Abstract
Alarmins are immune-activating factors released after cellular injury or death. By secreting alarmins, cells can interact with immune cells and induce a variety of inflammatory responses. The broad family of alarmins involves several members, such as high-mobility group box 1, S100 proteins, interleukin-33, and heat shock proteins, among others. Studies have found that the concentrations and expression profiles of alarmins are altered in immune-mediated diseases. Furthermore, they are involved in the pathogenesis of inflammatory conditions. The aim of this narrative review is to present the current evidence on the role of alarmins in rheumatoid arthritis, osteoarthritis, and psoriasis. We discuss their potential involvement in mechanisms underlying the progression of these diseases and whether they could become therapeutic targets. Moreover, we summarize the impact of pharmacological agents used in the treatment of these diseases on the expression of alarmins.
1. Introduction
Alarmins are considered danger-associated molecular patterns (DAMPs); they are endogenous immune-activating factors associated with cell injury and death. The main role of these molecules is to alert the immune system and initiate inflammatory responses [1]. Inflammatory mediators, such as tumor necrosis factor-α (TNF-α); interleukin (IL)-1β; IL-6; IL-8; and chemokine ligand (CC motif) 2 (CCL2), 3 (CCL3) and 4 (CCL4), as well as nitric oxide, are released by neutrophils and macrophages, the secretions of which are induced by alarmins [2,3,4,5]. Cells release alarmins in response to various stimuli, such as thermal, chemical, physical, metabolic, or infectious stimuli. Analysis of pathways stimulated by alarmins is fundamental to better understanding inflammatory processes, given that they may be biomarkers of immune-mediated diseases.
The pathogenesis of rheumatoid arthritis (RA) and inflammatory skin diseases, such as psoriasis, is associated with dysregulated immune responses and tissue damage. RA is a chronic inflammatory disease associated with multiple joint malfunctions. The pathogenesis of RA is complex and not fully understood; these mechanisms involve interactions between RA fibroblast-like synoviocytes (RA-FLSs) and immune cells, epigenetic factors, and the impact of environmental stimuli [6,7,8]. Similarly, the pathogenesis of psoriasis involves interactions between immune cells and keratinocytes. Abnormal inflammatory responses occurring in the skin lead to the excessive proliferation of keratinocytes, differentiation impairment, and the formation of pathological skin eruptions. Osteoarthritis (OA) is another disease with inflammatory components that play a role in its pathogenesis. It is a whole-joint disorder, and since it is associated with cartilage degeneration, alarmins may be involved in OA-related inflammatory mechanisms. The aim of the present review is to explore the current evidence on the role of alarmins in the pathogenesis of RA, OA, and psoriasis, with a focus on understanding their molecular mechanisms, involvement in disease progression, and potential therapeutic targets.
2. Materials and Methods
To perform this narrative review, we conducted a thorough literature search through the PubMed and Embase databases. We used the following keywords: ‘rheumatoid arthritis’, ‘osteoarthritis’, ‘psoriasis’, ‘high mobility group box 1’, ‘S100 proteins’, ‘interleukin-33’, ‘interleukin-1α’, ‘heat shock proteins’, ‘defensins’, cathelicidin’, ‘thymic stromal lymphopoietin’, and their combinations. We have discussed original articles that investigated the impact of alarmins on the pathogenesis of these diseases. Furthermore, we have analyzed studies that examined whether therapeutic agents used in the treatment of the abovementioned diseases affect the expression of alarmins.
3. Brief Overview of Alarmins
Alarmins represent a broad group of molecules, each with distinct structural and functional properties. Among the most common peptides mentioned are high-mobility group box 1 (HMGB1), S100 proteins, interleukin-33 (IL-33), interleukin-1α (IL-1α), heat shock proteins (HSPs), defensins, cathelicidin, thymic stromal lymphopoietin (TSLP), and others.
HMGB1, also known as amphoterin, is a non-histone chromatin-binding protein present in all cell types. The structure of human HMGB1 consists of 215 amino acid residues. They are arranged in two domains (HMG A box and HMG B box), a C-terminal acidic tail, and an N-terminal region [9]. Despite its subcellular location, HMGB1 exerts multiple functions. HMGB1 acts as a chaperone in the nucleus, maintaining chromosome structure and function. However, in the cytoplasm, by binding to the Beclin-1 (BECN1) protein, it can promote autophagy [9]. Extracellular HMBG1 can be passively released after cell death or actively secreted. It regulates inflammation and immune responses through various receptors or direct uptake, acting as DAMP. Many factors are responsible for regulating the secretion and release of HMGB1, such as post-translational modifications (e.g., acetylation, ADP-ribosylation, phosphorylation, and methylation) and the molecular mechanisms of cell death (e.g., apoptosis, pyroptosis, necroptosis, alcoptosis, and ferroptosis). The main receptors for HMGB1 are toll-like receptors (TLRs) and the receptor for advanced glycation endproducts (RAGE) [10]. Its potential role in immunological processes is exerted by the ability to activate nuclear factor kappa B (NF-kB) and subsequently stimulate the expression of IL-1 and TNF-α.
S100 proteins belong to a family of approximately 24 members. Each S100 subunit consists of four α-helices and two EF-hands (helix–loop–helix motifs), which are Ca2+-binding domains [11]. The term S100 is associated with their capacity to dissolve 100% of saturated ammonium sulfate solution [12]. The activation of S100 proteins consists of two mechanistic regulatory steps [13]. The initial step is to bind transition metals (Ca2+, Zn2+, Cu2+, and Mn2+) for folding [14,15]. The second step involves the synthesis of homo- and heterodimers [16]. After binding with Ca2+, the conformation changes, which allows the protein to exert its biological effects. The main functions of S100 proteins are regulations in cell apoptosis, proliferation, differentiation, gene expression, enzyme activation, inflammatory responses, and energy metabolism [17].
IL-1α is a pro-inflammatory cytokine that belongs to the IL-1 family. All 11 family members contain a single β-trefoil structure that binds to immunoglobulin-like receptors. IL-1α initiates an inflammatory process, but its isoform, IL-1β, propagates and maintains it. Both isoforms act via IL-1 receptors I and II (IL-1R-I and IL-1R-II). IL-1 plays an important role in responses to stress, regulating the expression of genes. Moreover, the IL-1 receptor antagonist (IL-1RA) is another molecule that binds to the IL-1 receptor. However, it inhibits inflammatory signals [18].
IL-33 is another member of the IL-1 family. Its structure is composed of two evolutionarily preserved domains: the N-terminal nuclear domain and the C-terminal IL-1-like cytokine domain. These domains are distinctly separated by a unique, divergent central segment [19]. It is expressed in the nuclei of keratinocytes, fibroblasts, vascular endothelial cells, dendritic cells, macrophages, and mast cells [20]. IL-33 mediates type 2 innate immune reactions and allergic inflammation. Furthermore, it acts as a chemokine, activating neutrophils and macrophages [21]. It exerts its functions by interacting with a range of target cells equipped with the suppression of the tumorigenicity 2 (ST2) receptor, such as mast cells and type 2 innate lymphoid cells [19]. Binding the IL-33 to ST2 leads to the transduction of inflammatory signals to Th2 cells, regulatory T cells, type 2 innate lymphocytes (ILC2), dendritic cells, macrophages, eosinophils, basophils, and mast cells. However, the soluble ST2 (sST2) receptor does not transduce the intracellular signals but rather acts as a decoy receptor, inhibiting IL-33 inflammatory signals. The transmembrane receptor for IL-33 has the ability to regulate genes by inducing the myeloid differentiation factor 88 (MyD88)-dependent activation of NF-kB and mitogen-activated protein kinase (MAPK).
TSLP belongs to the IL-2 cytokine family [22]. It is primarily produced by epithelial cells found in the gastrointestinal tract, lungs, and skin. Nevertheless, it is noteworthy that TSLP expression is also observable in various other cell types, including dendritic cells (DCs), basophils, and mast cells [23]. Various endogenous and environmental stimuli, including pro-inflammatory cytokines, tryptase, invading pathogens, allergens, irritants, pollutants, and cigarette smoke, can provoke epithelial cells at barrier surfaces to secrete TSLP.
4. Alarmins and Rheumatoid Arthritis
4.1. High-Mobility Group Box 1
Rheumatoid arthritis is an autoimmune disorder associated with joint swelling, as well as cartilage and bone damage. The pathogenesis of this disease involves numerous interactions between immune cells and FLSs that acquire an aggressive phenotype [24]. RA is a chronic disease with increasing prevalence [25], and both genetic (major histocompatibility complex alleles) and environmental (female sex, smoking, dust exposure, and diet) factors are considered risk factors [26]. The pathogenesis of RA is not entirely known, and thus, investigating the mechanisms associated with the development of RA may introduce novel treatment strategies. In this section, we will focus on the role of alarmins in this form of autoimmune arthritis.
To begin with, higher blood levels and the increased expression of HMGB1 in peripheral blood mononuclear cells have been detected in RA patients [27,28,29]. Furthermore, serum concentrations of HMGB1 are elevated in RA patients with an active disease [30]. Moreover, the level of HMGB1 will be greater in the synovial fluid of RA patients compared with those with OA [31]. Treatment of RA patients is associated with the reduced synovial expression of HMGB1 compared with untreated cohorts [32], thus suggesting that it could play a role in the pathogenesis of RA. Several studies have demonstrated various mechanisms involving a DNA-binding protein that could contribute to the disease’s progression. First, HMGB1 impacts the functionality and activity of RA-FLSs. For instance, it is associated with RA-FLS proliferation since siRNA-mediated silencing reduces synoviocyte proliferation. In addition, silencing HMGB1 reduces the migration and invasion of RA-FLSs and reduces MMP-13 and MMP-2 expression [32]. In another study, HMGB1 was found to synergize with lipopolysaccharide (LPS) to enhance RA-FLS proliferation and increase the expression of IL-6 and MMPs. Mechanistically, the combination of LPS with HMGB1 was found to stimulate the p38 and NF-κB pathways [33].
The pathogenesis of RA involves angiogenesis, as the formation of new blood vessels stimulates the infiltration of immune cells and drives synovial inflammation [34]. Importantly, HMGB1 has been associated with the enhanced process of angiogenesis [35]. The molecule can be internalized by the endothelial cells through RAGE, which eventually promotes the release of vascular endothelial growth factor (VEGF) [36]. Accordingly, in RA animal models, exogenous HMGB1 stimulates the development of blood vessels, which are reduced when an HMGB1 inhibitor is introduced [37]. In another study, it was demonstrated that HMGB1 could stimulate RA-FLSs to upregulate hypoxia-inducible factor 1α (HIF-1α) through NF-κB [38]. Furthermore, HMGB1 enhances inflammatory responses that promote the progression of RA. Specifically, it activates immune cells and mediates their migration and secretion of inflammatory cytokines. Macrophages are one of the key cells contributing to the pathogenesis of RA, and they do so by secreting a number of pro-inflammatory cytokines, including tumor necrosis factor-alpha (TNF-α), a molecule targeted by several biological agents used in the treatment of RA. Moreover, macrophages can stimulate T cells, another major cellular subtype involved in the pathogenesis of inflammatory arthritis [39]. In an early study by Andersson et al., the stimulation of peripheral blood mononuclear cells (PBMCs) with HMGB1 enhanced the gene expression and protein secretion of TNF-α [4]. Similarly, stimulating macrophages derived from synovial fluid with HMGB1 increases the production of inflammatory mediators, TNF-α, interleukin-1 beta (IL-1β), and IL-6. The blockade of the RAGE receptor suppresses the enhanced secretion of TNF-α [31]. Additionally, HMGB1 is involved in the process of macrophage migration. Studies have demonstrated that chemokines, which attract immune cells toward inflamed synovium, are upregulated in patients with RA. For instance, high levels of C-X-C motif chemokine ligand 12 (CXCL12) are found in the synovial fluid of patients in the active stage of RA [40]. CXCL12 can create a complex with HMGB1 to further enhance its chemotactic properties. In a study by Cecchinato and colleagues, the authors demonstrated that, in the presence of CXCL12, even low concentrations of HMGB1 induced the migration of monocytes derived from RA patients with active disease. Mechanistically, the authors found that the JAK/STAT and COX2 pathways take part in monocyte migratory responses [41]. Another macrophage-related mechanism occurring in RA is pyroptosis, a programmed cell death associated with the release of IL-18 and IL-1β [42]. Several studies have demonstrated that HMGB1 enhances macrophage pyroptosis [43,44]. In addition, different HMGB1 redox isoforms induce various responses. Disulfide HMGB1 was found to enhance the pro-inflammatory phenotype similar to the classic M1 subtype [45]. The suppression of HMGB1 expression is associated with reduced inflammatory responses in macrophages. Specifically, the transfection of microRNA-25 (miR-25) suppresses the expression of HMGB1 and reduces the production of pro-inflammatory mediators [46].
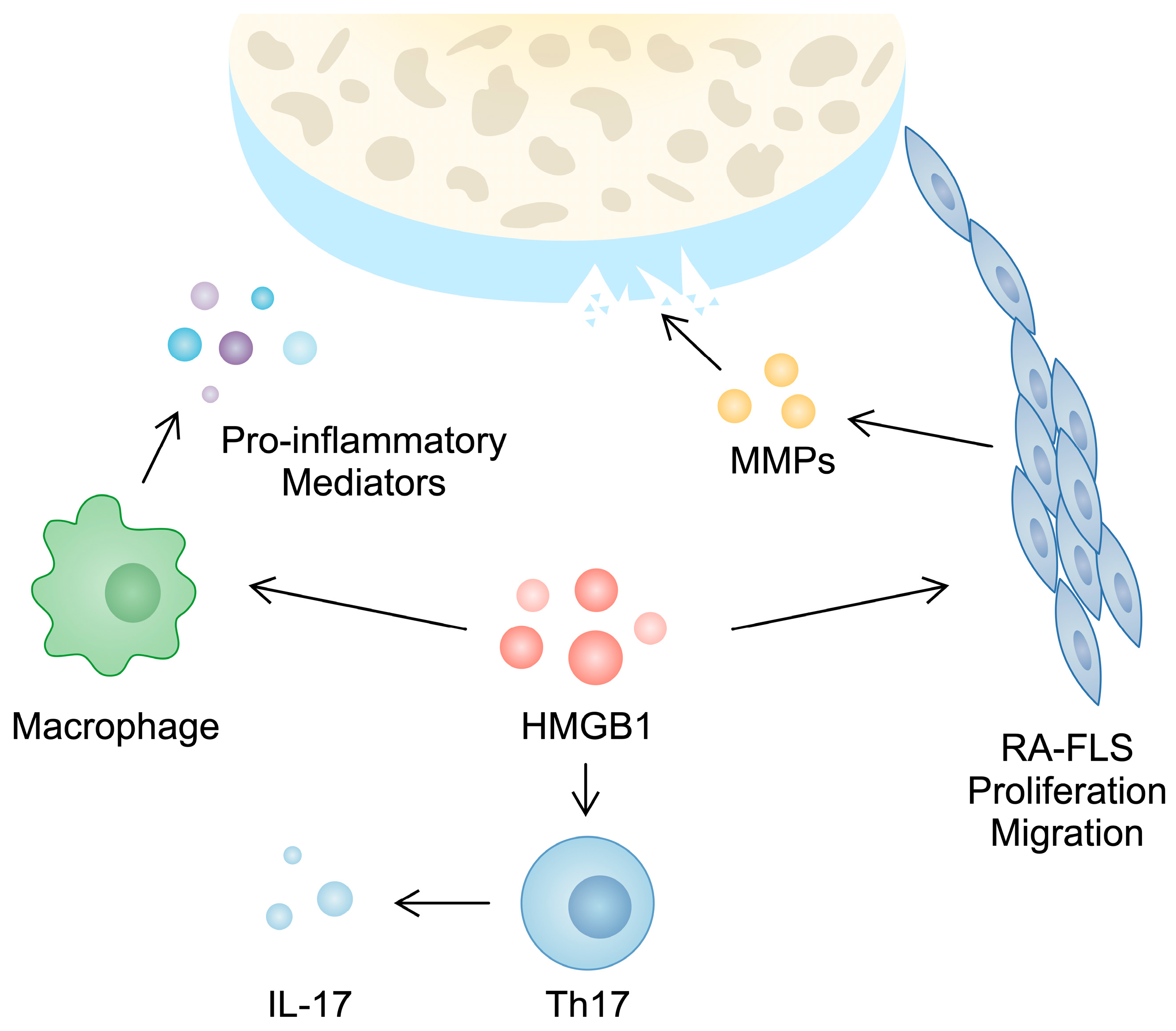
Furthermore, HMGB1 takes part in the activity of T cells, which play a major role in the progression of RA. For instance, a higher population of Th17 cells can be observed in the PBMCs of RA patients compared with controls [47]. These cells produce several cytokines, including IL-17, which is responsible for inducing angiogenesis, synovial inflammation, and cartilage and bone damage [48]. In PBMCs from RA patients, a positive correlation between HMGB1 and IL-17 gene expression has been observed. Moreover, in an in vitro experiment, the treatment of CD4+ T cells with HMGB1 enhanced the presence of Th17 markers [28]. This finding was confirmed in another in vitro analysis performed by Su and collaborators [49]. Taken together, these studies broadly demonstrate the role of HMGB1 in the pathogenesis and inflammatory responses typical of RA (Figure 1).
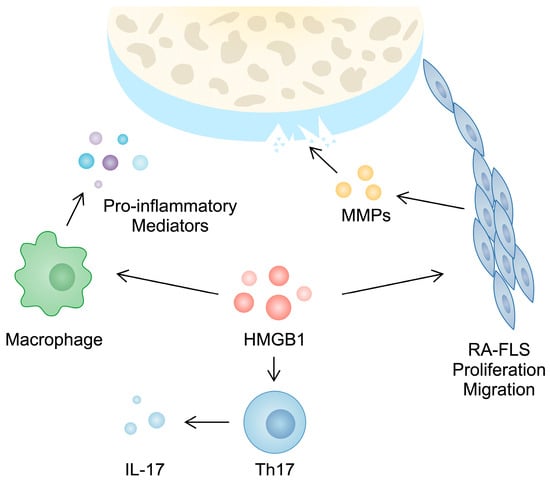
Figure 1.
Hypothetical involvement of HMGB1 in the pathogenesis of rheumatoid arthritis. Firstly, it is involved in the invasive features of RA-FLSs since its silencing is associated with reduced migration, proliferation, and the secretion of matrix metalloproteinases. Secondly, stimulating macrophages and CD4+ T cells with HMGB1 are associated with the enhanced production of pro-inflammatory cytokines and Th17 markers, respectively.
Importantly, it is interesting to note the role of drugs in the expression of HMGB1. Firstly, HMGB1 gene polymorphisms are associated with RA treatment responses. In a study by Xu et al., the authors found that allele G of the rs2249825 polymorphism was associated with refraction susceptibility to the treatment with conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) [50]. The synovial expression of HMGB1 decreased in patients treated with methotrexate (MTX), as compared with RA patients, who did not receive the antifolate [32]. MTX is broadly used in the treatment of RA, and few studies have investigated its effect on HMGB1. The treatment of monocytes with MTX in the presence of LPS/IFNγ does not change the secretion of TNF or HMGB1 [51]. However, when the RAGE receptor was stimulated with a truncated version of HMGB1 in a murine macrophage-like cell line, the introduction of MTX significantly reduced the secretion of TNF [52], indicating that MTX might affect HMGB1/RAGE interaction. In addition, HMGB1 is involved in the resistance of RA-FLSs to MTX. Specifically, MTX has been shown to enhance autophagy in RA-FLSs, a process that has been used to evade apoptosis. Moreover, the drug increases the expression of HMGB1 in RA-FLSs. Importantly, silencing HMGB1 significantly enhances apoptosis induced by MTX [53]. Recently, HMGB1 has been suggested to be involved in MTX-induced hepatotoxicity. The protein expression of alarmin is increased in the hepatic tissues of mice treated with MTX. HMGB1-knockdown experiments have demonstrated that this alarmin is involved in MTX-stimulated ferroptosis and autophagy [54].
Few studies have evaluated the associations between HMGB1 and other agents used in the treatment of RA. TNF (etanercept) and IL-1β (anakinara) inhibitors do not suppress the release of HMGB1 from monocytes [51]. Similarly, there are no significant differences in the protein and gene synovial expressions of HMGB1 in RA patients treated with infliximab, another monoclonal antibody-targeting TNF [55]. However, the serum level of HMGB1 significantly declines in RA patients treated with metformin [30], a biguanide derivative that was previously found to bind HMGB1 [56]. Taken together, HMGB1 seems to be involved in the pro-inflammatory mechanisms implicated in the pathogenesis of RA. Targeting HMGB1 could represent a future treatment strategy that needs to be further investigated.
4.2. S100 Proteins
S100 proteins represent another family of alarmins investigated in the context of RA. To begin with, many studies have evaluated the role and presence of calprotectin, a molecule composed of S100A8 and S100A9, in RA patients. In serum, significantly greater concentrations of calprotectin are detected in patients with RA compared with controls [57,58,59]. Thus, calprotectin has been evaluated as a diagnostic biomarker. The combination of serum calprotectin, anti-CCP3, and rheumatoid factor (RF) is associated with a promising positive predictive value for detecting RA occurrence within three years [60]. Furthermore, greater levels of calprotectin in the synovial fluid [61] and elevated expressions of S100A8 and S100A9 have been found in the monocytes of patients with RA [62]. Importantly, calprotectin levels are associated with disease activity, even in patients with low concentrations of CRP [63]. In a meta-analysis by Bae et al., circulating calprotectin was positively correlated with DAS28 and CRP [61]. Intriguingly, even a greater association with pro-inflammatory mediators was observed in ACPA-positive patients [64]. Using calprotectin as a marker of disease activity has been also suggested in patients in whom it would be difficult to measure inflammatory parameters due to the administration of tocilizumab [65]. Additionally, serum calprotectin concentrations could be used to identify patients with synovitis detected on power Doppler ultrasounds [66,67]. In line with these findings, serum levels of S100A8/A9 decrease in RA patients after three months of treatment, and lower levels have been detected in patients achieving remission [68].
