Differences in Plasma Extracellular Vesicles of Different Origin in On-Pump Versus Off-Pump Cardiac Surgery
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Characteristics
2.2. Anesthesia and CPB
2.3. Extracellular Vesicles
2.4. Cytokines
2.5. T-Cell Immunophenotyping by Flow Cytometry
2.6. B-Cell Immunophenotyping by Flow Cytometry
2.7. Statistics
3. Results
3.1. Laboratory Characteristics
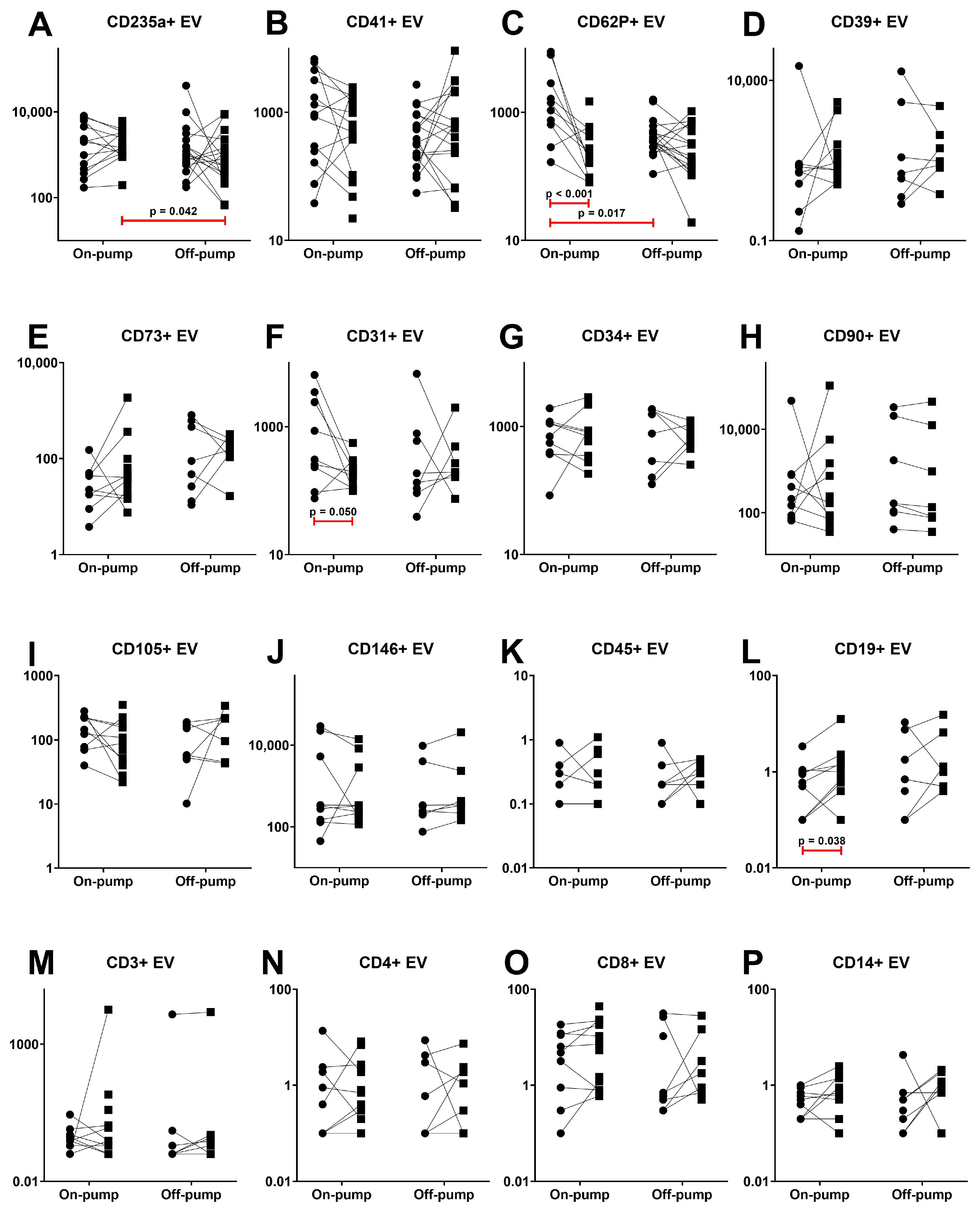
3.2. Extracellular Vesicles
3.3. T- and B-Cells
3.4. Cytokines
3.5. Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Paparella, D.; Yau, T.M.; Young, E. Cardiopulmonary bypass induced inflammation: Pathophysiology and treatment. An update. Eur. J. Cardio-Thorac. Surg. 2002, 21, 232–244. [Google Scholar] [CrossRef] [PubMed]
- Squiccimarro, E.; Labriola, C.; Malvindi, P.G.; Margari, V.; Guida, P.; Visicchio, G.; Kounakis, G.; Favale, A.; Dambruoso, P.; Mastrototaro, G.; et al. Prevalence and Clinical Impact of Systemic Inflammatory Reaction After Cardiac Surgery. J. Cardiothorac. Vasc. Anesth. 2019, 33, 1682–1690. [Google Scholar] [CrossRef] [PubMed]
- Squiccimarro, E.; Stasi, A.; Lorusso, R.; Paparella, D. Narrative review of the systemic inflammatory reaction to cardiac surgery and cardiopulmonary bypass. Artif. Organs 2022, 46, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.O.; Vasconcelos, V.W.; Lima, J.d.S.; Vieira Neto, J.R.; da Costa, G.E.; Esteves, J.d.C.; de Sousa, S.C.; Moura, J.A.; Santos, F.R.S.; Leitão Filho, J.M.; et al. Biochemical Changes in Cardiopulmonary Bypass in Cardiac Surgery: New Insights. J. Pers. Med. 2023, 13, 1506. [Google Scholar] [CrossRef] [PubMed]
- Giacinto, O.; Satriano, U.; Nenna, A.; Spadaccio, C.; Lusini, M.; Mastroianni, C.; Nappi, F.; Chello, M. Inflammatory Response and Endothelial Dysfunction Following Cardiopulmonary Bypass: Pathophysiology and Pharmacological Targets. Recent Pat. Inflamm. Allergy Drug Discov. 2019, 13, 158–173. [Google Scholar] [CrossRef]
- Lehmann, S.; Dieterlen, M.T.; Flister, A.; Klaeske, K.; Jawad, K.; Garbade, J.; Borger, M.A.; Kostelka, M. Differences of early immunological responses in on-pump versus off-pump cardiac surgery. Perfusion 2019, 34, 399–407. [Google Scholar] [CrossRef]
- Gaudriot, B.; Uhel, F.; Gregoire, M.; Gacouin, A.; Biedermann, S.; Roisne, A.; Flecher, E.; Le Tulzo, Y.; Tarte, K.; Tadié, J.M. Immune Dysfunction after Cardiac Surgery with Cardiopulmonary Bypass: Beneficial Effects of Maintaining Mechanical Ventilation. Shock 2015, 44, 228–233. [Google Scholar] [CrossRef]
- Sondekoppam, R.V.; Arellano, R.; Ganapathy, S.; Cheng, D. Pain and inflammatory response following off-pump coronary artery bypass grafting. Curr. Opin. Anaesthesiol. 2014, 27, 106–115. [Google Scholar] [CrossRef]
- Patel, V.; Unai, S.; Gaudino, M.; Bakaeen, F. Current Readings on Outcomes After Off-Pump Coronary Artery Bypass Grafting. Semin. Thorac. Cardiovasc. Surg. 2019, 31, 726–733. [Google Scholar] [CrossRef]
- Vedin, J.; Antovic, A.; Ericsson, A.; Vaage, J. Hemostasis in off-pump compared to on-pump coronary artery bypass grafting: A prospective, randomized study. Ann. Thorac. Surg. 2005, 80, 586–593. [Google Scholar] [CrossRef]
- Wehlin, L.; Vedin, J.; Vaage, J.; Lundahl, J. Activation of complement and leukocyte receptors during on- and off pump coronary artery bypass surgery. Eur. J. Cardio-Thorac. Surg. 2004, 25, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Sanwlani, R.; Gangoda, L. Role of extracellular vesicles in cell death and inflammation. Cells 2021, 10, 2663. [Google Scholar] [CrossRef] [PubMed]
- Buzas, E.I. The roles of extracellular vesicles in the immune system. Nat. Rev. Immunol. 2023, 23, 236–250. [Google Scholar] [CrossRef] [PubMed]
- Akhmerov, A.; Parimon, T. Extracellular Vesicles, Inflammation, and Cardiovascular Disease. Cells 2022, 11, 2229. [Google Scholar] [CrossRef]
- Ge, X.; Meng, Q.; Zhuang, R.; Yuan, D.; Liu, J.; Lin, F.; Fan, H.; Zhou, X. Circular RNA expression alterations in extracellular vesicles isolated from murine heart post ischemia/reperfusion injury. Int. J. Cardiol. 2019, 296, 136–140. [Google Scholar] [CrossRef]
- Ge, X.; Meng, Q.; Wei, L.; Liu, J.; Li, M.; Liang, X.; Lin, F.; Zhang, Y.; Li, Y.; Liu, Z.; et al. Myocardial ischemia-reperfusion induced cardiac extracellular vesicles harbour proinflammatory features and aggravate heart injury. J. Extracell. Vesicles 2021, 10, e12072. [Google Scholar] [CrossRef]
