Pathophysiology, Treatment, and Prognosis of Thrombocytopenia, Anasarca, Fever, Reticulin Fibrosis/Renal Failure, and Organomegaly (TAFRO) Syndrome: A Review
Abstract
1. Introduction
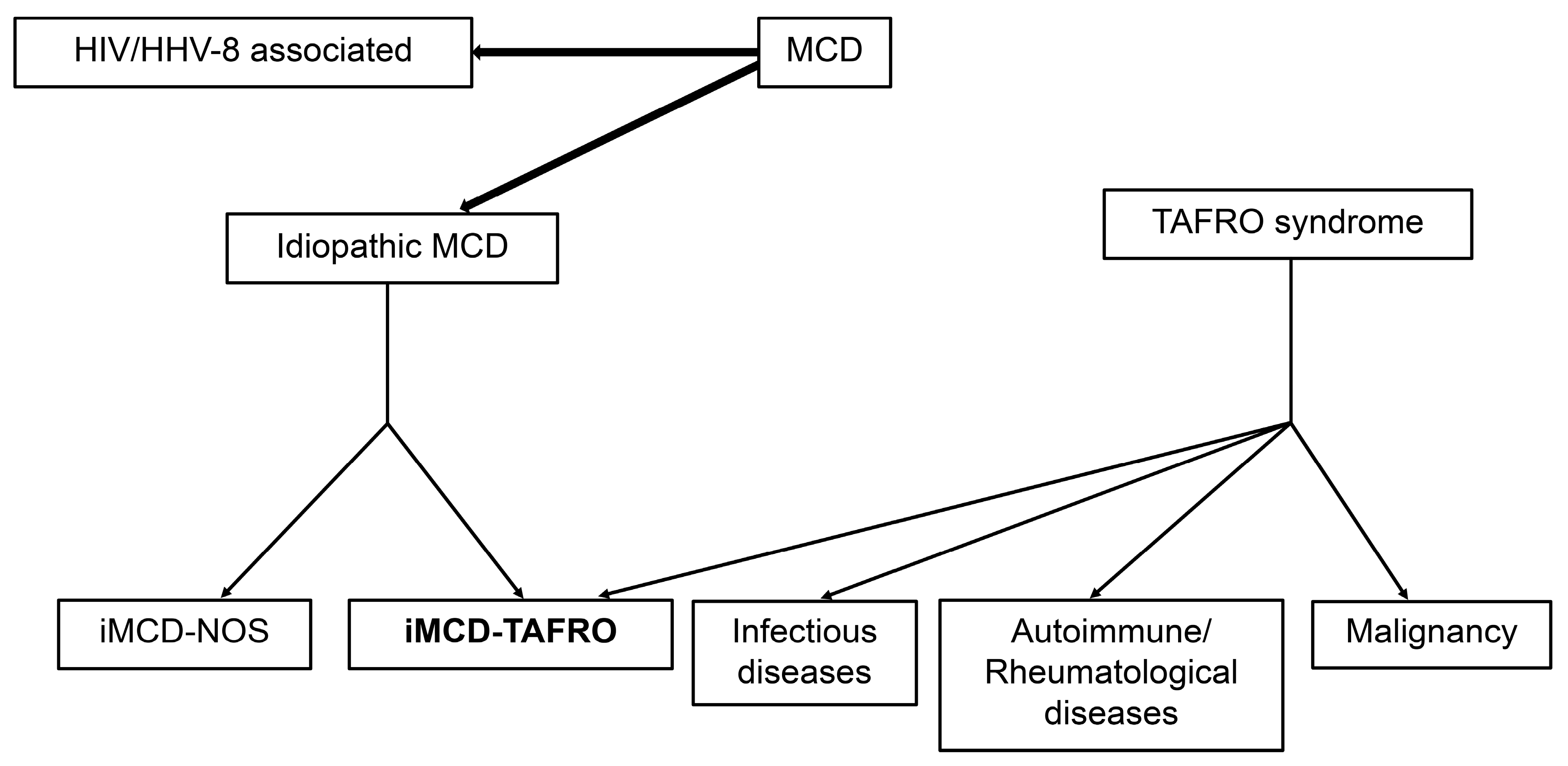
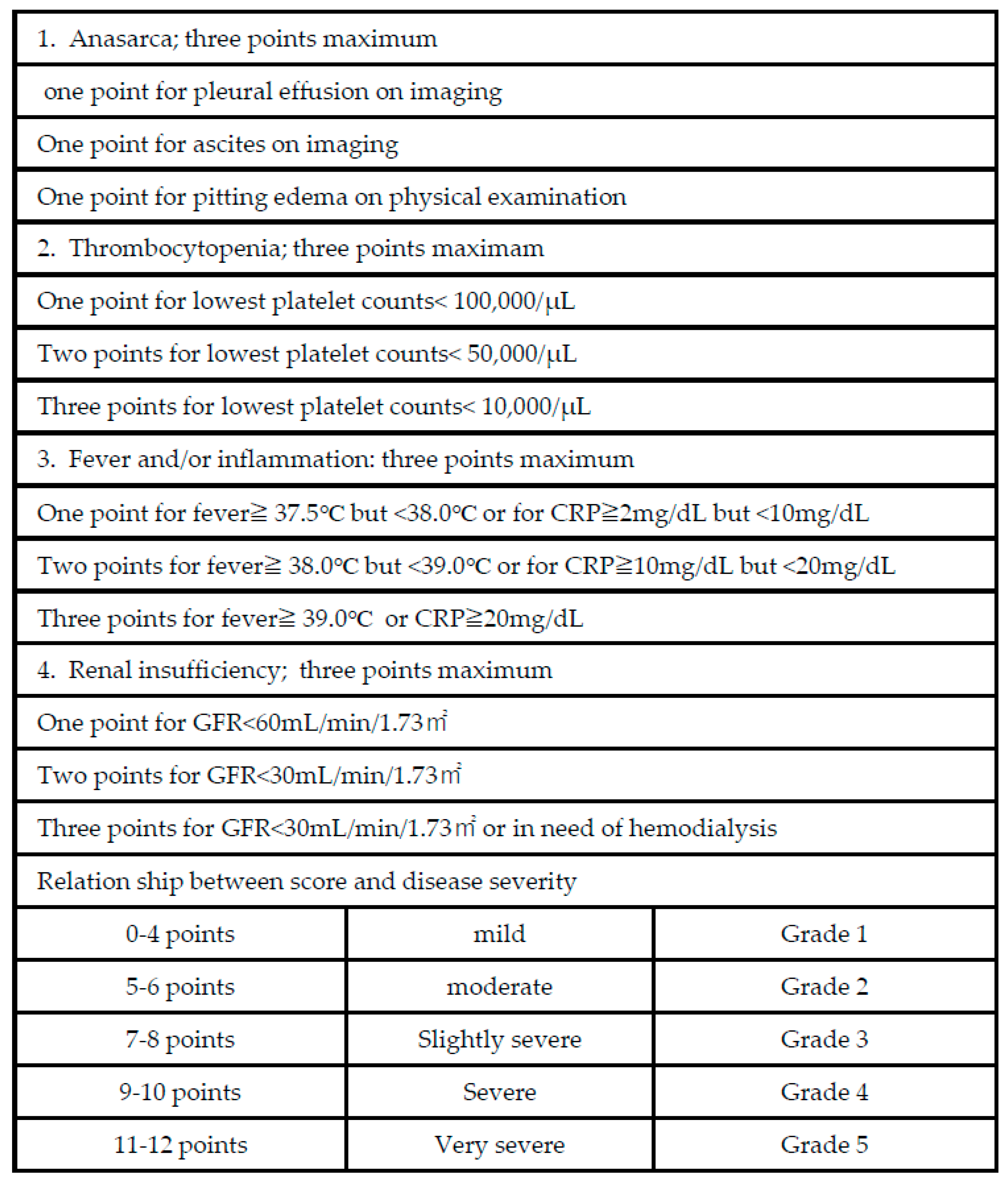
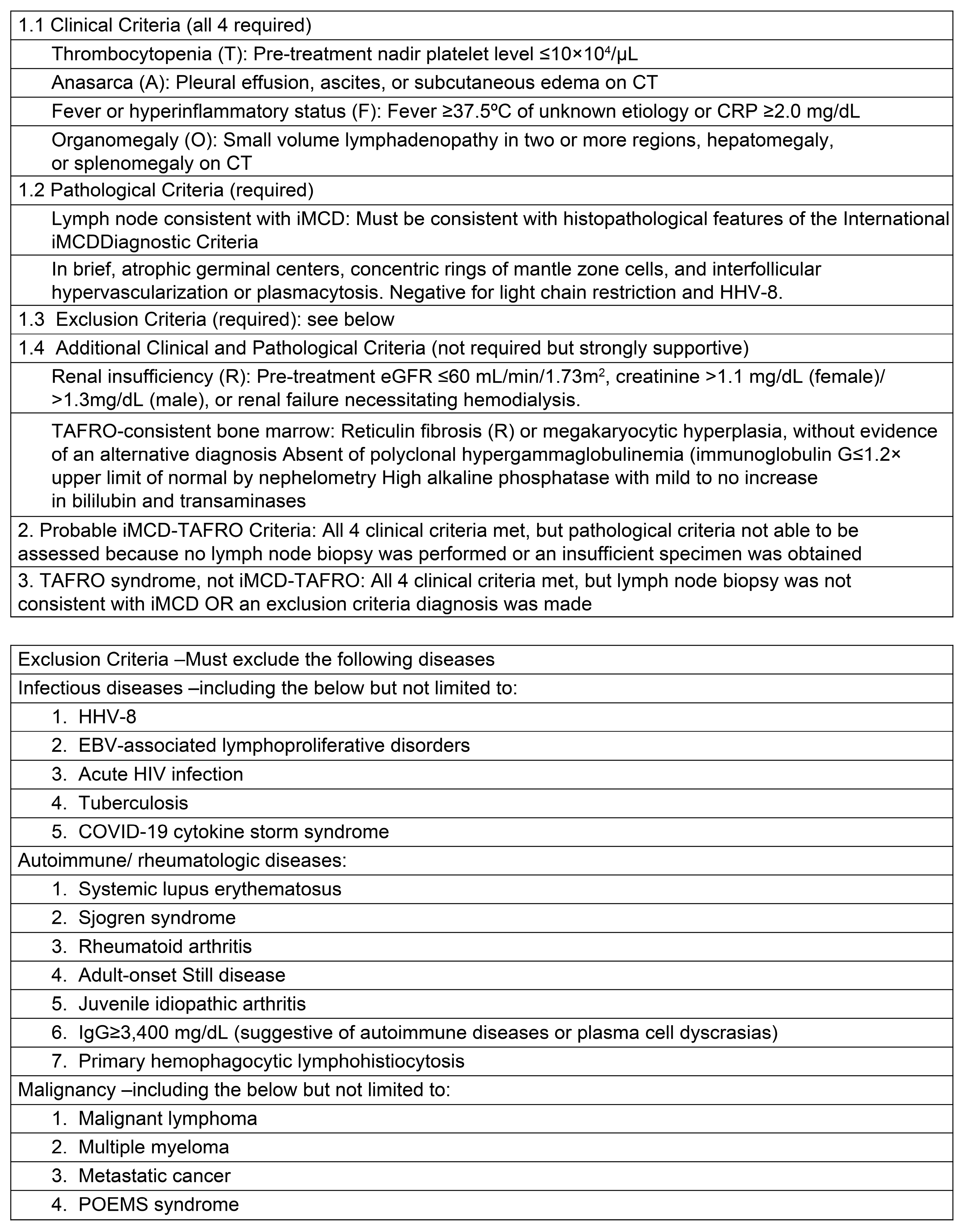
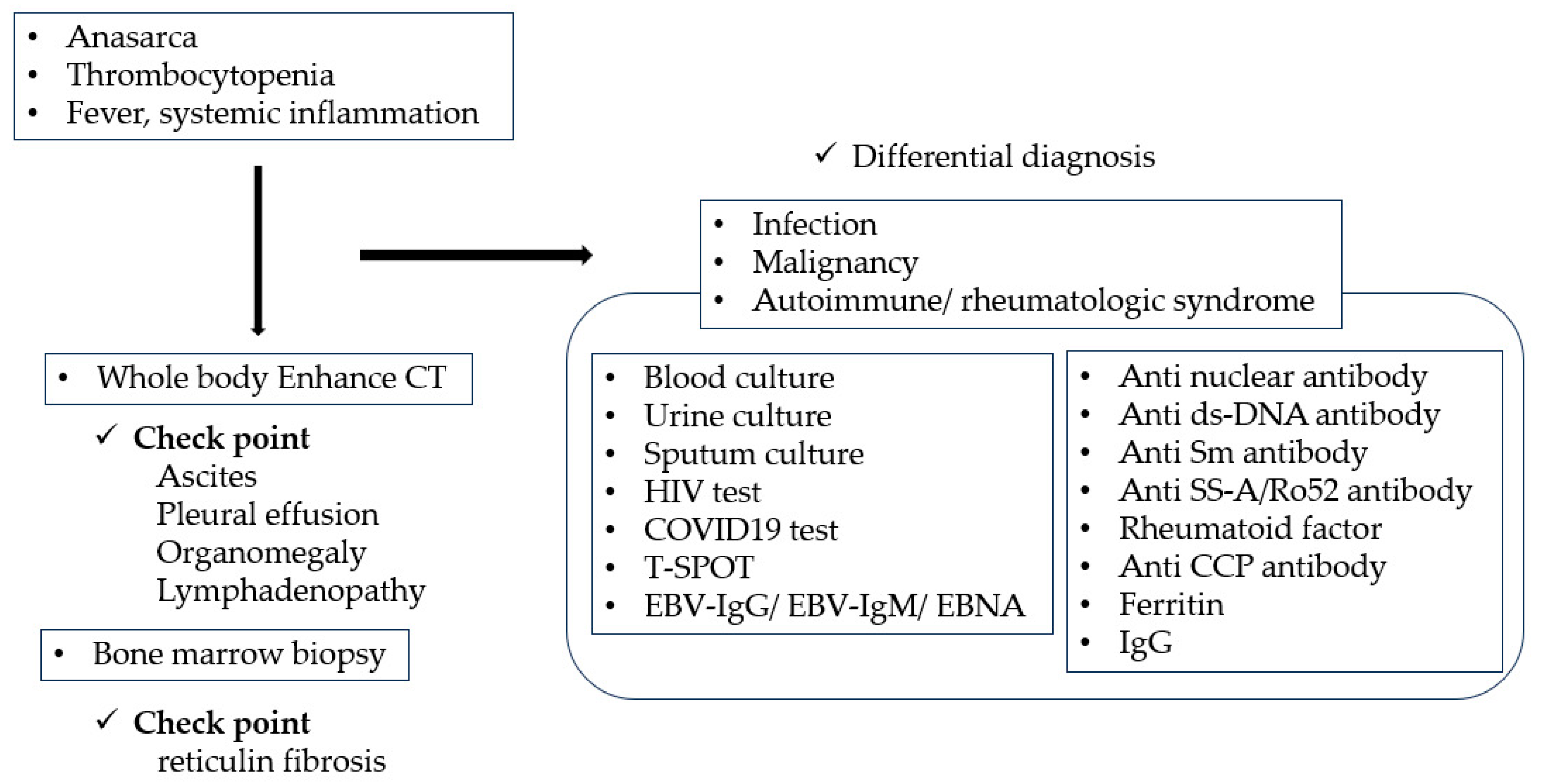
2. Classification Criteria for TAFRO Syndrome
MCD
3. Differences between TAFRO Syndrome and iMCD
4. Histopathological Findings Regarding TAFRO Syndrome
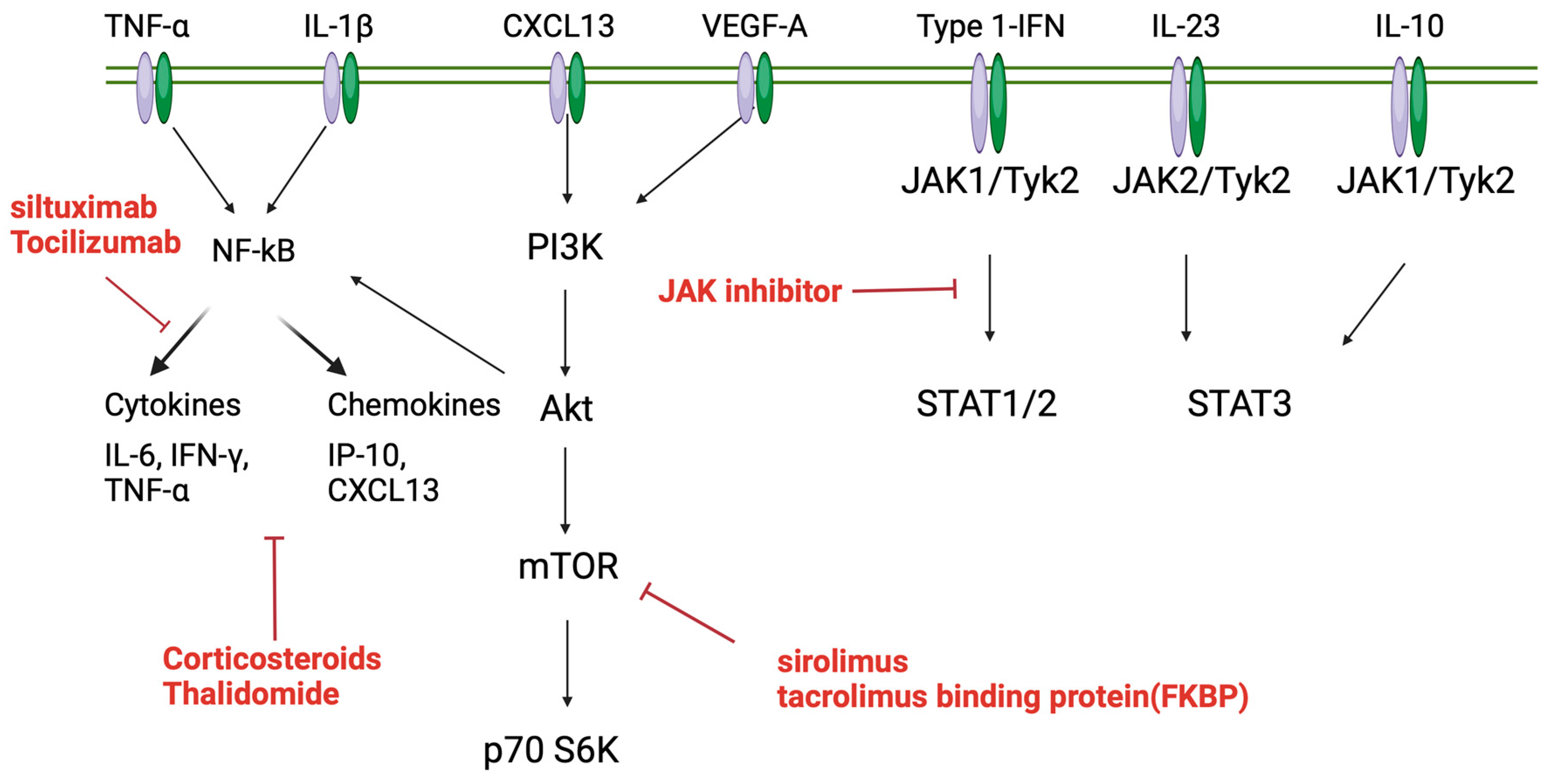
5. Pathophysiology of TAFRO Syndrome
5.1. Involvement of Type 1 Interferon
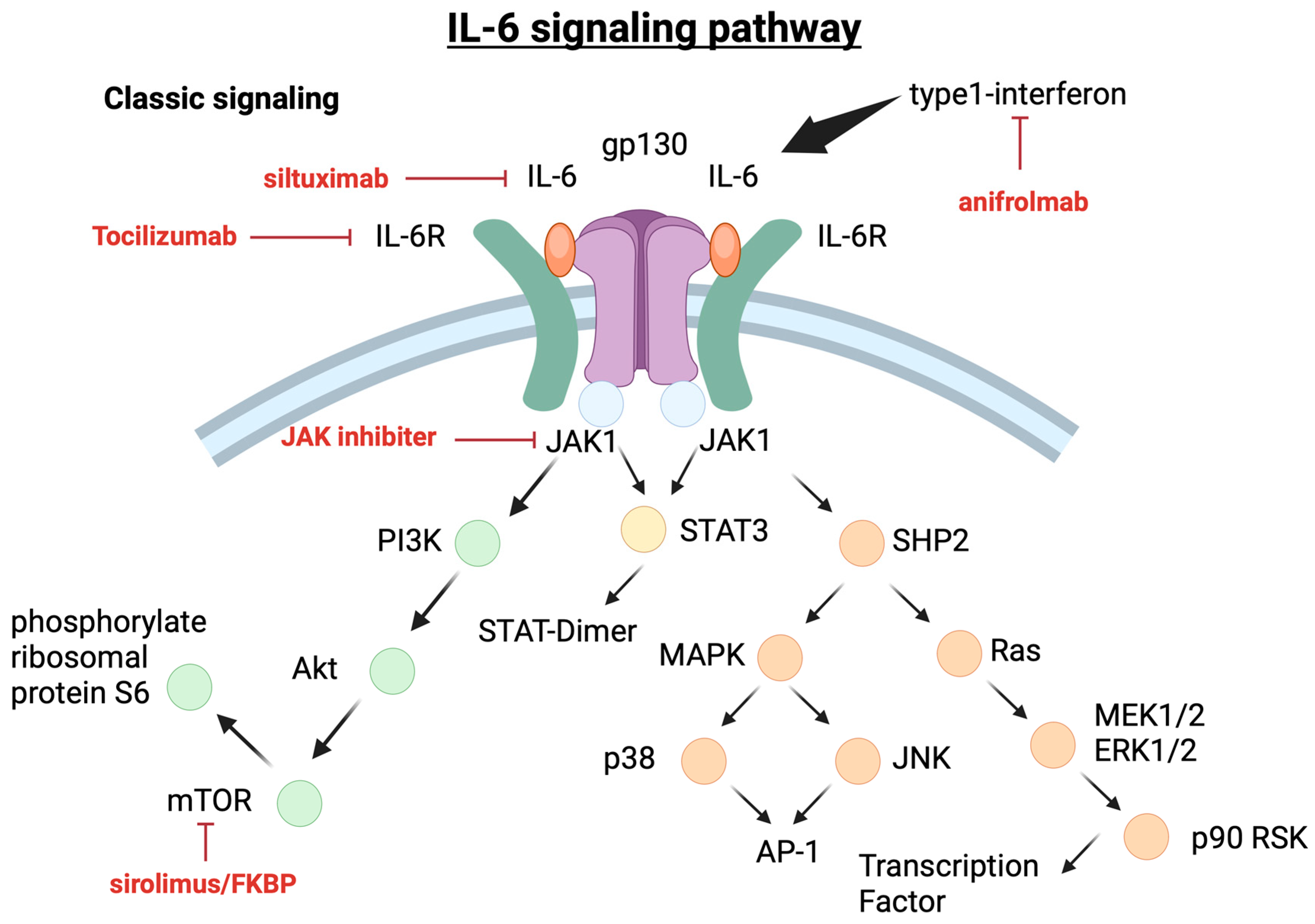
5.1.1. IL-6 Pathways
5.1.2. PI3K/Akt/mTOR Pathway
5.1.3. mTOR2 Pathway Activation of iMCD–TAFRO
5.1.4. JAK/STAT3 Pathway
6. Treatment of TAFRO Syndrome
6.1. Corticosteroids
6.2. Anti-IL6 Therapy
6.3. mTOR Inhibitor
6.4. JAK-1/2 Inhibitor
6.5. Anti-CD20 Therapies
6.6. Calcineurin Inhibitors
6.7. Cytotoxic Chemotherapy
6.8. Thalidomide
7. Prognosis
8. Conclusions—Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Takai, K.; Nikkuni, K.; Shibuya, H.; Hashidate, H. [Thrombocytopenia with mild bone marrow fibrosis accompanied by fever, pleural effusion, ascites and hepatosplenomegaly]. Rinsho Ketsueki 2010, 51, 320–325. [Google Scholar] [PubMed]
- Inoue, M.; Ankou, M.; Hua, J.; Iwaki, Y.; Hagihara, M.; Ota, Y. Complete resolution of TAFRO syndrome (thrombocytopenia, anasarca, fever, reticulin fibrosis and organomegaly) after immunosuppressive therapies using corticosteroids and cyclosporin A: A case report. J. Clin. Exp. Hematop. 2013, 53, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Iwaki, N.; Sato, Y.; Takata, K.; Kondo, E.; Ohno, K.; Takeuchi, M.; Orita, Y.; Nakao, S.; Yoshino, T. Atypical hyaline vascular-type Castleman’s disease with thrombocytopenia, anasarca, fever, and systemic lymphadenopathy. J. Clin. Exp. Hematop. 2013, 53, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Masaki, Y.; Nakajima, A.; Iwao, H.; Kurose, N.; Sato, T.; Nakamura, T.; Miki, M.; Sakai, T.; Kawanami, T.; Sawaki, T.; et al. Japanese variant of multicentric Castleman’s disease associated with serositis and thrombocytopenia—A report of two cases: Is TAFRO syndrome (Castleman- Kojima disease) a distinct clinicopathological entity? J. Clin. Exp. Hematop. 2013, 53, 79–85. [Google Scholar] [CrossRef]