Overall, multiple studies have demonstrated the potential role of calprotectin as a diagnostic biomarker, as well as a laboratory parameter, that could be used to monitor disease activity, even in patients with lower concentrations of other RA parameters, such as CRP. However, monitoring calprotectin to predict flares and relapses remains controversial. De Moel et al. showed that higher levels of circulating calprotectin at the moment of treatment tapering could indicate potential flares within twelve months [69]. By contrast, Romand and collaborators demonstrated that monitoring calprotectin levels does not assist in detecting RA relapses [70].
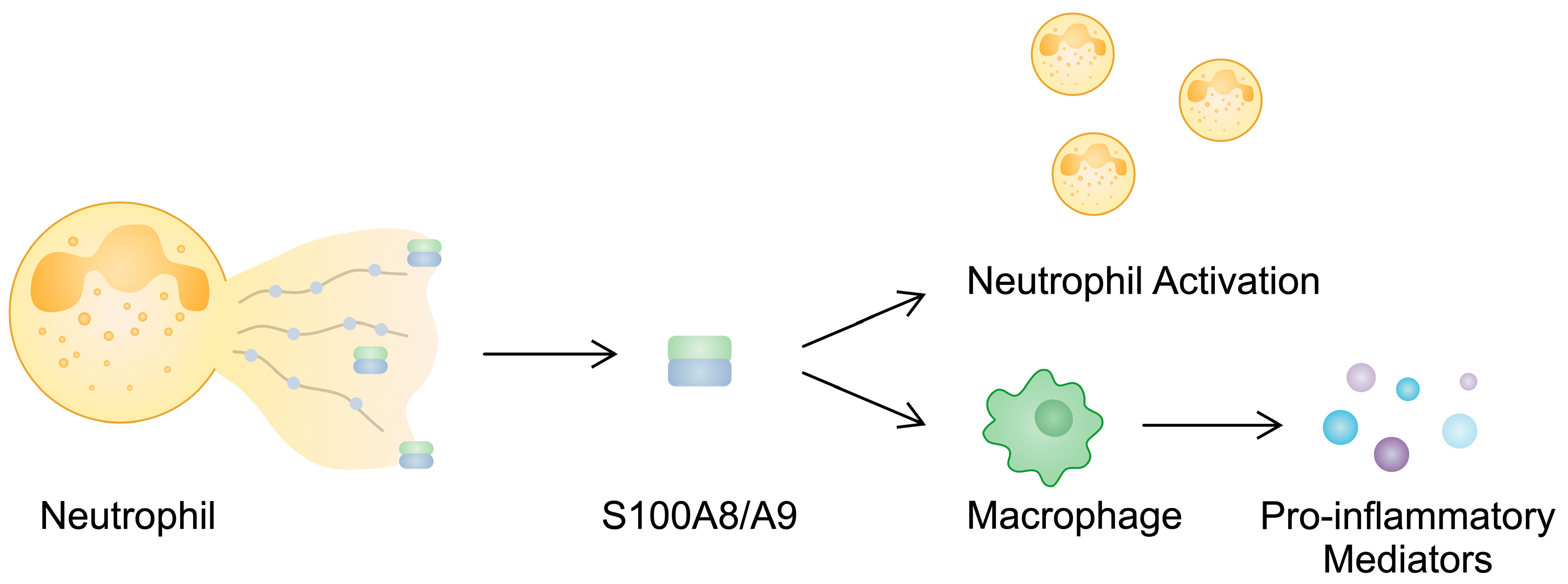
S100A8 and S100A9 impact the functionality of immune cells, including neutrophils and macrophages. Neutrophils play a significant role in the pathogenesis of RA since they release neutrophil extracellular traps (NETs), structures involved in the propagation of inflammation and a major source of citrullinated proteins [71]. Evidence suggests that S100A9/S100A9 can be released in NETs, indicating that it could be used as a marker of neutrophil-derived structures. Furthermore, it contributes to neutrophil activation [72]. The induction of pro-inflammatory cytokine secretion has been found to depend on the presence of phosphorylated S100A9 [73], and importantly, the phosphorylated protein is present in the synovial fluid of RA patients [74]. Calprotectin is also implicated in the activity of macrophages. The presence of S100A8/A9 has been found in macrophages from the synovial tissues of RA patients. Through the p38 and NF-κB pathways, S100A8/A9 can enhance the production of pro-inflammatory mediators in monocytes [75]. In addition, these alarmins stimulate NOD-, LRR-, and pyrin-domain-containing protein 3 (NLRP3) inflammasome, which is associated with inflammation and pyroptosis [76]. The stimulation of THP-1 macrophages with S100A8 enhances the protein expression of NLRP3 and its downstream elements, including GSDMD, IL-1β, and cleaved caspase-1, among others [77]. S100A8 and S100A9 are also implicated in other inflammatory pathways associated with T cells. The previously mentioned Th17 cells secrete IL-22 as well, which is implicated in the pathogenesis of RA [48]. In an in vitro experiment, the stimulation of RA-FLSs with IL-22 enhanced the mRNA expression of S100A8 and S100A9, which could then interact with immune cells to promote inflammation [78]. Additionally, these alarmins can be expressed by bone and cartilage cells [79]. In S100A9-/- antigen-induced arthritis, significantly reduced bone erosion has been observed. Moreover, the introduction of S100A8 promotes osteoclast formation during osteoclastogenesis [80]. By contrast, another study by Di Ceglie and colleagues suggested that the presence of S100A9 suppresses the differentiation of monocytes toward osteoclasts [81].
Taken together, S100A8 and S100A9 could play a role in RA progression (Figure 2). Importantly, the use of neutralizing antibody-targeting S100A9 results in decreased disease incidence and activity, as well as reduced cartilage and bone damage, in RA murine models [82]. In addition, the administration of the recombinant apoptosis inhibitor of macrophages (AIM) suppresses inflammatory responses in murine autoimmune arthritis models. Simultaneously, AIM has been suggested to reduce the expression of S100A9, but the difference is not significant (p = 0.065) [83]. These proteins could also serve as markers of responses. However, taking into account the relationship between S100A8/A9 and disease activity, it is intriguing that higher serum levels of S100A9 have been observed in responders to treatments with MTX ad etanercept [84]. Similarly, higher serum concentrations of calprotectin have been found in responders to treatments with adalimumab or etanercept [85].
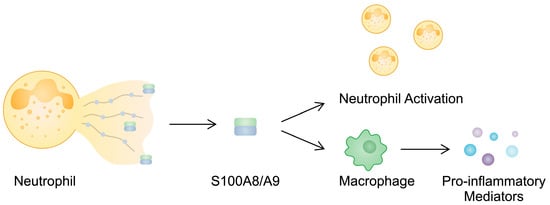
Figure 2.
S100A8/A9 is released during NETosis, which then activates other neutrophils and enhances macrophages so that they secrete pro-inflammatory cytokines.
The presence of other members of the S100 family has been studied as well. A greater expression of S100A11 (calgizzarin) has been found in the synovial tissue, and higher synovial fluid levels of S100A11 have been observed in patients with RA compared with OA [86]. Its levels positively correlate with disease activity and inflammatory markers [86,87], suggesting its involvement in disease progression. Similarly to the previously described proteins, S100A11 is associated with neutrophils. Specifically, the release of calgizzarin occurs during NETosis. In turn, the alarmin affects inflammation by enhancing IL-6 and TNF secretion in neutrophils derived from healthy patients. Intriguingly, S100A11 does not stimulate pro-inflammatory responses in neutrophils obtained from RA patients [87], suggesting that it might be involved in the early stages of RA pathogenesis or that RA cells exert some sort of tolerance. Another alarmin with elevated concentrations in the sera of RA patients is S100A12 [88]. Moreover, the levels of S100A12 are higher in the synovial fluids of RA patients compared with OA, where it was not detected [89]. Importantly, its concentrations are significantly correlated with ultrasonography scores, as well as clinical and inflammatory parameters [90]. Additionally, S100A12 stimulates the presence of neutrophils [89] and osteoclastogenesis [91], cell types highly implicated in RA progression.
4.3. Interleukin-33
Another alarmin studied in the context of RA is IL-33, a member of the IL-1 family. After being released from injured cells or tissues, an active and pro-inflammatory form of IL-33 acts through the suppression of the tumorigenicity 2 (ST2) receptor [92]. NF-κB, ERK, and MAPK are considered downstream elements of IL-33/ST2 signaling [93]. Several studies have investigated the involvement of IL-33 in RA. To begin with, different results have been obtained regarding the concentrations of this cytokine. In a study by Hong and collaborators, significantly higher serum levels of IL-33 were observed in RA patients compared with healthy controls. Furthermore, greater concentrations of IL-33 have been noted in the synovial fluids of RA patients when compared with patients with OA [94]. In line with these findings, Tang et al. also observed higher levels of IL-33 in RA synovial fluid, as compared with OA [95]. However, in another report by Talabot-Ayer and colleagues, the difference between the groups was not statistically significant. Furthermore, the authors demonstrated that the synovial expression of RA and OA does not differ significantly [96]. Importantly, positive correlations between serum and synovial fluid IL-33 levels with disease activity parameters, autoantibody concentrations, and inflammatory mediators have been found [94,95], which could suggest its involvement in the pathogenesis of the disease. Nevertheless, its involvement in mechanisms underlying the pathogenesis of the disease may be more complex. Recently, Poole and collaborators found an inverse correlation between IL-33 levels and the risk of RA-associated interstitial lung disease [97].
Firstly, the genetic polymorphism of IL-33 has been associated with disease susceptibility. Specifically, the rs10975519 CC genotype has been suggested to decrease the susceptibility to developing RA among females [98]. Secondly, in the autoantibody-induced arthritis animal models, the introduction of IL-33 was associated with significantly greater clinical scores for arthritis [99]. IL-33 is involved in various molecular mechanisms involving RA-related cells. It impacts the functionality of RA-FLSs by regulating the expression of pro-inflammatory mediators. Nonetheless, mechanisms induced by IL-33 might depend on the cellular context, as Wu et al. demonstrated that various doses of IL-33 can differently induce the expression of pro-inflammatory mediators [100]. By contrast, in an in vitro experiment, Lee and collaborators showed that the overexpression of IL-33 in stimulated RA-FLSs reduces the activity of NF-κB and pro-inflammatory cytokines [101]. As previously mentioned, the ERK pathway is a downstream element of IL-33 signaling. Intriguingly, it also seems to take part in the regulation of IL-33 secretion. Specifically, miR-483-3p is a molecule greatly expressed in RA-FLSs. The use of miR-483-3p mimics is associated with the greater production of IL-33 in RA-FLSs through the ERK pathway [102]. Subsequently, released IL-33 can interact with other immune cells to further regulate immune responses. The stimulation of peritoneal macrophages with IL-33 enhances the mRNA expression of MCP-1, IL-1β, and p-P65. Interestingly, a positive correlation between IL-33 and an anti-inflammatory IL-10 has been observed, which could suggest that, despite inducing inflammatory responses, IL-33 also enhances anti-inflammatory reactions [103]. Furthermore, macrophages respond to pro-inflammatory stimuli by secreting IL-33. The synovium of RA patients is associated with a greater presence of macrophage migration inhibitory factor (MIF) [104], a pro-inflammatory molecule that induces the expression of IL-33 in PBMC obtained from RA patients [105]. Moreover, IL-33 affects the behavior of dendritic cells, whose greater presence is found in the synovial fluids of RA patients [106]. This alarmin might modulate the functionality of dendritic cells to enhance the differentiation of Th17 cells [107], thus contributing to the progression of RA. IL-33 also enhances the migration of neutrophils through the CXCL1, CCL3, IL-1β, and TNFα mediators [108].
In addition, neutralizing IL-33 and the effect of systemic treatment further confirm its involvement in the pathogenesis of the disease. Despite ST2, there is a soluble form of the receptor (sST2) that can be used as a decoy for IL-33. In the CIA mouse model, the use of sST2 is associated with suppressed RA progression and reduced inflammation [109]. Moreover, treatment with TNFα inhibitors significantly reduces serum levels of IL-33 in responders [110,111], suggesting that IL-33 could be monitored to evaluate responses in patients treated with biologic agents, which has also been demonstrated in the case of rituximab [112]. A summary of the role of alarmins in the pathogenesis of RA is presented in Table 1.

Table 1.
Summary of mechanisms induced by alarmins that take part in the pathogenesis of rheumatoid arthritis.
5. Alarmins and Osteoarthritis
5.1. High-Mobility Group Box 1
Osteoarthritis is a degenerative chronic joint disease. Aging populations and a rising number of metabolic diseases are associated with an increasing number of OA patients. The clinical course usually involves the loss of joint function and pain. Multiple risk factors are associated with the development of OA, including age, obesity, prior joint trauma, genetic factors, and female sex [113]. It has been reported that OA affects nearly 27 million Americans, and this number is expected to double by 2030 [114,115]. This disease frequently involves the knee and hip joints, as well as the spine [116,117]. Articular cartilage is involved in smoothing the surface of the joint. Together with synovial fluid, these elements decrease friction during joint motion. As OA progresses, the cartilage becomes weaker, the space between joints narrows, and osteophytes may appear on bone surfaces [118,119]. Recent studies have found that the pathogenesis of OA is complex and involves immune mediators, such as alarmins.
To begin with, higher gene and protein expression of HMGB1 and RAGE have been detected in the synovial tissues of OA patients [120]. Moreover, the elevated expression of this alarmin has been observed in OA chondrocytes [121]. Li and colleagues found higher synovial fluid concentrations of HMGB1 in OA patients and that these levels positively correlated with OA severity [122].
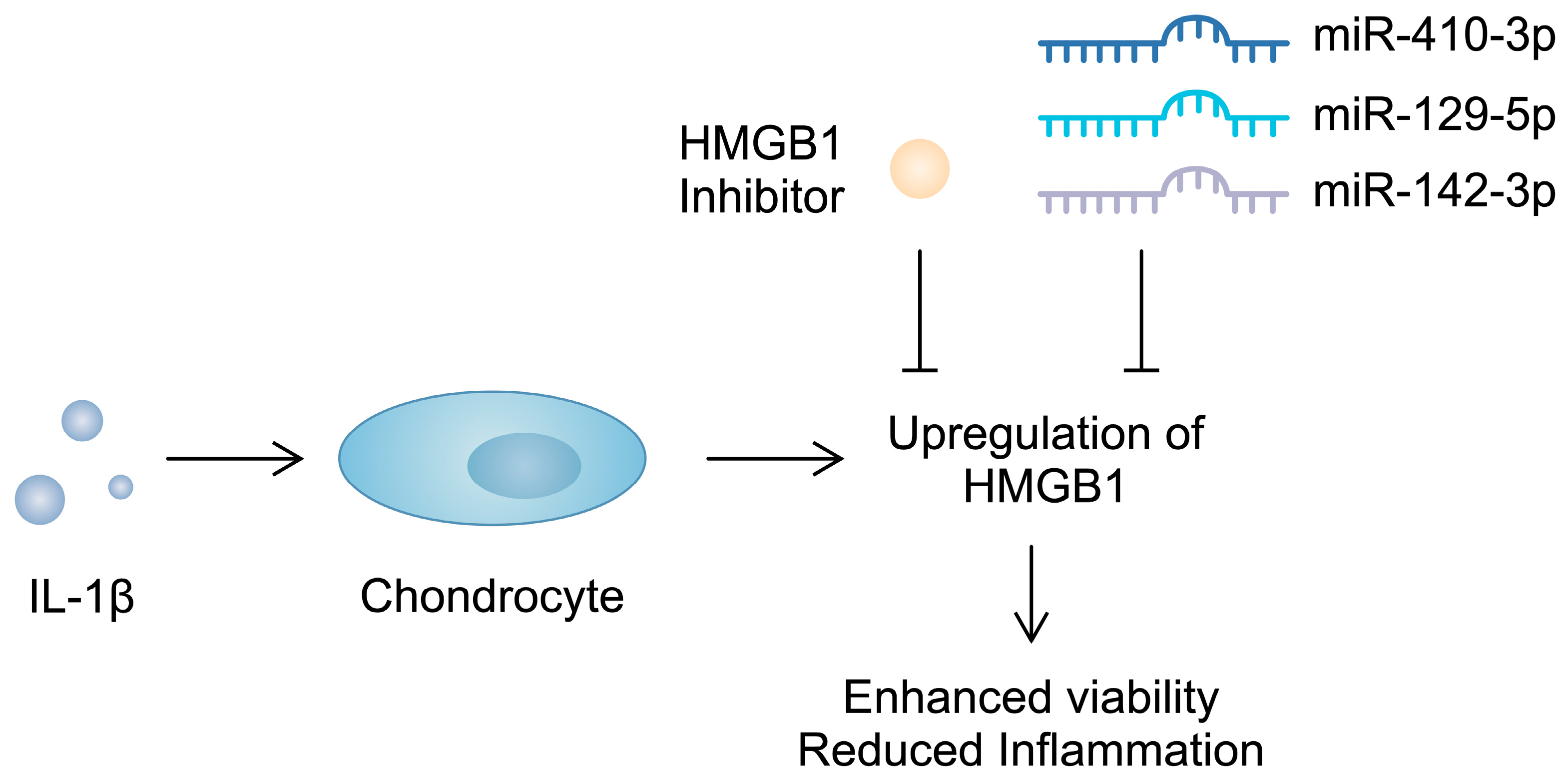
Importantly, apart from serving as a biomarker, HMGB1 contributes to the progression of OA. The intra-articular introduction of anti-HMGB1 antibodies significantly reduces the OARSI score in mice after anterior cruciate ligament transection (ACLT) [123]. Similarly, the use of glycyrrhizim, an HMGB1 inhibitor, is associated with the enhanced viability of IL-1β-pretreated chondrocytes. Importantly, this agent also suppresses the previously stimulated expression of inflammatory markers and cartilage-degrading enzymes [124]. Furthermore, several studies have demonstrated that non-coding RNA (ncRNA), molecules that regulate gene expression, can target alarmins and induce beneficial effects in chondrocytes. Specifically, miR-410-3p, miR-129-5p, and miR-142-3p have been found to directly target and downregulate HMGB1 in chondrocytes, which is associated with reduced inflammatory responses [125,126,127] (Figure 3). Consequently, the use of these molecules might be associated with protective mechanisms in OA. Importantly, in an in vivo experiment, the overexpression of miR-410-3p reduced cartilage damage in OA mouse models [125]. Additionally, an in vitro OA model is generated when chondrocytes are treated with IL-1β. During this process, an upregulation of HMGB1 can be observed, further confirming its involvement in the pathogenesis of OA [126]. Direct stimulation of mouse pre-chondral cell lines with recombinant HMGB1 has demonstrated a variety of effects induced by this alarmin. It stimulated the apoptosis of cartilage cells and enhanced the protein expression of a number of cartilage-degrading enzymes. Mechanistically, HMGB1 is associated with the Wnt/β-catenin signaling pathway; it enhances the expression of β-catenin [128]. Importantly, the enhanced activity of this pathway has been suggested to take part in the pathogenesis of OA, possibly through the upregulation of cartilage-degrading enzymes [129]. Therefore, HMGB1 seems to be involved in several mechanisms driving the progression of OA. Current evidence suggests that this alarmin enhances inflammatory responses and could be associated with the upregulation of cartilage-degrading enzymes, suggesting that HMGB1 could become a therapeutic target.
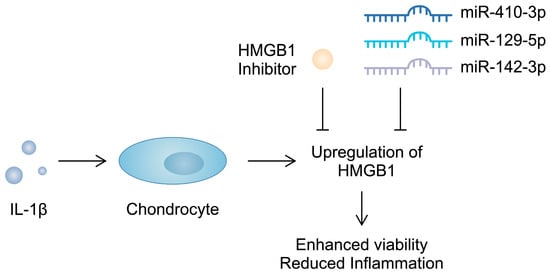
Figure 3.
Molecules targeting HMGB1 were found to suppress pro-inflammatory responses in chondrocytes induced by IL-1β.
5.2. S100 Proteins
S100A8 and S100A9 are involved in the pathogenesis of OA by regulating the activation of synovium and the degradation of cartilage [130]. Both alarmins are overexpressed in synovial fluid in OA, and they are positively correlated with radiological joint damage [131,132]. Furthermore, serum levels of S100A8/A9 are positively correlated with clinical symptoms, such as knee pain, as well as the serum concentrations of enzymes associated with cartilage degradation [131,133]. S100A8/A9 and S100A6 are believed to be diagnostic markers of OA [134,135,136]. However, in a study by Mahler and colleagues, the authors did not prove that calprotectin is a biomarker in patients with hand, knee, or hip OA [137].
S100 proteins are associated with macrophage phenotypes and behavior, which may depend on the stage of differentiation. Specifically, S100A8 expression has been correlated with M2 macrophage infiltration [134]. The stimulation of synovial tissues with recombinant S100A9 enhances the mRNA expression of pro-inflammatory mediators such as IL-6, IL-8, and IL-1β [138]. However, during macrophage differentiation, exposure to S100A9 leads to enhanced differentiation toward M2-like macrophages. Interestingly, this alarmin enhances the expression of both pro-inflammatory and anti-inflammatory mediators, together with MMPs [139]. Zreiqat et al. demonstrated that S100A9 from articular chondrocytes is involved in the early processes of cartilage degradation through the promotion of aggrecanases and MMPs. However, the authors found that they are not involved in the subsequent progression of OA [132]. Schelbergen et al. reported that S100A8/A9 can increase the formation of osteophytes and thus may be used as biomarkers of osteophyte development [140]. Similarly to HMGB1, S100A8 and S100A9 interact with the Wnt signaling pathway. Specifically, the intra-articular administration of S100A8 promotes canonical Wnt signaling. The enhanced expression of dickkopf-1 (Dkk-1) is suppressed, which is associated with suppressed signaling, resulting in the alleviated expression of MIP-1a, IL-6, and MMPs [141]. Importantly, the administration of adipose-derived stem cells in experimental OA reduces the levels of S100A8/A9 [142].
