The GgcxK325Q Mutation Does Not Affect the Calcium Homeostasis of the Epididymis and Male Fertility in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Generation of GgcxK325Q−/− Mice via CRISPR/Cas9 Technology
2.3. Fertility Assay
2.4. Total RNA Extraction and RT-qPCR
2.5. Western Blotting
2.6. Histological Analysis
2.7. Immunofluorescence and TUNEL
2.8. Computer-Assisted Sperm Analysis (CASA)
2.9. Ca2+ Measurement Assay
2.10. Phylogenetic Analyses
2.11. Statistical Analysis
3. Results
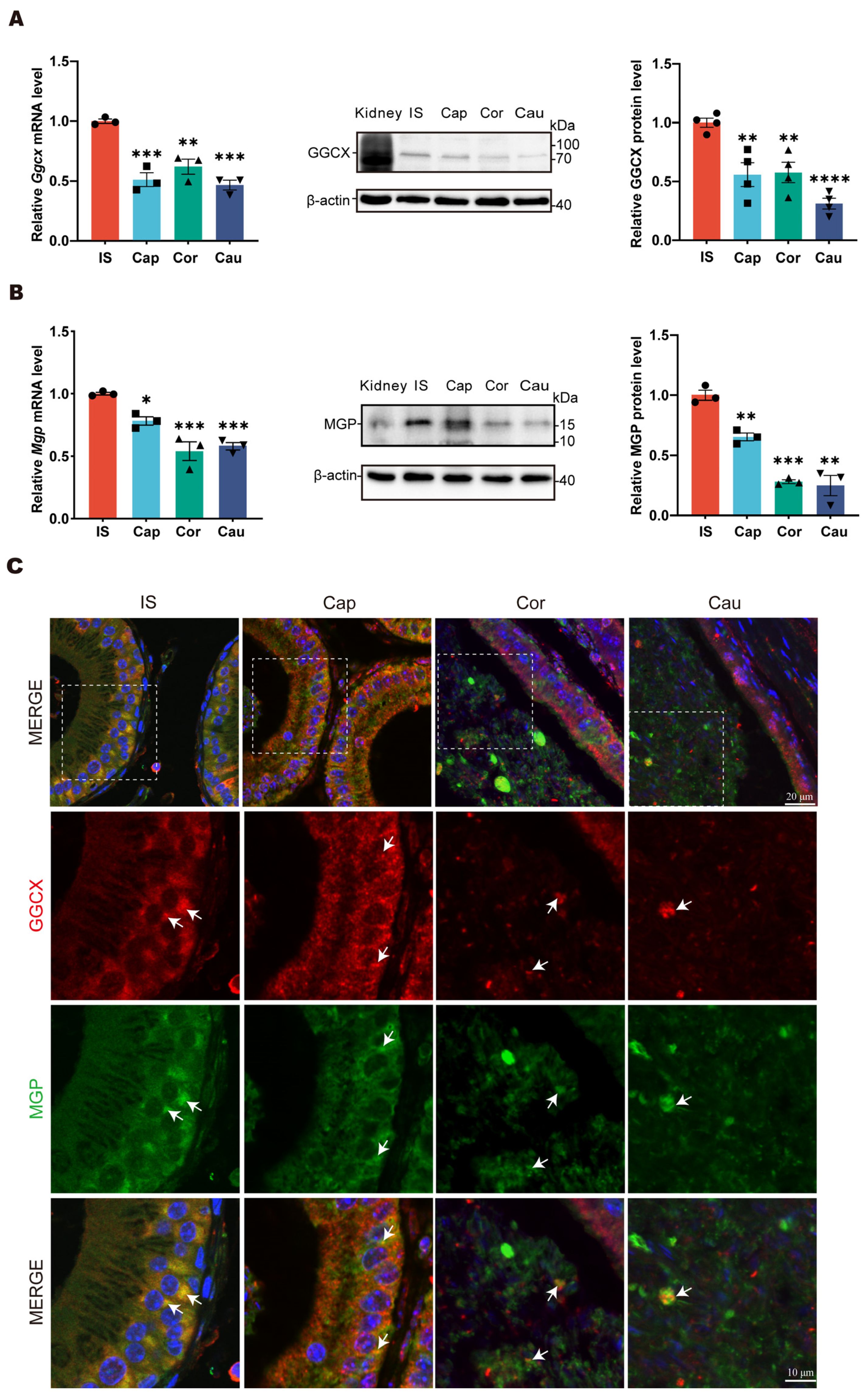
3.1. Expression Patterns of GGCX and MGP in Mouse Epididymis
3.2. Generation of GgcxK325Q Knock-in Mice
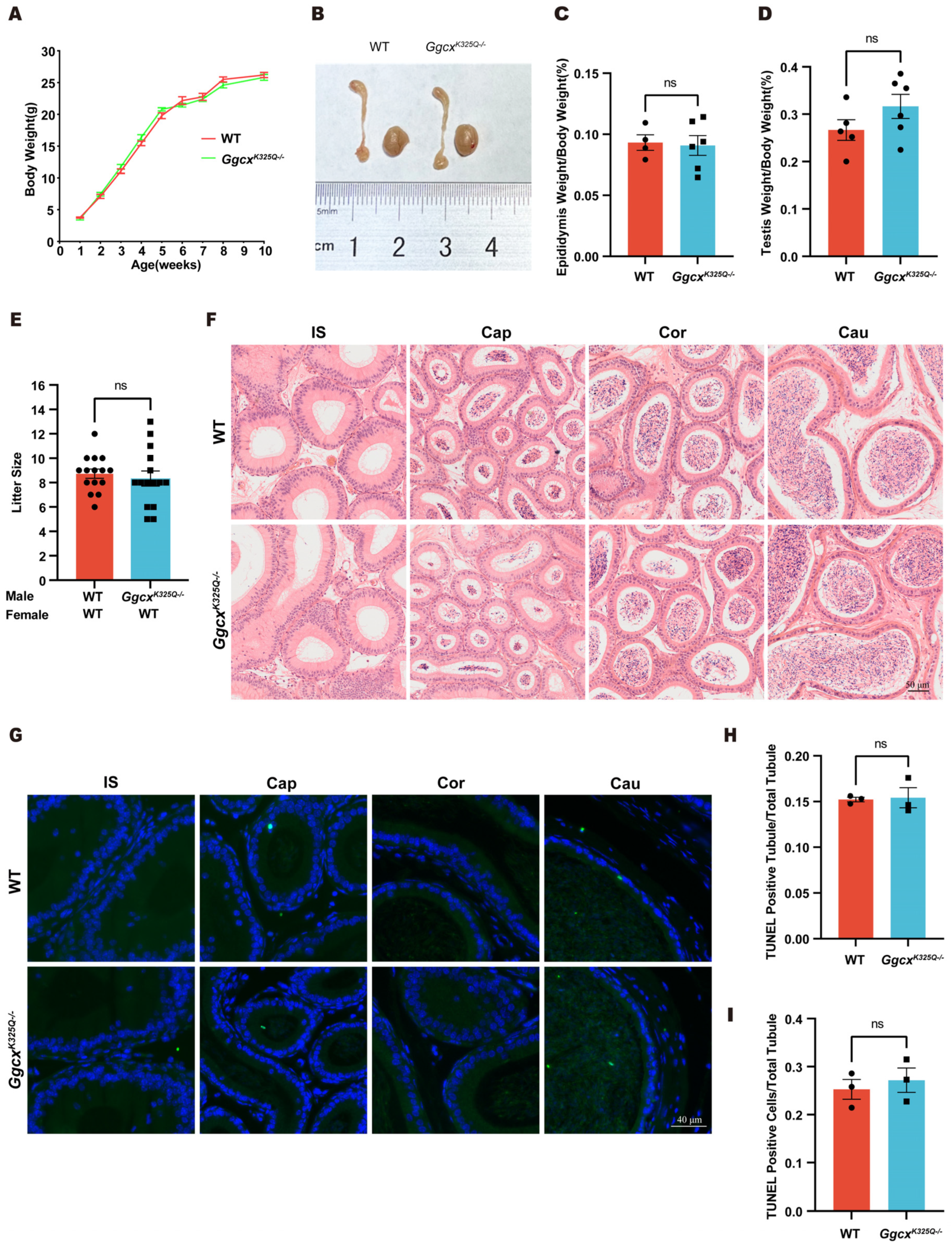
3.3. GgcxK325Q Does Not Affect the Normal Growth and Fertility of Mice
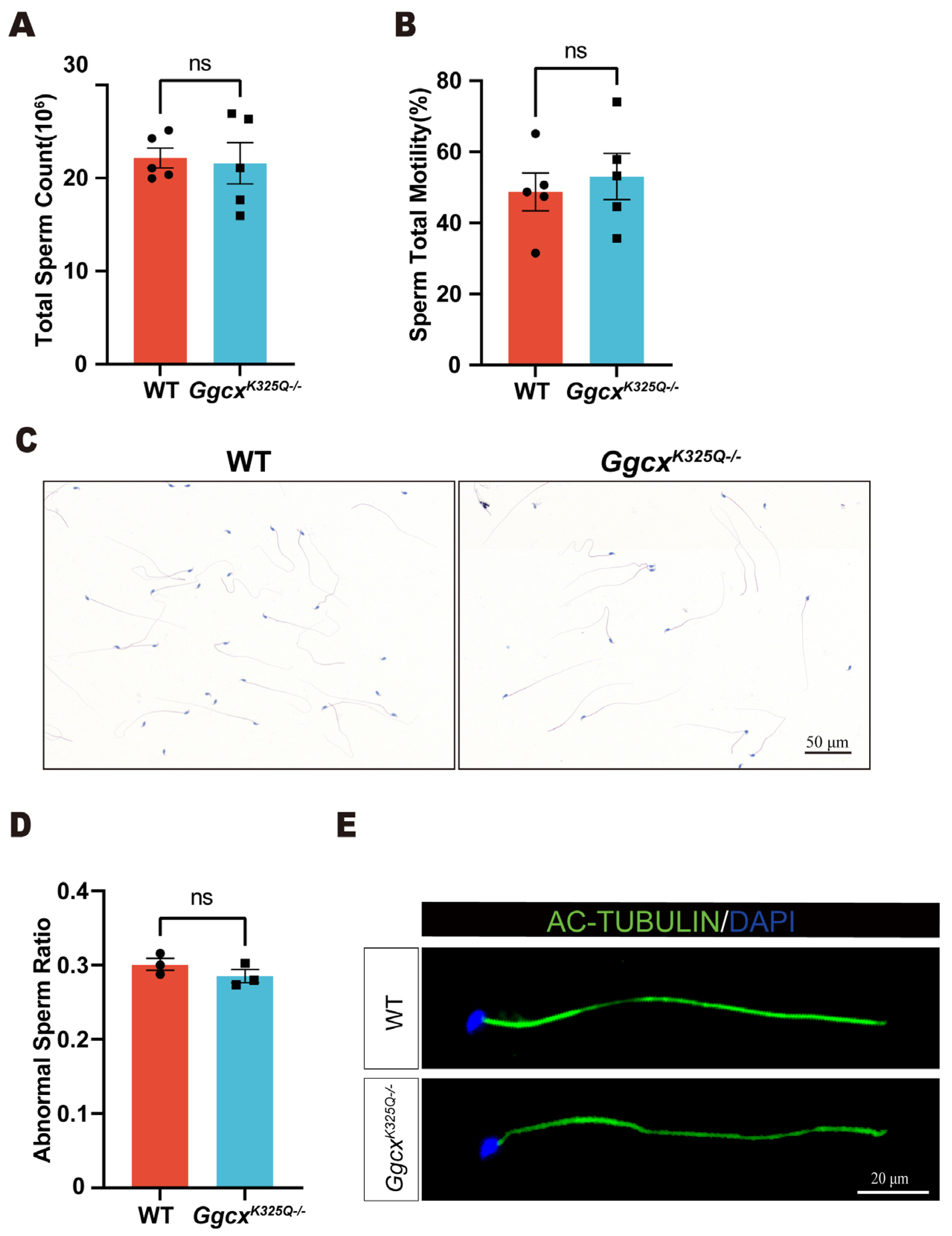
3.4. GgcxK325Q Does Not Affect Sperm Counts, Morphology, and Motility
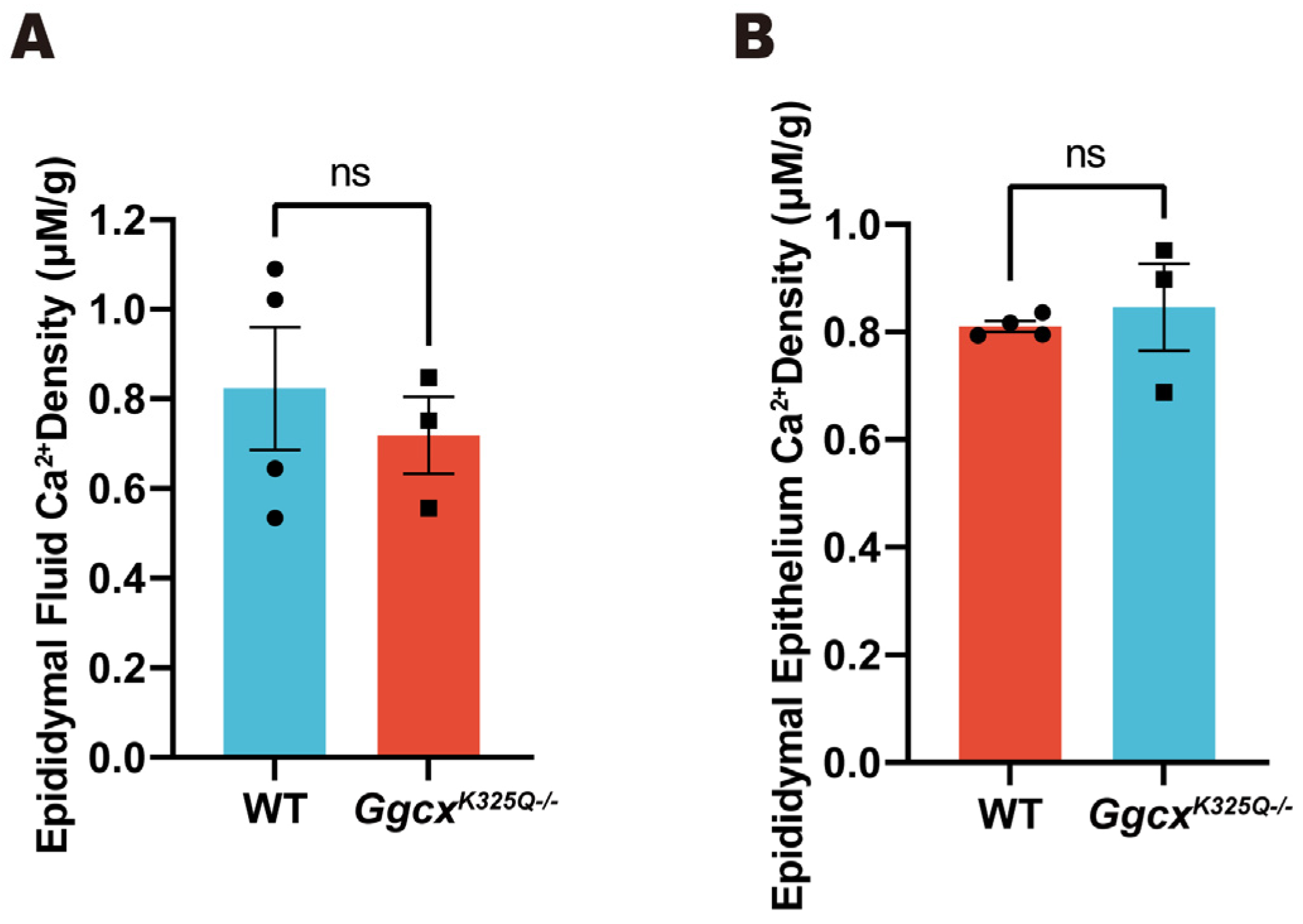
3.5. Calcium Homeostasis in Epididymal Luminal Is Normal in GgcxK325Q−/− Mice
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ali, W.; Deng, K.; Bian, Y.; Liu, Z.; Zou, H. Spectacular role of epididymis and bio-active cargo of nano-scale exosome in sperm maturation: A review. Biomed. Pharmacother. 2023, 164, 114889. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Ni, K.; Cai, Y.; Zhao, Q.; Shang, J.; Zhang, X.; Shen, S.; Xiong, C. Busulfan administration produces toxic effects on epididymal morphology and inhibits the expression of ZO-1 and vimentin in the mouse epididymis. Biosci. Rep. 2017, 37, BSR20171059. [Google Scholar] [CrossRef]
