Melanocortin Derivatives Induced Vascularization and Neuroglial Proliferation in the Rat Brain under Conditions of Cerebral Ischemia
Abstract
:1. Introduction
2. Materials and Methods
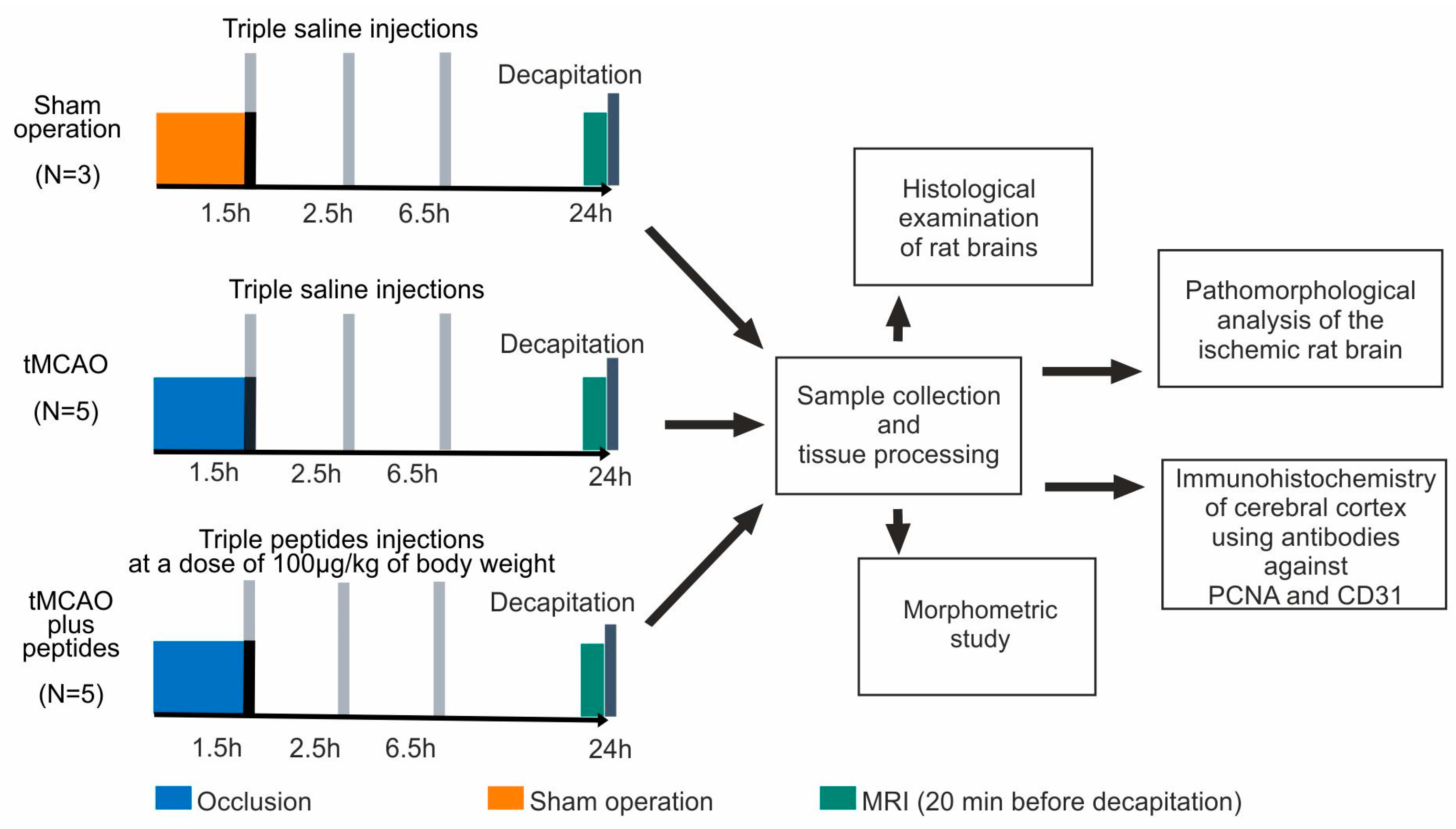
2.1. Animals and Experimental Groups
2.2. Rat Transient Middle Cerebral Artery Occlusion (tMCAO) Model
2.2.1. Operation
2.2.2. Peptide Administration
2.2.3. Magnetic Resonance Imaging
2.3. Sample Collection and Tissue Processing
2.4. Histological Examination of Rat Brains
2.5. Pathomorphological Analysis of the Ischemic Rat Brain
2.6. Immunohistochemistry
2.7. Morphometric Study
2.8. Data Analysis and Statistics
3. Results
3.1. MRI of Ischemic Foci after tMCAO
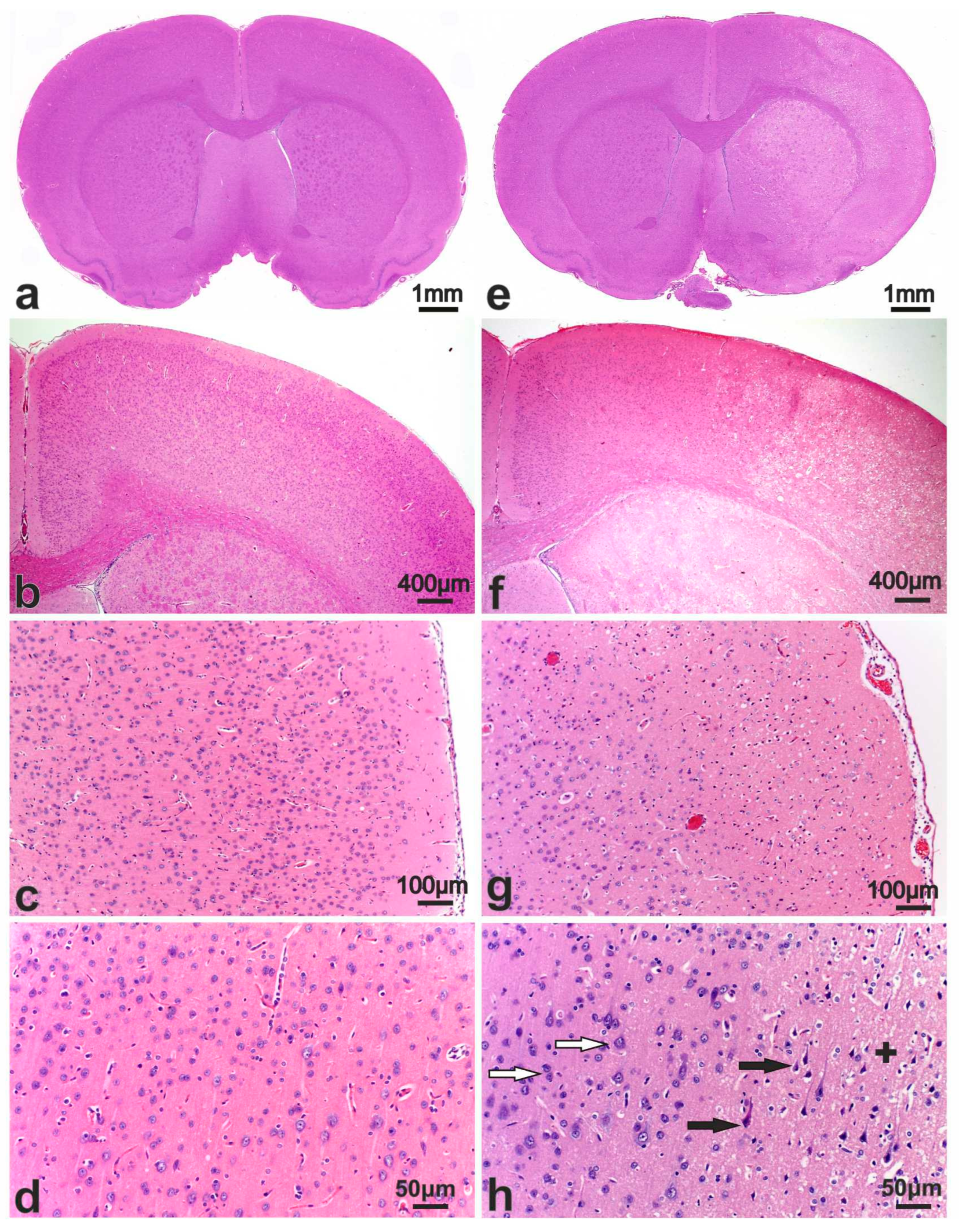
3.2. Morphological Study of the Brain after Sham Operation (SO)
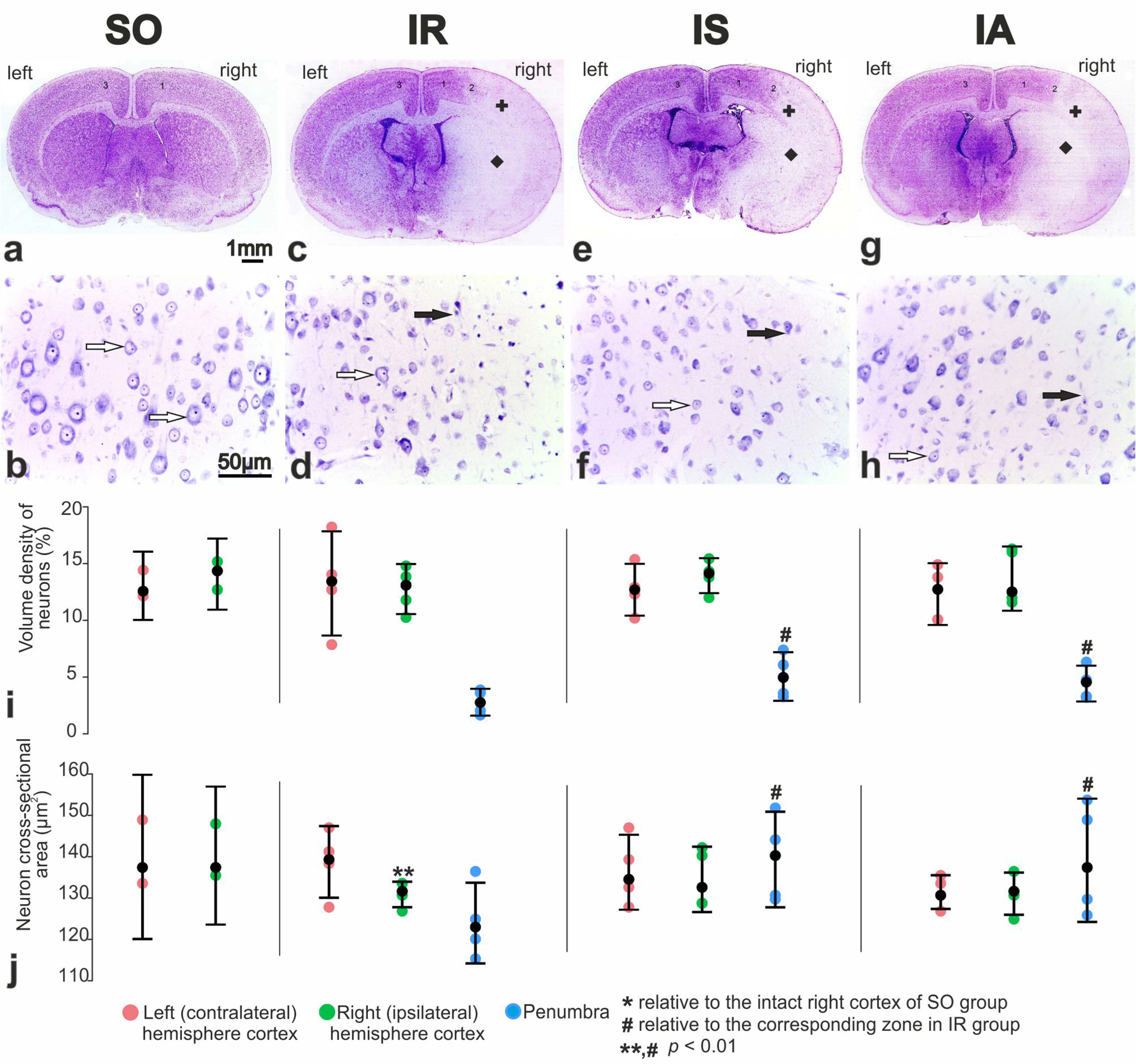
3.3. Pathomorphology of Focal Cerebral Ischemia and Morphofunctional Analysis of the Action of Peptide Drugs after tMCAO
3.4. Morphofunctional and Morphometric Analysis of the Action of Peptide Drugs after tMCAO
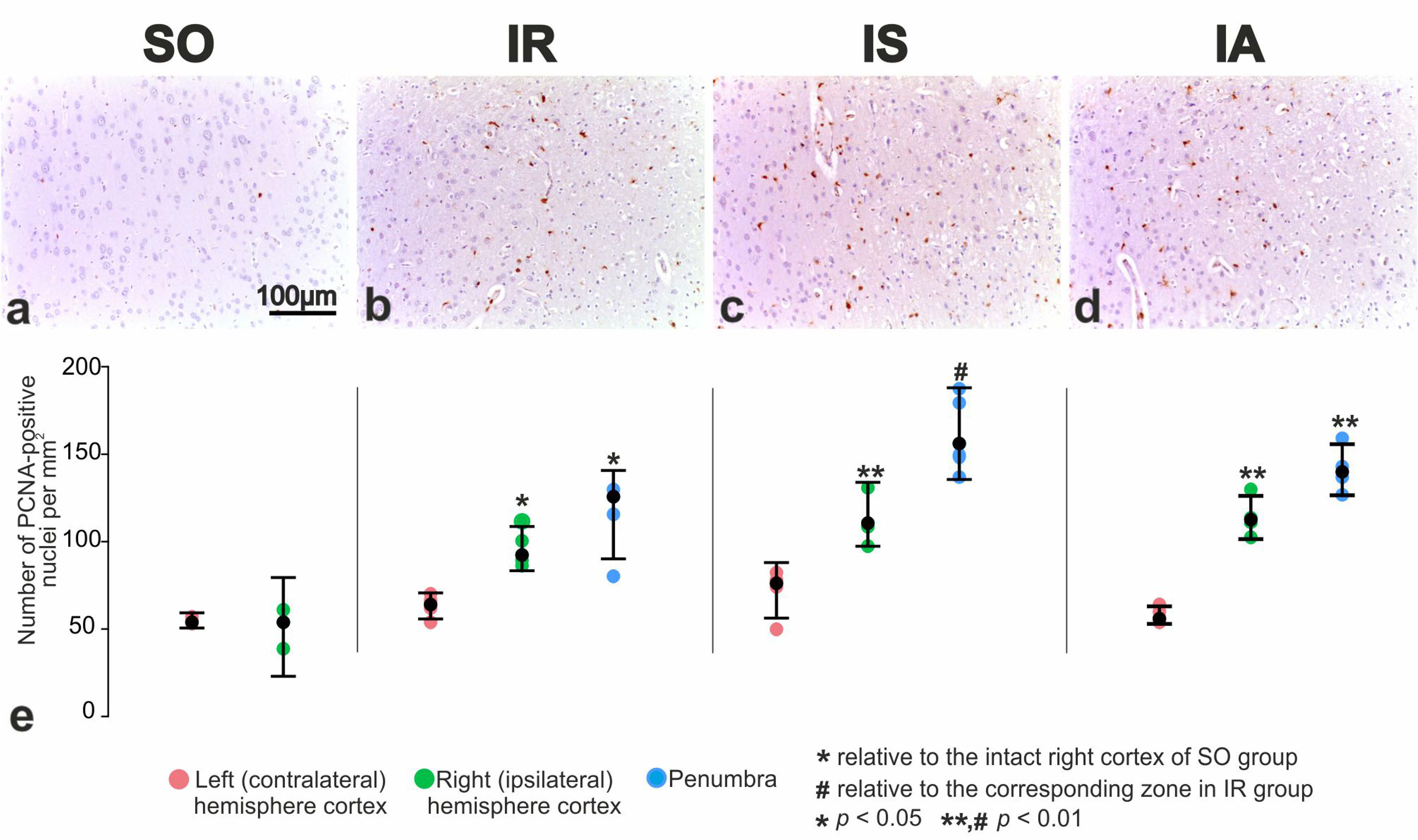
3.5. Immunohistochemical and Morphometric Study of the Effect of the Peptide Drugs on Proliferative Activity of Brain Neuroglial Cells under tMCAO Conditions
3.6. Immunohistochemical and Morphometric Study of the Effect of the Peptides on Cerebral Cortex Vascularization after tMCAO
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adams, H.P.; Adams, R.J.; Brott, T.; Del Zoppo, G.J.; Furlan, A.; Goldstein, L.B.; Grubb, R.L.; Higashida, R.; Kidwell, C.; Kwiatkowski, T.G.; et al. Guidelines for the early management of patients with ischemic stroke: A scientific statement from the Stroke Council of the American Stroke Association. Stroke 2003, 34, 1056–1083. [Google Scholar] [CrossRef]
- Kalogeris, T.; Baines, C.P.; Krenz, M.; Korthuis, R.J. Ischemia/Reperfusion. Compr. Physiol. 2016, 7, 113–170. [Google Scholar] [CrossRef]
- Xiao, M.; Xiao, Z.J.; Yang, B.; Lan, Z.; Fang, F. Blood-Brain Barrier: More Contributor to Disruption of Central Nervous System Homeostasis Than Victim in Neurological Disorders. Front. Neurosci. 2020, 14, 764. [Google Scholar] [CrossRef]
- Bogorad, M.I.; DeStefano, J.G.; Linville, R.M.; Wong, A.D.; Searson, P.C. Cerebrovascular plasticity: Processes that lead to changes in thearchitecture of brain microvessels. J. Cereb. Blood Flow Metab. 2019, 39, 1413. [Google Scholar] [CrossRef]
- Hachinski, V.; Donnan, G.A.; Gorelick, P.B.; Hacke, W.; Cramer, S.C.; Kaste, M.; Fisher, M.; Brainin, M.; Buchan, A.M.; Lo, E.H.; et al. Stroke: Working toward a prioritized world agenda. Stroke 2010, 41, 1084–1099. [Google Scholar] [CrossRef]
