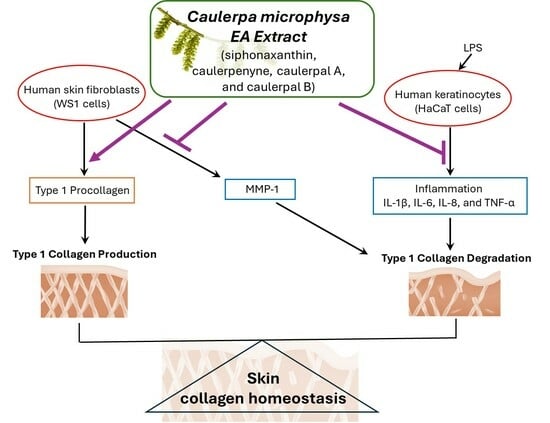
The Ethyl Acetate Extract of Caulerpa microphysa Promotes Collagen Homeostasis and Inhibits Inflammation in the Skin
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells
2.2. Preparation of Caulerpa microphysa Extracts
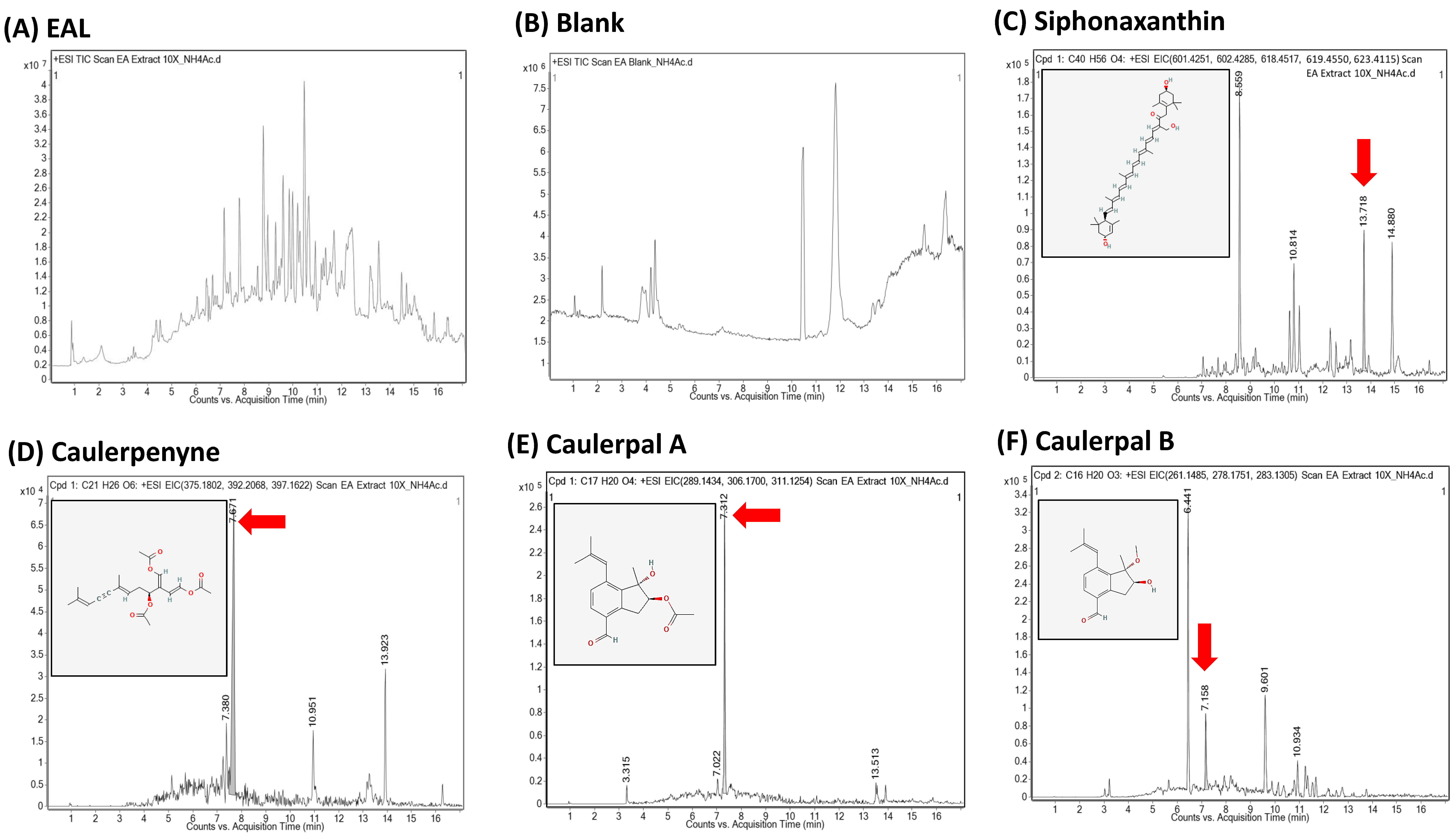
2.3. Ultra-Performance Liquid Chromatography (UPLC) and Quadrupole Time-of-Flight Mass Spectrometry (QTOF)
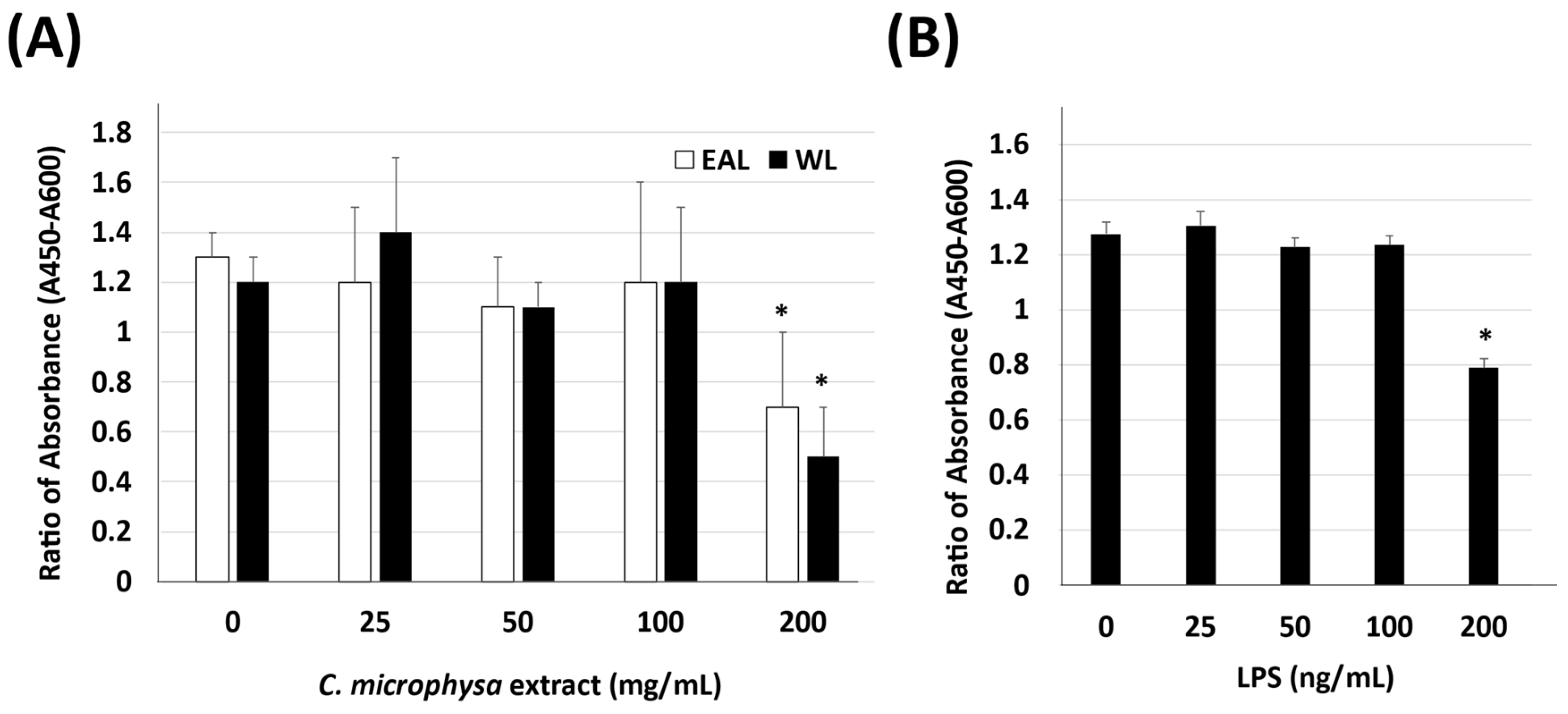
2.4. WST-1
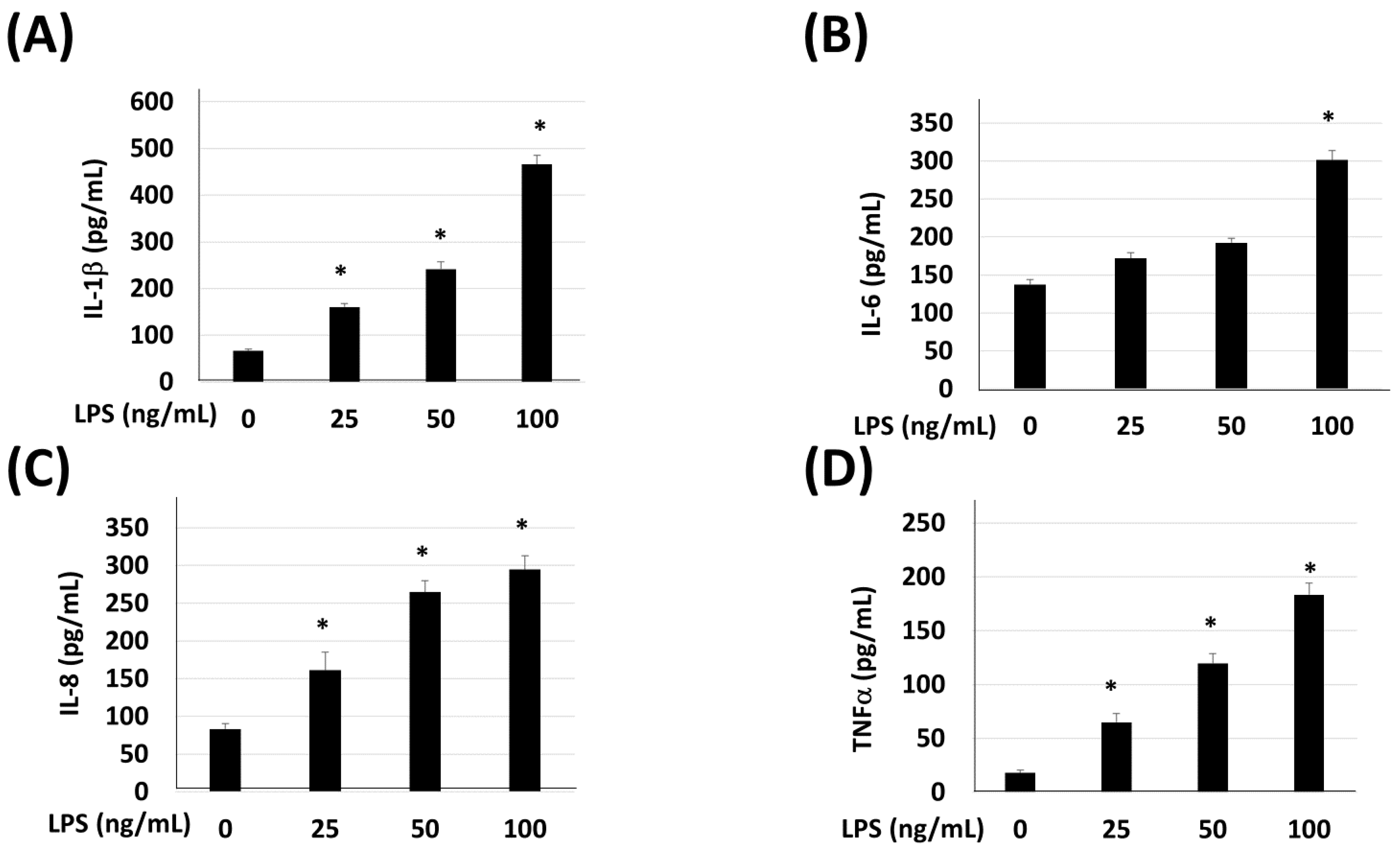
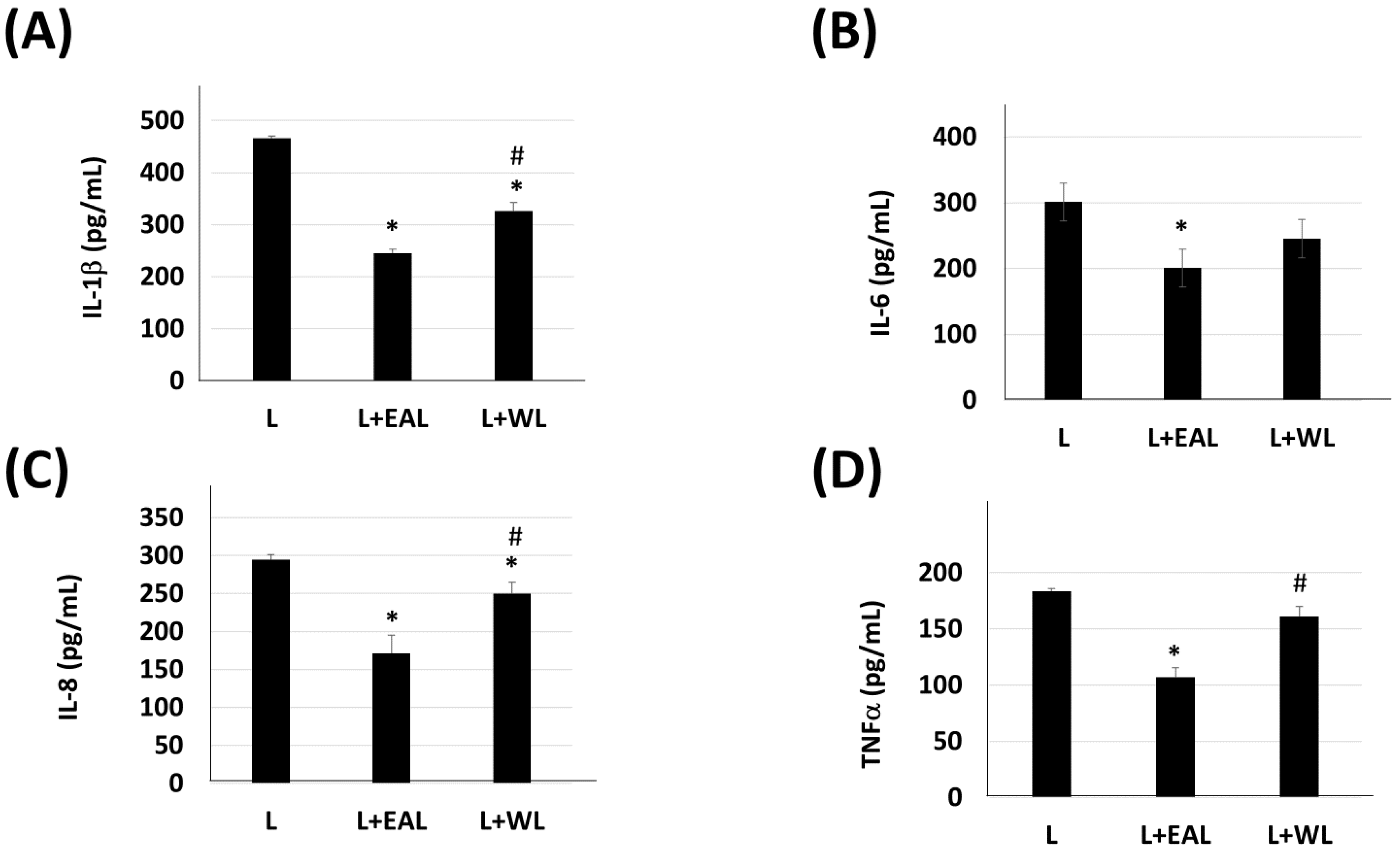
2.5. Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. Western Blot
2.7. Statistical Analyses
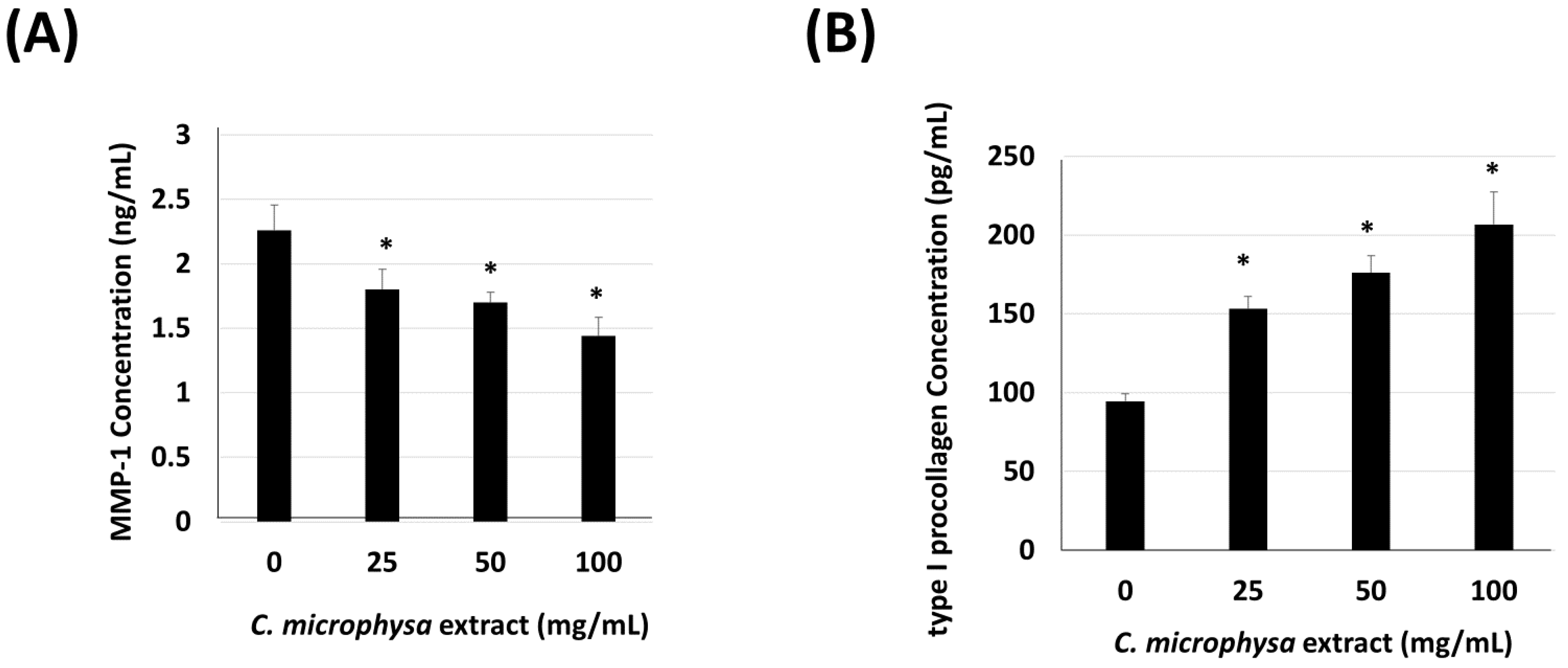
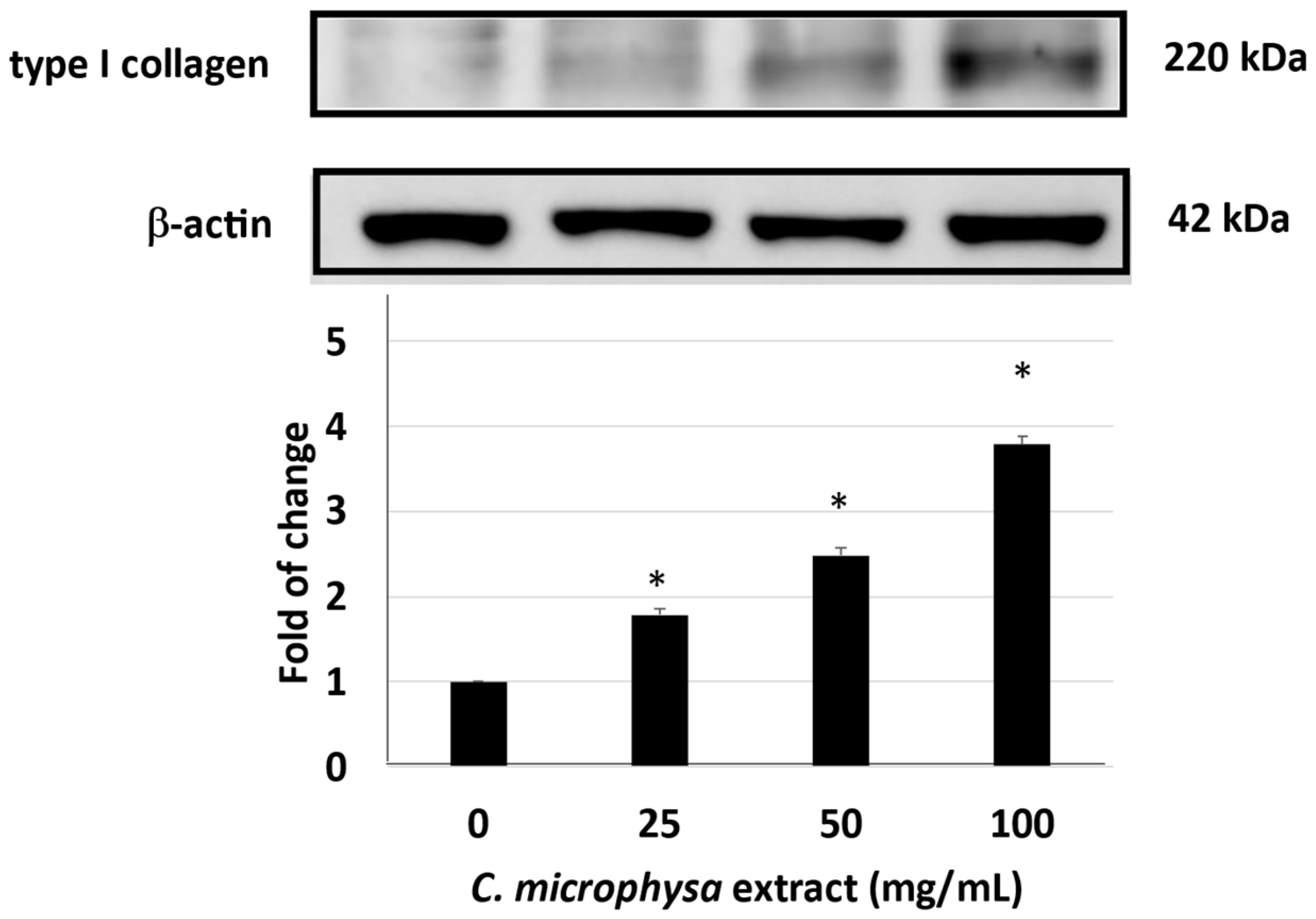
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Domozych, D.S.; LoRicco, J.G. The extracellular matrix of green algae. Plant Physiol. 2023, 194, 15–32. [Google Scholar] [CrossRef] [PubMed]
- Popper, Z.A.; Michel, G.; Hervé, C.; Domozych, D.S.; Willats, W.G.; Tuohy, M.G.; Kloareg, B.; Stengel, D.B. Evolution and diversity of plant cell walls: From algae to flowering plants. Annu. Rev. Plant Biol. 2011, 62, 567–590. [Google Scholar] [CrossRef] [PubMed]
- de Vries, J.; Stanton, A.; Archibald, J.M.; Gould, S.B. Streptophyte Terrestrialization in Light of Plastid Evolution. Trends Plant Sci. 2016, 21, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Abedin, M.M.; Singh, A.K.; Das, S. Role of phenolic compounds in plant-defensive mechanisms. In Plant Phenolics in Sustainable Agriculture; Lone, R., Shuab, R., Kamili, A., Eds.; Springer: Singapore, 2020; pp. 517–533. [Google Scholar]
