Glutathione in HIV-Associated Neurocognitive Disorders
Abstract
1. Introduction
2. Impact of HIV on the Brain
2.1. HIV: Accessing the Cell
2.2. HIV: Accessing the Brain
2.3. Destruction of the Blood–Brain Barrier
2.3.1. Endothelial Cells
2.3.2. Tight Junctions
2.3.3. Astrocyte End-Feet
2.4. HIV Reservoirs
2.5. Reservoir Reactivation
2.6. Inflammatory Processes
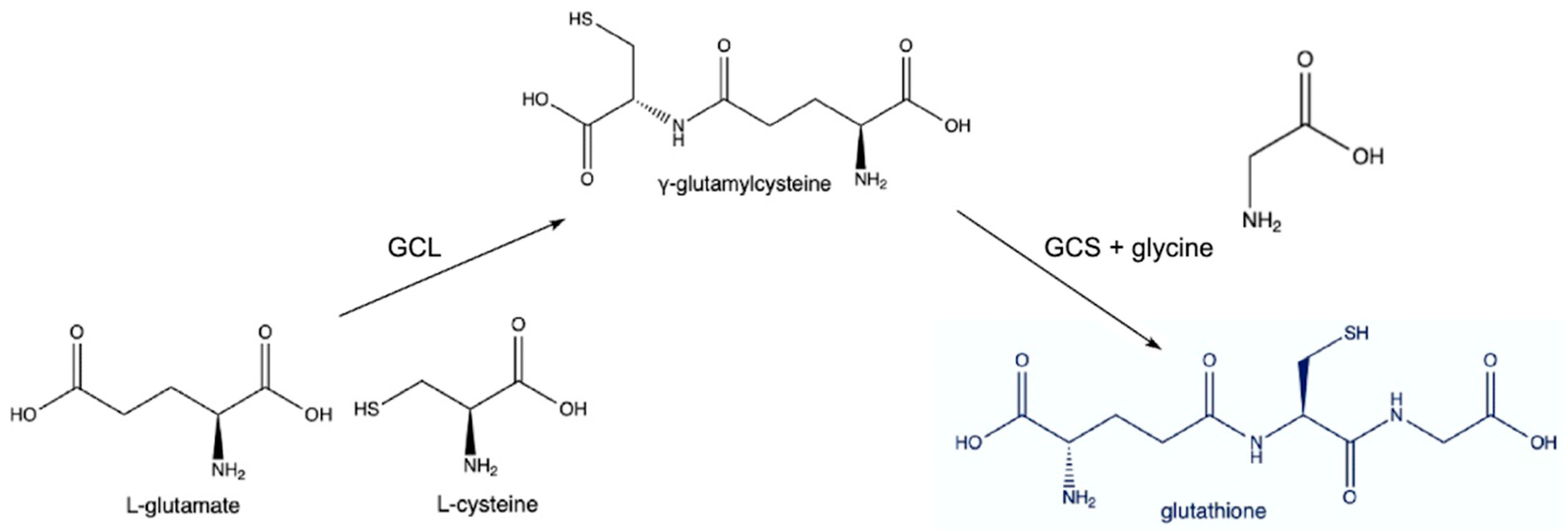
3. Brief Overview of Glutathione
3.1. Synthesis
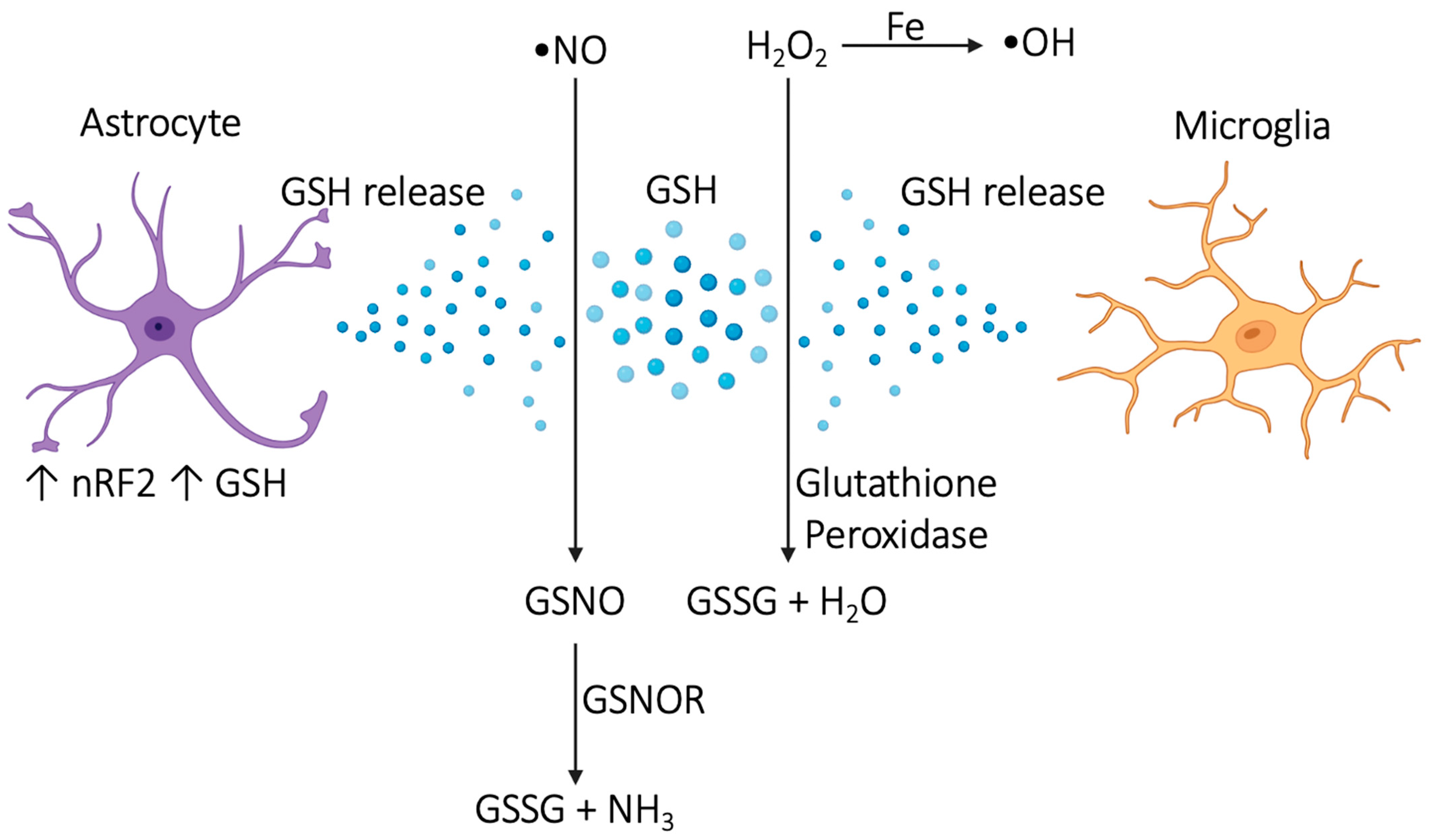
3.2. Antioxidant Mechanisms and Neuroprotection
3.3. Routes of Delivery
4. Glutathione in HIV-Associated Dementia (HIV Encephalitis)
5. Glutathione in HIV Peripheral Neuropathy
6. Glutathione in Lymphomas
7. Glutathione in Neurosyphilis
8. Glutathione in Secondary Viral, Fungal, and Parasitic Brain Infections
9. Glutathione in Vacuolar Myelopathy
10. Glutathione in Psychological Conditions Related to HIV
10.1. Post-Traumatic Stress Disorder (PTSD)
10.2. Substance Use Disorder
10.3. Sleep Disturbance
10.4. Psychosis
10.5. AIDS Mania
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oladeji, B.D.; Yosief, S.; Robertson, K. Global NeuroAIDS. In Encyclopedia of AIDS; Hope, T.J., Stevenson, M., Richman, D., Eds.; Springer: New York, NY, USA, 2015; pp. 1–10. ISBN 978-1-4614-9610-6. [Google Scholar]
- Wang, Y.; Liu, M.; Lu, Q.; Farrell, M.; Lappin, J.M.; Shi, J.; Lu, L.; Bao, Y. Global Prevalence and Burden of HIV-Associated Neurocognitive Disorder. Neurology 2020, 95, e2610–e2621. [Google Scholar] [CrossRef] [PubMed]
- Saloner, R.; Cysique, L.A. HIV-Associated Neurocognitive Disorders: A Global Perspective. J. Int. Neuropsychol. Soc. 2017, 23, 860–869. [Google Scholar] [CrossRef] [PubMed]
- Heaton, R.K.; Clifford, D.B.; Franklin, D.R.; Woods, S.P.; Ake, C.; Vaida, F.; Ellis, R.J.; Letendre, S.L.; Marcotte, T.D.; Atkinson, J.H.; et al. HIV-Associated Neurocognitive Disorders Persist in the Era of Potent Antiretroviral Therapy. Neurology 2010, 75, 2087–2096. [Google Scholar] [CrossRef] [PubMed]
- Nichols, S.L.; Bethel, J.; Kapogiannis, B.G.; Li, T.; Woods, S.P.; Patton, E.D.; Ren, W.; Thornton, S.E.; Major-Wilson, H.O.; Puga, A.M.; et al. Antiretroviral Treatment Initiation Does Not Differentially Alter Neurocognitive Functioning over Time in Youth with Behaviorally Acquired HIV. J. Neurovirol. 2016, 22, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Robertson, K.; Jiang, H.; Kumwenda, J.; Supparatpinyo, K.; Evans, S.; Campbell, T.B.; Price, R.; Tripathy, S.; Kumarasamy, N.; La Rosa, A.; et al. Improved Neuropsychological and Neurological Functioning Across Three Antiretroviral Regimens in Diverse Resource-Limited Settings: AIDS Clinical Trials Group Study A5199, the International Neurological Study. Clin. Infect. Dis. 2012, 55, 868–876. [Google Scholar] [CrossRef] [PubMed]
- Ly, J.; Lagman, M.; Saing, T.; Singh, M.K.; Tudela, E.V.; Morris, D.; Anderson, J.; Daliva, J.; Ochoa, C.; Patel, N.; et al. Liposomal Glutathione Supplementation Restores TH1 Cytokine Response to Mycobacterium Tuberculosis Infection in HIV-Infected Individuals. J. Interferon Cytokine Res. Off. J. Int. Soc. Interferon Cytokine Res. 2015, 35, 875–887. [Google Scholar] [CrossRef] [PubMed]