- Brandenburger, T.; Strehler, E.E.; Filoteo, A.G.; Caride, A.J.; Aumüller, G.; Post, H.; Schwarz, A.; Wilhelm, B. Switch of PMCA4 splice variants in bovine epididymis results in altered isoform expression during functional sperm maturation. J. Biol. Chem. 2011, 286, 7938–7946. [Google Scholar] [CrossRef]
- Weissgerber, P.; Kriebs, U.; Tsvilovskyy, V.; Olausson, J.; Kretz, O.; Stoerger, C.; Vennekens, R.; Wissenbach, U.; Middendorff, R.; Flockerzi, V.; et al. Male fertility depends on Ca2+ absorption by TRPV6 in epididymal epithelia. Sci. Signal. 2011, 4, ra27. [Google Scholar] [CrossRef]
- Laurentino, S.S.; Correia, S.; Cavaco, J.E.; Oliveira, P.F.; de Sousa, M.; Barros, A.; Socorro, S. Regucalcin, a calcium-binding protein with a role in male reproduction? Mol. Hum. Reprod. 2012, 18, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Miyata, H.; Satouh, Y.; Mashiko, D.; Muto, M.; Nozawa, K.; Shiba, K.; Fujihara, Y.; Isotani, A.; Inaba, K.; Ikawa, M. Sperm calcineurin inhibition prevents mouse fertility with implications for male contraceptive. Science 2015, 350, 442–445. [Google Scholar] [CrossRef]
- Tello-Mora, P.; Hernández-Cadena, L.; Pedraza, J.; López-Bayghen, E.; Quintanilla-Vega, B. Acrosome reaction and chromatin integrity as additional parameters of semen analysis to predict fertilization and blastocyst rates. Reprod. Biol. Endocrinol. RBE 2018, 16, 102. [Google Scholar] [CrossRef] [PubMed]
- Shum, W.; Zhang, B.L.; Cao, A.S.; Zhou, X.; Shi, S.M.; Zhang, Z.Y.; Gu, L.Y.; Shi, S. Calcium Homeostasis in the Epididymal Microenvironment: Is Extracellular Calcium a Cofactor for Matrix Gla Protein-Dependent Scavenging Regulated by Vitamins. Front. Cell Dev. Biol. 2022, 10, 827940. [Google Scholar] [CrossRef]