- Anrather, J.; Iadecola, C. Inflammation and Stroke: An Overview. Neurotherapeutics 2016, 13, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Filippenkov, I.B.; Stavchansky, V.V.; Denisova, A.E.; Yuzhakov, V.V.; Sevan’kaeva, L.E.; Sudarkina, O.Y.; Dmitrieva, V.G.; Gubsky, L.V.; Myasoedov, N.F.; Limborska, S.A.; et al. Novel Insights into the Protective Properties of ACTH(4-7)PGP (Semax) Peptide at the Transcriptome Level Following Cerebral Ischaemia–Reperfusion in Rats. Genes 2020, 11, 681. [Google Scholar] [CrossRef] [PubMed]
- Caligiuri, G. Mechanotransduction, immunoregulation, and metabolic functions of CD31 in cardiovascular pathophysiology. Cardiovasc. Res. 2019, 115, 1425–1434. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.Y.; Liu, L.; Yang, Q.W. Refocusing Neuroprotection in Cerebral Reperfusion Era: New Challenges and Strategies. Front. Neurol. 2018, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Dergunova, L.V.; Filippenkov, I.B.; Limborska, S.A.; Myasoedov, N.F. Neuroprotective Peptides and New Strategies for Ischemic Stroke Drug Discoveries. Genes 2023, 14, 953. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liao, J.; Xiong, L.; Xiao, Z.; Ye, F.; Wang, Y.; Chen, T.; Huang, L.; Chen, M.; Chen, Z.-S.; et al. Stepwise targeted strategies for improving neurological function by inhibiting oxidative stress levels and inflammation following ischemic stroke. J. Control. Release 2024, 3659, 38423472. [Google Scholar] [CrossRef]
- Zhou, H.; Yan, L.; Huang, H.; Li, X.; Xia, Q.; Zheng, L.; Shao, B.; Gao, Q.; Sun, N.; Shi, J. Tat-NTS peptide protects neurons against cerebral ischemia-reperfusion injury via ANXA1 SUMOylation in microglia. Theranostics 2023, 13, 5561–5583. [Google Scholar] [CrossRef]
- Lubell, W.D. Peptide-Based Drug Development. Biomedicines 2022, 10, 2037. [Google Scholar] [CrossRef]
- Apostolopoulos, V.; Bojarska, J.; Chai, T.T.; Elnagdy, S.; Kaczmarek, K.; Matsoukas, J.; New, R.; Parang, K.; Lopez, O.P.; Parhiz, H.; et al. A Global Review on Short Peptides: Frontiers and Perspectives. Molecules 2021, 26, 430. [Google Scholar] [CrossRef]
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Therapeutic peptides: Current applications and future directions. Signal Transduct. Target. Ther. 2022, 7, 48. [Google Scholar] [CrossRef]
- Mun, Y.; Kim, W.; Shin, D. Melanocortin 1 Receptor (MC1R): Pharmacological and Therapeutic Aspects. Int. J. Mol. Sci. 2023, 24, 12152. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Kahale, F.; Naderi, A.; Surico, P.; Yin, J.; Dohlman, T.; Chen, Y.; Dana, R. Therapeutic Effects of Stimulating the Melanocortin Pathway in Regulating Ocular Inflammation and Cell Death. Biomolecules 2024, 14, 169. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, A.; Tacchi, R.; Vergoni, A.V. Brain effects of melanocortins. Pharmacol. Res. 2009, 59, 13–47. [Google Scholar] [CrossRef] [PubMed]
- Leone, S.; Noera, G.; Bertolini, A. Developments and new vistas in the field of melanocortins. Biomol. Concepts 2015, 6, 361–382. [Google Scholar] [CrossRef] [PubMed]