- Deng, F.; Wang, S.; Zhang, L. Endothelial microparticles act as novel diagnostic and therapeutic biomarkers of circulatory hypoxia-related diseases: A literature review. J. Cell. Mol. Med. 2017, 21, 1698–1710. [Google Scholar] [CrossRef]
- Carrozzo, A.; Casieri, V.; Di Silvestre, D.; Brambilla, F.; De Nitto, E.; Sardaro, N.; Papini, G.; Storti, S.; Settanni, G.; Solinas, M.; et al. Plasma exosomes characterization reveals a perioperative protein signature in older patients undergoing different types of on-pump cardiac surgery. GeroScience 2021, 43, 773–789. [Google Scholar] [CrossRef]
- Baysa, A.; Fedorov, A.; Kondratov, K.; Ruusalepp, A.; Minasian, S.; Galagudza, M.; Popov, M.; Kurapeev, D.; Yakovlev, A.; Valen, G.; et al. Release of Mitochondrial and Nuclear DNA During On-Pump Heart Surgery: Kinetics and Relation to Extracellular Vesicles. J. Cardiovasc. Transl. Res. 2019, 12, 184–192. [Google Scholar] [CrossRef]
- Galeone, A.; Brunetti, G.; Rotunno, C.; Oranger, A.; Colucci, S.; Schinosa, L.d.L.T.; Zallone, A.; Grano, M.; Paparella, D. Activation of the receptor activator of the nuclear factor-κB ligand pathway during coronary bypass surgery: Comparison between on- and off-pump coronary artery bypass surgery procedures. Eur. J. Cardio-Thorac. Surg. 2013, 44, e141–e147. [Google Scholar] [CrossRef]
- Jankovicová, K.; Kudlová, M.T.; Kolácková, M.; Kunes, P.; Mand’ák, J.; Lonský, V.; Vlásková, D.; Andrýs, C.; Krejsek, J. The effect of cardiac surgery on peripheral blood lymphocyte populations. Acta Medica (Hradec Kralove) 2008, 51, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef] [PubMed]
- Lucien, F.; Gustafson, D.; Lenassi, M.; Li, B.; Teske, J.J.; Boilard, E.; von Hohenberg, K.C.; Falcón-Perez, J.M.; Gualerzi, A.; Reale, A.; et al. MIBlood-EV: Minimal information to enhance the quality and reproducibility of blood extracellular vesicle research. J. Extracell. Vesicles 2023, 12, 12385. [Google Scholar] [CrossRef]
- Goncharov, N.V.; Popova, P.I.; Kudryavtsev, I.V.; Golovkin, A.S.; Savitskaya, I.V.; Avdonin, P.P.; Korf, E.A.; Voitenko, N.G.; Belinskaia, D.A.; Serebryakova, M.K.; et al. Immunological Profile and Markers of Endothelial Dysfunction in Elderly Patients with Cognitive Impairments. Int. J. Mol. Sci. 2024, 25, 1888. [Google Scholar] [CrossRef]
- Petrova, T.; Kalinina, O.; Aquino, A.; Grigoryev, E.; Dubashynskaya, N.V.; Zubkova, K.; Kostareva, A.; Golovkin, A. Topographic Distribution of miRNAs (miR-30a, miR-223, miR-let-7a, miR-let-7f, miR-451, and miR-486) in the Plasma Extracellular Vesicles. Non-Coding RNA 2024, 10, 15. [Google Scholar] [CrossRef]
- Kudryavtsev, I.; Kalinina, O.; Bezrukikh, V.; Melnik, O.; Golovkin, A. The significance of phenotyping and quantification of plasma extracellular vesicles levels using high-sensitivity flow cytometry during covid-19 treatment. Viruses 2021, 13, 767. [Google Scholar] [CrossRef]
- Fedorov, A.; Kondratov, K.; Kishenko, V.; Mikhailovskii, V.; Kudryavtsev, I.; Belyakova, M.; Sidorkevich, S.; Vavilova, T.; Kostareva, A.; Sirotkina, O.; et al. Application of high-sensitivity flow cytometry in combination with low-voltage scanning electron microscopy for characterization of nanosized objects during platelet concentrate storage. Platelets 2020, 31, 226–235. [Google Scholar] [CrossRef]
- Kondratov, K.; Nikitin, Y.; Fedorov, A.; Kostareva, A.; Mikhailovskii, V.; Isakov, D.; Ivanov, A.; Golovkin, A. Heterogeneity of the nucleic acid repertoire of plasma extracellular vesicles demonstrated using high-sensitivity fluorescence-activated sorting. J. Extracell. Vesicles 2020, 9, 1743139. [Google Scholar] [CrossRef]
- Welsh, J.A.; Van Der Pol, E.; Arkesteijn, G.J.A.; Bremer, M.; Brisson, A.; Coumans, F.; Dignat-George, F.; Duggan, E.; Ghiran, I.; Giebel, B.; et al. MIFlowCyt-EV: A framework for standardized reporting of extracellular vesicle flow cytometry experiments. J. Extracell. Vesicles 2020, 9, 1713526. [Google Scholar] [CrossRef]
- Welsh, J.A.; Arkesteijn, G.J.A.; Bremer, M.; Cimorelli, M.; Dignat-George, F.; Giebel, B.; Görgens, A.; Hendrix, A.; Kuiper, M.; Lacroix, R.; et al. A compendium of single extracellular vesicle flow cytometry. J. Extracell. Vesicles 2023, 12, e12299. [Google Scholar] [CrossRef] [PubMed]