- Louis, C.; Vijgen, S.; Samii, K.; Chalandon, Y.; Terriou, L.; Launay, D.; Fajgenbaum, D.C.; Seebach, J.D.; Muller, Y.D. TAFRO syndrome in Caucasians: A case report and review of the literature. Front. Med. 2017, 4, 149. [Google Scholar] [CrossRef] [PubMed]
- Hiramatsu, S.; Ohmura, K.; Tsuji, H.; Kawabata, H.; Kitano, T.; Sogabe, A.; Hashimoto, M.; Murakami, K.; Imura, Y.; Yukawa, N.; et al. Successful treatment by rituximab in a patient with TAFRO syndrome with cardiomyopathy. Nihon Rinsho Meneki Gakkai Kaishi 2016, 39, 64–71. [Google Scholar] [CrossRef]
- José, F.F.; Kerbauy, L.N.; Perini, G.F.; Blumenschein, D.I.; Pasqualin, D.D.C.; Malheiros, D.M.A.C.; Campos Neto, G.C.; de Souza Santos, F.P.; Piovesan, R.; Hamerschlak, N. A life-threatening case of TAFRO syndrome with dramatic response to tocilizumab, rituximab, and pulse steroids: The first case report in Latin America. Medicine 2017, 96, e6271. [Google Scholar] [CrossRef]
- Morisawa, N.; Satoh, H.; Matsuyama, M.; Hayashi, N.; Adachi, A.; Satoh, J.I.; Yokoo, T.; Amemiya, M. [Usefulness of the treatment with corticosteroids and ciclosporin A for TAFRO syndrome]. Nihon Naika Gakkai Zasshi 2016, 105, 2432–2439. [Google Scholar] [CrossRef]
- Suzuki, K.; Nakamura, K.; Kasuya, T.; Yamasaki, K.; Tokinaga, K.; Tashiro, J.; Kimura, A.; Noro, M.; Akikusa, B.; Kojima, M.; et al. Case Report A case of TAFRO syndrome successfully treated with intravenous cyclophosphamide therapy. Nihon Naika Gakkai Zasshi 2016, 105, 2417–2425. [Google Scholar] [CrossRef]
- Masaki, Y.; Kawabata, H.; Fujimoto, S.; Kawano, M.; Iwaki, N.; Kotani, T.; Nakashima, A.; Kurose, N.; Takai, K.; Suzuki, R.; et al. Epidemiological analysis of multicentric and unicentric Castleman disease and TAFRO syndrome in Japan. J. Clin. Exp. Hematop. 2019, 59, 175–178. [Google Scholar] [CrossRef]
- Fujimoto, S.; Kawabata, H.; Sakai, T.; Yanagisawa, H.; Nishikori, M.; Nara, K.; Ohara, S.; Tsukamoto, N.; Kurose, N.; Yamada, S.; et al. Optimal treatments for TAFRO syndrome: A retrospective surveillance study in Japan. Int. J. Hematol. 2021, 113, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhang, X.; Qian, S.; Shi, C.; Li, X.; Feng, X.; Zhu, L.; Ge, J.; Li, Z.; Zhang, M. The experience of diagnosis and treatment for TAFRO syndrome. Ann. Hematol. 2023, 102, 3515–3520. [Google Scholar] [CrossRef]