- Abu-Ghannam, N.; Rajauria, G. Antimicrobial activity of compounds isolated from algae. In Functional Ingredients from Algae for Foods and Nutraceuticals; Domínguez, H., Ed.; Woodhead Publishing: Cambridge, UK, 2013; pp. 287–306. [Google Scholar]
- Pangestuti, R.; Kim, S.K. Biological activities and health benefit effects of natural pigments derived from marine algae. J. Funct. Foods 2011, 3, 255–2667. [Google Scholar] [CrossRef]
- Hegazi, M.M.; Perez-Ruzafa, A.; Almela, L.; María-Emilia, C. Separation and identification of chlorophylls and carotenoids from Caulerpa prolifera, Jania rubens and Padina pavonica by reversed-phase high-performance liquid chromatography. J. Chromatogr. A 1998, 829, 153–159. [Google Scholar] [CrossRef]
- Wahlström, N.; Nylander, F.; Malmhäll-Bah, E.; Sjövold, K.; Edlund, U.; Westman, G.; Albers, E. Composition and structure of cell wall ulvans recovered from Ulva spp. along the Swedish west coast. Carbohydr. Polym. 2020, 233, 115852. [Google Scholar] [CrossRef]
- Domozych, D.S.; Bagdan, K. The cell biology of charophytes: Exploring the past and models for the future. Plant Physiol. 2022, 190, 1588–1608. [Google Scholar] [CrossRef]
- Kloareg, B.; Badis, Y.; Cock, J.M.; Michel, G. Role and Evolution of the Extracellular Matrix in the Acquisition of Complex Multicellularity in Eukaryotes: A Macroalgal Perspective. Genes 2021, 12, 1059. [Google Scholar] [CrossRef]
- Takaichi, S. Carotenoids in algae: Distributions, biosyntheses and functions. Mar. Drugs 2011, 9, 1101–1118. [Google Scholar] [CrossRef]
- Sugawara, T.; Ganesan, P.; Li, Z.; Manabe, Y.; Hirata, T. Siphonaxanthin, a green algal carotenoid, as a novel functional compound. Mar. Drugs 2014, 12, 3660–3668. [Google Scholar] [CrossRef]