- Kalinina, O.; Golovkin, A.; Zaikova, E.; Aquino, A.; Bezrukikh, V.; Melnik, O.; Vasilieva, E.; Karonova, T.; Kudryavtsev, I.; Shlyakhto, E. Cytokine Storm Signature in Patients with Moderate and Severe COVID-19. Int. J. Mol. Sci. 2022, 23, 8879. [Google Scholar] [CrossRef] [PubMed]
- Golovkin, A.; Kalinina, O.; Bezrukikh, V.; Aquino, A.; Zaikova, E.; Karonova, T.; Melnik, O.; Vasilieva, E.; Kudryavtsev, I. Imbalanced Immune Response of T-Cell and B-Cell Subsets in Patients with Moderate and Severe COVID-19. Viruses 2021, 13, 1966. [Google Scholar] [CrossRef]
- Kudryavtsev, I.; Serebriakova, M.; Zhiduleva, E.; Murtazalieva, P.; Titov, V.; Malashicheva, A.; Shishkova, A.; Semenova, D.; Irtyuga, O.; Isakov, D.; et al. CD73 Rather Than CD39 Is Mainly Involved in Controlling Purinergic Signaling in Calcified Aortic Valve Disease. Front. Genet. 2019, 10, 604. [Google Scholar] [CrossRef] [PubMed]
- Karonova, T.L.; Kudryavtsev, I.V.; Golovatyuk, K.A.; Aquino, A.D.; Kalinina, O.V.; Chernikova, A.T.; Zaikova, E.K.; Lebedev, D.A.; Bykova, E.S.; Golovkin, A.S.; et al. Vitamin D Status and Immune Response in Hospitalized Patients with Moderate and Severe COVID-19. Pharmaceuticals 2022, 15, 305. [Google Scholar] [CrossRef] [PubMed]
- Kudryavtsev, I.; Benevolenskaya, S.; Serebriakova, M.; Grigor’yeva, I.; Kuvardin, E.; Rubinstein, A.; Golovkin, A.; Kalinina, O.; Zaikova, E.; Lapin, S.; et al. Circulating CD8+ T Cell Subsets in Primary Sjögren’s Syndrome. Biomedicines 2023, 11, 2778. [Google Scholar] [CrossRef]
- Ohayon, L.; Zhang, X.; Dutta, P. The role of extracellular vesicles in regulating local and systemic inflammation in cardiovascular disease. Pharmacol. Res. 2021, 170, 105692. [Google Scholar] [CrossRef]
- Fitzgerald, W.; Freeman, M.L.; Lederman, M.M.; Vasilieva, E.; Romero, R.; Margolis, L. A System of Cytokines Encapsulated in ExtraCellular Vesicles. Sci. Rep. 2018, 8, 8973. [Google Scholar] [CrossRef]
- Hussain, M.T.; Iqbal, A.J.; Norling, L.V. The role and impact of extracellular vesicles in the modulation and delivery of cytokines during autoimmunity. Int. J. Mol. Sci. 2020, 21, 7096. [Google Scholar] [CrossRef]
- Tushuizen, M.E.; Diamant, M.; Sturk, A.; Nieuwland, R. Cell-Derived Microparticles in the Pathogenesis of Cardiovascular Disease. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 4–9. [Google Scholar] [CrossRef][Green Version]
- Barry, O.P.; Praticò, D.; Savani, R.C.; FitzGerald, G.A. Modulation of monocyte-endothelial cell interactions by platelet microparticles. J. Clin. Investig. 1998, 102, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, M.; Martelli, N.; Manarini, S.; Mascetra, N.; Musiani, P.; Cerletti, C.; Aiello, F.B.; Evangelista, V. Polymorphonuclear leukocyte apoptosis is inhibited by platelet-released mediators, role of TGFβ-1. Thromb. Haemost. 2000, 84, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Słomka, A.; Urban, S.K.; Lukacs-Kornek, V.; Żekanowska, E.; Kornek, M. Large Extracellular Vesicles: Have We Found the Holy Grail of Inflammation? Front. Immunol. 2018, 9, 2723. [Google Scholar] [CrossRef] [PubMed]
- Lindemann, S.; Tolley, N.D.; Dixon, D.A.; McIntyre, T.M.; Prescott, S.M.; Zimmerman, G.A.; Weyrich, A.S. Activated platelets mediate inflammatory signaling by regulated interleukin 1β synthesis. J. Cell Biol. 2001, 154, 485–490. [Google Scholar] [CrossRef]
- Balvers, K.; Curry, N.; Kleinveld, D.J.B.; Böing, A.N.; Nieuwland, R.; Goslings, J.C.; Juffermans, N.P. Endogenous microparticles drive the proinflammatory host immune response in severely injured trauma patients. Shock 2015, 43, 317–321. [Google Scholar] [CrossRef]
- Tang, K.; Liu, J.; Yang, Z.; Zhang, B.; Zhang, H.; Huang, C.; Ma, J.; Shen, G.X.; Ye, D.; Huang, B. Microparticles mediate enzyme transfer from platelets to mast cells: A new pathway for lipoxin A4 biosynthesis. Biochem. Biophys. Res. Commun. 2010, 400, 432–436. [Google Scholar] [CrossRef]
- Sadallah, S.; Eken, C.; Martin, P.J.; Jürg, A.; Schifferli, J.A. Microparticles (Ectosomes) Shed by Stored Human Platelets Downregulate Macrophages and Modify the Development of Dendritic Cells. J. Immunol. 2011, 186, 6543–6552. [Google Scholar] [CrossRef]
- Dinkla, S.; Van Cranenbroek, B.; Van Der Heijden, W.A.; He, X.; Wallbrecher, R.; Dumitriu, I.E.; Van Der Ven, A.J.; Bosman, G.J.C.G.M.; Koenen, H.J.P.M.; Joosten, I. Platelet microparticles inhibit IL-17 production by regulatory T cells through P-selectin. Blood 2016, 127, 1976–1986. [Google Scholar] [CrossRef]