- Luo, G.; Ducy, P.; McKee, M.D.; Pinero, G.J.; Loyer, E.; Behringer, R.R.; Karsenty, G. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature 1997, 386, 78–81. [Google Scholar] [CrossRef]
- Marulanda, J.; Eimar, H.; McKee, M.D.; Berkvens, M.; Nelea, V.; Roman, H.; Borrás, T.; Tamimi, F.; Ferron, M.; Murshed, M. Matrix Gla protein deficiency impairs nasal septum growth, causing midface hypoplasia. J. Biol. Chem. 2017, 292, 11400–11412. [Google Scholar] [CrossRef]
- Ghosh, S.; Oldenburg, J.; Czogalla-Nitsche, K.J. The Role of GRP and MGP in the Development of Non-Hemorrhagic VKCFD1 Phenotypes. Int. J. Mol. Sci. 2022, 23, 798. [Google Scholar] [CrossRef]
- Ma, H.; Zhang, B.L.; Liu, B.Y.; Shi, S.; Gao, D.Y.; Zhang, T.C.; Shi, H.J.; Li, Z.; Shum, W.W. Vitamin K2-Dependent GGCX and MGP Are Required for Homeostatic Calcium Regulation of Sperm Maturation. iScience 2019, 14, 210–225. [Google Scholar] [CrossRef] [PubMed]
- Shiba, S.; Ikeda, K.; Horie-Inoue, K.; Azuma, K.; Hasegawa, T.; Amizuka, N.; Tanaka, T.; Takeiwa, T.; Shibata, Y.; Koji, T.; et al. Vitamin K-Dependent γ-Glutamyl Carboxylase in Sertoli Cells Is Essential for Male Fertility in Mice. Mol. Cell. Biol. 2021, 41, e00404-20. [Google Scholar] [CrossRef] [PubMed]
- Azuma, K.; Inoue, S. Multiple Modes of Vitamin K Actions in Aging-Related Musculoskeletal Disorders. Int. J. Mol. Sci. 2019, 20, 2844. [Google Scholar] [CrossRef] [PubMed]
