The Interplay between von Hippel–Lindau Tumor Suppressor Gene, Lon Protease, ROS Accumulation, and Inflammation in Clear Cell Renal Cell Carcinoma
Abstract
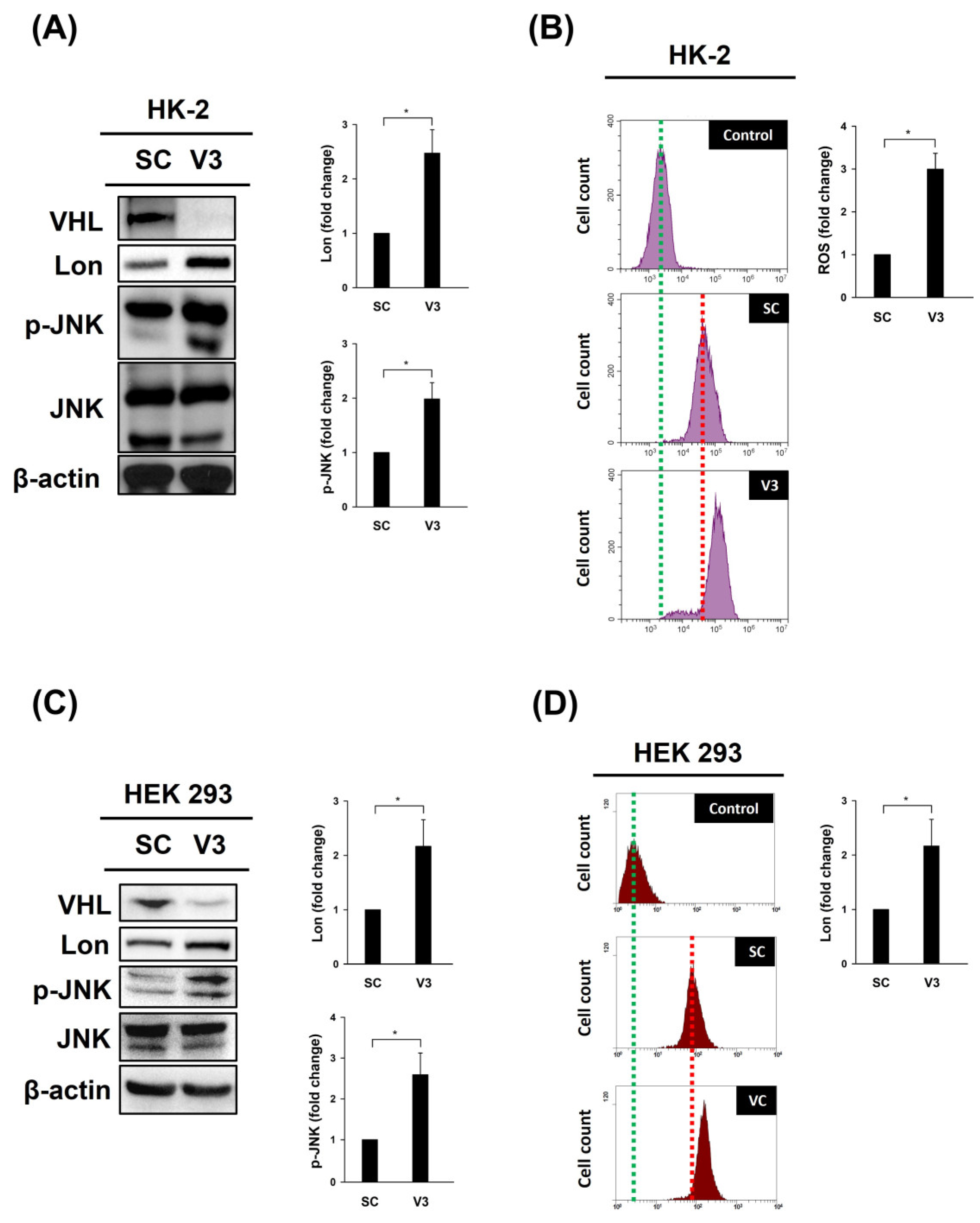
:1. Introduction
2. Materials and Methods
2.1. Antibodies
2.2. Cell Culture
2.3. Transfection
2.4. Detection of ROS Levels
2.5. Protein Extraction and Western Blot Analysis
2.6. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, J.; Tan, P.; Ishihara, M.; Bayley, N.A.; Schokrpur, S.; Reynoso, J.G.; Zhang, Y.; Lim, R.J.; Dumitras, C.; Yang, L.; et al. Tumor heterogeneity in VHL drives metastasis in clear cell renal cell carcinoma. Signal Transduct. Target. Ther. 2023, 8, 155. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, P.H.; Wiesener, M.S.; Chang, G.W.; Clifford, S.C.; Vaux, E.C.; Cockman, M.E.; Wykoff, C.C.; Pugh, C.W.; Maher, E.R.; Ratcliffe, P.J. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 1999, 399, 271–275. [Google Scholar] [CrossRef]
- Mazumder, S.; Higgins, P.J.; Samarakoon, R. Downstream Targets of VHL/HIF-alpha Signaling in Renal Clear Cell Carcinoma Progression: Mechanisms and Therapeutic Relevance. Cancers 2023, 15, 1316. [Google Scholar] [CrossRef]
- Kuo, C.Y.; Lin, C.H.; Hsu, T. VHL Inactivation in Precancerous Kidney Cells Induces an Inflammatory Response via ER Stress-Activated IRE1alpha Signaling. Cancer Res. 2017, 77, 3406–3416. [Google Scholar] [CrossRef]
- Kuo, C.Y.; Chiu, V.; Hsieh, P.C.; Hsu, T.; Lin, T.Y. Loss of Function of von Hippel-Lindau Trigger Lipocalin 2-Dependent Inflammatory Responses in Cultured and Primary Renal Tubular Cells. Oxid. Med. Cell Longev. 2021, 2021, 5571638. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.Y.; Hsieh, P.C.; Chiu, V.; Lan, C.C.; Lu, K.C. The von Hippel-Lindau Tumor Suppressor Gene Mutations Modulate Lipocalin-2 Expression in Ferroptotic-Inflammatory Pathways. Oxid. Med. Cell Longev. 2023, 2023, 7736638. [Google Scholar] [CrossRef]
- Bota, D.A.; Davies, K.J. Lon protease preferentially degrades oxidized mitochondrial aconitase by an ATP-stimulated mechanism. Nat. Cell Biol. 2002, 4, 674–680. [Google Scholar] [CrossRef]
- Quiros, P.M.; Barcena, C.; Lopez-Otin, C. Lon protease: A key enzyme controlling mitochondrial bioenergetics in cancer. Mol. Cell Oncol. 2014, 1, e968505. [Google Scholar] [CrossRef] [PubMed]
- Voos, W.; Pollecker, K. The Mitochondrial Lon Protease: Novel Functions off the Beaten Track? Biomol. 2020, 10, 253. [Google Scholar] [CrossRef]
- Ngo, J.K.; Pomatto, L.C.; Davies, K.J. Upregulation of the mitochondrial Lon Protease allows adaptation to acute oxidative stress but dysregulation is associated with chronic stress, disease, and aging. Redox Biol. 2013, 1, 258–264. [Google Scholar] [CrossRef]