- Arraud, N.; Linares, R.; Tan, S.; Gounou, C.; Pasquet, J.-M.M.; Mornet, S.; Brisson, A.R.; Linares, R.; Brisson, A.R.; Tan, S.; et al. Extracellular vesicles from blood plasma: Determination of their morphology, size, phenotype and concentration. J. Thromb. Haemost. 2014, 12, 614–627. [Google Scholar] [CrossRef]
- Boilard, E.; Duchez, A.C.; Brisson, A. The diversity of platelet microparticles. Curr. Opin. Hematol. 2015, 22, 437–444. [Google Scholar] [CrossRef]
- Flaumenhaft, R.; Dilks, J.R.; Richardson, J.; Alden, E.; Sunita, R.P.H.; Battinelli, E.; Klement, G.L.; Martha, S.V.; Italiano, J.E. Megakaryocyte-derived microparticles: Direct visualization and distinction from platelet-derived microparticles. Blood 2009, 113, 1112–1121. [Google Scholar] [CrossRef] [PubMed]
- Sinauridze, E.I.; Kireev, D.A.; Popenko, N.Y.; Pichugin, A.V.; Panteleev, M.A.; Krymskaya, O.V.; Ataullakhanov, F.I. Platelet microparticle membranes have 50- to 100-fold higher specific procoagulant activity than activated platelets. Thromb. Haemost. 2007, 97, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Muriithi, E.W.; Belcher, P.R.; Day, S.P.; Menys, V.C.; Wheatley, D.J. Heparin-induced platelet dysfunction and cardiopulmonary bypass. Ann. Thorac. Surg. 2000, 69, 1827–1832. [Google Scholar] [CrossRef] [PubMed]
- Wisgrill, L.; Lamm, C.; Hartmann, J.; Prei??ing, F.; Dragosits, K.; Bee, A.; Hell, L.; Thaler, J.; Ay, C.; Pabinger, I.; et al. Peripheral blood microvesicles secretion is influenced by storage time, temperature, and anticoagulants. Cytom. Part A 2016, 89, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Reston, J.T.; Tregear, S.J.; Turkelson, C.M. Meta-Analysis of Short-Term and Mid-Term Outcomes Following Off-Pump Coronary Artery Bypass Grafting. Ann. Thorac. Surg. 2003, 76, 1510–1515. [Google Scholar] [CrossRef]
- Tempo, J.A.; Englyst, N.A.; Holloway, J.A.; Smith, D.C. Platelet Microvesicles (Microparticles) in Cardiac Surgery. J. Cardiothorac. Vasc. Anesth. 2016, 30, 222–228. [Google Scholar] [CrossRef]
- Atherton, J.; Hampshire, T.; Englyst, N.; Holloway, J.; Clough, G.; Smith, D. The effect of circulating fresh blood through a micro-bypass circuit on platelet microparticles. Appl. Cardiopulm. Pathophysiol. 2012, 16, 176–177. [Google Scholar][Green Version]
- Xu, L.; Liang, Y.; Xu, X.; Xia, J.; Wen, C.; Zhang, P.; Duan, L. Blood cell-derived extracellular vesicles: Diagnostic biomarkers and smart delivery systems. Bioengineered 2021, 12, 7929–7940. [Google Scholar] [CrossRef]
- Rubin, O.; Delobel, J.; Prudent, M.; Lion, N.; Kohl, K.; Tucker, E.I.; Tissot, J.D.; Angelillo-Scherrer, A. Red blood cell-derived microparticles isolated from blood units initiate and propagate thrombin generation. Transfusion 2013, 53, 1744–1754. [Google Scholar] [CrossRef]
- Crawford, T.M.; Andersen, C.C.; Hodyl, N.A.; Robertson, S.A.; Stark, M.J. The contribution of red blood cell transfusion to neonatal morbidity and mortality. J. Paediatr. Child Health 2019, 55, 387–392. [Google Scholar] [CrossRef]
- Connor, J.P.; O’Shea, A.; McCool, K.; Sampene, E.; Barroilhet, L.M. Peri-operative allogeneic blood transfusion is associated with poor overall survival in advanced epithelial ovarian Cancer; potential impact of patient blood management on Cancer outcomes. Gynecol. Oncol. 2018, 151, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Danesh, A.; Inglis, H.C.; Jackman, R.P.; Wu, S.; Deng, X.; Muench, M.O.; Heitman, J.W.; Norris, P.J. Exosomes from red blood cell units bind to monocytes and induce proinflammatory cytokines, boosting T-cell responses in vitro. Blood 2014, 123, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Arraud, N.; Gounou, C.; Turpin, D.; Brisson, A.R. Fluorescence triggering: A general strategy for enumerating and phenotyping extracellular vesicles by flow cytometry. Cytom. Part A 2016, 89, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Niida, S.; Azuma, E.; Yanagibashi, T.; Muramatsu, M.; Huang, T.T.; Sagara, H.; Higaki, S.; Ikutani, M.; Nagai, Y.; et al. Inflammation-induced endothelial cell-derived extracellular vesicles modulate the cellular status of pericytes. Sci. Rep. 2015, 5, 8505. [Google Scholar] [CrossRef]
- Hamilton, K.K.; Hattori, R.; Esmon, C.T.; Sims, P.J. Complement proteins C5b-9 induce vesiculation of the endothelial plasma membrane and expose catalytic surface for assembly of the prothrombinase enzyme complex. J. Biol. Chem. 1990, 265, 3809–3814. [Google Scholar] [CrossRef]