- Tie, J.K.; Stafford, D.W. Structural and functional insights into enzymes of the vitamin K cycle. J. Thromb. Haemost. JTH 2016, 14, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.; Sun, H.; Raymond, R.M., Jr.; Furie, B.C.; Furie, B.; Bronstein, M.; Kaufman, R.J.; Westrick, R.; Ginsburg, D. Fatal hemorrhage in mice lacking gamma-glutamyl carboxylase. Blood 2007, 109, 5270–5275. [Google Scholar] [CrossRef]
- Dasi, M.A.; Gonzalez-Conejero, R.; Izquierdo, S.; Padilla, J.; Garcia, J.L.; Garcia-Barberá, N.; Argilés, B.; de la Morena-Barrio, M.E.; Hernández-Sánchez, J.M.; Hernández-Rivas, J.M.; et al. Uniparental disomy causes deficiencies of vitamin K-dependent proteins. J. Thromb. Haemost. JTH 2016, 14, 2410–2418. [Google Scholar] [CrossRef] [PubMed]
- Ferron, M.; Wei, J.; Yoshizawa, T.; Del Fattore, A.; DePinho, R.A.; Teti, A.; Ducy, P.; Karsenty, G. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell 2010, 142, 296–308. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.K.; Sowa, H.; Hinoi, E.; Ferron, M.; Ahn, J.D.; Confavreux, C.; Dacquin, R.; Mee, P.J.; McKee, M.D.; Jung, D.Y.; et al. Endocrine regulation of energy metabolism by the skeleton. Cell 2007, 130, 456–469. [Google Scholar] [CrossRef] [PubMed]
- Lacombe, J.; Ferron, M. VKORC1L1, An Enzyme Mediating the Effect of Vitamin K in Liver and Extrahepatic Tissues. Nutrients 2018, 10, 970. [Google Scholar] [CrossRef]
- Willems, B.A.; Vermeer, C.; Reutelingsperger, C.P.; Schurgers, L.J. The realm of vitamin K dependent proteins: Shifting from coagulation toward calcification. Mol. Nutr. Food Res. 2014, 58, 1620–1635. [Google Scholar] [CrossRef] [PubMed]