- Goit, R.K.; Ng, T.C.; Tam, K.C.; Tsang, J.K.W.; Taylor, A.W.; Lo, A.C.Y. Neuropeptide α-Melanocyte-Stimulating Hormone Promotes Neurological Recovery and Repairs Cerebral Ischemia/Reperfusion Injury in Type 1 Diabetes. Neurochem. Res. 2022, 47, 394–408. [Google Scholar] [CrossRef] [PubMed]
- Sudarkina, O.Y.; Filippenkov, I.B.; Stavchansky, V.V.; Denisova, A.E.; Yuzhakov, V.V.; Sevan’kaeva, L.E.; Valieva, L.V.; Remizova, J.A.; Dmitrieva, V.G.; Gubsky, L.V.; et al. Brain Protein Expression Profile Confirms the Protective Effect of the ACTH(4–7)PGP Peptide (Semax) in a Rat Model of Cerebral Ischemia–Reperfusion. Int. J. Mol. Sci. 2021, 22, 6179. [Google Scholar] [CrossRef]
- Wang, M.; Zhi, D.; Wang, H.; Ru, Y.; Ren, H.; Wang, N.; Liu, Y.; Li, Y.; Li, H. TAT-HSA-α-MSH fusion protein with extended half-life inhibits tumor necrosis factor-α in brain inflammation of mice. Appl. Microbiol. Biotechnol. 2016, 100, 5353–5361. [Google Scholar] [CrossRef]
- Yakovleva, E.V.; Kuzenkov, V.S.; Fedorov, V.N.; Skvortsova, V.I.; Koshelev, V.B.; Gusev, E.I.; Ashmarin, I.P. In vivo efficiency of semax in global cerebral ischemia. Bull. Exp. Biol. Med. 1999, 128, 806–807. [Google Scholar] [CrossRef]
- Storozhevykh, T.P.; Tukhbatova, G.R.; Senilova, Y.E.; Pinelis, V.G.; Andreeva, L.A.; Myasoyedov, N.F. Effects of semax and its Pro-Gly-Pro fragment on calcium homeostasis of neurons and their survival under conditions of glutamate toxicity. Bull. Exp. Biol. Med. 2007, 143, 601–604. [Google Scholar] [CrossRef]
- Bashkatova, V.G.; Koshelev, V.B.; Fadyukova, O.E.; Alexeev, A.A.; Vanin, A.F.; Rayevsky, K.S.; Ashmarin, I.P.; Armstrong, D.M. Novel synthetic analogue of ACTH 4-10 (Semax) but not glycine prevents the enhanced nitric oxide generation in cerebral cortex of rats with incomplete global ischemia. Brain Res. 2001, 894, 145–149. [Google Scholar] [CrossRef]
- Novosadova, E.V.; Arsenyeva, E.L.; Antonov, S.A.; Vanyushina, Y.N.; Malova, T.V.; Komissarov, A.A.; Illarioshkin, S.N.; Khaspekov, L.G.; Andreeva, L.A.; Myasoedov, N.F.; et al. The Use of Human Induced Pluripotent Stem Cells for Testing Neuroprotective Activity of Pharmacological Compounds. Biochemistry 2019, 84, 1296–1305. [Google Scholar] [CrossRef]
- Bakaeva, Z.V.; Surin, A.M.; Lizunova, N.V.; Zgodova, A.E.; Krasilnikova, I.A.; Fisenko, A.P.; Frolov, D.A.; Andreeva, L.A.; Myasoedov, N.F.; Pinelis, V.G. Neuroprotective Potential of Peptides HFRWPGP (ACTH 6-9 PGP), KKRRPGP, and PyrRP in Cultured Cortical Neurons at Glutamate Excitotoxicity. Dokl. Biochem. Biophys. 2020, 491, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Cherkasova, K.A.; Lyapina, L.A.; Ashmarin, I.P. Comparative study of modulatory effects of Semax and primary proline-containing peptides on hemostatic reactions. Bull. Exp. Biol. Med. 2001, 132, 625–626. [Google Scholar] [CrossRef] [PubMed]
- Khugaeva, V.K.; Aleksandrin, V.V. Relationship between therapeutic effect of the peptide preparation semax and severity of brain ischemia. Bull. Exp. Biol. Med. 1997, 124, 655–658. [Google Scholar] [CrossRef]