- Sureda, A.; Box, A.; Deudero, S.; Pons, A. Reciprocal effects of caulerpenyne and intense herbivorism on the antioxidant response of Bittium reticulatum and Caulerpa taxifolia. Ecotoxicol. Environ. Saf. 2009, 72, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Shao, P.; Pei, Y.; Fang, Z.; Sun, P. Effects of partial desulfation on antioxidant and inhibition of DLD cancer cell of Ulva fasciata polysaccharide. Int. J. Biol. Macromol. 2014, 65, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Peasura, N.; Laohakunjit, N.; Kerdchoechuen, O.; Vongsawasdi, P.; Chao, L.K. Assessment of biochemical and immunomodulatory activity of sulphated polysaccharides from Ulva intestinalis. Int. J. Biol. Macromol. 2016, 91, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Foote, C.S.; Chang, Y.C.; Denny, R.W. Chemistry of singlet oxygen. XI. Cis-trans isomerization of carotenoids by singlet oxygen and a probable quenching mechanism. J. Am. Chem. Soc. 1970, 92, 5218–5219. [Google Scholar] [CrossRef]
- Martin, H.D.; Ruck, C.; Schmidt, M.; Sell, S.; Beutner, S.; Mayer, B.; Walsh, R. Chemistry of carotenoid oxidation and free radical reactions. Pure Appl. Chem. 1999, 71, 2253–2262. [Google Scholar] [CrossRef]
- Ganesan, P.; Matsubara, K.; Sugawara, T.; Hirata, T. Marine algal carotenoids inhibit angiogenesis by down-regulating FGF-2-mediated intracellular signals in vascular endothelial cells. Mol. Cell Biochem. 2013, 380, 1–9. [Google Scholar] [CrossRef]
- Manabe, Y.; Takii, Y.; Sugawara, T. Siphonaxanthin, a carotenoid from green algae, suppresses advanced glycation end product-induced inflammatory responses. J. Nat. Med. 2020, 74, 127–134. [Google Scholar] [CrossRef]