- Schurgers, L.J.; Spronk, H.M.; Skepper, J.N.; Hackeng, T.M.; Shanahan, C.M.; Vermeer, C.; Weissberg, P.L.; Proudfoot, D. Post-translational modifications regulate matrix Gla protein function: Importance for inhibition of vascular smooth muscle cell calcification. J. Thromb. Haemost. JTH 2007, 5, 2503–2511. [Google Scholar] [CrossRef] [PubMed]
- Okubo, Y.; Masuyama, R.; Iwanaga, A.; Koike, Y.; Kuwatsuka, Y.; Ogi, T.; Yamamoto, Y.; Endo, Y.; Tamura, H.; Utani, A. Calcification in dermal fibroblasts from a patient with GGCX syndrome accompanied by upregulation of osteogenic molecules. PLoS ONE 2017, 12, e0177375. [Google Scholar] [CrossRef] [PubMed]
- Schurgers, L.J.; Uitto, J.; Reutelingsperger, C.P. Vitamin K-dependent carboxylation of matrix Gla-protein: A crucial switch to control ectopic mineralization. Trends Mol. Med. 2013, 19, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Proudfoot, D.; Shanahan, C.M. Molecular mechanisms mediating vascular calcification: Role of matrix Gla protein. Nephrology 2006, 11, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Watzka, M.; Geisen, C.; Scheer, M.; Wieland, R.; Wiegering, V.; Dörner, T.; Laws, H.J.; Gümrük, F.; Hanalioglu, S.; Unal, S.; et al. Bleeding and non-bleeding phenotypes in patients with GGCX gene mutations. Thromb. Res. 2014, 134, 856–865. [Google Scholar] [CrossRef] [PubMed]
- Vlasschaert, C.; Goss, C.J.; Pilkey, N.G.; McKeown, S.; Holden, R.M. Vitamin K Supplementation for the Prevention of Cardiovascular Disease: Where Is the Evidence? A Systematic Review of Controlled Trials. Nutrients 2020, 12, 2909. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Tu, G.; Wang, J.; Wang, Y.; Wu, P.; Yu, L.; Li, Z.; Yu, X. MLKL signaling regulates macrophage polarization in acute pancreatitis through CXCL10. Cell Death Dis. 2023, 14, 155. [Google Scholar] [CrossRef]