- Kenchappa, V.; Cao, R.; Venketaraman, V.; Betageri, G.V. Liposomes as Carriers for the Delivery of Efavirenz in Combination with Glutathione—An Approach to Combat Opportunistic Infections. Appl. Sci. 2022, 12, 1468. [Google Scholar] [CrossRef] [PubMed]
- Eligini, S.; Munno, M.; Atlas, D.; Banfi, C. N-Acetylcysteine Amide AD4/NACA and Thioredoxin Mimetic Peptides Inhibit Platelet Aggregation and Protect against Oxidative Stress. Antioxidants 2023, 12, 1395. [Google Scholar] [CrossRef] [PubMed]
- Rumbaugh, J.A.; Steiner, J.; Sacktor, N.; Nath, A. Developing Neuroprotective Strategies for Treatment of HIV-Associated Neurocognitive Dysfunction. Future HIV Ther. 2008, 2, 271–280. [Google Scholar] [CrossRef]
- Lipoic Acid Increases Glutathione Production and Enhances the Effect of Mercury in Human Cell Lines-PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/12049840/ (accessed on 6 May 2024).
- Kalamkar, S.; Acharya, J.; Kolappurath Madathil, A.; Gajjar, V.; Divate, U.; Karandikar-Iyer, S.; Goel, P.; Ghaskadbi, S. Randomized Clinical Trial of How Long-Term Glutathione Supplementation Offers Protection from Oxidative Damage and Improves HbA1c in Elderly Type 2 Diabetic Patients. Antioxidants 2022, 11, 1026. [Google Scholar] [CrossRef]
- HIV and AIDS. Available online: https://www.who.int/news-room/fact-sheets/detail/hiv-aids (accessed on 1 March 2024).
- Tegegne, D.; Mamo, G.; Negash, B.; Habte, S.; Gobena, T.; Letta, S. Poor Adherence to Highly Active Antiretroviral Therapy and Associated Factors among People Living with HIV in Eastern Ethiopia. SAGE Open Med. 2022, 10, 20503121221104429. [Google Scholar] [CrossRef] [PubMed]
- Burger, C.; Burger, R.; van Doorslaer, E. The Health Impact of Free Access to Antiretroviral Therapy in South Africa. Soc. Sci. Med. 2022, 299, 114832. [Google Scholar] [CrossRef] [PubMed]
- Badri, M.; Maartens, G.; Mandalia, S.; Bekker, L.-G.; Penrod, J.R.; Platt, R.W.; Wood, R.; Beck, E.J. Cost-Effectiveness of Highly Active Antiretroviral Therapy in South Africa. PLoS Med. 2006, 3, e4. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.A.; Tan, N.; Limaye, N.; Irungu, E.; Barnabas, R.V. The Cost of Differentiated HIV Antiretroviral Therapy Delivery strategies in Sub-Saharan Africa: A Systematic Review. J. Acquir. Immune Defic. Syndr. 1999 2019, 82, S339–S347. [Google Scholar] [CrossRef] [PubMed]
- Ivanoff, L.A.; Dubay, J.W.; Morris, J.F.; Roberts, S.J.; Gutshall, L.; Sternberg, E.J.; Hunter, E.; Matthews, T.J.; Petteway, S.R. V3 Loop Region of the HIV-1 Gp120 Envelope Protein Is Essential for Virus Infectivity. Virology 1992, 187, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Wilen, C.B.; Tilton, J.C.; Doms, R.W. HIV: Cell Binding and Entry. Cold Spring Harb. Perspect. Med. 2012, 2, a006866. [Google Scholar] [CrossRef]
- HIV Encephalitis-like Multinucleated Giant Cells in a Nodal Lymphoma in AIDS-GRAY-1990-Histopathology-Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1365-2559.1990.tb01149.x (accessed on 6 May 2024).