- Gusev, E.I.; Martynov, M.Y.; Kostenko, E.V.; Petrova, L.V.; Bobyreva, S.N. The efficacy of semax in the tretament of patients at different stages of ischemic stroke. Zhurnal Nevrol. Psihiatr. Im. S. S. Korsakova 2018, 118, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Filippenkov, I.B.; Remizova, J.A.; Denisova, A.E.; Stavchansky, V.V.; Golovina, K.D.; Gubsky, L.V.; Limborska, S.A.; Dergunova, L.V. Comparative Use of Contralateral and Sham-Operated Controls Reveals Traces of a Bilateral Genetic Response in the Rat Brain after Focal Stroke. Int. J. Mol. Sci. 2022, 23, 7308. [Google Scholar] [CrossRef]
- Filippenkov, I.B.; Remizova, J.A.; Stavchansky, V.V.; Denisova, A.E.; Gubsky, L.V.; Myasoedov, N.F.; Limborska, S.A.; Dergunova, L.V. Synthetic Adrenocorticotropic Peptides Modulate the Expression Pattern of Immune Genes in Rat Brain following the Early Post-Stroke Period. Genes 2023, 14, 1382. [Google Scholar] [CrossRef] [PubMed]
- Shpetko, Y.Y.; Filippenkov, I.B.; Denisova, A.E.; Stavchansky, V.V.; Gubsky, L.V.; Limborska, S.A.; Dergunova, L.V. Isoflurane Anesthesia’s Impact on Gene Expression Patterns of Rat Brains in an Ischemic Stroke Model. Genes 2023, 14, 1448. [Google Scholar] [CrossRef] [PubMed]
- Filippenkov, I.B.; Remizova, J.A.; Denisova, A.E.; Stavchansky, V.V.; Golovina, K.D.; Gubsky, L.V.; Limborska, S.A.; Dergunova, L.V. Differential gene expression in the contralateral hemisphere of the rat brain after focal ischemia. Sci. Rep. 2023, 13, 573. [Google Scholar] [CrossRef] [PubMed]
- Filippenkov, I.B.; Stavchansky, V.V.; Denisova, A.E.; Valieva, L.V.; Remizova, J.A.; Mozgovoy, I.V.; Zaytceva, E.I.; Gubsky, L.V.; Limborska, S.A.; Dergunova, L.V. Genome-Wide RNA-Sequencing Reveals Massive Circular RNA Expression Changes of the Neurotransmission Genes in the Rat Brain after Ischemia-Reperfusion. Genes 2021, 12, 1870. [Google Scholar] [CrossRef] [PubMed]
- Akimov, M.G.; Fomina-Ageeva, E.V.; Dudina, P.V.; Andreeva, L.A.; Myasoyedov, N.F.; Bezuglov, V.V. ACTH(6–9)PGP Peptide Protects SH-SY5Y Cells from H2O2, tert-Butyl Hydroperoxide, and Cyanide Cytotoxicity via Stimulation of Proliferation and Induction of Prosurvival-Related Genes. Molecules 2021, 26, 1878. [Google Scholar] [CrossRef] [PubMed]
- Levitskaya, N.G.; Glazova, N.Y.; Sebentsova, E.A.; Manchenko, D.M.; Andreeva, L.A.; Kamensky, A.A.; Myasoedov, N.F. Nootropic and anxiolytic effects of heptapeptide ACTH6-9Pro-Gly-Pro. Russ. J. Physiol. 2019, 6, 761–770. [Google Scholar]
- Koizumi, J.; Yoshida, Y.; Nakazawa, T.; Ooneda, G.; Koizumi, J.; Yoshida, Y.; Nakazawa, T.; Ooneda, G. Experimental studies of ischemic brain edema. Nosotchu 1986, 8, 1–8. [Google Scholar] [CrossRef]
- Garcia, J.H.; Liu, K.F.; Ho, K.L. Neuronal necrosis after middle cerebral artery occlusion in wistar rats progresses at different time intervals in the caudoputamen and the cortex. Stroke 1995, 26, 636–642. [Google Scholar] [CrossRef]