- Hu, S.G.; Liang, A.J.; Yao, G.X.; Li, X.Q.; Zou, M.; Liu, J.W.; Sun, Y. The dynamic metabolomic changes throughout mouse epididymal lumen fluid potentially contribute to sperm maturation. Andrology 2018, 6, 247–255. [Google Scholar] [CrossRef]
- Sallam, T.; Cheng, H.; Demer, L.L.; Tintut, Y. Regulatory circuits controlling vascular cell calcification. Cell. Mol. Life Sci. CMLS 2013, 70, 3187–3197. [Google Scholar] [CrossRef]
- Wan, Z.; Bai, X.; Wang, X.; Guo, X.; Wang, X.; Zhai, M.; Fu, Y.; Liu, Y.; Zhang, P.; Zhang, X.; et al. Mgp High-Expressing MSCs Orchestrate the Osteoimmune Microenvironment of Collagen/Nanohydroxyapatite-Mediated Bone Regeneration. Adv. Sci. 2024, e2308986. [Google Scholar] [CrossRef] [PubMed]
- Sarosiak, A.; Oziębło, D.; Udziela, M.; Vermeer, C.; Malejczyk, J.; Szaflik, J.P.; Ołdak, M. High expression of Matrix Gla Protein in Schnyder corneal dystrophy patients points to an active role of vitamin K in corneal health. Acta Ophthalmol. 2021, 99, e171–e177. [Google Scholar] [CrossRef] [PubMed]
- Shanahan, C.M.; Cary, N.R.; Metcalfe, J.C.; Weissberg, P.L. High expression of genes for calcification-regulating proteins in human atherosclerotic plaques. J. Clin. Investig. 1994, 93, 2393–2402. [Google Scholar] [CrossRef] [PubMed]
- Schuh, K.; Cartwright, E.J.; Jankevics, E.; Bundschu, K.; Liebermann, J.; Williams, J.C.; Armesilla, A.L.; Emerson, M.; Oceandy, D.; Knobeloch, K.P.; et al. Plasma membrane Ca2+ ATPase 4 is required for sperm motility and male fertility. J. Biol. Chem. 2004, 279, 28220–28226. [Google Scholar] [CrossRef] [PubMed]