- Li, Z.S.; Noda, K.; Fujita, E.; Manabe, Y.; Hirata, T.; Sugawara, T. The green algal carotenoid siphonaxanthin inhibits adipogenesis in 3T3-L1 preadipocytes and the accumulation of lipids in white adipose tissue of KK-Ay mice. J. Nutr. 2015, 145, 490–498. [Google Scholar] [CrossRef]
- Li, Z.S.; Zheng, J.W.; Manabe, Y.; Hirata, T.; Sugawara, T. Anti-Obesity Properties of the Dietary Green Alga, Codium cylindricum, in High-Fat Diet-Induced Obese Mice. J. Nutr. Sci. Vitaminol. 2018, 64, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Fischel, J.L.; Lemee, R.; Formento, P.; Caldani, C.; Moll, J.L.; Pesando, D.; Meinesz, A.; Grelier, P.; Pietra, P.; Guerriero, A.; et al. Cell growth inhibitory effects of caulerpenyne, a sesquiterpenoid from the marine algae Caulerpa taxifolia. Anticancer Res. 1995, 15, 2155–2160. [Google Scholar] [PubMed]
- Cavas, L.; Baskin, Y.; Yurdakoc, K.; Olgun, N. Antiproliferative and newly attributed apoptotic activities from an invasive marine alga: Caulerpa racemosa var. cylindracea. J. Exp. Mar. Biol. Ecol. 2006, 339, 111–119. [Google Scholar] [CrossRef]
- Nicoletti, E.; Della Pietà, F.; Calderone, V.; Bandecchi, P.; Pistello, M.; Morelli, I.; Cinelli, F. Antiviral properties of a crude extract from a green alga Caulerpa taxifolia (Vahl) C. Agardh. Phytother. Res. 1999, 13, 245–247. [Google Scholar] [CrossRef]
- Liu, A.H.; Liu, D.Q.; Liang, T.J.; Yu, X.Q.; Feng, M.T.; Yao, L.G.; Fang, Y.; Wang, B.; Feng, L.H.; Zhang, M.X.; et al. Caulerprenylols A and B, two rare antifungal prenylated para-xylenes from the green alga Caulerpa racemosa. Bioorg. Med. Chem. Lett. 2013, 23, 2491–2494. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.C.; Yeh, H.Y.; Shih, W.L. Extraction Procedure, Characteristics, and Feasibility of Caulerpa microphysa (Chlorophyta) Polysaccharide Extract as a Cosmetic Ingredient. Mar. Drugs 2021, 19, 524. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.S.S.P.; Costa, L.S.; Cordeiro, S.L.; Almeida-Lima, J.; Dantas-Santos, N.; Magalhães, K.D.; Sabry, D.A.; Albuquerque, I.R.L.; Pereira, M.R.; Leite, E.L.; et al. Evaluating the possible anticoagulant and antioxidant effects of sulfated polysaccharides from the tropical green alga Caulerpa cupressoides var. flabellata. J. Appl. Phycol. 2012, 24, 1159–1167. [Google Scholar] [CrossRef]
- Lin, H.C.; Chou, S.T.; Chuang, M.Y.; Liao, T.Y.; Tsai, W.S.; Chiu, T.H. The effects of Caulerpa microphysa enzyme-digested extracts on ACE-inhibitory activity and in vitro anti-tumour properties. Food Chem. 2012, 134, 2235–2241. [Google Scholar] [CrossRef]
- Henriksen, K.; Karsdal, M.A. Type I Collagen. In Biochemistry of Collagens, Laminins and Elastin: Structure, Function and Biomarkers; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 1–11. [Google Scholar]
- Osman, O.S.; Selway, J.L.; Harikumar, P.E.; Stocker, C.J.; Wargent, E.T.; Cawthorne, M.A.; Jassim, S.; Langlands, K. A novel method to assess collagen architecture in skin. BMC Bioinform. 2013, 14, 260. [Google Scholar] [CrossRef]
- Ivarsson, M.; McWhirter, A.; Borg, T.K.; Rubin, K. Type I collagen synthesis in cultured human fibroblasts: Regulation by cell spreading, platelet-derived growth factor and interactions with collagen fibers. Matrix Biol. 1998, 16, 409–425. [Google Scholar] [CrossRef]