- Masaki, Y.; Kawabata, H.; Takai, K.; Kojima, M.; Tsukamoto, N.; Ishigaki, Y.; Kurose, N.; Ide, M.; Murakami, J.; Nara, K.; et al. Proposed diagnostic criteria, disease severity classification and treatment strategy for TAFRO syndrome, 2015 version. Int. J. Hematol. 2016, 103, 686–692. [Google Scholar] [CrossRef]
- Masaki, Y.; Kawabata, H.; Takai, K.; Tsukamoto, N.; Fujimoto, S.; Ishigaki, Y.; Kurose, N.; Miura, K.; Nakamura, S.; Aoki, S.; et al. 2019 Updated diagnostic criteria and disease severity classification for TAFRO syndrome. Int. J. Hematol. 2020, 111, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, Y.; Fajgenbaum, D.C.; Pierson, S.K.; Iwaki, N.; Nishikori, A.; Kawano, M.; Nakamura, N.; Izutsu, K.; Takeuchi, K.; Nishimura, M.F.; et al. Validated international definition of the thrombocytopenia, anasarca, fever, reticulin fibrosis, renal insufficiency, and organomegaly clinical subtype (TAFRO) of idiopathic multicentric Castleman disease. Am. J. Hematol. 2021, 96, 1241–1252. [Google Scholar] [CrossRef] [PubMed]
- Castleman, B.; Iverson, L.; Menendez, V.P. Localized mediastinal lymphnode hyperplasia resembling thymoma. Cancer 1956, 9, 822–830. [Google Scholar] [CrossRef]
- Masaki, Y.; Ueda, Y.; Yanagisawa, H.; Arita, K.; Sakai, T.; Yamada, K.; Mizuta, S.; Fukushima, T.; Takai, K.; Aoki, S.; et al. TAFRO syndrome. Nippon Rinsho 2023, 81, 383–387. (In Japanese) [Google Scholar]
- Fajgenbaum, D.C.; van Rhee, F.; Nabel, C.S. HHV-8-negative, idiopathic multicentric Castleman disease: Novel insights into biology, pathogenesis, and therapy. Blood 2014, 123, 2924–2933. [Google Scholar] [CrossRef] [PubMed]
- Shirakashi, M.; Nishida, Y.; Nakashima, R.; Fujimoto, M.; Hiwa, R.; Tsuji, H.; Kitagori, K.; Akizuki, S.; Morinobu, A.; Yoshifuji, H. TAFRO syndrome is associated with anti-SSA/Ro60 antibodies, in contrast to idiopathic Castleman disease. Sci. Rep. 2024, 14, 2889. [Google Scholar] [CrossRef]
- Ide, M.; Yokoyama, T.; Ishikawa, M.; Kojima, K. Stepwise treatment for TAFRO syndrome. J. Med. Cases 2023, 14, 369–377. [Google Scholar] [CrossRef]