- Wang, J.M.; Wang, Y.; Huang, J.Y.; Yang, Z.; Chen, L.; Wang, L.C.; Tang, A.L.; Lou, Z.F.; Tao, J. C-reactive protein-induced endothelial microparticle generation in HUVECs is related to BH4-dependent NO formation. J. Vasc. Res. 2007, 44, 241–248. [Google Scholar] [CrossRef]
- Jy, W.; Minagar, A.; Jimenez, J.J.; Sheremata, W.A.; Mauro, L.M.; Horstman, L.L.; Bidot, C.; Ahn, Y.S. Endothelial microparticles (EMP) bind and activate monocytes: Elevated EMP-monocyte conjugates in multiple sclerosis. Front. Biosci. 2004, 9, 3137–3144. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, R.; Qu, H.; Wu, J.; Li, L.; Tang, Y. Endothelial microparticles activate endothelial cells to facilitate the inflammatory response. Mol. Med. Rep. 2017, 15, 1291–1296. [Google Scholar] [CrossRef]
- Weber, B.; Sturm, R.; Henrich, D.; Marzi, I.; Leppik, L. CD44+ and CD31+ extracellular vesicles (EVs) are significantly reduced in polytraumatized patients with hemorrhagic shock–evaluation of their diagnostic and prognostic potential. Front. Immunol. 2023, 14, 1196241. [Google Scholar] [CrossRef]
- Angelot, F.; Seillès, E.; Biichlé, S.; Berda, Y.; Gaugler, B.; Plumas, J.; Chaperot, L.; Dignat-George, F.; Tiberghien, P.; Saas, P.; et al. Endothelial cell-derived microparticles induce plasmacytoid dendritic cell maturation: Potential implications in inflammatory diseases. Haematologica 2009, 94, 1502–1512. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Orekhov, A.N.; Sobenin, I.A.; Bobryshev, Y.V. Plasmacytoid dendritic cells: Development, functions, and role in atherosclerotic inflammation. Front. Physiol. 2014, 5, 279. [Google Scholar] [CrossRef] [PubMed]
- Alculumbre, S.; Raieli, S.; Hoffmann, C.; Chelbi, R.; Danlos, F.X.; Soumelis, V. Plasmacytoid pre-dendritic cells (pDC): From molecular pathways to function and disease association. Semin. Cell Dev. Biol. 2019, 86, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Wheway, J.; Latham, S.L.; Combes, V.; Grau, G.E.R. Endothelial Microparticles Interact with and Support the Proliferation of T Cells. J. Immunol. 2014, 193, 3378–3387. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Li, L.; Yan, H.; Su, Q.; Huang, J.; Fu, C. Endothelial microparticles exert differential effects on functions of Th1 in patients with acute coronary syndrome. Int. J. Cardiol. 2013, 168, 5396–5404. [Google Scholar] [CrossRef] [PubMed]
- Park, M.S.; Owen, B.A.L.; Ballinger, B.A.; Sarr, M.G.; Schiller, H.J.; Zietlow, S.P.; Jenkins, D.H.; Ereth, M.H.; Owen, W.G.; Heit, J.A. Quantification of hypercoagulable state after blunt trauma: Microparticle and thrombin generation are increased relative to injury severity, while standard markers are not. Surgery 2012, 151, 831–836. [Google Scholar] [CrossRef]
- Lynch, S.F.; Ludlam, C.A. Plasma microparticles and vascular disorders. Br. J. Haematol. 2007, 137, 36–48. [Google Scholar] [CrossRef]
- Nieuwland, R.; Berckmans, R.J.; Rotteveel-Eijkman, R.C.; Maquelin, K.N.; Roozendaal, K.J.; Jansen, P.G.M.; Ten Have, K.T.; Eijsman, L.; Hack, C.E.; Sturk, A. Cell-derived microparticles generated in patients during cardiopulmonary bypass are highly procoagulant. Circulation 1997, 96, 3534–3541. [Google Scholar] [CrossRef]
- Fabre, O.; Vincentelli, A.; Corseaux, D.; Juthier, F.; Susen, S.; Bauters, A.; Van Belle, E.; Mouquet, F.; Le Tourneau, T.; Decoene, C.; et al. Comparison of Blood Activation in the Wound, Active Vent, and Cardiopulmonary Bypass Circuit. Ann. Thorac. Surg. 2008, 86, 537–541. [Google Scholar] [CrossRef]
- Fontaine, D.; Pradier, O.; Hacquebard, M.; Stefanidis, C.; Carpentier, Y.; De Canniere, D.; Fontaine, J.; Berkenboom, G. Oxidative stress produced by circulating microparticles in on-pump but not in off-pump coronary surgery. Acta Cardiol. 2009, 64, 715–722. [Google Scholar] [CrossRef]
- Mesri, M.; Altieri, D.C. Leukocyte microparticles stimulate endothelial cell cytokine release and tissue factor induction in a JNK1 signaling pathway. J. Biol. Chem. 1999, 274, 23111–23118. [Google Scholar] [CrossRef]
- Cerri, C.; Chimenti, D.; Conti, I.; Neri, T.; Paggiaro, P.; Celi, A. Monocyte/Macrophage-Derived Microparticles Up-Regulate Inflammatory Mediator Synthesis by Human Airway Epithelial Cells. J. Immunol. 2006, 177, 1975–1980. [Google Scholar] [CrossRef] [PubMed]