- Risteli, J.; Risteli, L. Analysing connective tissue metabolites in human serum. Biochemical, physiological and methodological aspects. J. Hepatol. 1995, 22 (Suppl. 2), 77–81. [Google Scholar]
- Risteli, L.; Risteli, J. Non-Invasive Methods for Detection of Organ Fibrosis; CRC Press: Boca Raton, FL, USA, 1990. [Google Scholar]
- Shoulders, M.D.; Raines, R.T. Collagen structure and stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef]
- Chang, S.W.; Buehler, M.J. Molecular biomechanics of collagen molecules. Mater. Today 2014, 17, 70–76. [Google Scholar] [CrossRef]
- Ricard-Blum, S. The collagen family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.; Little, E.; Quan, H.; Qin, Z.; Voorhees, J.J.; Fisher, G.J. Elevated matrix metalloproteinases and collagen fragmentation in photodamaged human skin: Impact of altered extracellular matrix microenvironment on dermal fibroblast function. J. Investig. Dermatol. 2013, 133, 1362–1366. [Google Scholar] [CrossRef] [PubMed]
- Lauer-Fields, J.L.; Juska, D.; Fields, G.B. Matrix metalloproteinases and collagen catabolism. Biopolymers 2002, 66, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Nagase, H.; Visse, R.; Murphy, G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 2006, 69, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Brennan, M.; Bhatti, H.; Nerusu, K.C.; Bhagavathula, N.; Kang, S.; Fisher, G.J.; Varani, J.; Voorhees, J.J. Matrix metalloproteinase-1 is the major collagenolytic enzyme responsible for collagen damage in UV-irradiated human skin. Photochem. Photobiol. 2003, 78, 43–48. [Google Scholar] [CrossRef]