- Wu, M.-C.; Wang, E.Y.; Lai, T.W. TAT Peptide at Treatment-Level Concentrations Crossed Brain Endothelial Cell Monolayer Independent of Receptor-Mediated Endocytosis or Peptide-Inflicted Barrier Disruption. PLoS ONE 2023, 18, e0292681. [Google Scholar] [CrossRef] [PubMed]
- Kanmogne, G.D.; Schall, K.; Leibhart, J.; Knipe, B.; Gendelman, H.E.; Persidsky, Y. HIV-1 Gp120 Compromises Blood-Brain Barrier Integrity and Enhances Monocyte Migration across Blood-Brain Barrier: Implication for Viral Neuropathogenesis. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2007, 27, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yang, S.; Luo, H.; Zeng, L.; Ye, L.; Lu, Y. Quantitative Evaluation of Monocyte Transmigration into the Brain Following Chemical Opening of the Blood–Brain Barrier in Mice. Brain Res. 2006, 1098, 79–85. [Google Scholar] [CrossRef]
- Gryshyna, A.; Chatterjee, T.; Arora, I.; Heath, S.; Goodin, B.; Aggarwal, S. Role of Mast Cells in HIV-Associated Chronic Widespread Pain. J. Pain 2022, 23, 3. [Google Scholar] [CrossRef]
- Huang, L.; Liu, M.; Jiang, W.; Ding, H.; Han, Y.; Wen, M.; Li, Y.; Liu, X.; Zeng, H. Bradykinin/Bradykinin 1 Receptor Promotes Brain Microvascular Endothelial Cell Permeability and Proinflammatory Cytokine Release by Downregulating Wnt3a. J. Biochem. Mol. Toxicol. 2022, 36, e23213. [Google Scholar] [CrossRef]
- Wallet, C.; De Rovere, M.; Van Assche, J.; Daouad, F.; De Wit, S.; Gautier, V.; Mallon, P.W.G.; Marcello, A.; Van Lint, C.; Rohr, O.; et al. Microglial Cells: The Main HIV-1 Reservoir in the Brain. Front. Cell Infect. Microbiol. 2019, 9, 362. [Google Scholar] [CrossRef] [PubMed]
- Kovalevich, J.; Langford, D. Neuronal Toxicity in HIV CNS Disease. Future Virol. 2012, 7, 687–698. [Google Scholar] [CrossRef]
- Ivey, N.S.; MacLean, A.G.; Lackner, A.A. AIDS and the Blood-Brain Barrier. J. Neurovirol. 2009, 15, 111–122. [Google Scholar] [CrossRef]
- Borgmann, K.; Ghorpade, A. Methamphetamine Augments Concurrent Astrocyte Mitochondrial Stress, Oxidative Burden, and Antioxidant Capacity: Tipping the Balance in HIV-Associated Neurodegeneration. Neurotox. Res. 2018, 33, 433–447. [Google Scholar] [CrossRef] [PubMed]
- Bozzelli, P.L.; Yin, T.; Avdoshina, V.; Mocchetti, I.; Conant, K.E.; Maguire-Zeiss, K.A. HIV-1 Tat Promotes Astrocytic Release of CCL2 through MMP/PAR-1 Signaling. Glia 2019, 67, 1719–1729. [Google Scholar] [CrossRef]
- Toborek, M.; Lee, Y.W.; Pu, H.; Malecki, A.; Flora, G.; Garrido, R.; Hennig, B.; Bauer, H.-C.; Nath, A. HIV-Tat Protein Induces Oxidative and Inflammatory Pathways in Brain Endothelium. J. Neurochem. 2003, 84, 169–179. [Google Scholar] [CrossRef]
- Wan, J.; Zhang, G.; Li, X.; Qiu, X.; Ouyang, J.; Dai, J.; Min, S. Matrix Metalloproteinase 3: A Promoting and Destabilizing Factor in the Pathogenesis of Disease and Cell Differentiation. Front. Physiol. 2021, 12, 663978. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, N.K.; Williams, R.; Callen, S.; Zien, C.; Narayan, O.; Buch, S. Roles of MCP-1 in Development of HIV-Dementia. Front. Biosci. J. Virtual Libr. 2008, 13, 3913–3918. [Google Scholar] [CrossRef]
- András, I.E.; Pu, H.; Deli, M.A.; Nath, A.; Hennig, B.; Toborek, M. HIV-1 Tat Protein Alters Tight Junction Protein Expression and Distribution in Cultured Brain Endothelial Cells. J. Neurosci. Res. 2003, 74, 255–265. [Google Scholar] [CrossRef]
- Qian, Y.-W.; Li, C.; Jiang, A.-P.; Ge, S.; Gu, P.; Fan, X.; Li, T.-S.; Jin, X.; Wang, J.-H.; Wang, Z.-L. HIV-1 Gp120 Glycoprotein Interacting with Dendritic Cell-Specific Intercellular Adhesion Molecule 3-Grabbing Non-Integrin (DC-SIGN) Down-Regulates Tight Junction Proteins to Disrupt the Blood Retinal Barrier and Increase Its Permeability. J. Biol. Chem. 2016, 291, 22977–22987. [Google Scholar] [CrossRef] [PubMed]
- Sreekumar, P.G.; Ferrington, D.A.; Kannan, R. Glutathione Metabolism and the Novel Role of Mitochondrial GSH in Retinal Degeneration. Antioxidants 2021, 10, 661. [Google Scholar] [CrossRef] [PubMed]
- Eugenin, E.A.; Clements, J.E.; Zink, M.C.; Berman, J.W. Human Immunodeficiency Virus Infection of Human Astrocytes Disrupts Blood–Brain Barrier Integrity by a Gap Junction-Dependent Mechanism. J. Neurosci. 2011, 31, 9456–9465. [Google Scholar] [CrossRef] [PubMed]
- Valdebenito, R.; Ruminot, I.; Garrido-Gerter, P.; Fernández-Moncada, I.; Forero-Quintero, L.; Alegría, K.; Becker, H.M.; Deitmer, J.W.; Barros, L.F. Targeting of Astrocytic Glucose Metabolism by Beta-Hydroxybutyrate. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2016, 36, 1813–1822. [Google Scholar] [CrossRef] [PubMed]
- Gray, L.R.; Roche, M.; Flynn, J.K.; Wesselingh, S.L.; Gorry, P.R.; Churchill, M.J. Is the Central Nervous System a Reservoir of HIV-1? Curr. Opin. HIV AIDS 2014, 9, 552–558. [Google Scholar] [CrossRef]
- Valdebenito, S.; Castellano, P.; Ajasin, D.; Eugenin, E.A. Astrocytes Are HIV Reservoirs in the Brain: A Cell Type with Poor HIV Infectivity and Replication but Efficient Cell-to-Cell Viral Transfer. J. Neurochem. 2021, 158, 429–443. [Google Scholar] [CrossRef] [PubMed]
- Brandmann, M.; Tulpule, K.; Schmidt, M.M.; Dringen, R. The Antiretroviral Protease Inhibitors Indinavir and Nelfinavir Stimulate Mrp1-Mediated GSH Export from Cultured Brain Astrocytes. J. Neurochem. 2012, 120, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Vainchtein, I.D.; Molofsky, A.V. Astrocytes and Microglia: In Sickness and in Health. Trends Neurosci. 2020, 43, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Green, M.V.; Thayer, S.A. HIV Gp120-Induced Neuroinflammation Potentiates NMDA Receptors to Overcome Basal Suppression of Inhibitory Synapses by P38 MAPK. J. Neurochem. 2019, 148, 499–515. [Google Scholar] [CrossRef]