- Masaki, Y.; Arita, K.; Sakai, T.; Takai, K.; Aoki, S.; Kawabata, H. Castleman disease and TAFRO syndrome. Ann. Hematol. 2022, 101, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, S.; Sakai, T.; Kawabata, H.; Kurose, N.; Yamada, S.; Takai, K.; Aoki, S.; Kuroda, J.; Ide, M.; Setoguchi, K.; et al. Is TAFRO syndrome a subtype of idiopathic multicentric Castleman disease? Am. J. Hematol. 2019, 94, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Kurose, N.; Futatsuya, C.; Mizutani, K.I.; Kumagai, M.; Shioya, A.; Guo, X.; Aikawa, A.; Nakada, S.; Fujimoto, S.; Kawabata, H.; et al. The clinicopathological comparison among nodal cases of idiopathic multicentric Castleman disease with and without TAFRO syndrome. Hum. Pathol. 2018, 77, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, M.F.; Nishimura, Y.; Nishikori, A.; Yoshino, T.; Sato, Y. Historical and pathological overview of Castleman disease. J. Clin. Exp. Hematop. 2022, 62, 60–72. [Google Scholar] [CrossRef]
- Pai, R.L.; Japp, A.S.; Gonzalez, M.; Rasheed, R.F.; Okumura, M.; Arenas, D.; Pierson, S.K.; Powers, V.; Layman, A.A.K.; Kao, C.; et al. Type I IFN response associated with mTOR activation in the TAFRO subtype of idiopathic multicentric Castleman disease. JCI Insight 2020, 5, e135031. [Google Scholar] [CrossRef] [PubMed]
- Sumiyoshi, R.; Koga, T.; Kawakami, A. Candidate biomarkers for idiopathic multicentric Castleman disease. J. Clin. Exp. Hematop. 2022, 62, 85–90. [Google Scholar] [CrossRef]
- Kishimoto, T.; Akira, S.; Narazaki, M.; Taga, T. Interleukin-6 family of cytokines and gp130. Blood 1995, 86, 1243–1254. [Google Scholar] [CrossRef]
- Yoshizaki, K.; Murayama, S.; Ito, H.; Koga, T. The role of interleukin-6 in Castleman disease. Hematol. Oncol. Clin. N. Am. 2018, 32, 23–36. [Google Scholar] [CrossRef]
- Leger-Ravet, M.B.; Peuchmaur, M.; Devergne, O.; Audouin, J.; Raphael, M.; Van Damme, J.; Galanaud, P.; Diebold, J.; Emilie, D. Interleukin-6 gene expression in Castleman’s disease. Blood 1991, 78, 2923–2930. [Google Scholar] [CrossRef]
- Suzuki, H.; Sano, T.; Shimasaki, Y.; Yamaguchi, M.; Ide, T.; Arinaga-Hino, T.; Kuwahara, R.; Amano, K.; Oshima, K.; Nagafuji, K.; et al. TAFRO syndrome that responded to prednisolone-only treatment: Evaluating changes in IL-6. Intern. Med. 2022, 61, 2967–2972. [Google Scholar] [CrossRef]
- Yoshizaki, K.; Matsuda, T.; Nishimoto, N.; Kuritani, T.; Taeho, L.; Aozasa, K.; Nakahata, T.; Kawai, H.; Tagoh, H.; Komori, T. Pathogenic significance of interleukin-6 (IL-6/BSF-2) in Castleman’s disease. Blood 1989, 74, 1360–1367. [Google Scholar] [CrossRef] [PubMed]