As the catabolic effects of S100A8/A9 are TLR-4-dependent [143], their interaction could be targeted. Paquinimod is an immunomodulatory drug that prevents S100A9 from binding to TLR-4. It suppresses the expression of IL-6, IL-8, and MMP1/3 in the human OA synovium. Importantly, prophylactic treatment with paquinimod reduces cartilage degradation and the formation of osteophytes [144].
The presence of S100A8/A9 is associated with several molecules implicated in the pathogenesis of OA. For instance, in a study by Corr and colleagues, the authors observed that basic calcium phosphate (BCP) upregulated the expression of MMP1 and S100A8 in macrophages [145]. BCP crystals are long-known contributors to OA development, as their presence correlates with radiographic progression. Mechanistically, BCP crystals enhance the production of IL-6 by chondrocytes, as well as osteoclastogenesis [146]. Similarly, alarmins seem to be involved in the biological effects induced by IL-22, a mediator that has been suggested as a therapeutic target in OA [147]. Specifically, IL-22 has been found to enhance the production of MMP-1 and calprotectin [78]. Overall, these studies demonstrate the involvement of S100 proteins in mechanisms and molecules associated with OA progression.
5.3. Interleukin-33
Few studies have investigated the presence and role of IL-33 in the context of OA. To begin with, a higher expression of the IL-33 gene has been observed in articular chondrocytes from OA patients, as compared with healthy controls [148]. Interestingly, IL-33 may be associated with vitamin D. Specifically, greater immunoexpression of IL-33 has been observed in vitamin D-deficient swine. Interestingly, in vitro experiments using human articular chondrocytes have shown that calcitriol could reduce the expression of IL-33, along with other pro-inflammatory mediators, their receptors, and MMPs [149]. Similarly, by using human chondrocytes, He et al. observed that recombinant IL-33 increases the gene and protein expressions of cartilage-degrading enzymes (ADAMTS-5; MMPs) and reduces the expression of chondrogenic factors such as aggrecan, COL2A1, and SOX-9. Importantly, the authors demonstrated the importance of IL-33 in the pathogenesis of OA, as a cartilage-specific knockout of this alarmin was associated with reduced pain, cartilage degradation, and synovitis in mice. Similar findings have been observed after introducing the ST2-neutralizing antibody [150]. The pro-inflammatory effects of IL-33 affect anti-inflammatory mediators, as the stimulation of chondrocytes with IL-33 reduces the expression of IL-37, a cytokine that has been suggested to be used in the treatment of OA [151,152]. Interestingly, IL-33 may affect the biological effects of other alarmins since it upregulates the expression of the RAGE receptor in chondrocytes [151]. Therefore, current evidence suggests that targeting IL-33 may be associated with reduced inflammation and the suppressed progression of OA. A summary of the role of alarmins in the pathogenesis of OA is presented in Table 2.

Table 2.
Summary of mechanisms induced by alarmins that take part in the pathogenesis of osteoarthritis.
6. Alarmins and Psoriasis
6.1. Typical Features and Epidemiology of Psoriasis
Psoriasis is a chronic inflammatory skin disease that lowers quality of life and is characterized by keratinocyte hyperproliferation and erythematous scaly plaques. It can develop in any part of the body. However, the most frequently affected are parts at risk of frequent injuries, such as elbows, knees, or loins. Psoriasis affects up to 3% of the world’s population, women and men equally, with variations in different regions. The disorder has a bimodal age distribution ranging from 18 to 39 years and 50 to 69 years [153].
Trauma to the skin is an important trigger that may precipitate the appearance of psoriatic lesions. It is widely known as Koebner’s phenomenon, and the triggering of the local inflammatory process is through the release of a nerve growth factor, a molecule that has been suggested to be involved in the pathogenesis of psoriasis. In addition, air pollution and bacterial, fungal, and viral infections are also linked to psoriasis. Despite the abovementioned factors, behavioral factors like dietary habits, smoking, and alcohol consumption are key risk factors. Regarding immunology, the skin is a fascinating milieu where the immune system is in a tight relationship with the endocrine and nervous systems. Consequently, mental status significantly influences the course of psoriasis, especially disorders like depression, anxiety, and chronic stress. Importantly, several drugs have been found to aggravate or induce the onset of psoriasis. These agents include beta-blockers, calcium-channel blockers, thiazide diuretics, lithium, anti-malarial drugs, interferons, imiquimod, angiotensin-converting enzyme inhibitors, terbinafine, tetracycline, NSAIDs, and fibrates [153]. Approximately 20% of patients with psoriasis develop psoriatic arthritis, further disfiguring and disabling them. These patients are also more often affected by other autoimmune diseases, such as rheumatoid arthritis, systemic lupus erythematosus, Sjogren syndrome, ulcerative colitis, vitiligo, and inflammatory bowel disease [154,155,156,157,158,159].
Studies claim that the pathogenesis of psoriasis is a combination of genetic predisposition with certain environmental factors. The evidence from the literature supports three main hypotheses on the origin of psoriasis; the first one considers it to be an autoimmune process; the second involves dysbiosis in the skin, as Toll-like receptors (TLR) have been found to be overexpressed in the keratinocytes and dendritic cells of psoriatic skin. TLRs induce the synthesis of antimicrobial peptides, leading to the activation of Th1 and Th17, which then release IL-18 and IL-23, respectively. The last theory assumes that an imbalance between oxidant and antioxidant mechanisms leads to the dysregulation of the immune system [153].
6.2. Pathogenesis of Psoriasis
Chronic inflammation involving an interplay between cytokines, chemokines, reactive oxygen species (ROS), antimicrobial peptides, and autoantigens is the main underlying mechanism in the pathogenesis of psoriasis. Inflammatory processes driving the progression of this disease involve both innate and adaptive immunity. Furthermore, a large number of cells are implicated in its pathogenesis. Keratinocytes are major responders to the skin’s inflammatory mediators, but other cells, such as dendritic cells, macrophages, mast cells, and neutrophils, are also involved in its pathogenesis. Their defensive role relies on phagocytosis and the presentation of antigens. These processes lead to the release of inflammatory mediators: IL-6, Il-1-β, TNF-α, IL-23, IL-12, CCL20, CXCL5, CXCL8, CXCL9, CXCL10, prostaglandins, leukotrienes, histamine, and ROS. Antimicrobial peptides, e.g., LL-37, beta-defensins, and S100 proteins, also play a key role in psoriasis, contributing to epidermal hyperproliferation, angiogenesis, and the activation of other cells of the immune system. Under pro-inflammatory conditions and the influence of IL-23 secreted by dendritic cells, naïve Th cells differentiate into Th17 and Th22 cells. Subsequently, these cells secrete IL-17A and IL-22, which enhance the migration of immune cells into the site of inflammation but also stimulate hyperproliferation and alter the maturation of keratinocytes. Moreover, IFN-gamma further stimulates antigen-presenting cells into producing pro-inflammatory mediators, which maintain the ongoing inflammatory process in the skin [153]. Regulatory T cells (Tregs) are also involved in the etiology of psoriasis. Their primary role is to inhibit the immune response and thus prevent the development of autoimmune diseases. However, the protective function of Tregs, both in the circulation and the skin, is impaired in patients with psoriasis, which inevitably alters the Th17/Treg balance [160].
6.3. High-Mobility Group Box 1
We previously mentioned that HMGB1 and its receptors are implicated in the pathogenesis of RA and OA. Importantly, current evidence suggests that HMGB1 also contributes to the progression of psoriasis. Higher levels of the alarmin have been noted in the serum of psoriasis patients [161,162]. Furthermore, a higher expression of this molecule in lesional skin, as compared with adjacent healthy tissue, has been detected. HMGB1 serum levels have been positively correlated with disease severity, as measured by the PASI score [162,163]. Moreover, the systemic treatment of psoriasis reduces the expression of HMGB1, which strongly suggests its involvement in the underlying mechanisms leading to the development of psoriasis [164]. Interestingly, different expression profiles of HMGB1 might point to different types of psoriasis. For instance, higher expression of this alarmin has been observed more among patients with generalized pustular psoriasis than those with psoriasis vulgaris. However, the difference was insignificant when serum levels of HMGB1 were compared [164]. Additionally, in an intriguing study by Mohamad et al., the authors found that patients with higher levels of HMGB1 had three vascular patterns of plaques when examined under dermoscopy and were more likely to have dotted and globular or looped vascular patterns, as well as ‘thick’ and ‘coarse’ scales. However, no significant difference was observed in scale distribution [165]. The described association between HMGB1 and vascular patterns provides an important correlation between HMGB1 levels and the severity of psoriasis, as the increase in the number of vascular patterns reflected the psoriasis severity.
Under normal circumstances, HMGB1 is expressed in the nucleus of epidermal cells, while in psoriasis vulgaris, it is located in the cell’s cytoplasm. According to previous data, HMGB1 translocates to the cytoplasm in response to different factors, such as LPS, TNF-alfa, and IFN-c [164]. This agrees with a previous study by Chen et al., who suggested that the relocalization of HMGB1 to the cytoplasm represents the signal for its secretion [162]. Secreted HMGB1 takes part in intercellular communication, which affects the functionality of immune cells. Interestingly, a significant relationship exists between HMGB1 and psoriasin (S100A7), which might suggest that they are released together [161]. Psoriasin is an antimicrobial peptide released by keratinocytes and leukocytes stimulated with IL-17, IL-22, and TNF-α. Its role involves chemotaxis for granulocytes, monocytes, and lymphocytes, and it is also said to contribute to psoriasis angiogenesis [166].
The secretion of HMGB1 affects keratinocytes by promoting their excessive proliferation and the expression of inflammatory cytokines, enhancing a vicious cycle. These observations confirm the involvement of both HMGB1 and keratinocytes in the pathogenesis of psoriasis, supporting the idea that keratinocytes secrete pro-inflammatory factors and respond to them [167]. Furthermore, as demonstrated in confocal fluorescence, HMGB1 co-localizes with keratins 1 and 10, molecules implicated in the pathogenesis of psoriasis, as these keratins are the markers of the early differentiation of keratinocytes [168]. Another mechanism in which HMGB1 is implicated is autophagy, a cellular recycling process. Wang et al. found a significant association between functionally active autophagy and psoriasis severity. Specifically, cytosolic HMGB1 acts as a Beclin 1-binding protein, thereby promoting autophagy. Beclin-1 (BECN1) plays an important role in autophagic programmed cell death, and its expression has been found to be higher in psoriatic epidermises than in healthy controls [169]. Therefore, the results of the study by Wang et al. strongly implicate the role of HMGB1 in autophagy as a crucial part of psoriasis etiology. Moreover, HMGB1 is more effective than other autosecretory proteins in regulating psoriasiform cutaneous inflammation. The specific ablation of HMGB1 in keratinocytes attenuates imiquimod-induced skin inflammation in a mouse model [169].
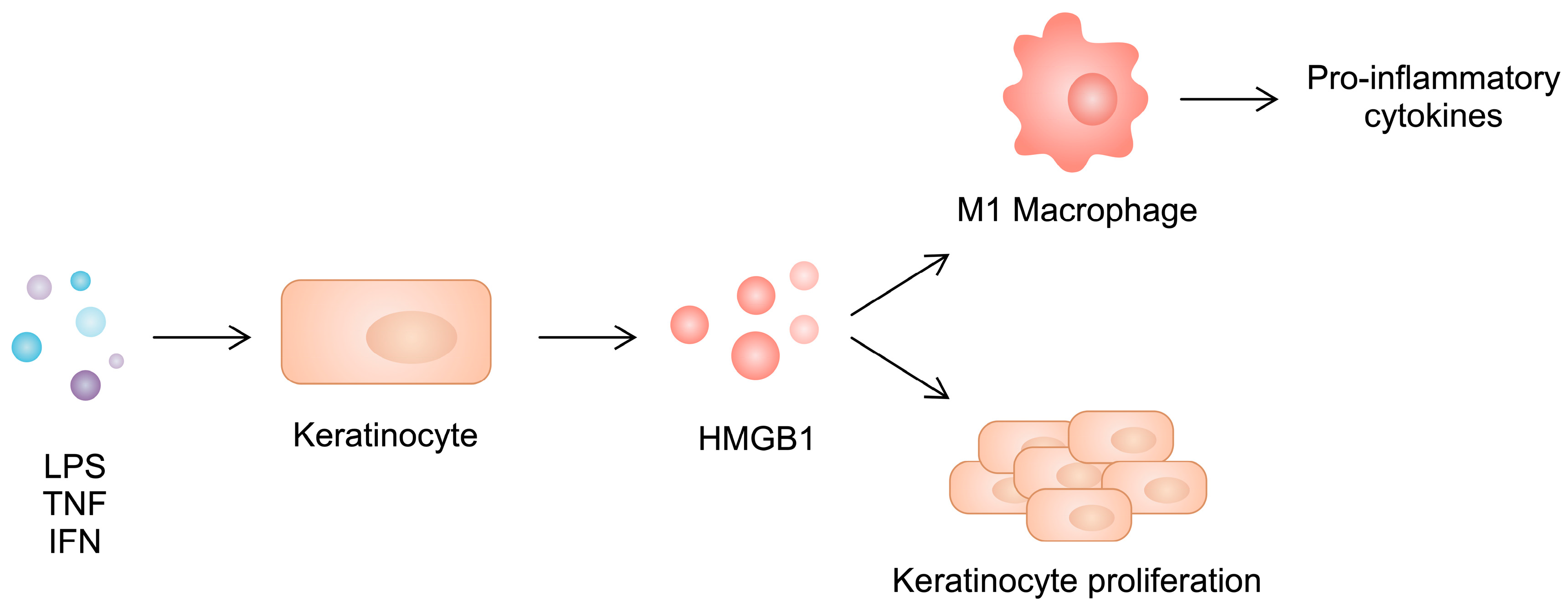
Similarly to RA, there is an imbalance between macrophage phenotypes in psoriatic skin. Specifically, a greater presence of the pro-inflammatory M1 subtype can be observed. HMGB1 released from keratinocytes shifts the polarization of macrophages toward the inflammatory phenotype, further enhancing the production of inflammatory cytokines such as IL-17, IL-1β, TNF-α, IL-12, and IL-6. At the same time, silencing HMGB1 in psoriatic keratinocytes inhibits the development of the M1 phenotype, diminishing their cytokine production (Figure 4). Interestingly, the M2 type is not affected. However, the level of IL-10 increases in M2 macrophages, with unchanged expression of TGF-β.
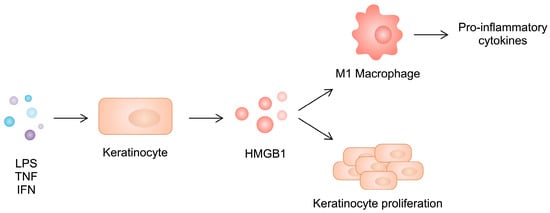
Figure 4.
Stimulated keratinocytes secrete HMGB1, which induces keratinocyte proliferation and enhances macrophage polarization toward the pro-inflammatory phenotype.
As previously mentioned, systemic treatment is associated with a reduction in HMGB1 expression. In animal models, Li et al. aimed to confirm whether the therapeutic effect of etanercept affected the HMGB1 signaling pathway. The authors found that administering etanercept decreased the protein expression of HMGB1 but also that of RAGE, TLR4, and p-P65. Overexpression of HMGB1 attenuated the inhibitory effect of etanercept. Mechanistically, TNF-α causes HMGB1 translocation to the cytoplasm, which, as mentioned before, is a signal for its secretion, enabling this alarmin to exert immune effects [170]. One study examined the level of alarmins after anti-psoriatic therapy in pediatric patients who underwent the Goeckerman regimen (GR), which consists of applying pharmaceutical crude tar and UVB light exposure. Administering GR resulted in a significant drop in HMGB1, together with a reduction in the PASI score [171].
6.4. S100 Proteins
S100 proteins exert multiple intra- and extracellular functions, such as initiating and maintaining inflammation, cell differentiation, proliferation, apoptosis, and microbial resistance. In psoriatic skin, higher expression of S100A2, S100A7, S100A8, S100A9, S100A12, and S100A8/A9 has been observed [161,172,173,174,175,176,177]. Furthermore, in the sera of psoriatic patients, the levels of S100A7, S100A8/A9, and S100A12 are increased compared with control groups [161,174]. Moreover, S100A7 and S100A12 have been positively correlated with the PASI score in patients with a severe disease course [161,166,174].
Studies suggest that S100 proteins are involved in the processes associated with the impaired skin barrier, as well as inflammatory responses. Laboratory studies investigating its underlying mechanism discovered the involvement of S100 family genes [173]. Specifically, a reduction in hydration stimuli decreased the expression of genes associated with epidermal barrier function and increased that of S100 family genes. Moreover, administering exogenous S100A8 and S100A9 decreased the expression of filaggrin and loricrin, proteins crucial for sustaining the skin barrier [173], indicating important interactions between S100 family members and proteins associated with the skin barrier.
Regarding inflammatory responses, S100 proteins are involved in feedback loops with cytokines involved in the pathogenesis of psoriasis. Firstly, the induced expression of S100A8/S100A9 results in an increase in the transcriptional level of IL-17A, mediating the development of autoreactive CD8+ T cells. In turn, IL-17A, together with IL-17F, IL-1α, and TNF, upregulates genes encoding both S100A8 and S100A9, and this induction is the most pronounced in the early stages of keratinocyte maturation [172]. In another study, S100A9 was positively correlated with the expression of Th17-related mediators, including IL-1-β, IL-6, IL-21, IL-22, IL-27, TNF, IL-12-β, IL-23A, and IL-17A [178]. In turn, IL-22 induces the expression of acute inflammatory proteins, which are associated with the upregulation of S100A9 in psoriatic skin [179]. Furthermore, S100A8 and S100A9 have been found to be associated with the expression of IL-8 and MCP-1 [180]. These pro-inflammatory responses could be associated with increased NF-κB activity [181]. Interestingly, in a study by Christmann et al., the authors showed that S100A8 and S100A9 do not induce keratinocyte differentiation or inflammatory responses. Rather, these proteins act as additional inflammatory mediators during the inflammatory process in the skin, mediated by IL-17, amplifying disease activity in psoriasis suggesting that these proteins may be involved in more complex signaling pathways. Nevertheless, the knockout of S100A9 is associated with the attenuation of psoriasis-like disease in mice, which further confirms its involvement in the pathophysiological mechanisms associated with this skin condition [172].
Apart from keratinocytes, monocytes represent another cell group involved in feedback loops between cytokines and alarmins. IL-6 is upregulated in psoriasis and is crucial to inflammatory crosstalk and pathways, acting synergistically with IL-1 and TNF-α. The effect of IL-6 on the induction of gene expression in monocytes is higher after stimulation with proteins from the S100 family [172]. A study by Berg et al. showed that S100A8 and S100A9 act not only locally but also play an important role in high-risk coronary plaque disease in patients with psoriasis and show a positive linear relationship with lipid-rich necrotic core (LRNC). Calprotectin causes surrounding neutrophils and monocytes to secrete NF-κB pathway products and drive the adhesion and migration of granulocytes through the endothelium. Fortunately, a therapy with biologics is associated with a 79% reduction in S100A7, S100A12, and S100A8/A9, as well as LRNC [176].
The treatment of psoriasis affects the expression of S100 proteins. Firstly, Benezeder et al. carried out a clinical trial aiming to elucidate the mechanism of action of topical dithranol (anthralin). The authors revealed that this caused a rapid decrease in S100A7 and S100A12 [182]. In another study, administering the previously mentioned GR regimen resulted in a significant drop in S100A7 and S100A9 [171]. Kruger et al. observed that treatment with the anti-IL-17a agent secukinumab decreased the expression of S100 proteins, further confirming the influence of IL-17 on the S100 protein family [172,183]. Similar effects have been observed after treatment with risankizumab [184], tacrolimus [185], and etanercept [174]. Additionally, the use of the novel TYK2 inhibitor deucravacitinib is associated with reducing the expression of S100A9 and S100A8 to that of healthy skin [186].
6.5. Cathelicidin
Cathelicidins are a family of small antimicrobial peptides (AMPs). They are an inseparable component of skin immunity, exhibiting a broad spectrum of antimicrobial activity against bacteria, viruses, and fungi. However, their role is not solely limited to defense against pathogens; they can trigger specific defense responses in the host [153]. Cathelicidin, also known as LL-37, comes from its precursor—hCAP18 (human cationic antimicrobial protein 18)—encoded by a single cathelicidin antimicrobial peptide gene (CAMP) in humans. Proteases cleave the hCAP18 into several active AMPs, including a 37-residue-long peptide cathelicidin, hence its abbreviation—LL-37 [153]. In 2014, cathelicidin was the first autoantigen discovered in psoriasis and remains the most widely researched one. At the same time, it was the first AMP in mammalian skin to be described [187].
Studies have proven the increased expression of LL-37 in the epidermis and dermis of psoriatic skin [188]. The expression of IgG autoantibodies against LL-37 autoantigen has been observed in the sera of patients with psoriasis, and this correlates with the PASI score. Also, it reflects disease progression in longitudinally collected serum samples from patients with psoriasis [189]. Autoantibodies against LL-37 are even higher in the sera of patients with psoriasis with psoriatic arthritis than in patients with only skin manifestations [189,190]. The role of LL-37 is not only limited to the permanent stimulation of innate immune cells but is also recognized as an autoantigen, circulating T cells in up to 75% of patients with moderate-to-severe psoriasis [188,190,191]. Whether cathelicidin comes from infiltrating neutrophils is still a subject of discussion; it is also thought to be produced by keratinocytes [192].