- Serantes, R.; Arnalich, F.; Figueroa, M.; Salinas, M.; Andrés-Mateos, E.; Codoceo, R.; Renart, J.; Matute, C.; Cavada, C.; Cuadrado, A.; et al. Interleukin-1beta Enhances GABAA Receptor Cell-Surface Expression by a Phosphatidylinositol 3-Kinase/Akt Pathway: Relevance to Sepsis-Associated Encephalopathy. J. Biol. Chem. 2006, 281, 14632–14643. [Google Scholar] [CrossRef]
- Ogino, K.; Wang, D.-H. Biomarkers of Oxidative/Nitrosative Stress: An Approach to Disease Prevention. Acta Med. Okayama 2007, 61, 181–189. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Rossi, R.; Colombo, R.; Giustarini, D.; Milzani, A. Biomarkers of Oxidative Damage in Human Disease. Clin. Chem. 2006, 52, 601–623. [Google Scholar] [CrossRef] [PubMed]
- Lyons, J.; Rauh-Pfeiffer, A.; Yu, Y.M.; Lu, X.-M.; Zurakowski, D.; Tompkins, R.G.; Ajami, A.M.; Young, V.R.; Castillo, L. Blood Glutathione Synthesis Rates in Healthy Adults Receiving a Sulfur Amino Acid-Free Diet. Proc. Natl. Acad. Sci. USA 2000, 97, 5071–5076. [Google Scholar] [CrossRef] [PubMed]
- Drukarch, B.; Schepens, E.; Jongenelen, C.A.; Stoof, J.C.; Langeveld, C.H. Astrocyte-Mediated Enhancement of Neuronal Survival Is Abolished by Glutathione Deficiency. Brain Res. 1997, 770, 123–130. [Google Scholar] [CrossRef] [PubMed]
- McGann, J.C.; Mandel, G. Neuronal Activity Induces Glutathione Metabolism Gene Expression in Astrocytes. Glia 2018, 66, 2024–2039. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Vartiainen, N.E.; Ying, W.; Chan, P.H.; Koistinaho, J.; Swanson, R.A. Astrocytes Protect Neurons from Nitric Oxide Toxicity by a Glutathione-Dependent Mechanism. J. Neurochem. 2001, 77, 1601–1610. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, K. Glutathione in the Brain. Int. J. Mol. Sci. 2021, 22, 5010. [Google Scholar] [CrossRef] [PubMed]
- Tirichen, H.; Yaigoub, H.; Xu, W.; Wu, C.; Li, R.; Li, Y. Mitochondrial Reactive Oxygen Species and Their Contribution in Chronic Kidney Disease Progression through Oxidative Stress. Front. Physiol. 2021, 12, 627837. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.-H.; Wang, H.-C.; Chang, C.-J.; Lee, S.-Y. Mitochondrial Glutathione in Cellular Redox Homeostasis and Disease Manifestation. Int. J. Mol. Sci. 2024, 25, 1314. [Google Scholar] [CrossRef]
- Simunkova, M.; Barbierikova, Z.; Jomova, K.; Hudecova, L.; Lauro, P.; Alwasel, S.H.; Alhazza, I.; Rhodes, C.J.; Valko, M. Antioxidant vs. Prooxidant Properties of the Flavonoid, Kaempferol, in the Presence of Cu(II) Ions: A ROS-Scavenging Activity, Fenton Reaction and DNA Damage Study. Int. J. Mol. Sci. 2021, 22, 1619. [Google Scholar] [CrossRef]
- Coco-Bassey, S.B.; Asemota, E.A.; Okoroiwu, H.U.; Etura, J.E.; Efiong, E.E.; Inyang, I.J.; Uko, E.K. Glutathione, Glutathione Peroxidase and Some Hematological Parameters of HIV-Seropositive Subjects Attending Clinic in University of Calabar Teaching Hospital, Calabar, Nigeria. BMC Infect. Dis. 2019, 19, 944. [Google Scholar] [CrossRef] [PubMed]
- Valdivia, A.; Ly, J.; Gonzalez, L.; Hussain, P.; Saing, T.; Islamoglu, H.; Pearce, D.; Ochoa, C.; Venketaraman, V. Restoring Cytokine Balance in HIV-Positive Individuals with Low CD4 T Cell Counts. AIDS Res. Hum. Retroviruses 2017, 33, 905–918. [Google Scholar] [CrossRef] [PubMed]
- Aebi, S.; Assereto, R.; Lauterburg, B.H. High-Dose Intravenous Glutathione in Man. Pharmacokinetics and Effects on Cyst(e)Ine in Plasma and Urine. Eur. J. Clin. Investig. 1991, 21, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Mischley, L.K.; Conley, K.E.; Shankland, E.G.; Kavanagh, T.J.; Rosenfeld, M.E.; Duda, J.E.; White, C.C.; Wilbur, T.K.; De La Torre, P.U.; Padowski, J.M. Central Nervous System Uptake of Intranasal Glutathione in Parkinson’s Disease. NPJ Park. Dis. 2016, 2, 16002. [Google Scholar] [CrossRef] [PubMed]
- Cornford, E.M.; Braun, L.D.; Crane, P.D.; Oldendorf, W.H. Blood-Brain Barrier Restriction of Peptides and the Low Uptake of Enkephalins. Endocrinology 1978, 103, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- Juhairiyah, F.; de Lange, E.C.M. Understanding Drug Delivery to the Brain Using Liposome-Based Strategies: Studies That Provide Mechanistic Insights Are Essential. AAPS J. 2021, 23, 114. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, C.; Kikuchi-Utsumi, K.; Aoyama, K.; Suzuki, R.; Okamoto, Y.; Matsumura, N.; Omata, D.; Maruyama, K.; Nakaki, T. Inhibition of miR-96-5p in the Mouse Brain Increases Glutathione Levels by Altering NOVA1 Expression. Commun. Biol. 2021, 4, 182. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.; Sinha, I.; Calcagnotto, A.; Trushin, N.; Haley, J.S.; Schell, T.D.; Richie, J.P. Oral Supplementation with Liposomal Glutathione Elevates Body Stores of Glutathione and Markers of Immune Function. Eur. J. Clin. Nutr. 2018, 72, 105–111. [Google Scholar] [CrossRef] [PubMed]
- To, K.; Cao, R.; Yegiazaryan, A.; Owens, J.; Nguyen, T.; Sasaninia, K.; Vaughn, C.; Singh, M.; Truong, E.; Medina, A.; et al. Effects of Oral Liposomal Glutathione in Altering the Immune Responses Against Mycobacterium Tuberculosis and the Mycobacterium Bovis BCG Strain in Individuals With Type 2 Diabetes. Front. Cell. Infect. Microbiol. 2021, 11, 657775. [Google Scholar] [CrossRef]