- Li, Y.; Powers, C.; Jiang, N.; Chopp, M. Intact, injured, necrotic and apoptotic cells after focal cerebral ischemia in the rat. J. Neurol. Sci. 1998, 156, 119–132. [Google Scholar] [CrossRef]
- Lipton, P. Ischemic cell death in brain neurons. Physiol. Rev. 1999, 79, 1431–1568. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 6th ed.; Academic Press: Cambridge, MA, USA, 2006. [Google Scholar]
- Avtandilov, G. Meditsinskaya Morfometriya [Medical Morphometry]; Meditsyna: Moscow, Russia, 1990. [Google Scholar]
- Weibel, E.R.; Kistler, G.S.; Scherle, W.F. Practical stereological methods for morphometric cytology. J. Cell Biol. 1966, 30, 23–38. [Google Scholar] [CrossRef] [PubMed]
- BioRender: Scientific Image and Illustration Software. Available online: https://app.biorender.com/ (accessed on 7 April 2023).
- Hermann, D.M.; Popa-Wagner, A.; Kleinschnitz, C.; Doeppner, T.R. Animal models of ischemic stroke and their impact on drug discovery. Expert Opin. Drug Discov. 2019, 14, 315–326. [Google Scholar] [CrossRef]
- Wang, C.; Liu, M.; Pan, Y.; Bai, B.; Chen, J. Global gene expression profile of cerebral ischemia-reperfusion injury in rat MCAO model. Oncotarget 2017, 8, 74607–74622. [Google Scholar] [CrossRef] [PubMed]
- Astrup, J.; Symon, L.; Branston, N.M.; Lassen, N.A. Cortical evoked potential and extracellular k+ and h+ at critical levels of brain ischemia. Stroke 1977, 8, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Ermine, C.M.; Bivard, A.; Parsons, M.W. Jean-Claude Baron The ischemic penumbra: From concept to reality. Int. J. Stroke 2021, 16, 497–509. [Google Scholar] [CrossRef]
- Baron, J.C. The core/penumbra model: Implications for acute stroke treatment and patient selection in 2021. Eur. J. Neurol. 2021, 28, 2794–2803. [Google Scholar] [CrossRef]
- Yang, S.H.; Liu, R. Four Decades of Ischemic Penumbra and Its Implication for Ischemic Stroke. Transl. Stroke Res. 2021, 12, 937–945. [Google Scholar] [CrossRef]
- Imai, H.; Harland, J.; McCulloch, J.; Graham, D.I.; Brown, S.M.; Macrae, I.M. Specific expression of the cell cycle regulation proteins, GADD34 and PCNA, in the peri-infarct zone after focal cerebral ischaemia in the rat. Eur. J. Neurosci. 2002, 15, 1929–1936. [Google Scholar] [CrossRef]
- Koizumi, T.; Kerkhofs, D.; Mizuno, T.; Steinbusch, H.W.M.; Foulquier, S. Vessel-Associated Immune Cells in Cerebrovascular Diseases: From Perivascular Macrophages to Vessel-Associated Microglia. Front. Neurosci. 2019, 13, 1291. [Google Scholar] [CrossRef]
- Haruwaka, K.; Ikegami, A.; Tachibana, Y.; Ohno, N.; Konishi, H.; Hashimoto, A.; Matsumoto, M.; Kato, D.; Ono, R.; Kiyama, H.; et al. Dual microglia effects on blood brain barrier permeability induced by systemic inflammation. Nat. Commun. 2019, 10, 5816. [Google Scholar] [CrossRef] [PubMed]