The role of LL-37 in the pathogenesis of psoriasis is exerted by activating dermal dendritic cells (dDCs) via TLR7 and TLR9. Upon the injury of keratinocytes, LL-37, self-DNA, and RNA are released [193]. Subsequently, cathelicidin can bind the nucleic acids and form a complex that activates LL-37-specific dDCs [188,194]. That action enables the whole inflammatory cascade, as activated dDCs produce excessive amounts of IFNα that can further activate conventional dendritic cells and other immune cells in the dermis [194,195]. At the same time, dermal dendritic cells release pro-inflammatory cytokines in lymph nodes such as IFNα, IL-6, IL-1β, TNFα, IL-12, and IL-23 [196,197]. Moreover, LL-37 has been shown to induce the proliferation of circulating CD3+ T cells in 24 out of 52 psoriasis patients. The vast majority of LL-37-reactive CD3+ T cells produce IL-17, and the capacity of their IL-17 production is associated with disease severity [198,199].
6.6. Heat Shock Proteins
The small heat shock protein (HSP) family includes molecules involved in cell survival, cytoskeleton dynamics, and cell differentiation. HSPs exhibit housekeeping and homeostatic functions in cellular stress. They are both pro-apoptotic and anti-apoptotic, depending on the conditions, and also play a crucial role in resistance to heat shock and oxidative treatments [200].
The psoriatic epidermises contain abundant levels of HSP27, HSP60, HSP70, and HSP90α [200,201]. Additionally, a higher expression of HSP27 has been observed in psoriatic patients with comorbid metabolic syndrome [202]. Serum analysis of psoriatic patients has revealed significantly greater concentrations of anti-Hsp90α antibodies compared with healthy controls. Moreover, the level of anti-Hsp90α antibodies positively correlates with the PASI score [203]. It has been found that 47% of psoriasis patients demonstrate significantly elevated antibody titers to HSP65. Additionally, a previous study demonstrated a positive correlation between antibody activity toward HSP65 and psoriasis disease activity [200].
Hsp27 is uniformly distributed throughout the healthy epidermis. In psoriatic skin, however, it is abundantly elevated. Owing to the molecular mimicry between human and microbial HSPs, the immune reaction against a microbial one may involve an attack against autologous HSPs, leading to autoimmunity [153]. HSP27 may also be an autoantigen associated with immune responses in streptococcal-induced psoriasis [204]. Moreover, studies have observed increased HSP27 expression in response to stress, explaining the role of a stressful environment in exacerbating psoriatic lesions and discovering antigenic determinants underlying its mechanism [153]. However, the data are inconsistent, as the phosphorylation of HSP27 by p38-MAPK is required for keratinocyte differentiation, and HSP27 inhibits the expression of IL-8 and PGE2 stimulated by TNF-α through NF-κB signaling. In addition, UV treatments upregulate HSP27 mRNA, suggesting the protective role of HSP27 in keratinocytes.
HSP90 is the most abundant chaperone protein in eukaryotic cells, playing an essential role in stress tolerance. It controls cellular processes such as hormonal signaling, cell cycle control, and the activation of many regulatory proteins [203]. HSP90 is prevalent in normal-appearing skin; however, certain isoforms, such as HSP90α, are overexpressed under stressful conditions [205]. As previously mentioned, Th1 and Th17 cytokines are involved in the pathogenesis of psoriasis. Act 1 adaptor protein (Act 1) is a molecule that is essential in IL-17-dependent signaling. HSP90 plays a key role in regulating Act 1, resulting in the facilitation of IL-17A signaling [206].
HSP70 is a family of proteins that are constitutively expressed in the skin. Previous studies have revealed that the HSP70 family can rescue DNA breaks from ROS-induced insults [200]. CD91 is one of the HSP70 receptors and is expressed predominantly by activated antigen-presenting cells (APCs). The accumulation of cells expressing CD91 has been observed in the early stages of psoriatic lesions [207]. However, some studies have revealed lower levels of HSP70 in basal, suprabasal, and superficial epidermal layers of psoriatic lesions when compared with those of normal skin [208]. The role of HSP70 in developing psoriasis seems to confirm the ongoing discussion on the association of the disease with Malassezia furfur. Baroni et al. demonstrated an increased level of HSP70 in Malassezia furfur-positive patients with psoriasis [209].
The role of HSP72 in psoriasis needs more elucidation as, in one study where systemic inflammation was induced by LPS, HSP72 induced by thermal pre-treatment inhibited IL-6 and TNF-α. When compared with healthy skin, both normal and psoriatic skin, when heat-stressed, showed higher levels of HSP72 in basal- and suprabasal-layer cells [200]. Moreover, the immunological role of HSP65 is of great importance [200]. Interestingly, HSP65 is also an antigen of Mycobacteria species, supporting the theory of molecular mimicry and underlying infection-induced psoriasis. Another example of linking immunological reaction to microbial factors and the pathogenesis of psoriasis was presented by Cancino-Diaz et al. The authors suggested the role of S. pyogenes in the pathogenesis of psoriasis as they observed an association between a high response to S. pyogenes HSP60 and the chronic form of psoriasis [210].
As members of the HSP family may be involved in the pathogenesis of psoriasis, several studies have investigated whether they could become therapeutic targets. Seifarth et al. found that the treatment of animal models with alfalfa-derived HSP70 had an anti-psoriatic effect, resulting in significantly lower PASI scores in treated individuals. Moreover, the therapeutic effect was exerted at the gene level, as alfalfa-derived HSP70 downregulated IL-4, IL-5, and IL-17F but it upregulated IL-17A and IL-22 [211]. A study by Tukaj et al. demonstrated that immunization with HSP70 led to clinical and histological improvement in imiquimod-induced, psoriasis-like skin inflammation in mouse models. At the same time, administering anti-HSP70 antibodies resulted in lower disease activity and the reduced presence of Th17 cells [212]. Furthermore, the use of RGRN-305, an HSP90 inhibitor, was associated with the downregulation of the TNF, IL-1β, IL-6, and CXCL8 in keratinocytes and mouse models. Mechanistically, the anti-inflammatory effect was suggested to occur due to the suppression of the NF-κB, ERK1/2, p38, and c-Jun signaling pathways [213]. The expression of HSP90 has also been analyzed after administering the widely used anti-psoriatic drug ustekinumab. A significant downregulation of HSP90 was observed after introducing treatment in [214]. In another study, in which an oral HSP90 inhibitor Debio 0932 was studied, it reduced epidermal thickness in a psoriasis xenograft transplantation model [215].
6.7. Defensins
Defensins are cationic microbial peptides. Like cathelicidin, they are expressed as propeptides [55]. However, in contrast to cathelicidin, which is encoded by a single gene, defensins have multiple genes that form several gene clusters. Defensins are host defense peptides involved in antimicrobial and immune signaling activities. Six human alpha-defensins (human neutrophil peptides 1-6 (HNPs)) and four human beta-defensins (hBDs 1-4) have been identified. They are produced by neutrophils and Paneth cells. Furthermore, mechanical stress increases the production of hBDs [216]. Other studies have shown that specific β-defensins might be endogenous pruritogens, directly stimulating sensory neurons [217].
In psoriatic skin, only HNP1-3, hBD-2, and hBD-3 are recognized [218,219,220]. In a study by Jansen et al., the authors found that hBD-2 serum levels were 400-fold higher in severe psoriasis than in healthy controls [221,222]. Furthermore, the skin of psoriatic patients showed higher hBD-2 expression than that of healthy subjects [223]. Their high expression may account for a lower prevalence of skin infections in psoriatic patients. Importantly, the risk for psoriasis is significantly associated with higher genomic copy numbers of hBD genes [224]. The expression of beta-defensins in psoriasis lesional skin is induced by TNF-α and IFN-gamma. Moreover, TNF-α promotes the secretion of hBD-2 synergistically with IL-17 via the induction of transcription factors (OCT-1, Nf-κB, and AP-1) [225]. Hence, it has been suggested that hBD is a marker of IL-17A-related skin diseases, thus predicting the response to anti-IL-17A treatment [226]. The role of hBD-2 in psoriasis, although not fully understood, can be attributed to the ability to act as a ligand for chemokine receptor 6 (CCR6), thereby inducing Th17.
In a randomized, placebo-controlled trial, a profile of gene expression after treatment with deucravacitinib in patients with moderate-to-severe plaque psoriasis was analyzed. The study revealed a return to non-lesional levels of beta-defensin 4A/B [186], and the impact of treatment was dose-dependent. Also, studies of patients receiving secukinumab or guselkumab have revealed a reduction in the expression of hBD2 in the skin [183,227]. However, not all studies are coherent regarding secukinumab’s effect on the level of this alarmin. A study by Wu et al. showed higher serum levels of hBD2 after treatment with secukinumab than in a normal population [228]. Another study by Cardner et al. analyzed the serum levels of beta-defensin in 2000 patients with psoriatic arthritis in a placebo-controlled phase-III clinical trial of secukinumab. It showed the baseline level of beta-defensin was quantitatively associated with the clinical response to secukinumab, as well as to adalimumab [229]. Also, treatment with risankizumab resulted in a decrease in hBD2 [184]. By contrast, treatment with narrow-band ultraviolet B (nb-UVB), a prevalent form of psoriasis treatment, did not affect the levels of hBD1 and hBD2, suggesting its mechanism of action is independent of influence on defensins [230].
6.8. Thymic Stromal Lymphopoietin
Thymic stromal lymphopoietin (TSLP) is an epithelial-derived cytokine from the IL-2 family resembling IL-7. It is known for promoting type-2 immune responses and being involved in many Th2-oriented diseases. The TSLP receptor (TSLPR) is expressed in the skin, gut, and lungs. After binding to the receptor, JAK1 and JAK2 are recruited and phosphorylated, and downstream STAT5 is activated. Consequently, the expression of pro-inflammatory genes is induced [231]. TSLP has two isoforms—the anti-inflammatory short-form TSLP (sfTSLP) and the pro-inflammatory long-form TSLP (lfTSLP). While sfTLSP is expressed constitutively, the lfTSLP is upregulated only in an inflammatory environment [231].
Several studies have examined the role of TSLP in the context of psoriasis. Firstly, serum levels of TSLP are significantly elevated in patients with psoriasis, and its concentrations are positively correlated with the PASI score. Furthermore, a greater expression of TSLP can be detected in psoriatic keratinocytes [227,232]. However, Suwarsa et al. found no significant difference between TSLP expression in the lesional vs. non-lesional skin of psoriasis vulgaris patients [233]. Also, the level of TSLP was higher in patients with psoriasis with psoriatic arthritis and smokers [232]. In addition, synovial fibroblasts from patients with PsA have shown elevated TSLP expression [234].
TSLP is produced by mutant bulge hair follicle stem cells and DCs, both dermal and myeloid, in patients with psoriasis [235]. Additionally, TSLP is expressed by keratinocytes [236]. This alarmin can stimulate the hyperproliferation of adjacent epidermal cells and induce the expression of VEGF-α, contributing to inflammation and epidermal hyperplasia in psoriatic skin, demonstrating that it can act in an autocrine manner [236]. TSLP is also responsible for the maturation of antigen-presenting cells and plays an essential role in promoting IL-23 production via CD40L.
Therefore, it is speculated that, by blocking TSLP, the DC activation and production of IL-23 can be hindered [231]. The injection of antibodies against TSLP in psoriasis-like mouse models has resulted in the regression of psoriasis, reduced epidermal hyperplasia, decreased VEGF α expression, and the epidermal inhibition of STAT-5 phosphorylation [236]. One of the possible directions of therapeutic strategies is the suppression of TSLP gene activation by ghrelin, which acts through the activation of glucocorticoid receptors [237]. Tashiro et al., studying the effects of hypoxia on TSLP, found an inhibition of the TNF-α -induced expression of TSLP in human and mouse keratinocyte cell lines. However, the expression of TNF-α, IL-6, IL-8, MCP-1, and VEGF-A was not inhibited. Interestingly, the inhibition of TSLP was reversed by introducing an HIF-2α antagonist but not by an HIF-1α inhibitor. Considering the abovementioned findings, HIF-2α may be a new future therapeutic strategy for treating psoriasis [238]. As the use of TNF-α inhibitors is associated with an elevated risk of infections, targeting TSLP may be more suitable for treating psoriasis [239].
6.9. Interleukin-33
Studies have proven that patients with moderate-to-severe psoriasis have increased levels of IL-33 in their sera and greater expression intra-epidermally [240,241]. However, the serum level of IL-33 does not correlate with psoriasis severity [161]. Conflicting results have been published regarding the role of IL-33 in psoriasis. Firstly, in one study, injecting IL-33 into an imiquimod-induced psoriasis model improved the disease. The authors suggested that IL-33 plays an anti-inflammatory and protective role [241]. By contrast, another study found that a mouse model of imiquimod-induced psoriasis that lacked epidermis-specific IL-33 displayed much milder psoriatic lesions than control mice [242].
The role of IL-33 in the pathogenesis of psoriasis is exerted via the stimulation of keratinocytes to produce CXCL1 and CXCL8, responsible for recruiting neutrophils and CCL20, which recruits IL-17-producing T cells to the epidermis [243]. Moreover, IL-33 lets DC mature, which enables the induction of differentiation into Th17 cells, producing IL-17A, strongly involved in the pathogenesis of psoriasis, by stimulating keratinocytes into proliferation and causing epidermal thickening. Under the influence of IL-17A, keratinocytes produce CXCL8 and IL-36G, which induce neutrophil chemotaxis [244]. There are contrasting results on whether TNF-alpha induces the production of IL-33 in human keratinocytes [21]. However, it has been demonstrated that its production can be induced by INF-gamma. That process is mediated via the epidermal growth factor receptor (EGFR), ERK, and p38 pathways [245]. It has also been speculated that the production of IL-33 is dependent on JAK/STAT, as JAK inhibitors diminish IL-33 expression induced by IFN-gamma [20]. Moreover, it has been reported that IL-17 is involved in the induction of IL-33 expression via the EGFR, ERK, p38, and JAK/STAT1 pathways [240]. The literature also provides evidence of IL-4 and IL-13 involvement in stimulating IL-33 expression [246,247]. However, the JAK inhibitor does not affect the IL-4-induced production of IL-33, but ERK inhibitors have confirmed the expected results [247].
It is being debated whether mast cells contribute to the inflammatory process in psoriasis. Several studies concluded that the secretion of IL-1, IL-6, and IL-13 by mast cells is activated by IL-33, highlighting its role in psoriasis [248]. Furthermore, psoriasis is associated with pruritus, lowering the quality of life. However, it is not fully understood which cytokines are responsible for stimulating nerves, as the subcutaneous injection of IL-17A, IL-23, and TNF-α does not induce scratching behavior [20]. However, IL-33 has been found to directly stimulate nerves in psoriasis, confirming that it is involved in the induction of pruritis [20]. Moreover, according to a study on atopic dermatitis, scratching damaged keratinocytes further secreted IL-33 in cutaneous lesions [243]. IL-33 could trigger Th2 cells to produce IL-4, IL-13, and IL-31 [249], which, along with IL-33, decrease filaggrin and loricrin expression in keratinocytes, resulting in an impaired skin barrier [20]. In psoriatic arthritis, IL-33 has been suggested to be involved in the stimulation of osteoclastogenesis, a process of monocyte differentiation toward osteoclasts [250]. The treatment of psoriasis affects the expression of IL-33. Specifically, Meephansan et al. observed that MTX treatment was associated with the reduced expression of IL-33. By contrast, NB-UVB therapy led to an increase in thin alarmin [251].
6.10. Interleukin-1α
IL-1α is constitutively expressed in the epidermis as a proIL-1α, playing a crucial role in skin homeostasis. It can be found in keratinocytes, Langerhans cells, and dermal fibroblasts. However, because the skin is made mostly of keratinocytes, these cells are the main source of that cytokine, making the skin the prime tissue for producing IL-1. Inflammatory stimuli further enhance the expression of IL-1α [18].
The mature form of IL-1 is secreted with the help of inflammasome and caspase-1. When hypoxia, injury, or acidosis leads to cell necrosis, its whole content with both proIL-1 and IL-1α is released. When localized extracellularly, proIL-1α recruits neutrophils and monocytes as an initial step of inflammation. Other factors known to dysregulate IL-1 production in the skin are UV light, HPV infection, and disruptions in the skin barrier [18].
The role of the IL-1 family in the pathogenesis of psoriasis has been widely discussed in the literature. It has been shown that IL-1 is a potent cytokine released by activated keratinocytes and that it can induce inflammation by regulating several genes in the skin [252,253]. IL-1 cytokines can also further stimulate the production of IL-6, TNF, and IL-33 by mast cells [254]. Furthermore, IL-1α acts synergistically with IL-17A, IL-22, OSM, and TNFα in inhibiting the differentiation of keratinocytes, being a fundamental part of the disease’s etiology [255]. Moreover, IL-1 stimulates macrophages into releasing another member of the IL-1 family—IL-36, mediating innate and adaptive immune responses that lead to a chronic pro-inflammatory state. Additionally, IL-1 activates dendritic cells, which, in response, release IL-12 and IL-23, leading, subsequently, to the differentiation of Th1 and Th17 cells, playing an essential role in psoriasis pathogenesis [253].
Conflicting results have been published regarding the expression of IL-1α in patients with psoriasis. Romero et al. found a higher expression of IL-1α in psoriatic skin [256]. Studies have shown that keratinocytes express a receptor for IL-1 on their surfaces, which explains why these cells can respond to this extracellular cytokine. The abovementioned findings led Portugal-Cohen et al. to identify IL-1 as a disease biomarker [257]. Conversely, several studies have found that the expression of IL-1α is lower in lesional psoriatic skin than in non-lesional regions, both in adult and pediatric populations [258,259,260]. In addition, a moderate negative correlation between IL-1α and the PASI score has been observed [259]. Moreover, the level of IL-1α in the sera of psoriatic patients has been reported to be lower [261]. However, according to Mee et al., there is no significant difference in IL-1α expression between lesional and non-lesional biopsies from psoriasis patients, with a small decrease in only some of them [262].
Although more studies are needed to find out why the level of IL-1α is lower in lesional psoriatic skin, a possible explanation for this is the observed simultaneous increase in the IL-1RA-antagonizing inflammatory action of IL-1α. IL-1RA is produced by many cells that the skin is made out of, such as keratinocytes and dermal fibroblasts, as well as by neutrophils and macrophages infiltrating the skin. It is known that IL-1α, along with TNF-α, can stimulate the expression of IL-1R. Given the fact that glucocorticosteroids are effective in treating psoriasis and that they reduce the expression of TNF-α and IL-1, it is possible that glucocorticoids might affect the IL-1R-to-IL-1 ratio through the regulation of these cytokines [263,264,265,266]. Therefore, addressing the restoration of that ratio might be a promising target in strategies for future anti-psoriatic therapies.
New data have also emerged evaluating the level of gut cytokines in patients with psoriasis. IL-1 alpha has been found to be significantly elevated compared with healthy controls. However, a study by Yegorov et al. found no correlation between the gut IL-1 alpha level and the PASI score. This study’s results support the important role of gut dysbiosis and the gut–brain axis in modulating the inflammatory response of the skin’s immune system. Moreover, it may help to find a possible molecular mechanism underlying inflammatory bowel disease, a frequent comorbidity of psoriasis. However, more evidence is required to establish whether the intestinal mucosa is the origin of this inflammation or the consequence of the systemic inflammation present in psoriasis itself [267].
As a major inflammatory player, the level of IL-1α in anti-psoriatic treatments has been the subject of research. Treatment with anti-TNF-α, anti-IL-17A, and anti-IL-12/23 agents contributes to an increase in the average level of lesional IL-1α in the skin [259]. Mechanistically, it has been suggested that preformed IL-1α is stored in the deep layers of the epidermis and is used for restoring impaired barrier function. Moreover, as previously mentioned, IL-1 is constitutively expressed in the skin, especially during the regeneration process, implicating the important role of this alarmin in immune defense mechanisms [268]. In one study, administering MTX in an in vitro psoriasis model decreased IL-1α release by inhibiting transport mediated by solute carrier family 46 (SLC46). The results not only confirmed the effectiveness of treating psoriasis with MTX but also provided the underlying molecular mechanism involving IL-1α [269]. A summary of the role of alarmins in the pathogenesis of psoriasis is presented in Table 3, while the impact of agents used in the treatment of psoriasis on the expression and concentrations of alarmins is depicted in Table 4.

Table 3.
Summary of mechanisms induced by alarmins that take part in the pathogenesis of psoriasis.

Table 4.
Summary of the impact of selected agents used in the treatment of psoriasis on the expression/concentration of alarmins.
7. Conclusions
Alarmins are a broad group of molecules that stimulate inflammatory responses. They can serve as diagnostic biomarkers or could be used to monitor disease activity and treatment response. Furthermore, current evidence suggests that alarmins are involved in the pathogenesis of immune-mediated diseases, such as RA, OA, and psoriasis, and thus are potential therapeutic targets. However, the role of some of these proteins remains inconclusive. For instance, IL-33 has been found to induce both pro- and anti-inflammatory responses in the context of OA. Further studies are required to investigate pathways induced by alarmins and their involvement in inflammatory disorders.