- Schmitt, B.; Vicenzi, M.; Garrel, C.; Denis, F.M. Effects of N-Acetylcysteine, Oral Glutathione (GSH) and a Novel Sublingual Form of GSH on Oxidative Stress Markers: A Comparative Crossover Study. Redox Biol. 2015, 6, 198–205. [Google Scholar] [CrossRef]
- Campolo, J.; Bernardi, S.; Cozzi, L.; Rocchiccioli, S.; Dellanoce, C.; Cecchettini, A.; Tonini, A.; Parolini, M.; De Chiara, B.; Micheloni, G.; et al. Medium-Term Effect of Sublingual l-Glutathione Supplementation on Flow-Mediated Dilation in Subjects with Cardiovascular Risk Factors. Nutrition 2017, 38, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Wade, L.A.; Brady, H.M. Cysteine and Cystine Transport at the Blood-Brain Barrier. J. Neurochem. 1981, 37, 730–734. [Google Scholar] [CrossRef] [PubMed]
- Holmay, M.J.; Terpstra, M.; Coles, L.D.; Mishra, U.; Ahlskog, M.; Öz, G.; Cloyd, J.C.; Tuite, P.J. N-Acetylcysteine Boosts Brain and Blood Glutathione in Gaucher and Parkinson Diseases. Clin. Neuropharmacol. 2013, 36, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Lindl, K.A.; Marks, D.R.; Kolson, D.L.; Jordan-Sciutto, K.L. HIV-Associated Neurocognitive Disorder: Pathogenesis and Therapeutic Opportunities. J. Neuroimmune Pharmacol. 2010, 5, 294. [Google Scholar] [CrossRef]
- Decloedt, E.H.; Freeman, C.; Howells, F.; Casson-Crook, M.; Lesosky, M.; Koutsilieri, E.; Lovestone, S.; Maartens, G.; Joska, J.A. Moderate to Severe HIV-Associated Neurocognitive Impairment: A Randomized Placebo-Controlled Trial of Lithium. Medicine 2016, 95, e5401. [Google Scholar] [CrossRef] [PubMed]
- Schifitto, G.; Navia, B.A.; Yiannoutsos, C.T.; Marra, C.M.; Chang, L.; Ernst, T.; Jarvik, J.G.; Miller, E.N.; Singer, E.J.; Ellis, R.J.; et al. Memantine and HIV-Associated Cognitive Impairment: A Neuropsychological and Proton Magnetic Resonance Spectroscopy Study. Aids 2007, 21, 1877–1886. [Google Scholar] [CrossRef]
- Nakasujja, N.; Miyahara, S.; Evans, S.; Lee, A.; Musisi, S.; Katabira, E.; Robertson, K.; Ronald, A.; Clifford, D.B.; Sacktor, N. Randomized Trial of Minocycline in the Treatment of HIV-Associated Cognitive Impairment. Neurology 2013, 80, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Simioni, S.; Cavassini, M.; Annoni, J.-M.; Rimbault Abraham, A.; Bourquin, I.; Schiffer, V.; Calmy, A.; Chave, J.-P.; Giacobini, E.; Hirschel, B.; et al. Cognitive Dysfunction in HIV Patients despite Long-Standing Suppression of Viremia. Aids 2010, 24, 1243–1250. [Google Scholar] [CrossRef]
- Gao, C.; Meng, J.; Xiao, X.; Wang, M.; Williams, A.B.; Wang, H. Antiretroviral Therapy Improves Neurocognitive Impairment in People Living with HIV? A Meta-Analysis. Int. J. Nurs. Sci. 2020, 7, 238–247. [Google Scholar] [CrossRef]
- Patel, K.; Ming, X.; Williams, P.L.; Robertson, K.R.; Oleske, J.M.; Seage, G.R. Impact of HAART and CNS-Penetrating Antiretroviral Regimens on HIV Encephalopathy among Perinatally Infected Children and Adolescents. Aids 2009, 23, 1893–1901. [Google Scholar] [CrossRef]
- Edén, A.; Price, R.W.; Spudich, S.; Fuchs, D.; Hagberg, L.; Gisslén, M. Immune Activation of the Central Nervous System Is Still Present after >4 Years of Effective Highly Active Antiretroviral Therapy. J. Infect. Dis. 2007, 196, 1779–1783. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, A.; Marwaha, P.K.; Worku, D.A. CD8 Encephalitis in HIV: A Review of This Emerging Entity. J. Clin. Med. 2023, 12, 770. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Spradley, T.; Campbell, M.; Biyani, S.; Singhal, P.; Elkhider, H.; Nalleballe, K.; Gokden, M.; Kumar, M.; Kapoor, N. CD8 Encephalitis: A Diagnostic Dilemma. Diagnostics 2022, 12, 2687. [Google Scholar] [CrossRef]
- Lucas, S.B.; Wong, K.T.; Nightingale, S.; Miller, R.F. HIV-Associated CD8 Encephalitis: A UK Case Series and Review of Histopathologically Confirmed Cases. Front. Neurol. 2021, 12, 628296. [Google Scholar] [CrossRef] [PubMed]
- Tütüncüler, F.; Eskiocak, S.; BaŞaran, Ü.N.; Ekuklu, G.; Ayvaz, S.; Vatansever, Ü. The Protective Role of Melatonin in Experimental Hypoxic Brain Damage. Pediatr. Int. 2005, 47, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Alconada, D.; Álvarez, A.; Arteaga, O.; Martínez-Ibargüen, A.; Hilario, E. Neuroprotective Effect of Melatonin: A Novel Therapy against Perinatal Hypoxia-Ischemia. Int. J. Mol. Sci. 2013, 14, 9379–9395. [Google Scholar] [CrossRef]
- Gitto, E.; Reiter, R.J.; Cordaro, S.P.; La Rosa, M.; Chiurazzi, P.; Trimarchi, G.; Gitto, P.; Calabrò, M.P.; Barberi, I. Oxidative and Inflammatory Parameters in Respiratory Distress Syndrome of Preterm Newborns: Beneficial Effects of Melatonin. Am. J. Perinatol. 2004, 21, 209–216. [Google Scholar] [CrossRef]
- Aoyama, K.; Wang, F.; Matsumura, N.; Kiyonari, H.; Shioi, G.; Tanaka, K.; Kinoshita, C.; Kikuchi-Utsumi, K.; Watabe, M.; Nakaki, T. Increased Neuronal Glutathione and Neuroprotection in GTRAP3-18-Deficient Mice. Neurobiol. Dis. 2012, 45, 973–982. [Google Scholar] [CrossRef] [PubMed]
- Rip, J.; Chen, L.; Hartman, R.; van den Heuvel, A.; Reijerkerk, A.; van Kregten, J.; van der Boom, B.; Appeldoorn, C.; de Boer, M.; Maussang, D.; et al. Glutathione PEGylated Liposomes: Pharmacokinetics and Delivery of Cargo across the Blood–Brain Barrier in Rats. J. Drug Target. 2014, 22, 460–467. [Google Scholar] [CrossRef]