- Szczepanowska, K.; Trifunovic, A. Mitochondrial matrix proteases: Quality control and beyond. FEBS J. 2022, 289, 7128–7146. [Google Scholar] [CrossRef] [PubMed]
- Bota, D.A.; Davies, K.J. Mitochondrial Lon protease in human disease and aging: Including an etiologic classification of Lon-related diseases and disorders. Free. Radic. Biol. Med. 2016, 100, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.W.; Kuo, C.Y.; Fan, C.C.; Fang, W.C.; Jiang, S.S.; Lo, Y.K.; Wang, T.Y.; Kao, M.C.; Lee, A.Y. Overexpression of Lon contributes to survival and aggressive phenotype of cancer cells through mitochondrial complex I-mediated generation of reactive oxygen species. Cell Death Dis. 2013, 4, e681. [Google Scholar] [CrossRef]
- Quiros, P.M.; Espanol, Y.; Acin-Perez, R.; Rodriguez, F.; Barcena, C.; Watanabe, K.; Calvo, E.; Loureiro, M.; Fernandez-Garcia, M.S.; Fueyo, A.; et al. ATP-dependent Lon protease controls tumor bioenergetics by reprogramming mitochondrial activity. Cell Rep. 2014, 8, 542–556. [Google Scholar] [CrossRef]
- Nie, X.; Li, M.; Lu, B.; Zhang, Y.; Lan, L.; Chen, L.; Lu, J. Down-regulating overexpressed human Lon in cervical cancer suppresses cell proliferation and bioenergetics. PLoS ONE 2013, 8, e81084. [Google Scholar] [CrossRef]
- Gildea, J.J.; Shah, I.; Weiss, R.; Casscells, N.D.; McGrath, H.E.; Zhang, J.; Jones, J.E.; Felder, R.A. HK-2 human renal proximal tubule cells as a model for G protein-coupled receptor kinase type 4-mediated dopamine 1 receptor uncoupling. Hypertens. 2010, 56, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Tam, S.Y.; Law, H.K. JNK in Tumor Microenvironment: Present Findings and Challenges in Clinical Translation. Cancers 2021, 13, 2196. [Google Scholar] [CrossRef]
- Han, M.S.; Barrett, T.; Brehm, M.A.; Davis, R.J. Inflammation Mediated by JNK in Myeloid Cells Promotes the Development of Hepatitis and Hepatocellular Carcinoma. Cell Rep. 2016, 15, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Han, X.; Zhang, T.; Tian, K.; Li, Z.; Luo, F. Reactive oxygen species (ROS) scavenging biomaterials for anti-inflammatory diseases: From mechanism to therapy. J. Hematol. Oncol. 2023, 16, 116. [Google Scholar] [CrossRef]
- Wolf, M.M.; Madden, M.Z.; Arner, E.N.; Bader, J.E.; Ye, X.; Vlach, L.; Tigue, M.L.; Landis, M.D.; Jonker, P.B.; Hatem, Z.; et al. VHL loss reprograms the immune landscape to promote an inflammatory myeloid microenvironment in renal tumorigenesis. J. Clin. Investig. 2024, 134, e173934. [Google Scholar] [CrossRef]
- Nguyen-Tran, H.H.; Nguyen, T.N.; Chen, C.Y.; Hsu, T. Endothelial Reprogramming Stimulated by Oncostatin M Promotes Inflammation and Tumorigenesis in VHL-Deficient Kidney Tissue. Cancer Res. 2021, 81, 5060–5073. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.N.; Nguyen-Tran, H.H.; Chen, C.Y.; Hsu, T. IL6 and CCL18 Mediate Cross-talk between VHL-Deficient Kidney Cells and Macrophages during Development of Renal Cell Carcinoma. Cancer Res. 2022, 82, 2716–2733. [Google Scholar] [CrossRef] [PubMed]