- Bardelli, C.; Amoruso, A.; Federici Canova, D.; Fresu, L.G.; Balbo, P.; Neri, T.; Celi, A.; Brunelleschi, S. Autocrine activation of human monocyte/macrophages by monocyte-derived microparticles and modulation by PPARγ ligands. Br. J. Pharmacol. 2012, 165, 716–728. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.L.; Midura, E.F.; Prakash, P.S.; Rice, T.C.; Kunz, N.; Kalies, K.; Caldwell, C.C. Neutrophil derived microparticles increase mortality and the counter-inflammatory response in a murine model of sepsis. Biochim. Biophys. Acta-Mol. Basis Dis. 2017, 1863, 2554–2563. [Google Scholar] [CrossRef] [PubMed]
- Prakash, P.S.; Caldwell, C.C.; Lentsch, A.B.; Pritts, T.A.; Robinson, B.R.H. Human microparticles generated during sepsis in patients with critical illness are neutrophil-derived and modulate the immune response. J. Trauma Acute Care Surg. 2012, 73, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Pliyev, B.K.; Kalintseva, M.V.; Abdulaeva, S.V.; Yarygin, K.N.; Savchenko, V.G. Neutrophil microparticles modulate cytokine production by natural killer cells. Cytokine 2014, 65, 126–129. [Google Scholar] [CrossRef]
- Scanu, A.; Molnarfi, N.; Brandt, K.J.; Gruaz, L.; Dayer, J.-M.; Burger, D. Stimulated T cells generate microparticles, which mimic cellular contact activation of human monocytes: Differential regulation of pro- and anti-inflammatory cytokine production by high-density lipoproteins. J. Leukoc. Biol. 2008, 83, 921–927. [Google Scholar] [CrossRef]
- Shefler, I.; Salamon, P.; Reshef, T.; Mor, A.; Mekori, Y.A. T Cell-Induced Mast Cell Activation: A Role for Microparticles Released from Activated T Cells. J. Immunol. 2010, 185, 4206–4212. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, X.; Zhao, T.; Li, W.; Xiang, J. Natural CD8+25+ regulatory T cell-secreted exosomes capable of suppressing cytotoxic T lymphocyte-mediated immunity against B16 melanoma. Biochem. Biophys. Res. Commun. 2013, 438, 152–155. [Google Scholar] [CrossRef]
- Tung, S.L.; Boardman, D.A.; Sen, M.; Letizia, M.; Peng, Q.; Cianci, N.; Dioni, L.; Carlin, L.M.; Lechler, R.; Bollati, V.; et al. Regulatory T cell-derived extracellular vesicles modify dendritic cell function. Sci. Rep. 2018, 8, 6065. [Google Scholar] [CrossRef]
- Zhang, F.; Li, R.; Yang, Y.; Shi, C.; Shen, Y.; Lu, C.; Chen, Y.; Zhou, W.; Lin, A.; Yu, L.; et al. Specific Decrease in B-Cell-Derived Extracellular Vesicles Enhances Post-Chemotherapeutic CD8+ T Cell Responses. Immunity 2019, 50, 738–750.e7. [Google Scholar] [CrossRef]
- Mostefai, H.A.; Agouni, A.; Carusio, N.; Mastronardi, M.L.; Heymes, C.; Henrion, D.; Andriantsitohaina, R.; Martinez, M.C. Phosphatidylinositol 3-Kinase and Xanthine Oxidase Regulate Nitric Oxide and Reactive Oxygen Species Productions by Apoptotic Lymphocyte Microparticles in Endothelial Cells. J. Immunol. 2008, 180, 5028–5035. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, L.; Ludwig, S.; Vahl, J.M.; Brunner, C.; Hoffmann, T.K.; Theodoraki, M.N. The emerging role of exosomes in diagnosis, prognosis, and therapy in head and neck cancer. Int. J. Mol. Sci. 2020, 21, 4072. [Google Scholar] [CrossRef] [PubMed]
- Golovkin, A.S.; Asadullina, I.A.; Kudryavtsev, I.V. Purinergic Regulation of Basic Physiological and Pathological Processes. Med. Immunol. 2018, 20, 463–476. [Google Scholar] [CrossRef]
- Delorme, A.S.; Laguide, A.; Tamagne, M.; Pinheiro, M.K.; Cagnet, L.; Neyrinck-Leglantier, D.; Khelfa, M.; Cleophax, S.; Pirenne, F.; Vingert, B. Immune interactions and regulation with CD39+ extracellular vesicles from platelet concentrates. Front. Immunol. 2024, 15, 1397967. [Google Scholar] [CrossRef]
- Smyth, L.A.; Ratnasothy, K.; Tsang, J.Y.S.S.; Boardman, D.; Warley, A.; Lechler, R.; Lombardi, G. CD73 expression on extracellular vesicles derived from CD4+CD25+Foxp3+ T cells contributes to their regulatory function. Eur. J. Immunol. 2013, 43, 2430–2440. [Google Scholar] [CrossRef]


| Parameters | On-Pump Surgery, n = 18 | Off-Pump Surgery, n = 18 |
|---|---|---|
| Median age | 64 (59; 69) | 68 (65; 75) |
| Median number of shunts (min–max) | 3 (2–5) | 3 (1–4) |
| Diabetes mellitus | 3 (16%) | 6 (33%) |
| Dyslipidemia | 8 (44%) | 9 (50%) |
| Obesity | 5 (28%) | 2 (11%) |
| Chronic gastritis, remission | 15 (83%) | 14 (78%) |
| Arterial hypertension | 18 (100%) | 18 (100%) |
| Myocardial infarction in anamnesis | 11 (61.1%) | 12 (66.7%) |
| Left ventricular ejection fraction, (%) | 63 (58; 71.5) | 60 (57; 68) |