- Lindner, D.; Zietsch, C.; Becher, P.M.; Schulze, K.; Schultheiss, H.P.; Tschöpe, C.; Westermann, D. Differential expression of matrix metalloproteases in human fibroblasts with different origins. Biochem. Res. Int. 2012, 2012, 875742. [Google Scholar] [CrossRef]
- Du, G.; Liu, C.; Li, X.; Chen, W.; He, R.; Wang, X.; Feng, P.; Lan, W. Induction of matrix metalloproteinase-1 by tumor necrosis factor-α is mediated by interleukin-6 in cultured fibroblasts of keratoconus. Exp. Biol. Med. 2016, 241, 2033–2041. [Google Scholar] [CrossRef]
- Sakamoto, T.; Ojima, N.; Yamashita, M. Induction of Mrnas in Response to Acclimation of Trout Cells to Different Osmolalities. Fish Physiol. Biochem. 2000, 22, 255–262. [Google Scholar] [CrossRef]
- Okazaki, M.; Yoshimura, K.; Uchida, G.; Harii, K. Correlation between age and the secretions of melanocyte-stimulating cytokines in cultured keratinocytes and fibroblasts. Br. J. Dermatol. 2005, 153 (Suppl. 2), 23–29. [Google Scholar] [CrossRef]
- Watt, F.M.; Fujiwara, H. Cell-extracellular matrix interactions in normal and diseased skin. Cold Spring Harb. Perspect. Biol. 2011, 3, a005124. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, O.; Ayyangar, U.; Kurbet, A.S.; Ashok, D.; Raghavan, S. Unraveling the ECM-Immune Cell Crosstalk in Skin Diseases. Front. Cell Dev. Biol. 2019, 7, 68. [Google Scholar] [CrossRef] [PubMed]
- Brandao-Rangel, M.A.R.; Oliveira, C.R.; da Silva Olímpio, F.R.; Aimbire, F.; Mateus-Silva, J.R.; Chaluppe, F.A.; Vieira, R.P. Hydrolyzed Collagen Induces an Anti-Inflammatory Response That Induces Proliferation of Skin Fibroblast and Keratinocytes. Nutrients 2022, 14, 4975. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, Z.; Song, S.; Zhu, B.; Zhao, L.; Jiang, J.; Liu, N.; Wang, J.; Chen, X. Anti-inflammatory activity and structural identification of a sulfated polysaccharide CLGP4 from Caulerpa lentillifera. Int. J. Biol. Macromol. 2020, 146, 931–938. [Google Scholar] [CrossRef]