- Iwaki, N.; Gion, Y.; Kondo, E.; Kawano, M.; Masunari, T.; Moro, H.; Nikkuni, K.; Takai, K.; Hagihara, M.; Hashimoto, Y.; et al. Elevated serum interferon γ-induced protein 10 kDa is associated with TAFRO syndrome. Sci. Rep. 2017, 7, 42316. [Google Scholar] [CrossRef] [PubMed]
- Pierson, S.K.; Stonestrom, A.J.; Shilling, D.; Ruth, J.; Nabel, C.S.; Singh, A.; Ren, Y.; Stone, K.; Li, H.; van Rhee, F.; et al. Plasma proteomics identifies a ‘chemokine storm’ in idiopathic multicentric Castleman disease. Am. J. Hematol. 2018, 93, 902–912. [Google Scholar] [CrossRef] [PubMed]
- Fajgenbaum, D.C.; Langan, R.A.; Japp, A.S.; Partridge, H.L.; Pierson, S.K.; Singh, A.; Arenas, D.J.; Ruth, J.R.; Nabel, C.S.; Stone, K.; et al. Identifying and targeting pathogenic PI3K/AKT/mTOR signaling in IL-6-blockade-refractory idiopathic multicentric Castleman disease. J. Clin. Investig. 2019, 129, 4451–4463. [Google Scholar] [CrossRef]
- Sumiyoshi, R.; Koga, T.; Furukawa, K.; Umeda, M.; Yamamoto, K.; Mori, R.; Kawakami, A. A case of tocilizumab-refractory idiopathic multicentric Castleman’s disease successfully treated with sirolimus. Clin. Immunol. 2021, 233, 108887. [Google Scholar] [CrossRef] [PubMed]
- Saxton, R.A.; Sabatini, D.M. MTOR signaling in growth, metabolism, and disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef]
- Koga, T.; Hagimori, N.; Takemori, S.; Morimoto, S.; Sumiyoshi, R.; Shimizu, T.; Hosogaya, N.; Fukushima, C.; Yamamoto, H.; Kawakami, A. Randomized, double-blind, placebo-controlled, parallel-group trial of sirolimus for tocilizumab-resistant idiopathic multicentric Castleman disease: Study protocol for clinical trial. Medicine 2020, 99, e20710. [Google Scholar] [CrossRef]
- Phillips, A.D.; Kakkis, J.J.; Tsao, P.Y.; Pierson, S.K.; Fajgenbaum, D.C. Increased mTORC2 pathway activation in lymph nodes of iMCD-TAFRO. J. Cell. Mol. Med. 2022, 26, 3147–3152. [Google Scholar] [CrossRef] [PubMed]
- García-Martínez, J.M.; Alessi, D.R. mTOR complex 2 (mTORC2) controls hydrophobic motif phosphorylation and activation of serum- and glucocorticoid-induced protein kinase 1 (SGK1). Biochem. J. 2008, 416, 375–385. [Google Scholar] [CrossRef]
- Kim, L.C.; Cook, R.S.; Chen, J. mTORC1 and mTORC2 in cancer and the tumor microenvironment. Oncogene 2017, 36, 2191–2201. [Google Scholar] [CrossRef]