Author Contributions
Conceptualization, A.P.; writing—original draft preparation, K.K., W.S., E.B., M.R., and A.P.; writing—review and editing, K.K., W.S., E.B., M.R., and A.P.; supervision, A.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Yang, D.; Han, Z.; Oppenheim, J.J. Alarmins and immunity. Immunol. Rev. 2017, 280, 41–56. [Google Scholar] [CrossRef]
- Oppenheim, J.J.; Yang, D. Alarmins: Chemotactic activators of immune responses. Curr. Opin. Immunol. 2005, 17, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Chaly, Y.V.; Paleolog, E.M.; Kolesnikova, T.S.; Tikhonov, I.I.; Petratchenko, E.V.; Voitenok, N.N. Neutrophil alpha-defensin human neutrophil peptide modulates cytokine production in human monocytes and adhesion molecule expression in endothelial cells. Eur. Cytokine Netw. 2000, 11, 257–266. [Google Scholar]
- Andersson, U.; Wang, H.; Palmblad, K.; Aveberger, A.C.; Bloom, O.; Erlandsson-Harris, H.; Janson, A.; Kokkola, R.; Zhang, M.; Yang, H.; et al. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J. Exp. Med. 2000, 192, 565–570. [Google Scholar] [CrossRef]
- Scaffidi, P.; Misteli, T.; Bianchi, M.E. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 2002, 418, 191–195. [Google Scholar] [CrossRef]
- De Almeida, D.E.; Ling, S.; Pi, X.; Hartmann-Scruggs, A.M.; Pumpens, P.; Holoshitz, J. Immune dysregulation by the rheumatoid arthritis shared epitope. J. Immunol. 2010, 185, 1927–1934. [Google Scholar] [CrossRef] [PubMed]
- Barik, R.R.; Bhatt, L.K. Emerging epigenetic targets in rheumatoid arthritis. Rheumatol. Int. 2021, 41, 2047–2067. [Google Scholar] [CrossRef]
- Klein, K.; Gay, S. Epigenetics in rheumatoid arthritis. Curr. Opin. Rheumatol. 2015, 27, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Kang, R.; Tang, D. The mechanism of HMGB1 secretion and release. Exp. Mol. Med. 2022, 54, 91–102. [Google Scholar] [CrossRef]
- Yang, H.; Wang, H.; Andersson, U. Targeting Inflammation Driven by HMGB1. Front. Immunol. 2020, 11, 484. [Google Scholar] [CrossRef]
- Kawasaki, H.; Nakayama, S.; Kretsinger, R.H. Classification and evolution of EF-hand proteins. Biometals 1998, 11, 277–295. [Google Scholar] [CrossRef] [PubMed]
- Marenholz, I.; Heizmann, C.W.; Fritz, G. S100 proteins in mouse and man: From evolution to function and pathology (including an update of the nomenclature). Biochem. Biophys. Res. Commun. 2004, 322, 1111–1122. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Ali, S.A. Multifunctional Role of S100 Protein Family in the Immune System: An Update. Cells 2022, 11, 2274. [Google Scholar] [CrossRef] [PubMed]
- Gilston, B.A.; Skaar, E.P.; Chazin, W.J. Binding of transition metals to S100 proteins. Sci. China Life Sci. 2016, 59, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, L.C.; Donor, M.T.; Prell, J.S.; Harms, M.J. Multiple Evolutionary Origins of Ubiquitous Cu2+ and Zn2+ Binding in the S100 Protein Family. PLoS ONE 2016, 11, e0164740. [Google Scholar] [CrossRef]
- Fritz, G.; Botelho, H.M.; Morozova-Roche, L.A.; Gomes, C.M. Natural and amyloid self-assembly of S100 proteins: Structural basis of functional diversity. FEBS J. 2010, 277, 4578–4590. [Google Scholar] [CrossRef] [PubMed]
- Donato, R.; Cannon, B.R.; Sorci, G.; Riuzzi, F.; Hsu, K.; Weber, D.J.; Geczy, C.L. Functions of S100 proteins. Curr. Mol. Med. 2013, 13, 24–57. [Google Scholar] [CrossRef] [PubMed]
- Bou-Dargham, M.J.; Khamis, Z.I.; Cognetta, A.B.; Sang, Q.A. The Role of Interleukin-1 in Inflammatory and Malignant Human Skin Diseases and the Rationale for Targeting Interleukin-1 Alpha. Med. Res. Rev. 2017, 37, 180–216. [Google Scholar] [CrossRef] [PubMed]
- Perez, F.; Ruera, C.N.; Miculan, E.; Carasi, P.; Dubois-Camacho, K.; Garbi, L.; Guzman, L.; Hermoso, M.A.; Chirdo, F.G. IL-33 Alarmin and Its Active Proinflammatory Fragments Are Released in Small Intestine in Celiac Disease. Front. Immunol. 2020, 11, 581445. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, G.; Yamamura, K.; Kawamura, K.; Kido-Nakahara, M.; Ito, T.; Nakahara, T. Regulatory Mechanism of the IL-33-IL-37 Axis via Aryl Hydrocarbon Receptor in Atopic Dermatitis and Psoriasis. Int. J. Mol. Sci. 2023, 24, 14633. [Google Scholar] [CrossRef]
- Borgia, F.; Custurone, P.; Peterle, L.; Pioggia, G.; Gangemi, S. Role of Epithelium-Derived Cytokines in Atopic Dermatitis and Psoriasis: Evidence and Therapeutic Perspectives. Biomolecules 2021, 11, 1843. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Ozaki, K.; Baumann, H.; Levin, S.D.; Puel, A.; Farr, A.G.; Ziegler, S.F.; Leonard, W.J.; Lodish, H.F. Cloning of a receptor subunit required for signaling by thymic stromal lymphopoietin. Nat. Immunol. 2000, 1, 59–64. [Google Scholar] [CrossRef]
- He, R.; Geha, R.S. Thymic stromal lymphopoietin. Ann. N. Y. Acad. Sci. 2010, 1183, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Smolen, J.S.; Aletaha, D.; McInnes, I.B. Rheumatoid arthritis. Lancet 2016, 388, 2023–2038. [Google Scholar] [CrossRef] [PubMed]
- Safiri, S.; Kolahi, A.A.; Hoy, D.; Smith, E.; Bettampadi, D.; Mansournia, M.A.; Almasi-Hashiani, A.; Ashrafi-Asgarabad, A.; Moradi-Lakeh, M.; Qorbani, M.; et al. Global, regional and national burden of rheumatoid arthritis 1990–2017: A systematic analysis of the Global Burden of Disease study 2017. Ann. Rheum. Dis. 2019, 78, 1463–1471. [Google Scholar] [CrossRef] [PubMed]
- Deane, K.D.; Demoruelle, M.K.; Kelmenson, L.B.; Kuhn, K.A.; Norris, J.M.; Holers, V.M. Genetic and environmental risk factors for rheumatoid arthritis. Best Pract. Res. Clin. Rheumatol. 2017, 31, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, R.S.; Bruchfeld, A.; Yang, L.; Qureshi, A.R.; Gallowitsch-Puerta, M.; Patel, N.B.; Huston, B.J.; Chavan, S.; Rosas-Ballina, M.; Gregersen, P.K.; et al. Cholinergic anti-inflammatory pathway activity and High Mobility Group Box-1 (HMGB1) serum levels in patients with rheumatoid arthritis. Mol. Med. 2007, 13, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Sandoghchian Shotorbani, S.; Su, Z.; Liu, Y.; Tong, J.; Zheng, D.; Chen, J.; Xu, Y.; Jiao, Z.; Wang, S.; et al. Enhanced HMGB1 expression may contribute to Th17 cells activation in rheumatoid arthritis. Clin. Dev. Immunol. 2012, 2012, 295081. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zeng, T.; Zhang, X.; Tian, Y.; Wu, Y.; Yu, J.; Pei, Z.; Liu, Y.; Hu, T.; Tan, L. Clinical diagnostic significance of 14-3-3η protein, high-mobility group box-1, anti-cyclic citrullinated peptide antibodies, anti-mutated citrullinated vimentin antibodies and rheumatoid factor in rheumatoid arthritis. Br. J. Biomed. Sci. 2020, 77, 19–23. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, Y.; Jiang, S.; Fan, Y.; Huang, J.; Xiao, B.; Rao, H.; Huang, L. Effects of metformin therapy on HMGB1 levels in rheumatoid arthritis patients. Eur. J. Med. Res. 2023, 28, 512. [Google Scholar] [CrossRef]
- Taniguchi, N.; Kawahara, K.; Yone, K.; Hashiguchi, T.; Yamakuchi, M.; Goto, M.; Inoue, K.; Yamada, S.; Ijiri, K.; Matsunaga, S.; et al. High mobility group box chromosomal protein 1 plays a role in the pathogenesis of rheumatoid arthritis as a novel cytokine. Arthritis Rheumatol. 2003, 48, 971–981. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.B.; Xu, P.; Xu, K.; Cai, Y.S.; Sun, M.Y.; Yang, L.; Sun, J.; Lu, S.M. Methotrexate affects HMGB1 expression in rheumatoid arthritis, and the downregulation of HMGB1 prevents rheumatoid arthritis progression. Mol. Cell. Biochem. 2016, 420, 161–170. [Google Scholar] [CrossRef] [PubMed]
- He, Z.W.; Qin, Y.H.; Wang, Z.W.; Chen, Y.; Shen, Q.; Dai, S.M. HMGB1 acts in synergy with lipopolysaccharide in activating rheumatoid synovial fibroblasts via p38 MAPK and NF-κB signaling pathways. Mediat. Inflamm. 2013, 2013, 596716. [Google Scholar] [CrossRef] [PubMed]
- Elshabrawy, H.A.; Chen, Z.; Volin, M.V.; Ravella, S.; Virupannavar, S.; Shahrara, S. The pathogenic role of angiogenesis in rheumatoid arthritis. Angiogenesis 2015, 18, 433–448. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.F.; Liu, Z.L.; Pan, C.; Yang, X.Q.; Ning, S.L.; Liu, H.D.; Guo, S.; Yu, J.M.; Zhang, Z.L. HMGB1 correlates with angiogenesis and poor prognosis of perihilar cholangiocarcinoma via elevating VEGFR2 of vessel endothelium. Oncogene 2019, 38, 868–880. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.; Luo, H.; Wu, R.; Wang, J.; Zhou, B.; Zhang, Y.; Jiang, Y.; Xu, J. Internalization of HMGB1 (High Mobility Group Box 1) Promotes Angiogenesis in Endothelial Cells. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 2922–2940. [Google Scholar] [CrossRef]
- Biscetti, F.; Flex, A.; Pecorini, G.; Angelini, F.; Arena, V.; Stigliano, E.; Gremese, E.; Tolusso, B.; Ferraccioli, G. The role of high-mobility group box protein 1 in collagen antibody-induced arthritis is dependent on vascular endothelial growth factor. Clin. Exp. Immunol. 2016, 184, 62–72. [Google Scholar] [CrossRef]
- Park, S.Y.; Lee, S.W.; Kim, H.Y.; Lee, W.S.; Hong, K.W.; Kim, C.D. HMGB1 induces angiogenesis in rheumatoid arthritis via HIF-1α activation. Eur. J. Immunol. 2015, 45, 1216–1227. [Google Scholar] [CrossRef]
- Siouti, E.; Andreakos, E. The many facets of macrophages in rheumatoid arthritis. Biochem. Pharmacol. 2019, 165, 152–169. [Google Scholar] [CrossRef]
- Peng, L.; Zhu, N.; Mao, J.; Huang, L.; Yang, Y.; Zhou, Z.; Wang, L.; Wu, B. Expression levels of CXCR4 and CXCL12 in patients with rheumatoid arthritis and its correlation with disease activity. Exp. Ther. Med. 2020, 20, 1925–1934. [Google Scholar] [CrossRef]
- Cecchinato, V.; D’Agostino, G.; Raeli, L.; Nerviani, A.; Schiraldi, M.; Danelon, G.; Manzo, A.; Thelen, M.; Ciurea, A.; Bianchi, M.E.; et al. Redox-Mediated Mechanisms Fuel Monocyte Responses to CXCL12/HMGB1 in Active Rheumatoid Arthritis. Front. Immunol. 2018, 9, 2118. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.Y.; Li, K.T.; Yang, H.X.; Yang, B.; Lu, X.; Zhao, L.D.; Fei, Y.Y.; Chen, H.; Wang, L.; Li, J.; et al. Complement C1q synergizes with PTX3 in promoting NLRP3 inflammasome over-activation and pyroptosis in rheumatoid arthritis. J. Autoimmun. 2020, 106, 102336. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Zhao, F.; Cao, Y.; Ping, F.; Wang, W.; Li, Y. HMGB1 mediates lipopolysaccharide-induced macrophage autophagy and pyroptosis. BMC Mol. Cell Biol. 2023, 24, 2. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Zhang, Q.; Tan, J.; Xiong, Y.; Liang, Y.; Yan, J.; Gu, F.; Xu, Y. HMGB1 induces macrophage pyroptosis in chronic endometritis. Int. Immunopharmacol. 2023, 123, 110706. [Google Scholar] [CrossRef]
- Salo, H.; Qu, H.; Mitsiou, D.; Aucott, H.; Han, J.; Zhang, X.M.; Aulin, C.; Erlandsson Harris, H. Disulfide and Fully Reduced HMGB1 Induce Different Macrophage Polarization and Migration Patterns. Biomolecules 2021, 11, 800. [Google Scholar] [CrossRef]
- Zhu, C.; Chen, T.; Liu, B. Inhibitory effects of miR-25 targeting HMGB1 on macrophage secretion of inflammatory cytokines in sepsis. Oncol. Lett. 2018, 16, 5027–5033. [Google Scholar] [CrossRef] [PubMed]
- Paradowska-Gorycka, A.; Wajda, A.; Romanowska-Próchnicka, K.; Walczuk, E.; Kuca-Warnawin, E.; Kmiolek, T.; Stypinska, B.; Rzeszotarska, E.; Majewski, D.; Jagodzinski, P.P.; et al. Th17/Treg-Related Transcriptional Factor Expression and Cytokine Profile in Patients with Rheumatoid Arthritis. Front. Immunol. 2020, 11, 572858. [Google Scholar] [CrossRef] [PubMed]
- Roeleveld, D.M.; Koenders, M.I. The role of the Th17 cytokines IL-17 and IL-22 in Rheumatoid Arthritis pathogenesis and developments in cytokine immunotherapy. Cytokine 2015, 74, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Sun, C.; Zhou, C.; Liu, Y.; Zhu, H.; Sandoghchian, S.; Zheng, D.; Peng, T.; Zhang, Y.; Jiao, Z.; et al. HMGB1 blockade attenuates experimental autoimmune myocarditis and suppresses Th17-cell expansion. Eur. J. Immunol. 2011, 41, 3586–3595. [Google Scholar] [CrossRef]
- Xu, K.; Ren, X.; Ju, B.; Aihaiti, Y.; Cai, Y.; Zhang, Y.; He, L.; Wang, J. Clinical markers combined with HMGB1 polymorphisms to predict efficacy of conventional DMARDs in rheumatoid arthritis patients. Clin. Immunol. 2020, 221, 108592. [Google Scholar] [CrossRef]
- Schierbeck, H.; Wähämaa, H.; Andersson, U.; Harris, H.E. Immunomodulatory drugs regulate HMGB1 release from activated human monocytes. Mol. Med. 2010, 16, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Kuroiwa, Y.; Takakusagi, Y.; Kusayanagi, T.; Kuramochi, K.; Imai, T.; Hirayama, T.; Ito, I.; Yoshida, M.; Sakaguchi, K.; Sugawara, F. Identification and characterization of the direct interaction between methotrexate (MTX) and high-mobility group box 1 (HMGB1) protein. PLoS ONE 2013, 8, e63073. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Cai, Y.S.; Lu, S.M.; Li, X.L.; Liu, L.; Li, Z.; Liu, H.; Xu, P. Autophagy induction contributes to the resistance to methotrexate treatment in rheumatoid arthritis fibroblast-like synovial cells through high mobility group box chromosomal protein 1. Arthritis Res. Ther. 2015, 17, 374. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Leng, M.; Ding, C.; Zhu, X.; Zhang, Y.; Sun, C.; Lou, P. Ferritinophagy-mediated ferroptosis facilitates methotrexate-induced hepatotoxicity by high-mobility group box 1 (HMGB1). Liver Int. 2024, 44, 691–705. [Google Scholar] [CrossRef] [PubMed]
- Sundberg, E.; Grundtman, C.; Af Klint, E.; Lindberg, J.; Ernestam, S.; Ulfgren, A.K.; Harris, H.E.; Andersson, U. Systemic TNF blockade does not modulate synovial expression of the pro-inflammatory mediator HMGB1 in rheumatoid arthritis patients--a prospective clinical study. Arthritis Res. Ther. 2008, 10, R33. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, T.; Sakata, N.; Narumi, Y.; Kimura, T.; Hayashi, T.; Nagano, K.; Liu, K.; Nishibori, M.; Tsukita, S.; Yamada, T.; et al. Metformin directly binds the alarmin HMGB1 and inhibits its proinflammatory activity. J. Biol. Chem. 2017, 292, 8436–8446. [Google Scholar] [CrossRef] [PubMed]
- Jarlborg, M.; Courvoisier, D.S.; Lamacchia, C.; Martinez Prat, L.; Mahler, M.; Bentow, C.; Finckh, A.; Gabay, C.; Nissen, M.J. Serum calprotectin: A promising biomarker in rheumatoid arthritis and axial spondyloarthritis. Arthritis Res. Ther. 2020, 22, 105. [Google Scholar] [CrossRef]
- Mansour, H.E.; Abdullrhman, M.A.; Mobasher, S.A.; El Mallah, R.; Abaza, N.; Hamed, F.; Khalil, A.A.F. Serum Calprotectin in Rheumatoid Arthritis: A Promising Diagnostic Marker, How Far Is It Related to Activity and Sonographic Findings? J. Med. Ultrasound. 2017, 25, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liang, Y. Clinical significance of serum calprotectin level for the disease activity in active rheumatoid arthritis with normal C-reactive protein. Int. J. Clin. Exp. Pathol. 2019, 12, 1009–1014. [Google Scholar] [PubMed]
- Bettner, L.F.; Peterson, R.A.; Bergstedt, D.T.; Kelmenson, L.B.; Demoruelle, M.K.; Mikuls, T.R.; Edison, J.D.; Parish, M.C.; Feser, M.L.; Frazer-Abel, A.A.; et al. Combinations of Anticyclic Citrullinated Protein Antibody, Rheumatoid Factor, and Serum Calprotectin Positivity Are Associated with the Diagnosis of Rheumatoid Arthritis within 3 Years. ACR Open Rheumatol. 2021, 3, 684–689. [Google Scholar] [CrossRef]
- Bae, S.C.; Lee, Y.H. Calprotectin levels in rheumatoid arthritis and their correlation with disease activity: A meta-analysis. Postgrad. Med. 2017, 129, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Roszkowski, L.; Jaszczyk, B.; Plebańczyk, M.; Ciechomska, M. S100A8 and S100A12 Proteins as Biomarkers of High Disease Activity in Patients with Rheumatoid Arthritis That Can Be Regulated by Epigenetic Drugs. Int. J. Mol. Sci. 2022, 24, 710. [Google Scholar] [CrossRef] [PubMed]
- Hurnakova, J.; Hulejova, H.; Zavada, J.; Komarc, M.; Cerezo, L.A.; Mann, H.; Vencovsky, J.; Pavelka, K.; Senolt, L. Serum calprotectin may reflect inflammatory activity in patients with active rheumatoid arthritis despite normal to low C-reactive protein. Clin. Rheumatol. 2018, 37, 2055–2062. [Google Scholar] [CrossRef]
- Sejersen, K.; Weitoft, T.; Knight, A.; Lysholm, J.; Larsson, A.; Rönnelid, J. Serum calprotectin correlates more strongly with inflammation and disease activity in ACPA positive than ACPA negative rheumatoid arthritis. Rheumatology, 2023; ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Gernert, M.; Schmalzing, M.; Tony, H.P.; Strunz, P.P.; Schwaneck, E.C.; Fröhlich, M. Calprotectin (S100A8/S100A9) detects inflammatory activity in rheumatoid arthritis patients receiving tocilizumab therapy. Arthritis Res. Ther. 2022, 24, 200. [Google Scholar] [CrossRef] [PubMed]
- Inciarte-Mundo, J.; Ramirez, J.; Hernández, M.V.; Ruiz-Esquide, V.; Cuervo, A.; Cabrera-Villalba, S.R.; Pascal, M.; Yagüe, J.; Cañete, J.D.; Sanmarti, R. Calprotectin and TNF trough serum levels identify power Doppler ultrasound synovitis in rheumatoid arthritis and psoriatic arthritis patients in remission or with low disease activity. Arthritis Res. Ther. 2016, 18, 160. [Google Scholar] [CrossRef] [PubMed]
- Hurnakova, J.; Zavada, J.; Hanova, P.; Hulejova, H.; Klein, M.; Mann, H.; Sleglova, O.; Olejarova, M.; Forejtova, S.; Ruzickova, O.; et al. Serum calprotectin (S100A8/9): An independent predictor of ultrasound synovitis in patients with rheumatoid arthritis. Arthritis Res. Ther. 2015, 17, 252. [Google Scholar] [CrossRef] [PubMed]
- Andrés Cerezo, L.; Mann, H.; Pecha, O.; Pleštilová, L.; Pavelka, K.; Vencovský, J.; Senolt, L. Decreases in serum levels of S100A8/9 (calprotectin) correlate with improvements in total swollen joint count in patients with recent-onset rheumatoid arthritis. Arthritis Res. Ther. 2011, 13, R122. [Google Scholar] [CrossRef] [PubMed]