- Samikkannu, T.; Rao, K.V.K.; Pilakka Kanthikeel, S.; Subba Rao Atluri, V.; Agudelo, M.; Roy, U.; Nair, M.P.N. Immunoneuropathogenesis of HIV-1 Clades B and C: Role of Redox Expression and Thiol Modification. Free Radic. Biol. Med. 2014, 69, 136–144. [Google Scholar] [CrossRef]
- Wang, S.X.; Ho, E.L.; Grill, M.; Lee, E.; Peterson, J.; Robertson, K.; Fuchs, D.; Sinclair, E.; Price, R.W.; Spudich, S. Peripheral Neuropathy in Primary HIV Infection Associates with Systemic and CNS Immune Activation. J. Acquir. Immune Defic. Syndr. 2014, 66, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.-J.; Fu, Y.-Y.; Wei, Q.-Q.; Zhang, Z.-J. Neuroinflammation in HIV-Related Neuropathic Pain. Front. Pharmacol. 2021, 12, 653852. [Google Scholar] [CrossRef] [PubMed]
- Gabbai, A.A.; Castelo, A.; Oliveira, A.S.B. Chapter 29-HIV Peripheral Neuropathy. In Handbook of Clinical Neurology; Said, G., Krarup, C., Eds.; Peripheral Nerve Disorders; Elsevier: Amsterdam, The Netherlands, 2013; Volume 115, pp. 515–529. [Google Scholar]
- Kieburtz, K.; Simpson, D.; Yiannoutsos, C.; Max, M.B.; Hall, C.D.; Ellis, R.J.; Marra, C.M.; McKendall, R.; Singer, E.; Dal Pan, G.J.; et al. A Randomized Trial of Amitriptyline and Mexiletine for Painful Neuropathy in HIV Infection. AIDS Clinical Trial Group 242 Protocol Team. Neurology 1998, 51, 1682–1688. [Google Scholar] [CrossRef] [PubMed]
- Shlay, J.C.; Chaloner, K.; Max, M.B.; Flaws, B.; Reichelderfer, P.; Wentworth, D.; Hillman, S.; Brizz, B.; Cohn, D.L. Acupuncture and Amitriptyline for Pain Due to HIV-Related Peripheral Neuropathy: A Randomized Controlled Trial. Terry Beirn Community Programs for Clinical Research on AIDS. JAMA 1998, 280, 1590–1595. [Google Scholar] [CrossRef] [PubMed]
- Simpson, D.M.; Rice, A.S.C.; Emir, B.; Landen, J.; Semel, D.; Chew, M.L.; Sporn, J. A Randomized, Double-Blind, Placebo-Controlled Trial and Open-Label Extension Study to Evaluate the Efficacy and Safety of Pregabalin in the Treatment of Neuropathic Pain Associated with Human Immunodeficiency Virus Neuropathy. Pain 2014, 155, 1943–1954. [Google Scholar] [CrossRef] [PubMed]
- Hahn, K.; Arendt, G.; Braun, J.S.; von Giesen, H.-J.; Husstedt, I.W.; Maschke, M.; Straube, M.E.; Schielke, E. German Neuro-AIDS Working Group A Placebo-Controlled Trial of Gabapentin for Painful HIV-Associated Sensory Neuropathies. J. Neurol. 2004, 251, 1260–1266. [Google Scholar] [CrossRef] [PubMed]
- Wulff, E.A.; Wang, A.K.; Simpson, D.M. HIV-Associated Peripheral Neuropathy. Drugs 2000, 59, 1251–1260. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, Z.; Yang, W.; Liao, A.; Zhang, R.; Wu, B.; Wang, H.; Yao, K.; Li, Y. Study on mechanism of bortezomib inducing peripheral neuropathy and the reversing effect of reduced glutathione. Zhonghua Xue Ye Xue Za Zhi Zhonghua Xueyexue Zazhi 2011, 32, 107–111. [Google Scholar]
- Huang, H.; Zhao, M.; Liu, X.; Song, J.; Liu, L.; Liu, Y.; Xiang, P.; Wang, Y.; Fang, B. Glutathione Combined with Mecobalamin in the Treatment of Chemotherapy-Induced Peripheral Neuropathy in Multiple Myeloma: A Retrospective Clinical Study. Ann. Palliat. Med. 2021, 10, 12335–12346. [Google Scholar] [CrossRef]
- Lee, M.; Cho, S.; Roh, K.; Chae, J.; Park, J.-H.; Park, J.; Lee, M.-A.; Kim, J.; Auh, C.-K.; Yeom, C.-H.; et al. Glutathione Alleviated Peripheral Neuropathy in Oxaliplatin-Treated Mice by Removing Aluminum from Dorsal Root Ganglia. Am. J. Transl. Res. 2017, 9, 926–939. [Google Scholar]
- Ezaka, M.; Marutani, E.; Miyazaki, Y.; Kanemaru, E.; Selig, M.K.; Boerboom, S.L.; Ostrom, K.F.; Stemmer-Rachamimov, A.; Bloch, D.B.; Brenner, G.J.; et al. Oral Administration of Glutathione Trisulfide Increases Reactive Sulfur Levels in Dorsal Root Ganglion and Ameliorates Paclitaxel-Induced Peripheral Neuropathy in Mice. Antioxidants 2022, 11, 2122. [Google Scholar] [CrossRef] [PubMed]
- Motwani, L.; Asif, N.; Patel, A.; Vedantam, D.; Poman, D.S. Neuropathy in Human Immunodeficiency Virus: A Review of the Underlying Pathogenesis and Treatment. Cureus 2022, 14, e25905. [Google Scholar] [CrossRef] [PubMed]
- Noy, A. Optimizing Treatment of HIV-Associated Lymphoma. Blood 2019, 134, 1385–1394. [Google Scholar] [CrossRef] [PubMed]
- Gopal, S.; Patel, M.R.; Yanik, E.L.; Cole, S.R.; Achenbach, C.J.; Napravnik, S.; Burkholder, G.A.; Reid, E.G.; Rodriguez, B.; Deeks, S.G.; et al. Temporal Trends in Presentation and Survival for HIV-Associated Lymphoma in the Antiretroviral Therapy Era. JNCI J. Natl. Cancer Inst. 2013, 105, 1221–1229. [Google Scholar] [CrossRef] [PubMed]
- Coté, T.R.; Biggar, R.J.; Rosenberg, P.S.; Devesa, S.S.; Percy, C.; Yellin, F.J.; Lemp, G.; Hardy, C.; Geodert, J.J.; Blattner, W.A. Non-Hodgkin’s Lymphoma among People with AIDS: Incidence, Presentation and Public Health Burden. Int. J. Cancer 1997, 73, 645–650. [Google Scholar] [CrossRef]
- Hansen, A.R.; Vardell, V.A.; Fitzgerald, L.A. Epidemiologic Characteristics, Treatment Patterns, and Survival Analysis of Plasmablastic Lymphoma in the United States: A SEER and NCDB Analysis. Clin. Lymphoma Myeloma Leuk. 2023, 24, e152–e160. [Google Scholar] [CrossRef] [PubMed]
- Harris, I.S.; Treloar, A.E.; Inoue, S.; Sasaki, M.; Gorrini, C.; Lee, K.C.; Yung, K.Y.; Brenner, D.; Knobbe-Thomsen, C.B.; Cox, M.A.; et al. Glutathione and Thioredoxin Antioxidant Pathways Synergize to Drive Cancer Initiation and Progression. Cancer Cell 2015, 27, 211–222. [Google Scholar] [CrossRef]