- Pierson, S.K.; Shenoy, S.; Oromendia, A.B.; Gorzewski, A.M.; Langan Pai, R.A.; Nabel, C.S.; Ruth, J.R.; Parente, S.A.T.; Arenas, D.J.; Guilfoyle, M.; et al. Discovery and validation of a novel subgroup and therapeutic target in idiopathic multicentric Castleman disease. Blood Adv. 2021, 5, 3445–3456. [Google Scholar] [CrossRef] [PubMed]
- Arenas, D.J.; Floess, K.; Kobrin, D.; Pai, R.L.; Srkalovic, M.B.; Tamakloe, M.A.; Rasheed, R.; Ziglar, J.; Khor, J.; Parente, S.A.T.; et al. Increased mTOR activation in idiopathic multicentric Castleman disease. Blood 2020, 135, 1673–1684. [Google Scholar] [CrossRef] [PubMed]
- Kadoba, K.; Waki, D.; Nishimura, K.; Mukoyama, H.; Saito, R.; Murabe, H.; Yokota, T. Development of severe thrombocytopenia with TAFRO syndrome-like features in a patient with rheumatoid arthritis treated with a Janus kinase inhibitor: A case report. Medicine 2020, 99, e22793. [Google Scholar] [CrossRef] [PubMed]
- Wakiya, R.; Kameda, T.; Takeuchi, Y.; Ozaki, H.; Nakashima, S.; Shimada, H.; Kadowaki, N.; Dobashi, H. Sequential change in serum VEGF levels in a case of tocilizumab-resistant TAFRO syndrome treated effectively with rituximab. Mod. Rheumatol. Case Rep. 2021, 5, 145–151. [Google Scholar] [CrossRef]
- Kikuchi, T.; Shimizu, T.; Toyama, T.; Abe, R.; Okamoto, S. Successful treatment of TAFRO syndrome with tocilizumab, prednisone, and cyclophosphamide. Intern. Med. 2017, 56, 2205–2211. [Google Scholar] [CrossRef]
- Williams, C.; Phillips, A.; Aggarwal, V.; Slonim, L.B.; Fajgenbaum, D.C.; Karmali, R. TAFRO syndrome and elusive diagnosis of idiopathic multicentric Castleman disease treated with empiric anti-interleukin-6 therapy. Case Rep. Oncol. 2021, 14, 1359–1365. [Google Scholar] [CrossRef]
- Kakutani, T.; Nunokawa, T.; Chinen, N.; Tamai, Y. Treatment-resistant idiopathic multicentric Castleman disease with thrombocytopenia, anasarca, fever, reticulin fibrosis, renal dysfunction, and organomegaly managed with Janus kinase inhibitors: A case report. Medicine 2022, 101, e32200. [Google Scholar] [CrossRef]
- Killian, M.; Viel, S.; Chalayer, E.; Forest, F.; Grange, S.; Bonnefoy, P.B.; Oksenhendler, E.; Cathébras, P.; Paul, S. JAK1/2 inhibition in severe TAFRO syndrome: A case report. Ann. Intern. Med. 2021, 174, 719–721. [Google Scholar] [CrossRef]
- van Rhee, F.; Wong, R.S.; Munshi, N.; Rossi, J.F.; Ke, X.Y.; Fosså, A.; Simpson, D.; Capra, M.; Liu, T.; Hsieh, R.K.; et al. Siltuximab for multicentric Castleman’s disease: A randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2014, 15, 966–974. [Google Scholar] [CrossRef]