- Luconi, M.; Krausz, C.; Forti, G.; Baldi, E. Extracellular calcium negatively modulates tyrosine phosphorylation and tyrosine kinase activity during capacitation of human spermatozoa. Biol. Reprod. 1996, 55, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Okunade, G.W.; Miller, M.L.; Pyne, G.J.; Sutliff, R.L.; O’Connor, K.T.; Neumann, J.C.; Andringa, A.; Miller, D.A.; Prasad, V.; Doetschman, T.; et al. Targeted ablation of plasma membrane Ca2+-ATPase (PMCA) 1 and 4 indicates a major housekeeping function for PMCA1 and a critical role in hyperactivated sperm motility and male fertility for PMCA4. J. Biol. Chem. 2004, 279, 33742–33750. [Google Scholar] [CrossRef] [PubMed]
- Jiao, S.Y.; Yang, Y.H.; Chen, S.R. Molecular genetics of infertility: Loss-of-function mutations in humans and corresponding knockout/mutated mice. Hum. Reprod. Update 2021, 27, 154–189. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Jin, D.Y.; Chen, X.; Schurgers, L.J.; Stafford, D.W.; Tie, J.K. γ-Glutamyl carboxylase mutations differentially affect the biological function of vitamin K-dependent proteins. Blood 2021, 137, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Ben Khelifa, M.; Coutton, C.; Zouari, R.; Karaouzène, T.; Rendu, J.; Bidart, M.; Yassine, S.; Pierre, V.; Delaroche, J.; Hennebicq, S.; et al. Mutations in DNAH1, which encodes an inner arm heavy chain dynein, lead to male infertility from multiple morphological abnormalities of the sperm flagella. Am. J. Hum. Genet. 