- Gamcsik, M.P.; Kasibhatla, M.S.; Teeter, S.D.; Colvin, O.M. Glutathione Levels in Human Tumors. Biomark. Biochem. Indic. Expo. Response Susceptibility Chem. 2012, 17, 671–691. [Google Scholar] [CrossRef] [PubMed]
- Nelson, B.P.; Nalesnik, M.A.; Bahler, D.W.; Locker, J.; Fung, J.J.; Swerdlow, S.H. Epstein-Barr Virus-Negative Post-Transplant Lymphoproliferative Disorders: A Distinct Entity? Am. J. Surg. Pathol. 2000, 24, 375–385. [Google Scholar] [CrossRef]
- Kennedy, L.; Sandhu, J.K.; Harper, M.-E.; Cuperlovic-Culf, M. Role of Glutathione in Cancer: From Mechanisms to Therapies. Biomolecules 2020, 10, 1429. [Google Scholar] [CrossRef]
- Kelly, R.A.; Leedale, J.; Calleja, D.; Enoch, S.J.; Harrell, A.; Chadwick, A.E.; Webb, S. Modelling Changes in Glutathione Homeostasis as a Function of Quinone Redox Metabolism. Sci. Rep. 2019, 9, 6333. [Google Scholar] [CrossRef] [PubMed]
- Mehrabian, A.; Dadpour, S.; Mashreghi, M.; Zarqi, J.; Askarizadeh, A.; Badiee, A.; Arabi, L.; Moosavian, S.A.; Jaafari, M.R. The Comparison of Biodistribution of Glutathione PEGylated Nanoliposomal Doxorubicin Formulations Prepared by Pre-Insertion and Post-Insertion Methods for Brain Delivery in Normal Mice. IET Nanobiotechnol. 2023, 17, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Dashwood, T.; Walmsley, S. The Intersection of HIV and Syphilis: Update on the Key Considerations in Testing and Management. Curr. HIV/AIDS Rep. 2021, 18, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Bordón, J.; Martínez-Vázquez, C.; Alvarez, M.; Miralles, C.; Ocampo, A.; de la Fuente-Aguado, J.; Sopeña-Perez Arguelles, B. Neurosyphilis in HIV-Infected Patients. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 1995, 14, 864–869. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-J.; Chi, C.-Y.; Chou, C.-H.; Ho, C.-M.; Lin, P.-C.; Liao, C.-H.; Ho, M.-W.; Wang, J.-H. Syphilis and Neurosyphilis in Human Immunodeficiency Virus-Infected Patients: A Retrospective Study at a Teaching Hospital in Taiwan. J. Microbiol. Immunol. Infect. 2012, 45, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Honda, Y.; Kessoku, T.; Sumida, Y.; Kobayashi, T.; Kato, T.; Ogawa, Y.; Tomeno, W.; Imajo, K.; Fujita, K.; Yoneda, M.; et al. Efficacy of Glutathione for the Treatment of Nonalcoholic Fatty Liver Disease: An Open-Label, Single-Arm, Multicenter, Pilot Study. BMC Gastroenterol. 2017, 17, 96. [Google Scholar] [CrossRef] [PubMed]
- Venketaraman, V.; Rodgers, T.; Linares, R.; Reilly, N.; Swaminathan, S.; Hom, D.; Millman, A.C.; Wallis, R.; Connell, N.D. Glutathione and Growth Inhibition of Mycobacterium Tuberculosis in Healthy and HIV Infected Subjects. AIDS Res. Ther. 2006, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.; Nguyen, J.; Blair, L.; Banjanin, M.; Grewal, B.; Bowman, S.; Boyd, H.; Gerstner, G.; Cho, H.J.; Panfilov, D.; et al. Pathogenesis of Human Immunodeficiency Virus-Mycobacterium Tuberculosis Co-Infection. J. Clin. Med. 2020, 9, 3575. [Google Scholar] [CrossRef] [PubMed]
- Chiacchio, T.; Petruccioli, E.; Vanini, V.; Cuzzi, G.; La Manna, M.P.; Orlando, V.; Pinnetti, C.; Sampaolesi, A.; Antinori, A.; Caccamo, N.; et al. Impact of Antiretroviral and Tuberculosis Therapies on CD4+ and CD8+ HIV/M. Tuberculosis-Specific T-Cell in Co-Infected Subjects. Immunol. Lett. 2018, 198, 33–43. [Google Scholar] [CrossRef]
- Wen, L.; Shi, L.; Wan, S.-S.; Xu, T.; Zhang, L.; Zhou, Z.-G. Changes in the Balance of Th17/Treg Cells and Oxidative Stress Markers in Patients with HIV-associated Pulmonary Tuberculosis Who Develop IRIS. Exp. Ther. Med. 2023, 25, 271. [Google Scholar] [CrossRef]
- Zhao, Y.; Ye, L.; Zhao, F.; Zhang, L.; Lu, Z.; Chu, T.; Wang, S.; Liu, Z.; Sun, Y.; Chen, M.; et al. Cryptococcus Neoformans, a Global Threat to Human Health. Infect. Dis. Poverty 2023, 12, 20. [Google Scholar] [CrossRef] [PubMed]
- Rajasingham, R.; Govender, N.P.; Jordan, A.; Loyse, A.; Shroufi, A.; Denning, D.W.; Meya, D.B.; Chiller, T.M.; Boulware, D.R. The Global Burden of HIV-Associated Cryptococcal Infection in Adults in 2020: A Modelling Analysis. Lancet Infect. Dis. 2022, 22, 1748–1755. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Lin, D.; Tu, S.; Gao, S.; Shao, A.; Sheng, J. Is Ferroptosis a Future Direction in Exploring Cryptococcal Meningitis? Front. Immunol. 2021, 12, 598601. [Google Scholar] [CrossRef] [PubMed]
- Di Rocco, A. Diseases of the Spinal Cord in Human Immunodeficiency Virus Infection. Semin. Neurol. 1999, 19, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Dal Pan, G.J.; Glass, J.D.; McArthur, J.C. Clinicopathologic Correlations of HIV-1-Associated Vacuolar Myelopathy: An Autopsy-Based Case-Control Study. Neurology 1994, 44, 2159–2164. [Google Scholar] [CrossRef] [PubMed]
- Rezaie, A.; Parmar, R.; Rendon, C.; Zell, S.C. HIV-Associated Vacuolar Myelopathy: A Rare Initial Presentation of HIV. SAGE Open Med. Case Rep. 2020, 8, 2050313X20945562. [Google Scholar] [CrossRef] [PubMed]
- Prakhova, L.N.; Ilves, A.G.; Kizhlo, S.N.; Savintseva, Z.I. Successful Treatment of Human Immunodeficiency Virus-Associated Highly Active Antiretroviral Therapy-Resistant Vacuolar Myelopathy with Intravenous Immunoglobulin. Ann. Indian Acad. Neurol. 2020, 23, 220–222. [Google Scholar] [CrossRef] [PubMed]
- HIV Psychiatry. Available online: https://www.psychiatry.org:443/psychiatrists/practice/professional-interests/hiv-psychiatry (accessed on 2 March 2024).