- Shetty, R.; Noland, R.; Nandi, G.; Suzuki, C.K. Powering down the mitochondrial LonP1 protease: A novel strategy for anticancer therapeutics. Expert. Opin. Ther. Targets 2024, 28, 9–15. [Google Scholar] [CrossRef]
- Bulteau, A.L.; Bayot, A. Mitochondrial proteases and cancer. Biochim. Biophys. Acta 2011, 1807, 595–601. [Google Scholar] [CrossRef]
- Kuo, C.L.; Ponneri Babuharisankar, A.; Lin, Y.C.; Lien, H.W.; Lo, Y.K.; Chou, H.Y.; Tangeda, V.; Cheng, L.C.; Cheng, A.N.; Lee, A.Y. Mitochondrial oxidative stress in the tumor microenvironment and cancer immunoescape: Foe or friend? J. Biomed. Sci. 2022, 29, 74. [Google Scholar] [CrossRef] [PubMed]
- Simmons, S.; Peng, J.; Bienkowska, J.; Berger, B. Discovering What Dimensionality Reduction Really Tells Us About RNA-Seq Data. J. Comput. Biol. 2015, 22, 715–728. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Dai, Y. Principal component analysis based methods in bioinformatics studies. Brief. Bioinform. 2011, 12, 714–722. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Cai, N.; Pacheco, P.P.; Narrandes, S.; Wang, Y.; Xu, W. Applications of Support Vector Machine (SVM) Learning in Cancer Genomics. Cancer Genom. Proteom. 2018, 15, 41–51. [Google Scholar] [CrossRef]
- Kourou, K.; Exarchos, T.P.; Exarchos, K.P.; Karamouzis, M.V.; Fotiadis, D.I. Machine learning applications in cancer prognosis and prediction. Comput. Struct. Biotechnol. J. 2015, 13, 8–17. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, Y.-C.; Kuo, C.-Y. The Interplay between von Hippel–Lindau Tumor Suppressor Gene, Lon Protease, ROS Accumulation, and Inflammation in Clear Cell Renal Cell Carcinoma. Curr. Issues Mol. Biol. 2024, 46, 11296-11302. https://doi.org/10.3390/cimb46100671
Tsai Y-C, Kuo C-Y. The Interplay between von Hippel–Lindau Tumor Suppressor Gene, Lon Protease, ROS Accumulation, and Inflammation in Clear Cell Renal Cell Carcinoma. Current Issues in Molecular Biology. 2024; 46(10):11296-11302. https://doi.org/10.3390/cimb46100671
Chicago/Turabian StyleTsai, Yao-Chou, and Chan-Yen Kuo. 2024. "The Interplay between von Hippel–Lindau Tumor Suppressor Gene, Lon Protease, ROS Accumulation, and Inflammation in Clear Cell Renal Cell Carcinoma" Current Issues in Molecular Biology 46, no. 10: 11296-11302. https://doi.org/10.3390/cimb46100671
APA StyleTsai, Y.-C., & Kuo, C.-Y. (2024). The Interplay between von Hippel–Lindau Tumor Suppressor Gene, Lon Protease, ROS Accumulation, and Inflammation in Clear Cell Renal Cell Carcinoma. Current Issues in Molecular Biology, 46(10), 11296-11302. https://doi.org/10.3390/cimb46100671