- Barbier, P.; Guise, S.; Huitorel, P.; Amade, P.; Pesando, D.; Briand, C.; Peyrot, V. Caulerpenyne from Caulerpa taxifolia has an antiproliferative activity on tumor cell line SK-N-SH and modifies the microtubule network. Life Sci. 2001, 70, 415–429. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, K.-Y.; Cheng, L.-C.; Hung, Z.-C.; Chen, Z.-Y.; Wang, C.-W.; Hou, H.-H. The Ethyl Acetate Extract of Caulerpa microphysa Promotes Collagen Homeostasis and Inhibits Inflammation in the Skin. Curr. Issues Mol. Biol. 2024, 46, 2701-2712. https://doi.org/10.3390/cimb46030170
Lu K-Y, Cheng L-C, Hung Z-C, Chen Z-Y, Wang C-W, Hou H-H. The Ethyl Acetate Extract of Caulerpa microphysa Promotes Collagen Homeostasis and Inhibits Inflammation in the Skin. Current Issues in Molecular Biology. 2024; 46(3):2701-2712. https://doi.org/10.3390/cimb46030170
Chicago/Turabian StyleLu, Kuo-Yun, Li-Ching Cheng, Zheng-Ci Hung, Ze-Ying Chen, Chuang-Wei Wang, and Hsin-Han Hou. 2024. "The Ethyl Acetate Extract of Caulerpa microphysa Promotes Collagen Homeostasis and Inhibits Inflammation in the Skin" Current Issues in Molecular Biology 46, no. 3: 2701-2712. https://doi.org/10.3390/cimb46030170
APA StyleLu, K.-Y., Cheng, L.-C., Hung, Z.-C., Chen, Z.-Y., Wang, C.-W., & Hou, H.-H. (2024). The Ethyl Acetate Extract of Caulerpa microphysa Promotes Collagen Homeostasis and Inhibits Inflammation in the Skin. Current Issues in Molecular Biology, 46(3), 2701-2712. https://doi.org/10.3390/cimb46030170