- Igawa, T.; Sato, Y. TAFRO syndrome. Hematol. Oncol. Clin. N. Am. 2018, 32, 107–118. [Google Scholar] [CrossRef]
- Lust, H.; Gong, S.; Remiker, A.; Rossoff, J. Idiopathic multicentric Castleman disease with TAFRO clinical subtype responsive to IL-6/JAK inhibition: A pediatric case series. Pediatr. Blood Cancer 2021, 68, e29261. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.R.; Tian, M. Glucocorticoids combined with tofacitinib in the treatment of Castleman’s disease: A case report. World J. Clin. Cases 2022, 10, 10794–10802. [Google Scholar] [CrossRef] [PubMed]
- Sakashita, K.; Murata, K.; Takamori, M. TAFRO syndrome: Current perspectives. J. Blood Med. 2018, 9, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Ocio, E.M.; Sanchez-Guijo, F.M.; Diez-Campelo, M.; Castilla, C.; Blanco, O.J.; Caballero, D.; San Miguel, J.F. Efficacy of rituximab in an aggressive form of multicentric Castleman disease associated with immune phenomena. Am. J. Hematol. 2005, 78, 302–305. [Google Scholar] [CrossRef]
- Watanabe, M.; Haji, Y.; Hozumi, M.; Amari, Y.; Mizuno, Y.; Ito, T.; Kato, M.; Okada, M. Combined B-cell immunomodulation with rituximab and Belimumab in severe, refractory TAFRO syndrome associated with Sjögren’s syndrome: A case report. Mod. Rheumatol. Case Rep. 2023, 7, 475–479. [Google Scholar] [CrossRef]
- Dufour, J.H.; Dziejman, M.; Liu, M.T.; Leung, J.H.; Lane, T.E.; Luster, A.D. IFN-γ-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J. Immunol. 2002, 168, 3195–3204. [Google Scholar] [CrossRef]
- Chronowski, G.M.; Ha, C.S.; Wilder, R.B.; Cabanillas, F.; Manning, J.; Cox, J.D. Treatment of unicentric and multicentric Castleman disease and the role of radiotherapy. Cancer 2001, 92, 670–676. [Google Scholar] [CrossRef]
- Nishi, J.; Arimura, K.; Utsunomiya, A.; Yonezawa, S.; Kawakami, K.; Maeno, N.; Ijichi, O.; Ikarimoto, N.; Nakata, M.; Kitajima, I.; et al. Expression of vascular endothelial growth factor in sera and lymph nodes of the plasma cell type of Castleman’s disease. Br. J. Haematol. 1999, 104, 482–485. [Google Scholar] [CrossRef]
- Stary, G.N.; Kohrgruber, N.; Herneth, A.M.; Gaiger, A.; Stingl, G.; Rieger, A. Complete regression of HIV-associated multicentric castleman disease treated with rituximab and thalidomide. Aids 2008, 22, 1232–1234. [Google Scholar] [CrossRef]