- Wicks, E.E.; Ran, K.R.; Kim, J.E.; Xu, R.; Lee, R.P.; Jackson, C.M. The Translational Potential of Microglia and Monocyte-Derived Macrophages in Ischemic Stroke. Front. Immunol. 2022, 13, 2976. [Google Scholar] [CrossRef] [PubMed]
- Perego, C.; Fumagalli, S.; De Simoni, M.G. Temporal pattern of expression and colocalization of microglia/macrophage phenotype markers following brain ischemic injury in mice. J. Neuroinflamm. 2011, 8, 174. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, P.; Guo, Y.; Wang, H.; Leak, R.K.; Chen, S.; Gao, Y.; Chen, J. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke 2012, 43, 3063–3070. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Leak, R.K.; Cao, G. Microglia-mediated neuroinflammation and neuroplasticity after stroke. Front. Cell. Neurosci. 2022, 16, 980722. [Google Scholar] [CrossRef] [PubMed]
- Rajan, W.D.; Wojtas, B.; Gielniewski, B.; Gieryng, A.; Zawadzka, M.; Kaminska, B. Dissecting functional phenotypes of microglia and macrophages in the rat brain after transient cerebral ischemia. Glia 2019, 67, 232–245. [Google Scholar] [CrossRef]
- Jurga, A.M.; Paleczna, M.; Kuter, K.Z. Overview of General and Discriminating Markers of Differential Microglia Phenotypes. Front. Cell. Neurosci. 2020, 14, 198. [Google Scholar] [CrossRef]
- Raffaele, S.; Gelosa, P.; Bonfanti, E.; Lombardi, M.; Castiglioni, L.; Cimino, M.; Sironi, L.; Abbracchio, M.P.; Verderio, C.; Fumagalli, M. Microglial vesicles improve post-stroke recovery by preventing immune cell senescence and favoring oligodendrogenesis. Mol. Ther. 2021, 29, 1439. [Google Scholar] [CrossRef]
- Jin, W.N.; Shi, S.X.Y.; Li, Z.; Li, M.; Wood, K.; Gonzales, R.J.; Liu, Q. Depletion of microglia exacerbates postischemic inflammation and brain injury. J. Cereb. Blood Flow Metab. 2017, 37, 2224–2236. [Google Scholar] [CrossRef] [PubMed]
- Otxoa-de-Amezaga, A.; Miró-Mur, F.; Pedragosa, J.; Gallizioli, M.; Justicia, C.; Gaja-Capdevila, N.; Ruíz-Jaen, F.; Salas-Perdomo, A.; Bosch, A.; Calvo, M.; et al. Microglial cell loss after ischemic stroke favors brain neutrophil accumulation. Acta Neuropathol. 2019, 137, 321–341. [Google Scholar] [CrossRef]
- Rawlinson, C.; Jenkins, S.; Thei, L.; Dallas, M.L.; Chen, R. Post-Ischaemic Immunological Response in the Brain: Targeting Microglia in Ischaemic Stroke Therapy. Brain Sci. 2020, 10, 159. [Google Scholar] [CrossRef]
- Kim, S.; Son, Y. Astrocytes Stimulate Microglial Proliferation and M2 Polarization In Vitro through Crosstalk between Astrocytes and Microglia. Int. J. Mol. Sci. 2021, 22, 8800. [Google Scholar] [CrossRef]
- Wang, J.; Guo, Y.; Xu, D.; Cui, J.; Wang, Y.; Su, Y.; Liu, Y.; Shen, Y.; Jing, X.; Bai, W. The immunolocalization of cluster of differentiation 31, phalloidin and alpha smooth muscle actin on vascular network of normal and ischemic rat brain. Sci. Rep. 2022, 12, 22288. [Google Scholar] [CrossRef] [PubMed]