- de Moel, E.C.; Rech, J.; Mahler, M.; Roth, J.; Vogl, T.; Schouffoer, A.; Goekoop, R.J.; Huizinga, T.W.J.; Allaart, C.F.; Toes, R.E.M.; et al. Circulating calprotectin (S100A8/A9) is higher in rheumatoid arthritis patients that relapse within 12 months of tapering anti-rheumatic drugs. Arthritis Res. Ther. 2019, 21, 268. [Google Scholar] [CrossRef]
- Romand, X.; Clapasson, M.; Chuong, M.V.; Paclet, M.H.; Fautrel, B.; Baillet, A. Serum calprotectin levels do not predict subsequent relapse in rheumatoid arthritis in remission: A post-hoc analysis of STRASS study. RMD Open 2023, 9, e003198. [Google Scholar] [CrossRef]
- Chen, W.; Wang, Q.; Ke, Y.; Lin, J. Neutrophil Function in an Inflammatory Milieu of Rheumatoid Arthritis. J. Immunol. Res. 2018, 2018, 8549329. [Google Scholar] [CrossRef] [PubMed]
- Sprenkeler, E.G.G.; Zandstra, J.; van Kleef, N.D.; Goetschalckx, I.; Verstegen, B.; Aarts, C.E.M.; Janssen, H.; Tool, A.T.J.; van Mierlo, G.; van Bruggen, R.; et al. S100A8/A9 Is a Marker for the Release of Neutrophil Extracellular Traps and Induces Neutrophil Activation. Cells 2022, 11, 236. [Google Scholar] [CrossRef] [PubMed]
- Schenten, V.; Plançon, S.; Jung, N.; Hann, J.; Bueb, J.L.; Bréchard, S.; Tschirhart, E.J.; Tolle, F. Secretion of the Phosphorylated Form of S100A9 from Neutrophils Is Essential for the Proinflammatory Functions of Extracellular S100A8/A9. Front. Immunol. 2018, 9, 447. [Google Scholar] [CrossRef] [PubMed]
- Jung, N.; Schenten, V.; Bueb, J.L.; Tolle, F.; Bréchard, S. miRNAs Regulate Cytokine Secretion Induced by Phosphorylated S100A8/A9 in Neutrophils. Int. J. Mol. Sci. 2019, 20, 5699. [Google Scholar] [CrossRef] [PubMed]
- Sunahori, K.; Yamamura, M.; Yamana, J.; Takasugi, K.; Kawashima, M.; Yamamoto, H.; Chazin, W.J.; Nakatani, Y.; Yui, S.; Makino, H. The S100A8/A9 heterodimer amplifies proinflammatory cytokine production by macrophages via activation of nuclear factor kappa B and p38 mitogen-activated protein kinase in rheumatoid arthritis. Arthritis Res. Ther. 2006, 8, R69. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.V.; Deng, M.; Ting, J.P. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Kong, X.; You, Y.; Xiang, L.; Zhang, Y.; Wu, R.; Zhou, L.; Duan, L. S100A8-Mediated NLRP3 Inflammasome-Dependent Pyroptosis in Macrophages Facilitates Liver Fibrosis Progression. Cells 2022, 11, 3579. [Google Scholar] [CrossRef] [PubMed]
- Carrión, M.; Juarranz, Y.; Martínez, C.; González-Álvaro, I.; Pablos, J.L.; Gutiérrez-Cañas, I.; Gomariz, R.P. IL-22/IL-22R1 axis and S100A8/A9 alarmins in human osteoarthritic and rheumatoid arthritis synovial fibroblasts. Rheumatology 2013, 52, 2177–2186. [Google Scholar] [CrossRef] [PubMed]
- Zreiqat, H.; Howlett, C.R.; Gronthos, S.; Hume, D.; Geczy, C.L. S100A8/S100A9 and their association with cartilage and bone. J. Mol. Histol. 2007, 38, 381–391. [Google Scholar] [CrossRef]
- Grevers, L.C.; de Vries, T.J.; Vogl, T.; Abdollahi-Roodsaz, S.; Sloetjes, A.W.; Leenen, P.J.; Roth, J.; Everts, V.; van den Berg, W.B.; van Lent, P.L. S100A8 enhances osteoclastic bone resorption in vitro through activation of Toll-like receptor 4: Implications for bone destruction in murine antigen-induced arthritis. Arthritis Rheum. 2011, 63, 1365–1375. [Google Scholar] [CrossRef]
- Di Ceglie, I.; Blom, A.B.; Davar, R.; Logie, C.; Martens, J.H.A.; Habibi, E.; Böttcher, L.M.; Roth, J.; Vogl, T.; Goodyear, C.S.; et al. The alarmin S100A9 hampers osteoclast differentiation from human circulating precursors by reducing the expression of RANK. FASEB J. 2019, 33, 10104–10115. [Google Scholar] [CrossRef]
- Cesaro, A.; Anceriz, N.; Plante, A.; Pagé, N.; Tardif, M.R.; Tessier, P.A. An inflammation loop orchestrated by S100A9 and calprotectin is critical for development of arthritis. PLoS ONE 2012, 7, e45478. [Google Scholar] [CrossRef]
- Yasuda, K.; Shimodan, S.; Maehara, N.; Hirota, A.; Iijima, R.; Nishijima, A.; Mori, H.; Toyama, R.; Ito, A.; Yoshikawa, Y.; et al. AIM/CD5L ameliorates autoimmune arthritis by promoting removal of inflammatory DAMPs at the lesions. J. Autoimmun. 2023, 142, 103149. [Google Scholar] [CrossRef] [PubMed]
- Obry, A.; Lequerré, T.; Hardouin, J.; Boyer, O.; Fardellone, P.; Philippe, P.; Le Loët, X.; Cosette, P.; Vittecoq, O. Identification of S100A9 as biomarker of responsiveness to the methotrexate/etanercept combination in rheumatoid arthritis using a proteomic approach. PLoS ONE 2014, 9, e115800. [Google Scholar] [CrossRef]
- Tweehuysen, L.; den Broeder, N.; van Herwaarden, N.; Joosten, L.A.B.; van Lent, P.L.; Vogl, T.; van den Hoogen, F.H.J.; Thurlings, R.M.; den Broeder, A.A. Predictive value of serum calprotectin (S100A8/A9) for clinical response after starting or tapering anti-TNF treatment in patients with rheumatoid arthritis. RMD Open 2018, 4, e000654. [Google Scholar] [CrossRef]
- Andrés Cerezo, L.; Šumová, B.; Prajzlerová, K.; Veigl, D.; Damgaard, D.; Nielsen, C.H.; Pavelka, K.; Vencovský, J.; Šenolt, L. Calgizzarin (S100A11): A novel inflammatory mediator associated with disease activity of rheumatoid arthritis. Arthritis Res. Ther. 2017, 19, 79. [Google Scholar] [CrossRef]
- Navrátilová, A.; Bečvář, V.; Baloun, J.; Damgaard, D.; Nielsen, C.H.; Veigl, D.; Pavelka, K.; Vencovský, J.; Šenolt, L.; Andrés Cerezo, L. S100A11 (calgizzarin) is released via NETosis in rheumatoid arthritis (RA) and stimulates IL-6 and TNF secretion by neutrophils. Sci. Rep. 2021, 11, 6063. [Google Scholar] [CrossRef] [PubMed]
- Foell, D.; Kane, D.; Bresnihan, B.; Vogl, T.; Nacken, W.; Sorg, C.; Fitzgerald, O.; Roth, J. Expression of the pro-inflammatory protein S100A12 (EN-RAGE) in rheumatoid and psoriatic arthritis. Rheumatology 2003, 42, 1383–1389. [Google Scholar] [CrossRef]
- Rouleau, P.; Vandal, K.; Ryckman, C.; Poubelle, P.E.; Boivin, A.; Talbot, M.; Tessier, P.A. The calcium-binding protein S100A12 induces neutrophil adhesion, migration, and release from bone marrow in mouse at concentrations similar to those found in human inflammatory arthritis. Clin. Immunol. 2003, 107, 46–54. [Google Scholar] [CrossRef]
- Nordal, H.H.; Brun, J.G.; Halse, A.K.; Jonsson, R.; Fagerhol, M.K.; Hammer, H.B. The neutrophil protein S100A12 is associated with a comprehensive ultrasonographic synovitis score in a longitudinal study of patients with rheumatoid arthritis treated with adalimumab. BMC Musculoskelet. Disord. 2014, 15, 335. [Google Scholar] [CrossRef]
- Nishida, M.; Saegusa, J.; Tanaka, S.; Morinobu, A. S100A12 facilitates osteoclast differentiation from human monocytes. PLoS ONE 2018, 13, e0204140. [Google Scholar] [CrossRef] [PubMed]
- Liew, F.Y.; Girard, J.P.; Turnquist, H.R. Interleukin-33 in health and disease. Nat. Rev. Immunol. 2016, 16, 676–689. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, R.; Lee, R.T. The IL-33/ST2 pathway: Therapeutic target and novel biomarker. Nat. Rev. Drug Discov. 2008, 7, 827–840. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.S.; Moon, S.J.; Joo, Y.B.; Jeon, C.H.; Cho, M.L.; Ju, J.H.; Oh, H.J.; Heo, Y.J.; Park, S.H.; Kim, H.Y.; et al. Measurement of interleukin-33 (IL-33) and IL-33 receptors (sST2 and ST2L) in patients with rheumatoid arthritis. J. Korean Med. Sci. 2011, 26, 1132–1139. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Huang, H.; Hu, F.; Zhou, W.; Guo, J.; Jiang, H.; Mu, R.; Li, Z. Increased IL-33 in synovial fluid and paired serum is associated with disease activity and autoantibodies in rheumatoid arthritis. Clin. Dev. Immunol. 2013, 2013, 985301. [Google Scholar] [CrossRef] [PubMed]
- Talabot-Ayer, D.; McKee, T.; Gindre, P.; Bas, S.; Baeten, D.L.; Gabay, C.; Palmer, G. Distinct serum and synovial fluid interleukin (IL)-33 levels in rheumatoid arthritis, psoriatic arthritis and osteoarthritis. Joint Bone Spine 2012, 79, 32–37. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Poole, J.A.; England, B.R.; Sayles, H.; Johnson, T.M.; Duryee, M.J.; Hunter, C.D.; Baker, J.F.; Kerr, G.S.; Kunkel, G.; Cannon, G.W.; et al. Serum alarmins and the risk of incident interstitial lung disease in rheumatoid arthritis. Rheumatology, 2023; ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Iwaszko, M.; Wielińska, J.; Świerkot, J.; Kolossa, K.; Sokolik, R.; Bugaj, B.; Chaszczewska-Markowska, M.; Jeka, S.; Bogunia-Kubik, K. Gene Polymorphisms as Potential Biomarkers of Disease Susceptibility and Response to TNF Inhibitors in Rheumatoid Arthritis, Ankylosing Spondylitis, and Psoriatic Arthritis Patients. Front. Immunol. 2021, 12, 631603. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Jiang, H.R.; Li, Y.; Pushparaj, P.N.; Kurowska-Stolarska, M.; Leung, B.P.; Mu, R.; Tay, H.K.; McKenzie, A.N.; McInnes, I.B.; et al. IL-33 exacerbates autoantibody-induced arthritis. J. Immunol. 2010, 184, 2620–2626. [Google Scholar] [CrossRef]
- Wu, J.; Li, Q.; Deng, J.; Zhao, J.J.; Yu, Q.H. Association between IL-33 and other inflammatory factors in patients with rheumatoid arthritis and in fibroblast-like synoviocytes. Exp. Ther. Med. 2021, 21, 161. [Google Scholar] [CrossRef]
- Lee, E.J.; So, M.W.; Hong, S.; Kim, Y.G.; Yoo, B.; Lee, C.K. Interleukin-33 acts as a transcriptional repressor and extracellular cytokine in fibroblast-like synoviocytes in patients with rheumatoid arthritis. Cytokine 2016, 77, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Fu, W.; Zhao, S.; Jiao, T.; Wu, D.; Wang, Y. miR-483-3p Promotes IL-33 Production from Fibroblast-Like Synoviocytes by Regulating ERK Signaling in Rheumatoid Arthritis. Inflammation 2021, 44, 2302–2308. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Chen, B.; Wen, Z.; Huang, Z.; Ye, L. IL-33/ST2-mediated inflammation in macrophages is directly abrogated by IL-10 during rheumatoid arthritis. Oncotarget 2017, 8, 32407–32418. [Google Scholar] [CrossRef] [PubMed]
- Onodera, S.; Tanji, H.; Suzuki, K.; Kaneda, K.; Mizue, Y.; Sagawa, A.; Nishihira, J. High expression of macrophage migration inhibitory factor in the synovial tissues of rheumatoid joints. Cytokine 1999, 11, 163–167. [Google Scholar] [CrossRef] [PubMed]
- García-Arellano, S.; Hernández-Palma, L.A.; Cerpa-Cruz, S.; Sánchez-Zuno, G.A.; Herrera-Godina, M.G.; Muñoz-Valle, J.F. The Novel Role of MIF in the Secretion of IL-25, IL-31, and IL-33 from PBMC of Patients with Rheumatoid Arthritis. Molecules 2021, 26, 4968. [Google Scholar] [CrossRef] [PubMed]
- Lutzky, V.; Hannawi, S.; Thomas, R. Cells of the synovium in rheumatoid arthritis. Dendritic cells. Arthritis Res. Ther. 2007, 9, 219. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Kim, M.S.; Lim, H.X.; Cho, D.; Kim, T.S. IL-33-matured dendritic cells promote Th17 cell responses via IL-1β and IL-6. Cytokine 2017, 99, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Verri, W.A.; Souto, F.O.; Vieira, S.M.; Almeida, S.C.; Fukada, S.Y.; Xu, D.; Alves-Filho, J.C.; Cunha, T.M.; Guerrero, A.T.; Mattos-Guimaraes, R.B.; et al. IL-33 induces neutrophil migration in rheumatoid arthritis and is a target of anti-TNF therapy. Ann. Rheum. Dis. 2010, 69, 1697–1703. [Google Scholar] [CrossRef] [PubMed]
- Leung, B.P.; Xu, D.; Culshaw, S.; McInnes, I.B.; Liew, F.Y. A novel therapy of murine collagen-induced arthritis with soluble T1/ST2. J. Immunol. 2004, 173, 145–150. [Google Scholar] [CrossRef]
- Kageyama, Y.; Torikai, E.; Tsujimura, K.; Kobayashi, M. Involvement of IL-33 in the pathogenesis of rheumatoid arthritis: The effect of etanercept on the serum levels of IL-33. Mod. Rheumatol. 2012, 22, 89–93. [Google Scholar] [CrossRef]
- Matsuyama, Y.; Okazaki, H.; Hoshino, M.; Onishi, S.; Kamata, Y.; Nagatani, K.; Nagashima, T.; Iwamoto, M.; Yoshio, T.; Ohto-Ozaki, H.; et al. Sustained elevation of interleukin-33 in sera and synovial fluids from patients with rheumatoid arthritis non-responsive to anti-tumor necrosis factor: Possible association with persistent IL-1β signaling and a poor clinical response. Rheumatol. Int. 2012, 32, 1397–1401. [Google Scholar] [CrossRef] [PubMed]
- Sellam, J.; Rivière, E.; Courties, A.; Rouzaire, P.O.; Tolusso, B.; Vital, E.M.; Emery, P.; Ferraccioli, G.; Soubrier, M.; Ly, B.; et al. Serum IL-33, a new marker predicting response to rituximab in rheumatoid arthritis. Arthritis Res. Ther. 2016, 18, 294. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Farooqi, A. Osteoarthritis. Best Pract. Res. Clin. Rheumatol. 2008, 22, 657–675. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, R.C.; Felson, D.T.; Helmick, C.G.; Arnold, L.M.; Choi, H.; Deyo, R.A.; Gabriel, S.; Hirsch, R.; Hochberg, M.C.; Hunder, G.G.; et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008, 58, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Ashford, S.; Williard, J. Osteoarthritis: A review. Nurse Pract. 2014, 39, 1–8. [Google Scholar] [CrossRef]
- Coaccioli, S.; Sarzi-Puttini, P.; Zis, P.; Rinonapoli, G.; Varrassi, G. Osteoarthritis: New Insight on Its Pathophysiology. J. Clin. Med. 2022, 11, 6013. [Google Scholar] [CrossRef] [PubMed]
- Maccagnano, G.; Pesce, V.; Noia, G.; Coviello, M.; Vicenti, G.; Vitiello, R.; Ziranu, A.; Spinarelli, A.; Moretti, B. The effects of a new protocol on blood loss in total knee arthroplasty. Orthop. Rev. 2022, 14, 37625. [Google Scholar] [CrossRef] [PubMed]
- Glauser, T.A.; Salinas, G.D.; Roepke, N.L.; Williamson, J.C.; Reese, A.; Gutierrez, G.; Abdolrasulnia, M. Management of mild-to-moderate osteoarthritis: A study of the primary care perspective. Postgrad. Med. 2011, 123, 126–134. [Google Scholar] [CrossRef]
- Shelton, L.R. A closer look at osteoarthritis. Nurse Pract. 2013, 38, 30–36, quiz 36–37. [Google Scholar] [CrossRef]
- Sun, X.H.; Liu, Y.; Han, Y.; Wang, J. Expression and Significance of High-Mobility Group Protein B1 (HMGB1) and the Receptor for Advanced Glycation End-Product (RAGE) in Knee Osteoarthritis. Med. Sci. Monit. 2016, 22, 2105–2112. [Google Scholar] [CrossRef]
- Wagner, G.; Lehmann, C.; Bode, C.; Miosge, N.; Schubert, A. High Mobility Group Box 1 Protein in Osteoarthritic Knee Tissue and Chondrogenic Progenitor Cells: An ex vivo and in vitro study. Cartilage 2021, 12, 484–495. [Google Scholar] [CrossRef]
- Li, Z.-C.; Cheng, G.-Q.; Hu, K.-Z.; Li, M.-Q.; Zang, W.-P.; Dong, Y.-Q.; Wang, W.-L.; Liu, Z.-D. Correlation of Synovial Fluid HMGB-1 Levels with Radiographic Severity of Knee Osteoarthritis. Clin. Investig. Med. 2011, 34, 298. [Google Scholar] [CrossRef] [PubMed]
- Aulin, C.; Lassacher, T.; Palmblad, K.; Erlandsson Harris, H. Early stage blockade of the alarmin HMGB1 reduces cartilage destruction in experimental OA. Osteoarthr. Cartil. 2020, 28, 698–707. [Google Scholar] [CrossRef]
- Zhou, S.; Liu, G.; Si, Z.; Yu, L.; Hou, L. Glycyrrhizin, an HMGB1 inhibitor, Suppresses Interleukin-1β-Induced Inflammatory Responses in Chondrocytes from Patients with Osteoarthritis. Cartilage 2021, 13, 947S–955S. [Google Scholar] [CrossRef]
- Pan, H.; Dai, H.; Wang, L.; Lin, S.; Tao, Y.; Zheng, Y.; Jiang, R.; Fang, F.; Wu, Y. MicroRNA-410-3p modulates chondrocyte apoptosis and inflammation by targeting high mobility group box 1 (HMGB1) in an osteoarthritis mouse model. BMC Musculoskelet. Disord. 2020, 21, 486. [Google Scholar] [CrossRef]
- Qiu, M.; Liu, D.; Fu, Q. MiR-129-5p shuttled by human synovial mesenchymal stem cell-derived exosomes relieves IL-1β induced osteoarthritis via targeting HMGB1. Life Sci. 2021, 269, 118987. [Google Scholar] [CrossRef]
- Wang, X.; Guo, Y.; Wang, C.; Yu, H.; Yu, X. MicroRNA-142-3p Inhibits Chondrocyte Apoptosis and Inflammation in Osteoarthritis by Targeting HMGB1. Inflammation 2016, 39, 1718–1728. [Google Scholar] [CrossRef] [PubMed]
- Shu, Z.; Miao, X.; Tang, T.; Zhan, P.; Zeng, L.; Jiang, Y. The GSK-3β/β-catenin signaling pathway is involved in HMGB1-induced chondrocyte apoptosis and cartilage matrix degradation. Int. J. Mol. Med. 2020, 45, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Zhang, Q.; Pu, F.; Zhu, Z.; Lu, K.; Lu, W.W.; Tong, L.; Yu, H.; Chen, D. Signalling interaction between β-catenin and other signalling molecules during osteoarthritis development. Cell Prolif. 2024, e13600. [Google Scholar] [CrossRef]
- Foell, D.; Wittkowski, H.; Vogl, T.; Roth, J. S100 proteins expressed in phagocytes: A novel group of damage-associated molecular pattern molecules. J. Leukoc. Biol. 2007, 81, 28–37. [Google Scholar] [CrossRef]
- van Lent, P.L.; Blom, A.B.; Schelbergen, R.F.; Slöetjes, A.; Lafeber, F.P.; Lems, W.F.; Cats, H.; Vogl, T.; Roth, J.; van den Berg, W.B. Active involvement of alarmins S100A8 and S100A9 in the regulation of synovial activation and joint destruction during mouse and human osteoarthritis. Arthritis Rheum. 2012, 64, 1466–1476. [Google Scholar] [CrossRef] [PubMed]
- Zreiqat, H.; Belluoccio, D.; Smith, M.M.; Wilson, R.; Rowley, L.A.; Jones, K.; Ramaswamy, Y.; Vogl, T.; Roth, J.; Bateman, J.F.; et al. S100A8 and S100A9 in experimental osteoarthritis. Arthritis Res. Ther. 2010, 12, R16. [Google Scholar] [CrossRef] [PubMed]
- Ruan, G.; Xu, J.; Wang, K.; Zheng, S.; Wu, J.; Ren, J.; Bian, F.; Chang, B.; Zhu, Z.; Han, W.; et al. Associations between serum S100A8/S100A9 and knee symptoms, joint structures and cartilage enzymes in patients with knee osteoarthritis. Osteoarthr. Cartil. 2019, 27, 99–105. [Google Scholar] [CrossRef]
- Huang, X.; Liu, J.; Huang, W. Identification of S100A8 as a common diagnostic biomarkers and exploring potential pathogenesis for osteoarthritis and metabolic syndrome. Front. Immunol. 2023, 14, 1185275. [Google Scholar] [CrossRef]
- Lourido, L.; Balboa-Barreiro, V.; Ruiz-Romero, C.; Rego-Pérez, I.; Camacho-Encina, M.; Paz-González, R.; Calamia, V.; Oreiro, N.; Nilsson, P.; Blanco, F.J. A clinical model including protein biomarkers predicts radiographic knee osteoarthritis: A prospective study using data from the Osteoarthritis Initiative. Osteoarthr. Cartil. 2021, 29, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Lourido, L.; Ayoglu, B.; Fernández-Tajes, J.; Oreiro, N.; Henjes, F.; Hellström, C.; Schwenk, J.M.; Ruiz-Romero, C.; Nilsson, P.; Blanco, F.J. Discovery of circulating proteins associated to knee radiographic osteoarthritis. Sci. Rep. 2017, 7, 137. [Google Scholar] [CrossRef]