| Antibody | Manufacture | Receptor Specification | Cell Origin |
|---|---|---|---|
| Panel 1 | |||
| CD235a PE | BioLegend, San Diego, CA, USA | glycophorin A | Erythroid precursors and erythrocytes |
| CD41 AF488 | BioLegend, San Diego, CA, USA | α subunit of the gpIIb/IIIa (CD41/CD61) complex | Platelets and megakaryocytes |
| Panel 2 | |||
| CD41 AF488 | BioLegend, San Diego, CA, USA | α subunit of the gpIIb/IIIa (CD41/CD61) complex | Platelets and megakaryocytes |
| CD62P PE | BD Pharmingen, Franklin Lakes, NJ, USA | Type I transmembrane glycoprotein; P-selectin; platelet activation-dependent granule membrane protein (PADGEM); GMP-140 | Activated platelets, megakaryocytes, and endothelial cells |
| Panel 3 | |||
| CD39 FITC | BioLegend, San Diego, CA, USA | Ecto nucleotidase that can hydrolyze both nucleoside triphosphates and diphosphates | Activated lymphocytes, regulatory T-cells, a subset of T-cells and B-cells, and dendritic cells |
| CD73 PE | BD Pharmingen, Franklin Lakes, NJ, USA | Ecto-5′-nucleotidase that converts adenosine monophosphate (AMP) to adenosine | Regulatory T-cells, subsets of T- and B-cells, mesenchymal stem cells, follicular dendritic cells, endothelial cells, and epithelial cells |
| Panel 4 | |||
| CD31 FITC | BioLegend, San Diego, CA, USA | Platelet endothelial cell adhesion molecule-1 (PECAM-1) | Monocytes, platelets, granulocytes, endothelial cells and lymphocyte subsets |
| CD34 PE/Dazzle | BioLegend, San Diego, CA, USA | Type I monomeric sialomucin-like glycophosphoprotein | hematopoietic stem/progenitor cells, bone marrow stromal cells, capillary endothelial cells, embryonic fibroblasts, some nervous tissue |
| CD90 PE/Cy7 | BioLegend, San Diego, CA, USA | GPI-anchored protein, Thy-1 | Neuronal cells, a subset of CD34+ cells, fibroblasts, activated endothelial cells |
| CD105 APC | BioLegend, San Diego, CA, USA | Type I integral membrane homodimer protein, a component of the TGF-β receptor system | vascular endothelial cells, activated monocytes, tissue macrophages, activated endothelium |
| CD146 PE | BioLegend, San Diego, CA, USA | Integral transmembrane glycoprotein that belongs to the immunoglobulin superfamily | Epithelial cells, endothelial cells, fibroblasts, activated T-cells, multipotent mesenchymal stromal cells, and activated keratinocytes |
| Panel 5 | |||
| CD45 KrO | Beckman Coulter, West Sacramento, CA, USA | Single chain type I membrane glycoprotein, leukocyte common antigen (LCA) | All hematopoietic cells, except erythrocytes and platelets |
| CD3 AlexaFluor750 | Beckman Coulter, West Sacramento, CA, USA | CD3/T-cell receptor (TCR) complex, member of the immunoglobulin superfamily | All mature T-cells, NKT cells, and some thymocytes |
| CD4 PE | Beckman Coulter, West Sacramento, CA, USA | Single-chain type I transmembrane glycoprotein, member of the Ig superfamily | Most thymocytes, a subset of T-cells, and monocytes/macrophages, T-helper cells marker |
| CD8 PC5.5 | Beckman Coulter, West Sacramento, CA, USA | Type I glycoprotein, a member of the immunoglobulin superfamily | Majority of thymocytes, a subset of peripheral blood T-cells, and NK cells, T-cytotoxic cells marker |
| CD19 FITC | BioLegend, San Diego, CA, USA | Type I transmembrane glycoprotein, a member of the immunoglobulin superfamily | B-cells, follicular dendritic cells |
| CD14 APC | Beckman Coulter, West Sacramento, CA, USA | Glycosylphosphatidylinositol (GPI)-linked membrane glycoprotein, LPS receptor | High levels on monocytes and macrophages, and at lower levels on granulocytes |
| Parameter, Units | On-Pump Surgery | Off-Pump Surgery | Significance | ||
|---|---|---|---|---|---|
| Before | After | Before | After | ||
| RBC, ×1012 | 4.17 (3.89; 4.28) | 3.42 (3.19; 3.93) | 4.26 (4.11; 4.46) | 3.55 (3.11; 4.18) | p1,2 = 0.05 p3,4 < 0.01 |
| Hemoglobin, g/L | 125 (124; 128) | 106 (99; 117) | 134 (119; 135) | 112 (92; 123) | p1,2 = 0.05 p3,4 < 0.01 |
| Platelets, ×109 | 151 (134; 203) | 125 (114; 161) | 177 (149; 219) | 137 (115; 207) | p1,2 = 0.03 |
| WBC, ×109 | 5.56 (5.18; 6.68) | 13.97 (13.10; 15.28) | 6.13 (5.78; 7.51) | 12.20 (9.20; 14.30) | p1,2 = 0.01 p3,4 < 0.01 |
| Lymphocytes, ×109 | 1.76 (1.338; 2.03) | 1.38 (0.97; 1.96) | 1.81 (1.41; 2.79) | 1.05 (0.71; 1.74) | p3,4 < 0.01 |
| Neutrophils, ×109 | 3.24 (2.55; 3.75) | 11.12 (10.61; 12.10) | 3.37 (2.35; 3.99) | 9.70 (8.03:10.70) | p1,2 = 0.01 p3,4 < 0.01 |