- Nedelcovych, M.T.; Manning, A.A.; Semenova, S.; Gamaldo, C.; Haughey, N.J.; Slusher, B.S. The Psychiatric Impact of HIV. ACS Chem. Neurosci. 2017, 8, 1432–1434. [Google Scholar] [CrossRef] [PubMed]
- Blank, M.B.; Mandell, D.S.; Aiken, L.; Hadley, T.R. Co-Occurrence of HIV and Serious Mental Illness Among Medicaid Recipients. Psychiatr. Serv. 2002, 53, 868–873. [Google Scholar] [CrossRef]
- Mijch, A.M.; Judd, F.K.; Lyketsos, C.G.; Ellen, S.; Cockram, A. Secondary Mania in Patients With HIV Infection. J. Neuropsychiatry Clin. Neurosci. 1999, 11, 475–480. [Google Scholar] [CrossRef]
- Lyketsos, C.G.; Schwartz, J.; Fishman, M.; Treisman, G. AIDS Mania. J. Neuropsychiatry Clin. Neurosci. 1997, 9, 277–279. [Google Scholar] [CrossRef] [PubMed]
- Shungu, D.C. N-Acetylcysteine for the Treatment of Glutathione Deficiency and Oxidative Stress in Schizophrenia. Biol. Psychiatry 2012, 71, 937–938. [Google Scholar] [CrossRef] [PubMed]
- Lapidus, K.A.B.; Gabbay, V.; Mao, X.; Johnson, A.; Murrough, J.W.; Mathew, S.J.; Shungu, D.C. In Vivo 1H MRS Study of Potential Associations between Glutathione, Oxidative Stress and Anhedonia in Major Depressive Disorder. Neurosci. Lett. 2014, 569, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Godlewska, B.R.; Near, J.; Cowen, P.J. Neurochemistry of Major Depression: A Study Using Magnetic Resonance Spectroscopy. Psychopharmacology 2015, 232, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Freed, R.D.; Hollenhorst, C.N.; Weiduschat, N.; Mao, X.; Kang, G.; Shungu, D.C.; Gabbay, V. A Pilot Study of Cortical Glutathione in Youth with Depression. Psychiatry Res. Neuroimaging 2017, 270, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Zalachoras, I.; Hollis, F.; Ramos-Fernández, E.; Trovo, L.; Sonnay, S.; Geiser, E.; Preitner, N.; Steiner, P.; Sandi, C.; Morató, L. Therapeutic Potential of Glutathione-Enhancers in Stress-Related Psychopathologies. Neurosci. Biobehav. Rev. 2020, 114, 134–155. [Google Scholar] [CrossRef] [PubMed]
- George, M.C.; Wongmek, A.; Kaku, M.; Nmashie, A.; Robinson-Papp, J. A Mixed-Methods Pilot Study of Mindfulness Based Stress Reduction for HIV-Associated Chronic Pain. Behav. Med. Wash. DC 2017, 43, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Dell’Oste, V.; Fantasia, S.; Gravina, D.; Palego, L.; Betti, L.; Dell’Osso, L.; Giannaccini, G.; Carmassi, C. Metabolic and Inflammatory Response in Post-Traumatic Stress Disorder (PTSD): A Systematic Review on Peripheral Neuroimmune Biomarkers. Int. J. Environ. Res. Public Health 2023, 20, 2937. [Google Scholar] [CrossRef] [PubMed]
- Ande, A.; Sinha, N.; Rao, P.S.S.; McArthur, C.P.; Ayuk, L.; Achu, P.N.; Njinda, A.; Kumar, A.; Kumar, S. Enhanced Oxidative Stress by Alcohol Use in HIV+ Patients: Possible Involvement of Cytochrome P450 2E1 and Antioxidant Enzymes. AIDS Res. Ther. 2015, 12, 29. [Google Scholar] [CrossRef]
- Castellano, P.; Nwagbo, C.; Martinez, L.R.; Eugenin, E.A. Methamphetamine Compromises Gap Junctional Communication in Astrocytes and Neurons. J. Neurochem. 2016, 137, 561–575. [Google Scholar] [CrossRef]
- Taibi, D.M. Sleep Disturbances in Persons Living with HIV. J. Assoc. Nurses AIDS Care JANAC 2013, 24, S72–S85. [Google Scholar] [CrossRef] [PubMed]
- Neculicioiu, V.S.; Colosi, I.A.; Costache, C.; Toc, D.A.; Sevastre-Berghian, A.; Colosi, H.A.; Clichici, S. Sleep Deprivation-Induced Oxidative Stress in Rat Models: A Scoping Systematic Review. Antioxidants 2023, 12, 1600. [Google Scholar] [CrossRef] [PubMed]
- Villafuerte, G.; Miguel-Puga, A.; Murillo Rodríguez, E.; Machado, S.; Manjarrez, E.; Arias-Carrión, O. Sleep Deprivation and Oxidative Stress in Animal Models: A Systematic Review. Oxid. Med. Cell Longev. 2015, 2015, 234952. [Google Scholar] [CrossRef] [PubMed]
- Bushana, P.N.; Schmidt, M.A.; Chang, K.M.; Vuong, T.; Sorg, B.A.; Wisor, J.P. Effect of N-Acetylcysteine on Sleep: Impacts of Sex and Time of Day. Antioxidants 2023, 12, 1124. [Google Scholar] [CrossRef]
- Mullier, E.; Roine, T.; Griffa, A.; Xin, L.; Baumann, P.S.; Klauser, P.; Cleusix, M.; Jenni, R.; Alemàn-Gómez, Y.; Gruetter, R.; et al. N-Acetyl-Cysteine Supplementation Improves Functional Connectivity Within the Cingulate Cortex in Early Psychosis: A Pilot Study. Int. J. Neuropsychopharmacol. 2019, 22, 478–487. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erdos, T.; Masuda, M.; Venketaraman, V. Glutathione in HIV-Associated Neurocognitive Disorders. Curr. Issues Mol. Biol. 2024, 46, 5530-5549. https://doi.org/10.3390/cimb46060330
Erdos T, Masuda M, Venketaraman V. Glutathione in HIV-Associated Neurocognitive Disorders. Current Issues in Molecular Biology. 2024; 46(6):5530-5549. https://doi.org/10.3390/cimb46060330
Chicago/Turabian StyleErdos, Thomas, Mika Masuda, and Vishwanath Venketaraman. 2024. "Glutathione in HIV-Associated Neurocognitive Disorders" Current Issues in Molecular Biology 46, no. 6: 5530-5549. https://doi.org/10.3390/cimb46060330
APA StyleErdos, T., Masuda, M., & Venketaraman, V. (2024). Glutathione in HIV-Associated Neurocognitive Disorders. Current Issues in Molecular Biology, 46(6), 5530-5549. https://doi.org/10.3390/cimb46060330