- Mahler, E.A.; Zweers, M.C.; van Lent, P.L.; Blom, A.B.; van den Hoogen, F.H.; van den Berg, W.B.; Roth, J.; Vogl, T.; Bijlsma, J.W.; van den Ende, C.H.; et al. Association between serum levels of the proinflammatory protein S100A8/A9 and clinical and structural characteristics of patients with established knee, hip, and hand osteoarthritis. Scand. J. Rheumatol. 2015, 44, 56–60. [Google Scholar] [CrossRef]
- van den Bosch, M.H.; Blom, A.B.; Schelbergen, R.F.; Koenders, M.I.; van de Loo, F.A.; van den Berg, W.B.; Vogl, T.; Roth, J.; van der Kraan, P.M.; van Lent, P.L. Alarmin S100A9 Induces Proinflammatory and Catabolic Effects Predominantly in the M1 Macrophages of Human Osteoarthritic Synovium. J. Rheumatol. 2016, 43, 1874–1884. [Google Scholar] [CrossRef]
- van Kooten, N.J.T.; Blom, A.B.; Teunissen van Manen, I.J.; Theeuwes, W.F.; Roth, J.; Gorris, M.A.J.; Walgreen, B.; Sloetjes, A.W.; Helsen, M.M.; Vitters, E.L.; et al. S100A8/A9 drives monocytes towards M2-like macrophage differentiation and associates with M2-like macrophages in osteoarthritic synovium. Rheumatology, 2024; ahead of pint. [Google Scholar] [CrossRef]
- Schelbergen, R.F.; de Munter, W.; van den Bosch, M.H.; Lafeber, F.P.; Sloetjes, A.; Vogl, T.; Roth, J.; van den Berg, W.B.; van der Kraan, P.M.; Blom, A.B.; et al. Alarmins S100A8/S100A9 aggravate osteophyte formation in experimental osteoarthritis and predict osteophyte progression in early human symptomatic osteoarthritis. Ann. Rheum. Dis. 2016, 75, 218–225. [Google Scholar] [CrossRef]
- van den Bosch, M.H.; Blom, A.B.; Schelbergen, R.F.; Vogl, T.; Roth, J.P.; Slöetjes, A.W.; van den Berg, W.B.; van der Kraan, P.M.; van Lent, P.L. Induction of Canonical Wnt Signaling by the Alarmins S100A8/A9 in Murine Knee Joints: Implications for Osteoarthritis. Arthritis Rheumatol. 2016, 68, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Schelbergen, R.F.; van Dalen, S.; ter Huurne, M.; Roth, J.; Vogl, T.; Noël, D.; Jorgensen, C.; van den Berg, W.B.; van de Loo, F.A.; Blom, A.B.; et al. Treatment efficacy of adipose-derived stem cells in experimental osteoarthritis is driven by high synovial activation and reflected by S100A8/A9 serum levels. Osteoarthr. Cartil. 2014, 22, 1158–1166. [Google Scholar] [CrossRef] [PubMed]
- Schelbergen, R.F.; Blom, A.B.; van den Bosch, M.H.; Slöetjes, A.; Abdollahi-Roodsaz, S.; Schreurs, B.W.; Mort, J.S.; Vogl, T.; Roth, J.; van den Berg, W.B.; et al. Alarmins S100A8 and S100A9 elicit a catabolic effect in human osteoarthritic chondrocytes that is dependent on Toll-like receptor 4. Arthritis Rheum. 2012, 64, 1477–1487. [Google Scholar] [CrossRef] [PubMed]
- Schelbergen, R.F.; Geven, E.J.; van den Bosch, M.H.; Eriksson, H.; Leanderson, T.; Vogl, T.; Roth, J.; van de Loo, F.A.; Koenders, M.I.; van der Kraan, P.M.; et al. Prophylactic treatment with S100A9 inhibitor paquinimod reduces pathology in experimental collagenase-induced osteoarthritis. Ann. Rheum. Dis. 2015, 74, 2254–2258. [Google Scholar] [CrossRef] [PubMed]
- Corr, E.M.; Cunningham, C.C.; Helbert, L.; McCarthy, G.M.; Dunne, A. Osteoarthritis-associated basic calcium phosphate crystals activate membrane proximal kinases in human innate immune cells. Arthritis Res. Ther. 2017, 19, 23. [Google Scholar] [CrossRef]
- Stack, J.; McCarthy, G. Basic calcium phosphate crystals and osteoarthritis pathogenesis: Novel pathways and potential targets. Curr. Opin. Rheumatol. 2016, 28, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Yi, C.; Yi, Y.; Wei, J.; Jin, Q.; Li, J.; Sacitharan, P.K. Targeting IL-22 and IL-22R protects against experimental osteoarthritis. Cell. Mol. Immunol. 2021, 18, 1329–1331. [Google Scholar] [CrossRef]
- Li, C.; Chen, K.; Kang, H.; Yan, Y.; Liu, K.; Guo, C.; Qi, J.; Yang, K.; Wang, F.; Guo, L.; et al. Double-stranded RNA released from damaged articular chondrocytes promotes cartilage degeneration via Toll-like receptor 3-interleukin-33 pathway. Cell Death Dis. 2017, 8, e3165. [Google Scholar] [CrossRef]
- Rai, V.; Radwan, M.M.; Agrawal, D.K. IL-33, IL-37, and Vitamin D Interaction Mediate Immunomodulation of Inflammation in Degenerating Cartilage. Antibodies 2021, 10, 41. [Google Scholar] [CrossRef]
- He, Z.; Song, Y.; Yi, Y.; Qiu, F.; Wang, J.; Li, J.; Jin, Q.; Sacitharan, P.K. Blockade of IL-33 signalling attenuates osteoarthritis. Clin. Transl. Immunol. 2020, 9, e1185. [Google Scholar] [CrossRef]
- Rai, V.; Dilisio, M.F.; Samadi, F.; Agrawal, D.K. Counteractive Effects of IL-33 and IL-37 on Inflammation in Osteoarthritis. Int. J. Environ. Res. Public Health 2022, 19, 5690. [Google Scholar] [CrossRef] [PubMed]
- van Geffen, E.W.; van Caam, A.P.M.; Schreurs, W.; van de Loo, F.A.; van Lent, P.L.E.M.; Koenders, M.I.; Thudium, C.S.; Bay-Jensen, A.C.; Blaney Davidson, E.N.; van der Kraan, P.M. IL-37 diminishes proteoglycan loss in human OA cartilage: Donor-specific link between IL-37 and MMP-3. Osteoarthr. Cartil. 2019, 27, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Nicols, B.F.; Robledo-Pulido, J.J.; Alvarado-Navarro, A. Etiopathogenesis of Psoriasis: Integration of Proposed Theories. Immunol. Investig. 2024, 53, 348–415. [Google Scholar] [CrossRef]
- Oliveira, M.e.F.; Rocha, B.e.O.; Duarte, G.V. Psoriasis: Classical and emerging comorbidities. An. Bras. Dermatol. 2015, 90, 9–20. [Google Scholar] [CrossRef]
- Yen, H.; Chi, C.C. Association between Psoriasis and Vitiligo: A Systematic Review and Meta-Analysis. Am. J. Clin. Dermatol. 2019, 20, 31–40. [Google Scholar] [CrossRef]
- Wu, J.J.; Nguyen, T.U.; Poon, K.Y.; Herrinton, L.J. The association of psoriasis with autoimmune diseases. J. Am. Acad. Dermatol. 2012, 67, 924–930. [Google Scholar] [CrossRef]
- Najarian, D.J.; Gottlieb, A.B. Connections between psoriasis and Crohn’s disease. J. Am. Acad. Dermatol. 2003, 48, 805–821, quiz 822–804. [Google Scholar] [CrossRef]
- Ayala-Fontánez, N.; Soler, D.C.; McCormick, T.S. Current knowledge on psoriasis and autoimmune diseases. Psoriasis 2016, 6, 7–32. [Google Scholar] [CrossRef] [PubMed]
- Makredes, M.; Robinson, D.; Bala, M.; Kimball, A.B. The burden of autoimmune disease: A comparison of prevalence ratios in patients with psoriatic arthritis and psoriasis. J. Am. Acad. Dermatol. 2009, 61, 405–410. [Google Scholar] [CrossRef]
- Nussbaum, L.; Chen, Y.L.; Ogg, G.S. Role of regulatory T cells in psoriasis pathogenesis and treatment. Br. J. Dermatol. 2021, 184, 14–24. [Google Scholar] [CrossRef]
- Borsky, P.; Fiala, Z.; Andrys, C.; Beranek, M.; Hamakova, K.; Malkova, A.; Svadlakova, T.; Krejsek, J.; Palicka, V.; Borska, L.; et al. Alarmins HMGB1, IL-33, S100A7, and S100A12 in Psoriasis Vulgaris. Mediat. Inflamm. 2020, 2020, 8465083. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Guo, Z.-P.; Li, L.; Wang, L.; Jia, R.-Z.; Cao, N.; Qin, S.; Li, M.-M. Increased HMGB1 serum levels and altered HMGB1 expression in patients with psoriasis vulgaris. Arch. Dermatol. Res. 2013, 305, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, C.; Strohbuecker, L.; Lotfi, R.; Sucker, A.; Joosten, I.; Koenen, H.; Korber, A. High mobility group box 1 is increased in the sera of psoriatic patients with disease progression. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Yamaguchi, Y.; Watanabe, Y.; Takamura, N.; Aihara, M. Increased level of high mobility group box 1 in the serum and skin in patients with generalized pustular psoriasis. J. Dermatol. 2020, 47, 1033–1036. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, N.E.; Hashem, N.; Eltahlawy, S.; Gomaa, E. Involvement of high-mobility group box-1 and Dermoscopy in diagnosis of psoriasis severity. J. Cosmet. Dermatol. 2022, 21, 6336–6342. [Google Scholar] [CrossRef] [PubMed]
- Maurelli, M.; Gisondi, P.; Danese, E.; Gelati, M.; Papagrigoraki, A.; Del Giglio, M.; Lippi, G.; Girolomoni, G. Psoriasin (S100A7) is increased in the serum of patients with moderate-to-severe psoriasis. Br. J. Dermatol. 2020, 182, 1502–1503. [Google Scholar] [CrossRef]
- Chen, J.; Fu, Y.; Xiong, S. Keratinocyte derived HMGB1 aggravates psoriasis dermatitis via facilitating inflammatory polarization of macrophages and hyperproliferation of keratinocyte. Mol. Immunol. 2023, 163, 1–12. [Google Scholar] [CrossRef]
- Jeon, S.; Song, J.; Lee, D.; Kim, G.T.; Park, S.H.; Shin, D.Y.; Shin, K.O.; Park, K.; Shim, S.M.; Park, T.S. Inhibition of sphingosine 1-phosphate lyase activates human keratinocyte differentiation and attenuates psoriasis in mice. J. Lipid Res. 2020, 61, 20–32. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, H.; Zheng, H.; Zhou, X.; Shen, G.; Teng, X.; Liu, X.; Zhang, J.; Wei, X.; Hu, Z.; et al. Autophagy-based unconventional secretion of HMGB1 by keratinocytes plays a pivotal role in psoriatic skin inflammation. Autophagy 2021, 17, 529–552. [Google Scholar] [CrossRef]
- Li, S.; Li, G.; Li, X.; Wu, F.; Li, L. Etanercept ameliorates psoriasis progression through regulating high mobility group box 1 pathway. Ski. Res. Technol. 2023, 29, e13329. [Google Scholar] [CrossRef] [PubMed]
- Holmannová, D.; Císařová, B.; Borský, P.; Fiala, Z.; Andrýs, C.; Hamáková, K.; Švadláková, T.; Krejsek, J.; Palička, V.; Kotingová, L.; et al. Goeckerman Regimen Reduces Alarmin Levels and PASI Score in Paediatric Patients with Psoriasis. Acta Med. 2021, 64, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Christmann, C.; Zenker, S.; Martens, L.; Hubner, J.; Loser, K.; Vogl, T.; Roth, J. Interleukin 17 Promotes Expression of Alarmins S100A8 and S100A9 During the Inflammatory Response of Keratinocytes. Front. Immunol. 2020, 11, 599947. [Google Scholar] [CrossRef] [PubMed]
- Dolivo, D.; Rodrigues, A.; Sun, L.; Galiano, R.; Mustoe, T.; Hong, S.J. Reduced hydration regulates pro-inflammatory cytokines via CD14 in barrier function-impaired skin. Biochim. Biophys. Acta Mol. Basis Dis. 2022, 1868, 166482. [Google Scholar] [CrossRef] [PubMed]
- Wilsmann-Theis, D.; Wagenpfeil, J.; Holzinger, D.; Roth, J.; Koch, S.; Schnautz, S.; Bieber, T.; Wenzel, J. Among the S100 proteins, S100A12 is the most significant marker for psoriasis disease activity. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1165–1170. [Google Scholar] [CrossRef] [PubMed]
- Schonthaler, H.B.; Guinea-Viniegra, J.; Wculek, S.K.; Ruppen, I.; Ximénez-Embún, P.; Guío-Carrión, A.; Navarro, R.; Hogg, N.; Ashman, K.; Wagner, E.F. S100A8-S100A9 protein complex mediates psoriasis by regulating the expression of complement factor C3. Immunity 2013, 39, 1171–1181. [Google Scholar] [CrossRef] [PubMed]
- Berg, A.R.; Hong, C.G.; Svirydava, M.; Li, H.; Parel, P.M.; Florida, E.; O’Hagan, R.; Pantoja, C.J.; Lateef, S.S.; Anzenberg, P.; et al. Association of S100A8/A9 with Lipid-Rich Necrotic Core and Treatment with Biologic Therapy in Patients with Psoriasis: Results from an Observational Cohort Study. J. Investig. Dermatol. 2022, 142, 2909–2919. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, M.; Sawada, Y.; Saito-Sasaki, N.; Yoshioka, H.; Hama, K.; Omoto, D.; Ohmori, S.; Okada, E.; Nakamura, M. High S100A2 expression in keratinocytes in patients with drug eruption. Sci. Rep. 2021, 11, 5493. [Google Scholar] [CrossRef]
- da Costa, L.C.O.; Gardinassi, L.G.; Veras, F.P.; Milanezi, C.; Ramalho, L.N.Z.; Benevides, L.; Alves-Filho, J.C.; da Silva, J.S.; da Silva Souza, C. Expression of B lymphocyte-induced maturation protein 1 (Blimp-1) in keratinocyte and cytokine signalling drives human Th17 response in psoriasis. Arch. Dermatol. Res. 2023, 315, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Fritz, Y.; Klenotic, P.A.; Swindell, W.R.; Yin, Z.Q.; Groft, S.G.; Zhang, L.; Baliwag, J.; Camhi, M.I.; Diaconu, D.; Young, A.B.; et al. Induction of Alternative Proinflammatory Cytokines Accounts for Sustained Psoriasiform Skin Inflammation in IL-17C+IL-6KO Mice. J. Investig. Dermatol. 2017, 137, 696–705. [Google Scholar] [CrossRef]
- Kim, M.J.; Im, M.A.; Lee, J.S.; Mun, J.Y.; Kim, D.H.; Gu, A.; Kim, I.S. Effect of S100A8 and S100A9 on expressions of cytokine and skin barrier protein in human keratinocytes. Mol. Med. Rep. 2019, 20, 2476–2483. [Google Scholar] [CrossRef]
- Benedyk, M.; Sopalla, C.; Nacken, W.; Bode, G.; Melkonyan, H.; Banfi, B.; Kerkhoff, C. HaCaT keratinocytes overexpressing the S100 proteins S100A8 and S100A9 show increased NADPH oxidase and NF-kappaB activities. J. Investig. Dermatol. 2007, 127, 2001–2011. [Google Scholar] [CrossRef]
- Benezeder, T.; Painsi, C.; Patra, V.; Dey, S.; Holcmann, M.; Lange-Asschenfeldt, B.; Sibilia, M.; Wolf, P. Dithranol targets keratinocytes, their crosstalk with neutrophils and inhibits the IL-36 inflammatory loop in psoriasis. Elife 2020, 9, e56991. [Google Scholar] [CrossRef]
- Krueger, J.G.; Wharton, K.A., Jr.; Schlitt, T.; Suprun, M.; Torene, R.I.; Jiang, X.; Wang, C.Q.; Fuentes-Duculan, J.; Hartmann, N.; Peters, T.; et al. IL-17A inhibition by secukinumab induces early clinical, histopathologic, and molecular resolution of psoriasis. J. Allergy Clin. Immunol. 2019, 144, 750–763. [Google Scholar] [CrossRef]
- Visvanathan, S.; Baum, P.; Vinisko, R.; Schmid, R.; Flack, M.; Lalovic, B.; Kleiner, O.; Fuentes-Duculan, J.; Garcet, S.; Davis, J.W.; et al. Psoriatic skin molecular and histopathologic profiles after treatment with risankizumab versus ustekinumab. J. Allergy Clin. Immunol. 2019, 143, 2158–2169. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Guo, J.; Yin, L.; Tu, J.; Yin, Z. Tacrolimus Inhibits TNF-alpha/IL-17A-Produced pro-Inflammatory Effect on Human Keratinocytes by Regulating IkappaBzeta. Inflammation 2020, 43, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Catlett, I.M.; Hu, Y.; Gao, L.; Banerjee, S.; Gordon, K.; Krueger, J.G. Molecular and clinical effects of selective tyrosine kinase 2 inhibition with deucravacitinib in psoriasis. J. Allergy Clin. Immunol. 2022, 149, 2010–2020.e2018. [Google Scholar] [CrossRef] [PubMed]
- Ten Bergen, L.L.; Petrovic, A.; Aarebrot, A.K.; Appel, S. Current knowledge on autoantigens and autoantibodies in psoriasis. Scand. J. Immunol. 2020, 92, e12945. [Google Scholar] [CrossRef]
- Lande, R.; Gregorio, J.; Facchinetti, V.; Chatterjee, B.; Wang, Y.H.; Homey, B.; Cao, W.; Wang, Y.H.; Su, B.; Nestle, F.O.; et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature 2007, 449, 564–569. [Google Scholar] [CrossRef]
- Yuan, Y.; Qiu, J.; Lin, Z.T.; Li, W.; Haley, C.; Mui, U.N.; Ning, J.; Tyring, S.K.; Wu, T. Identification of Novel Autoantibodies Associated with Psoriatic Arthritis. Arthritis Rheumatol. 2019, 71, 941–951. [Google Scholar] [CrossRef]
- Emmungil, H.; Ilgen, U.; Direskeneli, R.H. Autoimmunity in psoriatic arthritis: Pathophysiological and clinical aspects. Turk. J. Med. Sci. 2021, 51, 1601–1614. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, K.; Schauber, J.; Coda, A.; Lin, H.; Dorschner, R.A.; Schechter, N.M.; Bonnart, C.; Descargues, P.; Hovnanian, A.; Gallo, R.L. Kallikrein-mediated proteolysis regulates the antimicrobial effects of cathelicidins in skin. FASEB J. 2006, 20, 2068–2080. [Google Scholar] [CrossRef] [PubMed]
- Ong, P.Y.; Ohtake, T.; Brandt, C.; Strickland, I.; Boguniewicz, M.; Ganz, T.; Gallo, R.L.; Leung, D.Y. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N. Engl. J. Med. 2002, 347, 1151–1160. [Google Scholar] [CrossRef] [PubMed]
- Herster, F.; Bittner, Z.; Archer, N.K.; Dickhofer, S.; Eisel, D.; Eigenbrod, T.; Knorpp, T.; Schneiderhan-Marra, N.; Loffler, M.W.; Kalbacher, H.; et al. Neutrophil extracellular trap-associated RNA and LL37 enable self-amplifying inflammation in psoriasis. Nat. Commun. 2020, 11, 105. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, D.; Chamilos, G.; Lande, R.; Gregorio, J.; Meller, S.; Facchinetti, V.; Homey, B.; Barrat, F.J.; Zal, T.; Gilliet, M. Self-RNA-antimicrobial peptide complexes activate human dendritic cells through TLR7 and TLR8. J. Exp. Med. 2009, 206, 1983–1994. [Google Scholar] [CrossRef] [PubMed]
- Nestle, F.O.; Conrad, C.; Tun-Kyi, A.; Homey, B.; Gombert, M.; Boyman, O.; Burg, G.; Liu, Y.J.; Gilliet, M. Plasmacytoid predendritic cells initiate psoriasis through interferon-alpha production. J. Exp. Med. 2005, 202, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Di Meglio, P.; Perera, G.K.; Nestle, F.O. The multitasking organ: Recent insights into skin immune function. Immunity 2011, 35, 857–869. [Google Scholar] [CrossRef] [PubMed]
- Alwan, W.; Nestle, F.O. Pathogenesis and treatment of psoriasis: Exploiting pathophysiological pathways for precision medicine. Clin. Exp. Rheumatol. 2015, 33, S2–S6. [Google Scholar]
- Furue, M.; Kadono, T. The contribution of IL-17 to the development of autoimmunity in psoriasis. Innate Immun. 2019, 25, 337–343. [Google Scholar] [CrossRef]
- Lande, R.; Botti, E.; Jandus, C.; Dojcinovic, D.; Fanelli, G.; Conrad, C.; Chamilos, G.; Feldmeyer, L.; Marinari, B.; Chon, S.; et al. The antimicrobial peptide LL37 is a T-cell autoantigen in psoriasis. Nat. Commun. 2014, 5, 5621. [Google Scholar] [CrossRef]
- Wang, W.M.; Jin, H.Z. Heat shock proteins and psoriasis. Eur. J. Dermatol. 2019, 29, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Curry, J.L.; Qin, J.Z.; Bonish, B.; Carrick, R.; Bacon, P.; Panella, J.; Robinson, J.; Nickoloff, B.J. Innate immune-related receptors in normal and psoriatic skin. Arch. Pathol. Lab. Med. 2003, 127, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, S.; Korkmaz, H. Effect of alterations in apoptotic pathway on development of metabolic syndrome in patients with psoriasis vulgaris. Br. J. Dermatol. 2017, 176, 1549–1557. [Google Scholar] [CrossRef] [PubMed]