2014, 94, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Neesen, J.; Kirschner, R.; Ochs, M.; Schmiedl, A.; Habermann, B.; Mueller, C.; Holstein, A.F.; Nuesslein, T.; Adham, I.; Engel, W. Disruption of an inner arm dynein heavy chain gene results in asthenozoospermia and reduced ciliary beat frequency. Hum. Mol. Genet. 2001, 10, 1117–1128. [Google Scholar] [CrossRef]
- Papoti, D.; Yen, C.C.; Mackel, J.B.; Merkle, H.; Silva, A.C. An embedded four-channel receive-only RF coil array for fMRI experiments of the somatosensory pathway in conscious awake marmosets. NMR Biomed. 2013, 26, 1395–1402. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, M.; Cheng, P.; Liu, B.; Zhao, Y.; Gao, T.; Li, Z. The GgcxK325Q Mutation Does Not Affect the Calcium Homeostasis of the Epididymis and Male Fertility in Mice. Curr. Issues Mol. Biol. 2024, 46, 5052-5065. https://doi.org/10.3390/cimb46060303
Xiong M, Cheng P, Liu B, Zhao Y, Gao T, Li Z. The GgcxK325Q Mutation Does Not Affect the Calcium Homeostasis of the Epididymis and Male Fertility in Mice. Current Issues in Molecular Biology. 2024; 46(6):5052-5065. https://doi.org/10.3390/cimb46060303
Chicago/Turabian StyleXiong, Mingxiang, Pang Cheng, Bo Liu, Yanqiu Zhao, Ting Gao, and Zhen Li. 2024. "The GgcxK325Q Mutation Does Not Affect the Calcium Homeostasis of the Epididymis and Male Fertility in Mice" Current Issues in Molecular Biology 46, no. 6: 5052-5065. https://doi.org/10.3390/cimb46060303
APA StyleXiong, M., Cheng, P., Liu, B., Zhao, Y., Gao, T., & Li, Z. (2024). The GgcxK325Q Mutation Does Not Affect the Calcium Homeostasis of the Epididymis and Male Fertility in Mice. Current Issues in Molecular Biology, 46(6), 5052-5065. https://doi.org/10.3390/cimb46060303






