. Oshihmina, Julia S. Snegireva, Svetlana G. Gamaley, Galina M. Sysoeva, and et al. 2024. "Production of GcMAF with Anti-Inflammatory Properties and Its Effect on Models of Induced Arthritis in Mice and Cystitis in Rats" Current Issues in Molecular Biology 46, no. 10: 10934-10959. https://doi.org/10.3390/cimb46100650
APA StyleKirikovich, S. S., Levites, E. V., Proskurina, A. S., Ritter, G. S., Dolgova, E. V., Ruzanova, V. S., Oshihmina, S. G., Snegireva, J. S., Gamaley, S. G., Sysoeva, G. M., Danilenko, E. D., Taranov, O. S., Ostanin, A. A., Chernykh, E. R., Kolchanov, N. A., & Bogachev, S. S. (2024). Production of GcMAF with Anti-Inflammatory Properties and Its Effect on Models of Induced Arthritis in Mice and Cystitis in Rats. Current Issues in Molecular Biology, 46(10), 10934-10959. https://doi.org/10.3390/cimb46100650