- Damasiewicz-Bodzek, A.; Szumska, M.; Tyrpien-Golder, K. Antibodies to Heat Shock Proteins 90alpha and 90beta in Psoriasis. Arch. Immunol. Ther. Exp. 2020, 68, 9. [Google Scholar] [CrossRef]
- Besgen, P.; Trommler, P.; Vollmer, S.; Prinz, J.C. Ezrin, maspin, peroxiredoxin 2, and heat shock protein 27: Potential targets of a streptococcal-induced autoimmune response in psoriasis. J. Immunol. 2010, 184, 5392–5402. [Google Scholar] [CrossRef] [PubMed]
- Puig, L.; Fernandez-Figueras, M.T.; Ferrandiz, C.; Ribera, M.; de Moragas, J.M. Epidermal expression of 65 and 72 kd heat shock proteins in psoriasis and AIDS-associated psoriasiform dermatitis. J. Am. Acad. Dermatol. 1995, 33, 985–989. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wu, L.; Bulek, K.; Martin, B.N.; Zepp, J.A.; Kang, Z.; Liu, C.; Herjan, T.; Misra, S.; Carman, J.A.; et al. The psoriasis-associated D10N variant of the adaptor Act1 with impaired regulation by the molecular chaperone hsp90. Nat. Immunol. 2013, 14, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Boyman, O.; Conrad, C.; Dudli, C.; Kielhorn, E.; Nickoloff, B.J.; Nestle, F.O. Activation of dendritic antigen-presenting cells expressing common heat shock protein receptor CD91 during induction of psoriasis. Br. J. Dermatol. 2005, 152, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Bayramgurler, D.; Ozkara, S.K.; Apaydin, R.; Ercin, C.; Bilen, N. Heat shock proteins 60 and 70 expression of cutaneous lichen planus: Comparison with normal skin and psoriasis vulgaris. J. Cutan. Pathol. 2004, 31, 586–594. [Google Scholar] [CrossRef]
- Baroni, A.; Paoletti, I.; Ruocco, E.; Agozzino, M.; Tufano, M.A.; Donnarumma, G. Possible role of Malassezia furfur in psoriasis: Modulation of TGF-beta1, integrin, and HSP70 expression in human keratinocytes and in the skin of psoriasis-affected patients. J. Cutan. Pathol. 2004, 31, 35–42. [Google Scholar] [CrossRef]
- Cancino-Diaz, M.E.; Ruiz-Gonzalez, V.; Ramirez-Resendiz, L.; Ortiz, B.; Dominguez-Lopez, M.L.; Paredes-Cabrera, G.C.; Leon-Dorantes, G.; Blancas-Gonzalez, F.; Jimenez-Zamudio, L.; Garcia-Latorre, E. IgG class antibodies from psoriasis patients recognize the 60-KDa heat-shock protein of Streptococcus pyogenes. Int. J. Dermatol. 2004, 43, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Seifarth, F.G.; Lax, J.E.; Harvey, J.; DiCorleto, P.E.; Husni, M.E.; Chandrasekharan, U.M.; Tytell, M. Topical heat shock protein 70 prevents imiquimod-induced psoriasis-like inflammation in mice. Cell Stress Chaperones 2018, 23, 1129–1135. [Google Scholar] [CrossRef] [PubMed]
- Tukaj, S.; Sitko, K. Heat Shock Protein 90 (Hsp90) and Hsp70 as Potential Therapeutic Targets in Autoimmune Skin Diseases. Biomolecules 2022, 12, 1153. [Google Scholar] [CrossRef] [PubMed]
- Ben Abdallah, H.; Seeler, S.; Bregnhoj, A.; Ghatnekar, G.; Kristensen, L.S.; Iversen, L.; Johansen, C. Heat shock protein 90 inhibitor RGRN-305 potently attenuates skin inflammation. Front. Immunol. 2023, 14, 1128897. [Google Scholar] [CrossRef] [PubMed]
- Kakeda, M.; Arock, M.; Schlapbach, C.; Yawalkar, N. Increased expression of heat shock protein 90 in keratinocytes and mast cells in patients with psoriasis. J. Am. Acad. Dermatol. 2014, 70, 683–690.e681. [Google Scholar] [CrossRef] [PubMed]
- Stenderup, K.; Rosada, C.; Gavillet, B.; Vuagniaux, G.; Dam, T.N. Debio 0932, a new oral Hsp90 inhibitor, alleviates psoriasis in a xenograft transplantation model. Acta Derm. Venereol. 2014, 94, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Qiao, P.; Guo, W.; Ke, Y.; Fang, H.; Zhuang, Y.; Jiang, M.; Zhang, J.; Shen, S.; Qiao, H.; Dang, E.; et al. Mechanical Stretch Exacerbates Psoriasis by Stimulating Keratinocyte Proliferation and Cytokine Production. J. Investig. Dermatol. 2019, 139, 1470–1479. [Google Scholar] [CrossRef] [PubMed]
- Tseng, P.Y.; Hoon, M.A. Specific beta-Defensins Stimulate Pruritus through Activation of Sensory Neurons. J. Investig. Dermatol. 2022, 142, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Harder, J.; Schroder, J.M. Psoriatic scales: A promising source for the isolation of human skin-derived antimicrobial proteins. J. Leukoc. Biol. 2005, 77, 476–486. [Google Scholar] [CrossRef]
- Harder, J.; Bartels, J.; Christophers, E.; Schroder, J.M. A peptide antibiotic from human skin. Nature 1997, 387, 861. [Google Scholar] [CrossRef]
- Harder, J.; Bartels, J.; Christophers, E.; Schroder, J.M. Isolation and characterization of human beta -defensin-3, a novel human inducible peptide antibiotic. J. Biol. Chem. 2001, 276, 5707–5713. [Google Scholar] [CrossRef] [PubMed]
- Tampa, M.; Sarbu, M.I.; Mitran, M.I.; Mitran, C.I.; Matei, C.; Georgescu, S.R. The Pathophysiological Mechanisms and the Quest for Biomarkers in Psoriasis, a Stress-Related Skin Disease. Dis. Markers 2018, 2018, 5823684. [Google Scholar] [CrossRef] [PubMed]
- Jansen, P.A.; Rodijk-Olthuis, D.; Hollox, E.J.; Kamsteeg, M.; Tjabringa, G.S.; de Jongh, G.J.; van Vlijmen-Willems, I.M.; Bergboer, J.G.; van Rossum, M.M.; de Jong, E.M.; et al. Beta-defensin-2 protein is a serum biomarker for disease activity in psoriasis and reaches biologically relevant concentrations in lesional skin. PLoS ONE 2009, 4, e4725. [Google Scholar] [CrossRef] [PubMed]
- Kolbinger, F.; Loesche, C.; Valentin, M.A.; Jiang, X.; Cheng, Y.; Jarvis, P.; Peters, T.; Calonder, C.; Bruin, G.; Polus, F.; et al. β-Defensin 2 is a responsive biomarker of IL-17A-driven skin pathology in patients with psoriasis. J. Allergy Clin. Immunol. 2017, 139, 923–932.e928. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Yamasaki, K. Psoriasis and Antimicrobial Peptides. Int. J. Mol. Sci. 2020, 21, 6791. [Google Scholar] [CrossRef] [PubMed]
- Johansen, C.; Bertelsen, T.; Ljungberg, C.; Mose, M.; Iversen, L. Characterization of TNF-alpha- and IL-17A-Mediated Synergistic Induction of DEFB4 Gene Expression in Human Keratinocytes through IkappaBzeta. J. Investig. Dermatol. 2016, 136, 1608–1616. [Google Scholar] [CrossRef] [PubMed]
- Pourani, M.R.; Abdollahimajd, F.; Zargari, O.; Shahidi Dadras, M. Soluble biomarkers for diagnosis, monitoring, and therapeutic response assessment in psoriasis. J. Dermatolog. Treat. 2022, 33, 1967–1974. [Google Scholar] [CrossRef] [PubMed]
- Schakel, K.; Reich, K.; Asadullah, K.; Pinter, A.; Jullien, D.; Weisenseel, P.; Paul, C.; Gomez, M.; Wegner, S.; Personke, Y.; et al. Early disease intervention with guselkumab in psoriasis leads to a higher rate of stable complete skin clearance (‘clinical super response’): Week 28 results from the ongoing phase IIIb randomized, double-blind, parallel-group, GUIDE study. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 2016–2027. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.T.; Qiao, Z.H.; Tian, J.B.; Lin, J.L.; Hou, S.C.; Liu, X.M. The effect of secukinumab treatment for psoriasis on serum cytokines and correlation with disease severity. Skin Res. Technol. 2023, 29, e13405. [Google Scholar] [CrossRef]
- Cardner, M.; Tuckwell, D.; Kostikova, A.; Forrer, P.; Siegel, R.M.; Marti, A.; Vandemeulebroecke, M.; Ferrero, E. Analysis of serum proteomics data identifies a quantitative association between beta-defensin 2 at baseline and clinical response to IL-17 blockade in psoriatic arthritis. RMD Open 2023, 9, e003042. [Google Scholar] [CrossRef]
- Uzuncakmak, T.K.; Karadag, A.S.; Ozkanli, S.; Akbulak, O.; Ozlu, E.; Akdeniz, N.; Oguztuzun, S. Alteration of tissue expression of human beta defensin-1 and human beta defensin-2 in psoriasis vulgaris following phototherapy. Biotech. Histochem. 2020, 95, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Ham, J.; Shin, J.W.; Ko, B.C.; Kim, H.Y. Targeting the Epithelium-Derived Innate Cytokines: From Bench to Bedside. Immune Netw. 2022, 22, e11. [Google Scholar] [CrossRef] [PubMed]
- El-Ghareeb, M.I.; Helmy, A.; Al Kazzaz, S.; Samir, H. Serum TSLP is a potential biomarker of psoriasis vulgaris activity. Psoriasis 2019, 9, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Suwarsa, O.; Dharmadji, H.P.; Sutedja, E.; Herlina, L.; Sori, P.R.; Hindritiani, R.; Dwiyana, R.F.; Gunawan, H. Skin tissue expression and serum level of thymic stromal lymphopoietin in patients with psoriasis vulgaris. Dermatol. Rep. 2019, 11, 8006. [Google Scholar] [CrossRef] [PubMed]
- Fromm, S.; Cunningham, C.C.; Dunne, M.R.; Veale, D.J.; Fearon, U.; Wade, S.M. Enhanced angiogenic function in response to fibroblasts from psoriatic arthritis synovium compared to rheumatoid arthritis. Arthritis Res. Ther. 2019, 21, 297. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.H.; Zuo, Y.G. Thymic Stromal Lymphopoietin in Cutaneous Immune-Mediated Diseases. Front. Immunol. 2021, 12, 698522. [Google Scholar] [CrossRef] [PubMed]
- Gago-Lopez, N.; Mellor, L.F.; Megias, D.; Martin-Serrano, G.; Izeta, A.; Jimenez, F.; Wagner, E.F. Role of bulge epidermal stem cells and TSLP signaling in psoriasis. EMBO Mol. Med. 2019, 11, e10697. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Chong, H.J.; So, J.; Jo, Y.; Yune, T.Y.; Ju, B.G. Ghrelin Represses Thymic Stromal Lymphopoietin Gene Expression through Activation of Glucocorticoid Receptor and Protein Kinase C Delta in Inflamed Skin Keratinocytes. Int. J. Mol. Sci. 2022, 23, 3977. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, N.; Segawa, R.; Tobita, R.; Asakawa, S.; Mizuno, N.; Hiratsuka, M.; Hirasawa, N. Hypoxia inhibits TNF-alpha-induced TSLP expression in keratinocytes. PLoS ONE 2019, 14, e0224705. [Google Scholar] [CrossRef]
- Segawa, R.; Shigeeda, K.; Hatayama, T.; Dong, J.; Mizuno, N.; Moriya, T.; Hiratsuka, M.; Hirasawa, N. EGFR transactivation is involved in TNF-alpha-induced expression of thymic stromal lymphopoietin in human keratinocyte cell line. J. Dermatol. Sci. 2018, 89, 290–298. [Google Scholar] [CrossRef]
- Meephansan, J.; Komine, M.; Tsuda, H.; Karakawa, M.; Tominaga, S.; Ohtsuki, M. Expression of IL-33 in the epidermis: The mechanism of induction by IL-17. J. Dermatol. Sci. 2013, 71, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Hu, Y.; Gong, Y.; Zhang, X.; Cui, L.; Chen, R.; Yu, Y.; Yu, Q.; Chen, Y.; Diao, H.; et al. Interleukin-33 alleviates psoriatic inflammation by suppressing the T helper type 17 immune response. Immunology 2020, 160, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Chen, H.; Chen, L.; Mao, J.; Cai, S.; Xiao, Y.; Li, J.; Shi, J.; Li, B.; Xu, Y.; et al. An Autocrine Circuit of IL-33 in Keratinocytes Is Involved in the Progression of Psoriasis. J. Investig. Dermatol. 2021, 141, 596–606.e597. [Google Scholar] [CrossRef] [PubMed]
- Ampawong, S.; Kengkoom, K.; Sukphopetch, P.; Aramwit, P.; Muangkaew, W.; Kanjanapruthipong, T.; Buaban, T. Evaluating the effect of rice (Oryza sativa L.: SRNC05053-6-2) crude extract on psoriasis using in vitro and in vivo models. Sci. Rep. 2020, 10, 17618. [Google Scholar] [CrossRef] [PubMed]
- Barr, T.P.; Garzia, C.; Guha, S.; Fletcher, E.K.; Nguyen, N.; Wieschhaus, A.J.; Ferrer, L.; Covic, L.; Kuliopulos, A. PAR2 Pepducin-Based Suppression of Inflammation and Itch in Atopic Dermatitis Models. J. Investig. Dermatol. 2019, 139, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Meephansan, J.; Tsuda, H.; Komine, M.; Tominaga, S.; Ohtsuki, M. Regulation of IL-33 expression by IFN-gamma and tumor necrosis factor-alpha in normal human epidermal keratinocytes. J. Investig. Dermatol. 2012, 132, 2593–2600. [Google Scholar] [CrossRef] [PubMed]
- Du, H.Y.; Fu, H.Y.; Li, D.N.; Qiao, Y.; Wang, Q.W.; Liu, W. The Expression and Regulation of Interleukin-33 in Human Epidermal Keratinocytes: A New Mediator of Atopic Dermatitis and Its Possible Signaling Pathway. J. Interferon Cytokine Res. 2016, 36, 552–562. [Google Scholar] [CrossRef]
- Tsuji, G.; Hashimoto-Hachiya, A.; Yen, V.H.; Miake, S.; Takemura, M.; Mitamura, Y.; Ito, T.; Murata, M.; Furue, M.; Nakahara, T. Aryl Hydrocarbon Receptor Activation Downregulates IL-33 Expression in Keratinocytes via Ovo-Like 1. J. Clin. Med. 2020, 9, 891. [Google Scholar] [CrossRef] [PubMed]
- Cannavo, S.P.; Bertino, L.; Di Salvo, E.; Papaianni, V.; Ventura-Spagnolo, E.; Gangemi, S. Possible Roles of IL-33 in the Innate-Adaptive Immune Crosstalk of Psoriasis Pathogenesis. Mediat. Inflamm. 2019, 2019, 7158014. [Google Scholar] [CrossRef]
- Imai, Y. ILC2s in skin disorders. Allergol. Int. 2023, 72, 201–206. [Google Scholar] [CrossRef]
- Raimondo, A.; Lembo, S.; Di Caprio, R.; Donnarumma, G.; Monfrecola, G.; Balato, N.; Ayala, F.; Balato, A. Psoriatic cutaneous inflammation promotes human monocyte differentiation into active osteoclasts, facilitating bone damage. Eur. J. Immunol. 2017, 47, 1062–1074. [Google Scholar] [CrossRef] [PubMed]
- Meephansan, J.; Subpayasarn, U.; Ponnikorn, S.; Chakkavittumrong, P.; Juntongjin, P.; Komine, M.; Ohtsuki, M.; Poovorawan, Y. Methotrexate, but not narrowband ultraviolet B radiation, suppresses interleukin-33 mRNA levels in psoriatic plaques and protein levels in serum of patients with psoriasis. J. Dermatol. 2018, 45, 322–325. [Google Scholar] [CrossRef] [PubMed]
- Guilloteau, K.; Paris, I.; Pedretti, N.; Boniface, K.; Juchaux, F.; Huguier, V.; Guillet, G.; Bernard, F.X.; Lecron, J.C.; Morel, F. Skin Inflammation Induced by the Synergistic Action of IL-17A, IL-22, Oncostatin M, IL-1{alpha}, and TNF-{alpha} Recapitulates Some Features of Psoriasis. J. Immunol. 2010, 184, 5263–5270. [Google Scholar] [CrossRef] [PubMed]
- Conti, P.; Pregliasco, F.E.; Bellomo, R.G.; Gallenga, C.E.; Caraffa, A.; Kritas, S.K.; Lauritano, D.; Ronconi, G. Mast Cell Cytokines IL-1, IL-33, and IL-36 Mediate Skin Inflammation in Psoriasis: A Novel Therapeutic Approach with the Anti-Inflammatory Cytokines IL-37, IL-38, and IL-1Ra. Int. J. Mol. Sci. 2021, 22, 8076. [Google Scholar] [CrossRef] [PubMed]
- Iznardo, H.; Puig, L. IL-1 Family Cytokines in Inflammatory Dermatoses: Pathogenetic Role and Potential Therapeutic Implications. Int. J. Mol. Sci. 2022, 23, 9479. [Google Scholar] [CrossRef] [PubMed]
- Rabeony, H.; Petit-Paris, I.; Garnier, J.; Barrault, C.; Pedretti, N.; Guilloteau, K.; Jegou, J.F.; Guillet, G.; Huguier, V.; Lecron, J.C.; et al. Inhibition of keratinocyte differentiation by the synergistic effect of IL-17A, IL-22, IL-1α, TNFα and oncostatin M. PLoS ONE 2014, 9, e101937. [Google Scholar] [CrossRef] [PubMed]
- Romero, L.I.; Ikejima, T.; Pincus, S.H. In Situ Localization of Interleukin-1 in Normal and Psoriatic Skin. J. Investig. Dermatol. 1989, 93, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Portugal-Cohen, M.; Horev, L.; Ruffer, C.; Schlippe, G.; Voss, W.; Ma’or, Z.; Oron, M.; Soroka, Y.; Frušić-Zlotkin, M.; Milner, Y.; et al. Non-invasive skin biomarkers quantification of psoriasis and atopic dermatitis: Cytokines, antioxidants and psoriatic skin auto-fluorescence. Biomed. Pharmacother. 2012, 66, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Schaap, M.J.; Bruins, F.M.; He, X.; Orro, K.; Peppelman, M.; van Erp, P.E.J.; de Jong, E.M.G.J.; Koenen, H.J.P.M.; van den Bogaard, E.H.; Seyger, M.M.B. Skin Surface Protein Detection by Transdermal Analysis Patches in Pediatric Psoriasis. Skin Pharmacol. Physiol. 2021, 34, 271–280. [Google Scholar] [CrossRef]
- Orro, K.; Salk, K.; Merkulova, A.; Abram, K.; Karelson, M.; Traks, T.; Neuman, T.; Spee, P.; Kingo, K. Non-Invasive Assessment of Skin Surface Proteins of Psoriasis Vulgaris Patients in Response to Biological Therapy. Int. J. Mol. Sci. 2023, 24, 16248. [Google Scholar] [CrossRef]
- Orro, K.; Salk, K.; Abram, K.; Arshavskaja, J.; Meikas, A.; Karelson, M.; Neuman, T.; Kingo, K.; Spee, P. Assessment of soluble skin surface protein levels for monitoring. Front. Med. 2023, 10, 1072160. [Google Scholar] [CrossRef]
- Tamilselvi, E.; Haripriya, D.; Hemamalini, M.; Pushpa, G.; Swapna, S. Association of disease severity with IL-1 levels in methotrexate-treated psoriasis patients. Scand. J. Immunol. 2013, 78, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Mee, J.B.; Cork, M.J.; di Giovine, F.S.; Duff, G.W.; Groves, R.W. Interleukin-1: A key inflammatory mediator in psoriasis? Cytokine 2006, 33, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Krzesicki, R.F.; Hatfield, C.A.; Bienkowski, M.J.; McGuire, J.C.; Winterrowd, G.E.; Chapman, D.L.; Berger, A.E.; McEwan, R.N.; Carter, D.B.; Chosay, J.G. Regulation of expression of IL-1 receptor antagonist protein in human synovial and dermal fibroblasts. J. Immunol. 1993, 150, 4008–4018. [Google Scholar] [CrossRef] [PubMed]
- McColl, S.R.; Paquin, R.; Ménard, C.; Beaulieu, A.D. Human neutrophils produce high levels of the interleukin 1 receptor antagonist in response to granulocyte/macrophage colony-stimulating factor and tumor necrosis factor alpha. J. Exp. Med. 1992, 176, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Janson, R.W.; Hance, K.R.; Arend, W.P. Production of IL-1 receptor antagonist by human in vitro-derived macrophages. Effects of lipopolysaccharide and granulocyte-macrophage colony-stimulating factor. J. Immunol. 1991, 147, 4218–4223. [Google Scholar] [CrossRef] [PubMed]
- Arend, W.P. Interleukin 1 receptor antagonist. A new member of the interleukin 1 family. J. Clin. Investig. 1991, 88, 1445–1451. [Google Scholar] [CrossRef] [PubMed]
- Yegorov, S.; Babenko, D.; Kozhakhmetov, S.; Akhmaltdinova, L.; Kadyrova, I.; Nurgozhina, A.; Nurgaziyev, M.; Good, S.V.; Hortelano, G.H.; Yermekbayeva, B.; et al. Psoriasis Is Associated with Elevated Gut IL-1α and Intestinal Microbiome Alterations. Front. Immunol. 2020, 11, 571319. [Google Scholar] [CrossRef]
- Boraschi, D.; Tagliabue, A. The interleukin-1 receptor family. Semin. Immunol. 2013, 25, 394–407. [Google Scholar] [CrossRef]
- Lücke, G. Interplay of a well-known medication and newly discovered transporters driving bacteria-induced inflammation in psoriasis. Allergy, 2024; online ahead of print. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).