| Monocytes, ×109 | 0.50 (0.44; 0.65) | 1.00 (0.65; 1.35) | 0.56 (0.49; 0.69) | 0.80 (0.36; 1.07) | p1,2 = 0.01 |
| Eosinophils, ×109 | 0.09 (0.07; 0.11) | 0 (0; 0) | 0.07 (0.04; 0.14) | 0 (0; 0.01) | p1,2 = 0.01 p3,4 < 0.01 |
| Basophils, ×109 | 0.05 (0.01; 0.09) | 0.02 (0.01; 0.09) | 0.06 (0.03; 0.09) | 0.04 (0.03; 0.05) | |
| T-Cell Subsets | On-Pump | Off-Pump | Significance | |||
|---|---|---|---|---|---|---|
| Before Surgery | After Surgery | Before Surgery | After Surgery | |||
| Th | % | 45.1 (37.2; 51.3) | 43.7 (34.2; 47.5) | 43.8 (38.6; 52.4) | 37.4 (30.4; 43.2) | p3,4 = 0.01 |
| # | 741.4 (707.8; 784.6) | 470.0 (331.7; 830.4) | 809.8 (536.9; 991.1) | 476.3 (345.0; 723.5) | p1,2 = 0.02 p3,4 = 0.04 | |
| Tcyt | % | 21.3 (16.8; 36.0) | 21.9 (15.4; 29.1) | 23.8 (13.8; 27.1) | 20.5 (14.9; 27.9) | p 1,2 = 0.01 |
| # | 417.2 (241.2; 677.9) | 301.9 (258.3; 336.0) | 415.9 (217.8; 537.9) | 221.0 (106.8; 452.0) | p1,2 = 0.04 p3,4 < 0.01 | |
| B-cells | % | 8.7 (5.9; 9.4) | 21.0 (12.6; 27.1) | 9.5 (6.8; 11.4) | 19.5 (7.5; 20.3) | p1,2 = 0.01 p3,4 = 0.04 |
| # | 130.5 (99.4; 183.7) | 236.5 (119.4; 370.4) | 155.7 (81.5; 300.8) | 209.1 (107.8; 353.1) | p1,2 = 0.01 | |
| Cytokine, pg/mL | On-Pump | Off-Pump |
|---|---|---|
| sCD40L | 111.1 (89.5; 128.5) | 111.1 (89.5; 126.3) |
| EGF | 0 (0; 0) | 0 (0; 0) |
| Eotaxin | 168.1 (158.1; 200.7) | 178.6 (145.2; 184.5) |
| FGF-2 | 120.5 (0; 138.9) | 0 (0; 0) |
| FLT-3Ligand | 0 (0; 28.5) | 0 (0; 0) |
| Fractalkine/CX3CL1 | 0 (0; 0) | 0 (0; 0) |
| G-CSF | 209 (199; 233) | 199 (141; 238) |
| GM-CSF | 0 (0; 0) | 0 (0; 0) |
| GROa/CXCL1 | 661 (344; 949) | 586 (395; 950) |
| IFN α2 | 0 (0; 0) | 0 (0; 0) |
| IFN γ | 0 (0; 0) | 0 (0; 0) |
| IL-1α | 0 (0; 7.0) | 0 (0; 0) |
| IL-1β | 0 (0; 0) | 0 (0;0) |
| IL-1RA | 10.4 (7.1; 10.4) | 7.1 (0; 14.7) |
| IL-2 | 0 (0; 0) | 0 (0; 0) |
| IL-3 | 0 (0; 0) | 0 (0; 0) |
| IL-4 | 119.4 (0; 157.2) | 0 (0; 146.0) |
| IL-5 | 0 (0; 0) | 0 (0; 0) |
| IL-6 | 136.1 (129.6; 160.6) | 151.7 (141.2; 180.6) |
| IL-7 | 0 (0; 7.5) | 0 (0; 6.7) |
| IL-8/CXCL8 | 94.6 (87.9; 102.8) | 94.3 (87.5; 101.5) |
| IL-9 | 0 (0; 0) | 0 (0; 0) |
| IL-10 | 55.9 (33.5; 62.5) | 59.6 (33.5; 73.6) |
| IL-12 p40 | 0 (0; 0) | 0 (0; 0) |
| IL-12 p70 | 0 (0; 0) | 0 (0; 0) |
| IL-13 | 0 (0; 0) | 0 (0; 0) |
| IL-15 | 7.1 (4.1; 10.4) * | 4.9 (2.6; 6.8) * |
| IL-17A | 0 (0; 0) | 0 (0; 0) |
| IP-10/CXCL10 | 327 (241; 508) | 368 (234; 443) |
| MCP-1/CCL2 | 273 (234; 462) | 297 (271; 357) |
| MCP-3/CCL7 | 93.3 (0; 97.3) | 0 (0; 105.8) |
| MDC/CCL22 | 462 (398; 577) | 382 (344; 470) |
| MIP-1a/CCL3 | 0 (0; 0) | 0 (0; 0) |
| MIP-1b/CCL4 | 27.8 (18.5; 30.8) | 25.2 (19.2; 39.4) |
| TGF α | 0 (0; 0) | 0 (0; 0) |
| TNF α | 19.9 (15.9; 21.3) | 14.1 (11.6; 20.9) |
| TNF β | 0 (0; 0) | 0 (0; 3.2) |
| VEGF-A | 0 (0; 0) | 0 (0; 0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aquino, A.; Abutalimova, N.; Ma, Y.; Ismail-zade, I.; Grebennik, V.; Rubinstein, A.; Kudryavtsev, I.; Zaikova, E.; Sambur, D.; Marichev, A.; et al. Differences in Plasma Extracellular Vesicles of Different Origin in On-Pump Versus Off-Pump Cardiac Surgery. Curr. Issues Mol. Biol. 2024, 46, 13058-13077. https://doi.org/10.3390/cimb46110779
Aquino A, Abutalimova N, Ma Y, Ismail-zade I, Grebennik V, Rubinstein A, Kudryavtsev I, Zaikova E, Sambur D, Marichev A, et al. Differences in Plasma Extracellular Vesicles of Different Origin in On-Pump Versus Off-Pump Cardiac Surgery. Current Issues in Molecular Biology. 2024; 46(11):13058-13077. https://doi.org/10.3390/cimb46110779
Chicago/Turabian StyleAquino, Arthur, Napisat Abutalimova, Yi Ma, Imran Ismail-zade, Vadim Grebennik, Artem Rubinstein, Igor Kudryavtsev, Ekatherina Zaikova, Darina Sambur, Alexander Marichev, and et al. 2024. "Differences in Plasma Extracellular Vesicles of Different Origin in On-Pump Versus Off-Pump Cardiac Surgery" Current Issues in Molecular Biology 46, no. 11: 13058-13077. https://doi.org/10.3390/cimb46110779
APA StyleAquino, A., Abutalimova, N., Ma, Y., Ismail-zade, I., Grebennik, V., Rubinstein, A., Kudryavtsev, I., Zaikova, E., Sambur, D., Marichev, A., Kalinina, O., Bautin, A., Kostareva, A., Vaage, J., & Golovkin, A. (2024). Differences in Plasma Extracellular Vesicles of Different Origin in On-Pump Versus Off-Pump Cardiac Surgery. Current Issues in Molecular Biology, 46(11), 13058-13077. https://doi.org/10.3390/cimb46110779