- Erdener, Ş.E.; Tang, J.; Kılıç, K.; Postnov, D.; Giblin, J.T.; Kura, S.; Chen, I.C.A.; Vayisoğlu, T.; Sakadžić, S.; Schaffer, C.B.; et al. Dynamic capillary stalls in reperfused ischemic penumbra contributeto injury: A hyperacute role for neutrophils in persistent trafficjams. J. Cereb. Blood Flow Metab. 2021, 41, 236. [Google Scholar] [CrossRef] [PubMed]
- El Amki, M.; Wegener, S. Improving Cerebral Blood Flow after Arterial Recanalization: A Novel Therapeutic Strategy in Stroke. Int. J. Mol. Sci. 2017, 18, 2669. [Google Scholar] [CrossRef]
- Schaper, W. Collateral circulation: Past and present. Basic Res. Cardiol. 2009, 104, 5–21. [Google Scholar] [CrossRef]
- Ma, J.; Ma, Y.; Shuaib, A.; Winship, I.R. Impaired Collateral Flow in Pial Arterioles of Aged Rats During Ischemic Stroke. Transl. Stroke Res. 2020, 11, 243–253. [Google Scholar] [CrossRef]
- Sato, Y.; Falcone-Juengert, J.; Tominaga, T.; Su, H.; Liu, J. Remodeling of the Neurovascular Unit Following Cerebral Ischemia and Hemorrhage. Cells 2022, 11, 2823. [Google Scholar] [CrossRef] [PubMed]
- Biose, I.J.; Dewar, D.; Macrae, I.M.; McCabe, C. Impact of stroke co-morbidities on cortical collateral flow followingischaemic stroke. J. Cereb. Blood Flow Metab. 2020, 40, 978. [Google Scholar] [CrossRef]
- Sheth, S.A.; Liebeskind, D.S. Imaging Evaluation of Collaterals in the Brain: Physiology and Clinical Translation. Curr. Radiol. Rep. 2014, 2, 29. [Google Scholar] [CrossRef]
- Williamson, M.R.; Franzen, R.L.; Fuertes, C.J.A.; Dunn, A.K.; Drew, M.R.; Jones, T.A. A window of vascular plasticity coupled to behavioral recovery after stroke. J. Neurosci. 2020, 40, 7651–7667. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Fu, S.; Liu, Y.; Luo, H.; Li, F.; Wang, Y.; Gao, M.; Cheng, Y.; Xie, Z. NDP-MSH binding melanocortin-1 receptor ameliorates neuroinflammation and BBB disruption through CREB/Nr4a1/NF-κB pathway after intracerebral hemorrhage in mice. J. Neuroinflamm. 2019, 16, 192. [Google Scholar] [CrossRef] [PubMed]
- Szabó, I.; Biri-Kovács, B.; Vári, B.; Ranđelović, I.; Vári-Mező, D.; Juhász, É.; Halmos, G.; Bősze, S.; Tóvári, J.; Mező, G. Targeting the Melanocortin 1 Receptor in Melanoma: Biological Activity of α-MSH–Peptide Conjugates. Int. J. Mol. Sci. 2024, 25, 1095. [Google Scholar] [CrossRef] [PubMed]
- Ehrhardt, R.A.; Giesy, S.L.; Hileman, S.M.; Houseknecht, K.L.; Boisclair, Y.R. Effects of the central melanocortin system on feed intake, metabolic hormones and insulin action in the sheep. J. Anim. Sci. 2023, 101, 38035762. [Google Scholar] [CrossRef]
- Vyunova, T.V.; Andreeva, L.A.; Shevchenko, K.V.; Myasoedov, N.F. Synacton and individual activity of synthetic and natural corticotropins. J. Mol. Recognit. 2017, 30, e2597. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information, BioProject PRJNA472446. Available online: https://www.ncbi.nlm.nih.gov/Traces/study/?acc=SRP148632 (accessed on 6 February 2019).