- Miltenyi, Z.; Toth, J.; Gonda, A.; Tar, I.; Remenyik, E.; Illes, A. Successful immunomodulatory therapy in castleman disease with paraneoplastic pemphigus vulgaris. Pathol. Oncol. Res. 2009, 15, 375–381. [Google Scholar] [CrossRef]
- Jung, C.P.; Emmerich, B.; Goebel, F.D.; Bogner, J.R. Successful treatment of a patient with HIV-associated multicentric castleman disease (MCD) with thalidomide. Amm. J. Hematol. 2004, 75, 176–177. [Google Scholar] [CrossRef] [PubMed]
- Tatekawa, S.; Umemura, K.; Fukuyama, R.; Kohno, A.; Taniwaki, M.; Kuroda, J.; Morishita, Y. Thalidomide for tocilizumab-resistant ascites with TAFRO syndrome. Clin. Case Rep. 2015, 3, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Feng, C.; Zhang, X.; Zhou, J. TAFRO syndrome: A disease that known is half cured. Hematol. Oncol. 2023, 41, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Caballero, J.C.; Conejero, N.; Solan, L.; de la Pinta, F.J.D.; Cordoba, R.; Lopez-Garcia, A. Unraveling TAFRO syndrome: An In-Depth Look at the Pathophysiology, Management, and Future Perspectives. Biomedicines 2024, 12, 1076. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kakutani, T.; Kamada, R.; Tamai, Y. Pathophysiology, Treatment, and Prognosis of Thrombocytopenia, Anasarca, Fever, Reticulin Fibrosis/Renal Failure, and Organomegaly (TAFRO) Syndrome: A Review. Curr. Issues Mol. Biol. 2024, 46, 11255-11269. https://doi.org/10.3390/cimb46100668
Kakutani T, Kamada R, Tamai Y. Pathophysiology, Treatment, and Prognosis of Thrombocytopenia, Anasarca, Fever, Reticulin Fibrosis/Renal Failure, and Organomegaly (TAFRO) Syndrome: A Review. Current Issues in Molecular Biology. 2024; 46(10):11255-11269. https://doi.org/10.3390/cimb46100668
Chicago/Turabian StyleKakutani, Takuya, Riko Kamada, and Yotaro Tamai. 2024. "Pathophysiology, Treatment, and Prognosis of Thrombocytopenia, Anasarca, Fever, Reticulin Fibrosis/Renal Failure, and Organomegaly (TAFRO) Syndrome: A Review" Current Issues in Molecular Biology 46, no. 10: 11255-11269. https://doi.org/10.3390/cimb46100668
APA StyleKakutani, T., Kamada, R., & Tamai, Y. (2024). Pathophysiology, Treatment, and Prognosis of Thrombocytopenia, Anasarca, Fever, Reticulin Fibrosis/Renal Failure, and Organomegaly (TAFRO) Syndrome: A Review. Current Issues in Molecular Biology, 46(10), 11255-11269. https://doi.org/10.3390/cimb46100668