- National Center for Biotechnology Information, BioProject PRJNA491404. Available online: https://www.ncbi.nlm.nih.gov/sra/PRJNA491404 (accessed on 10 May 2020).


| Index | SO | IR | IS | IA |
|---|---|---|---|---|
| Area of the frontal brain section at the level of the rostral part of the lateral ventricles (mm2) | 71.1 ± 0.8 | 76.5 ± 5.1 | 76.7 ± 0.7 | 82.6 ± 3.4 |
| Area of the right hemisphere (mm2) | 35.7 ± 0.5 | 40.1 ± 2.3 | 43.2 ± 0.7 | 45.0 ± 2.1 |
| Percentage of the total area that the right hemisphere occupies (%) | 50.3 ± 0.4 | 52.6 ± 0.9 | 55.5 ± 1.1 | 54.5 ± 1.4 |
| Percentage comparison of the right hemisphere area to the left hemisphere area (%) | 100.1 ± 0.4 | 111.2 ± 4.2 | 125.4 ± 5.9 | 120.6 ± 6.9 |
| Area of the infarction zone in the right hemisphere (mm2) | 25.5 ± 1.1 | 25.5 ± 1.5 | 24.5 ± 1.9 | |
| Percentage of the total area that the infarction zone in the right hemisphere occupies (%) | 64.0 ± 2.6 | 59.0 ± 3.3 | 54.2 ± 2.3 # | |
| Area of the right hemisphere cortex (mm2) | 17.1 ± 0.1 | 19.9 ± 1.2 | 20.0 ± 0.8 | 20.1 ± 0.6 |
| Percentage of the intact cortex area in the right hemisphere (%) | 18.9 ± 1.4 | 20.1 ± 1.9 | 22.1 ± 3.0 | |
| Percentage of the infarction area in the right hemisphere cortex (%) | 75.6 ± 2.8 | 71.7 ± 3.9 | 58.1 ± 8.2 | |
| Percentage of the penumbra area in the right hemisphere cortex (%) | 5.4 ± 1.6 | 8.2 ± 2.4 | 15.2 ± 3.9 # | |
| Area of the right hemisphere’s striatum (mm2) | 8.9 ± 0.1 | 12.5 ± 0.9 * | 13.3 ± 0.8 | 10.3 ± 0.6 |
| Percentage of the intact striatum area in the right hemisphere (%) | 1.3 ± 0.9 | 1.0 ± 0.6 | 1.5 ± 0.9 | |
| Percentage of the infarction area in the striatum of the right hemisphere (%) | 89.1 ± 4.9 | 88.3 ± 4.5 | 79.1 ± 5.1 | |
| Percentage of the penumbra area in the striatum of the right hemisphere (%) | 9.6 ± 4.6 | 10.7 ± 4.0 | 19.4 ± 5.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stavchansky, V.V.; Yuzhakov, V.V.; Sevan’kaeva, L.E.; Fomina, N.K.; Koretskaya, A.E.; Denisova, A.E.; Mozgovoy, I.V.; Gubsky, L.V.; Filippenkov, I.B.; Myasoedov, N.F.; et al. Melanocortin Derivatives Induced Vascularization and Neuroglial Proliferation in the Rat Brain under Conditions of Cerebral Ischemia. Curr. Issues Mol. Biol. 2024, 46, 2071-2092. https://doi.org/10.3390/cimb46030133
Stavchansky VV, Yuzhakov VV, Sevan’kaeva LE, Fomina NK, Koretskaya AE, Denisova AE, Mozgovoy IV, Gubsky LV, Filippenkov IB, Myasoedov NF, et al. Melanocortin Derivatives Induced Vascularization and Neuroglial Proliferation in the Rat Brain under Conditions of Cerebral Ischemia. Current Issues in Molecular Biology. 2024; 46(3):2071-2092. https://doi.org/10.3390/cimb46030133
Chicago/Turabian StyleStavchansky, Vasily V., Vadim V. Yuzhakov, Larisa E. Sevan’kaeva, Natalia K. Fomina, Anastasia E. Koretskaya, Alina E. Denisova, Ivan V. Mozgovoy, Leonid V. Gubsky, Ivan B. Filippenkov, Nikolay F. Myasoedov, and et al. 2024. "Melanocortin Derivatives Induced Vascularization and Neuroglial Proliferation in the Rat Brain under Conditions of Cerebral Ischemia" Current Issues in Molecular Biology 46, no. 3: 2071-2092. https://doi.org/10.3390/cimb46030133
APA StyleStavchansky, V. V., Yuzhakov, V. V., Sevan’kaeva, L. E., Fomina, N. K., Koretskaya, A. E., Denisova, A. E., Mozgovoy, I. V., Gubsky, L. V., Filippenkov, I. B., Myasoedov, N. F., Limborska, S. A., & Dergunova, L. V. (2024). Melanocortin Derivatives Induced Vascularization and Neuroglial Proliferation in the Rat Brain under Conditions of Cerebral Ischemia. Current Issues in Molecular Biology, 46(3), 2071-2092. https://doi.org/10.3390/cimb46030133








