The Role of Licorice Chalcones as Molecular Genes and Signaling Pathways Modulator—A Review of Experimental Implications for Nicotine-Induced Non-Small Cell Lung Cancer Treatment
Abstract
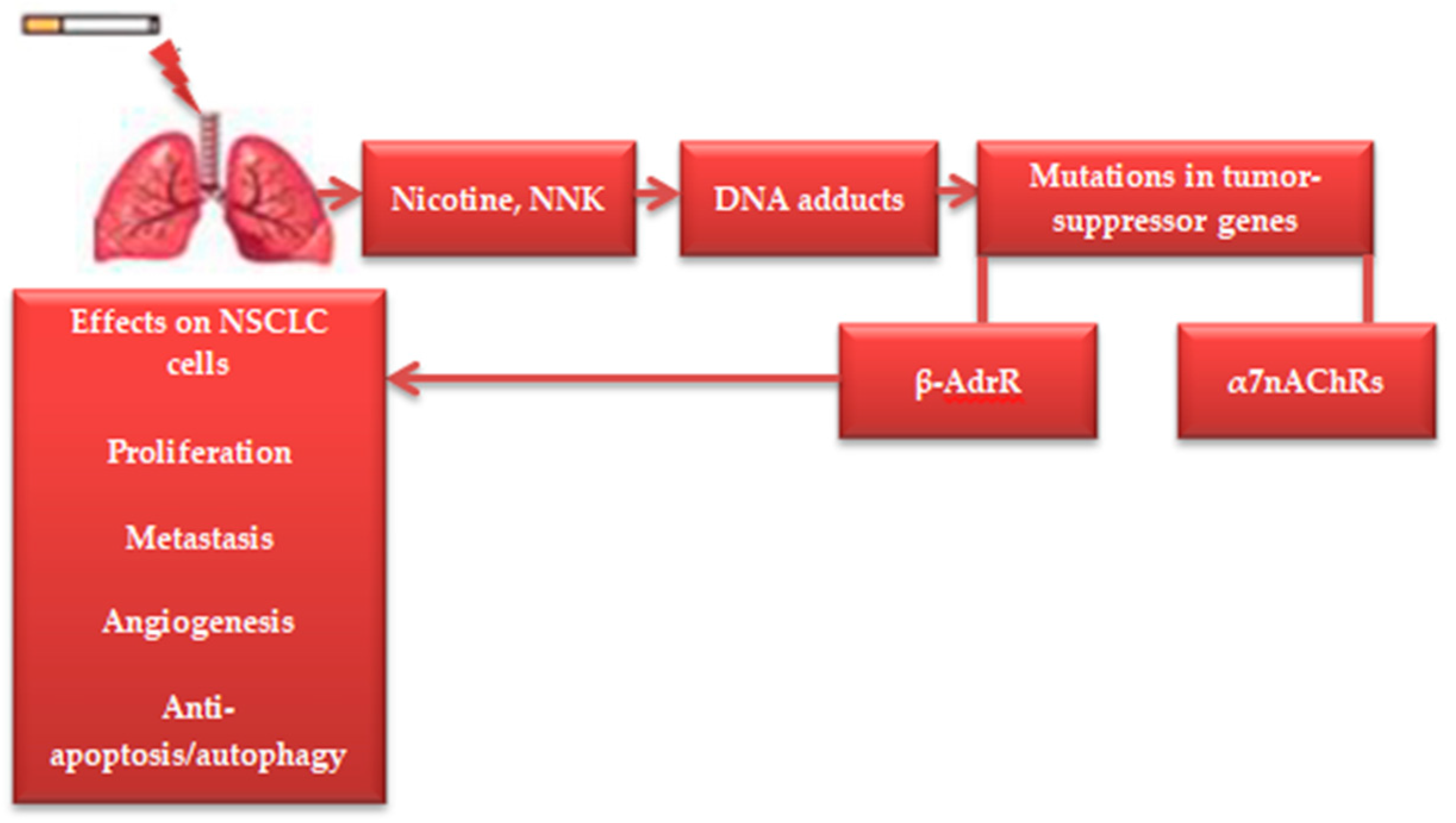
1. Introduction
2. Methods
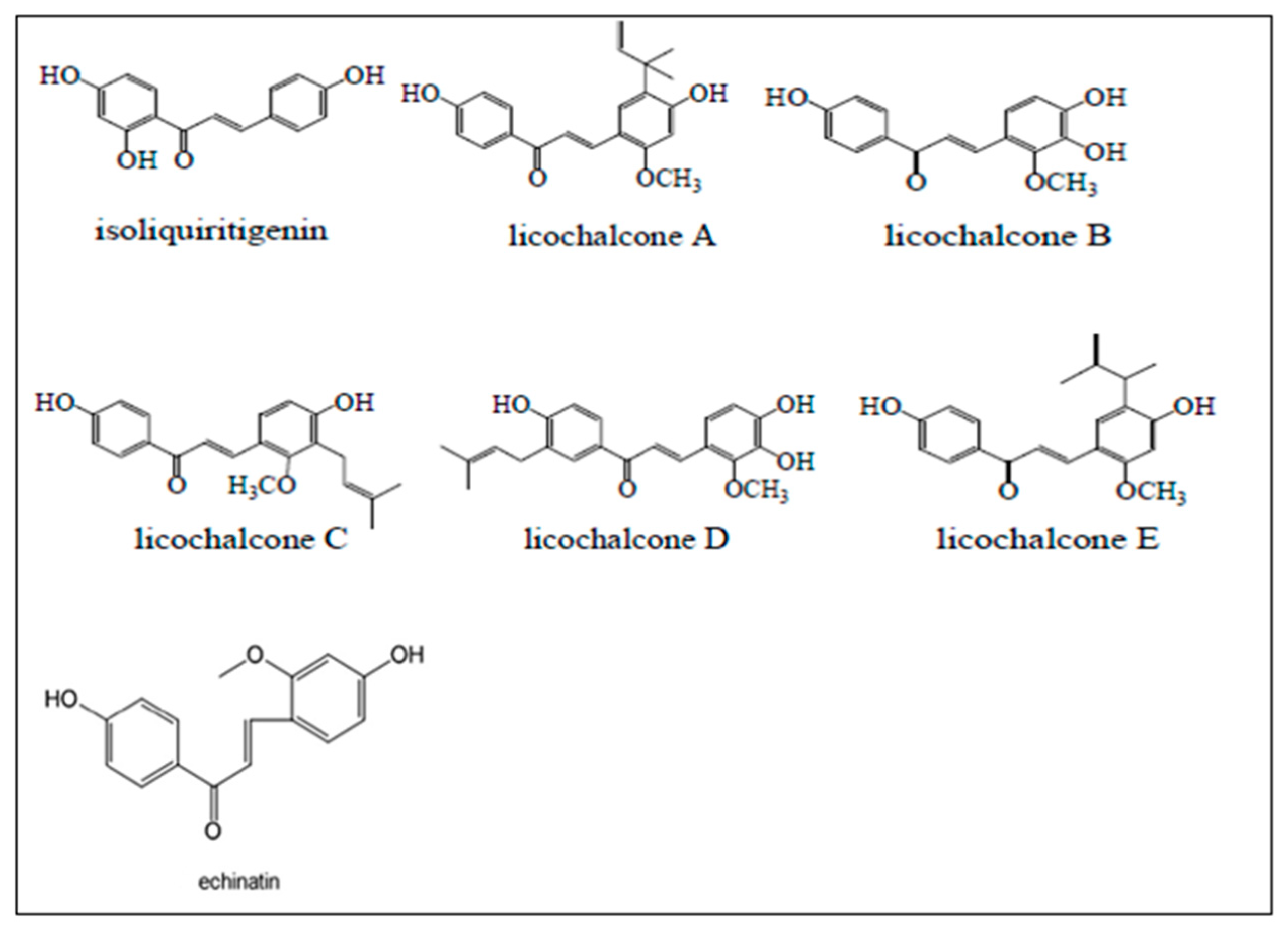
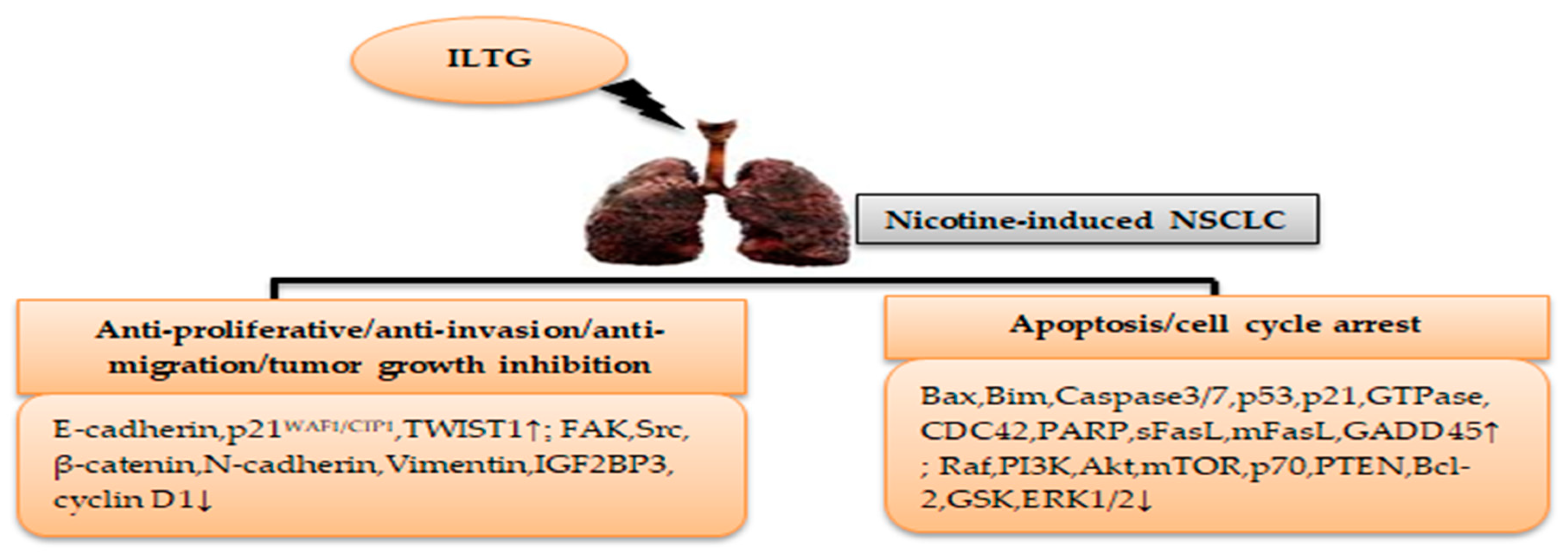
3. Licorice Isoliquiritigenin in Nicotine-Induced NSCLC Treatment
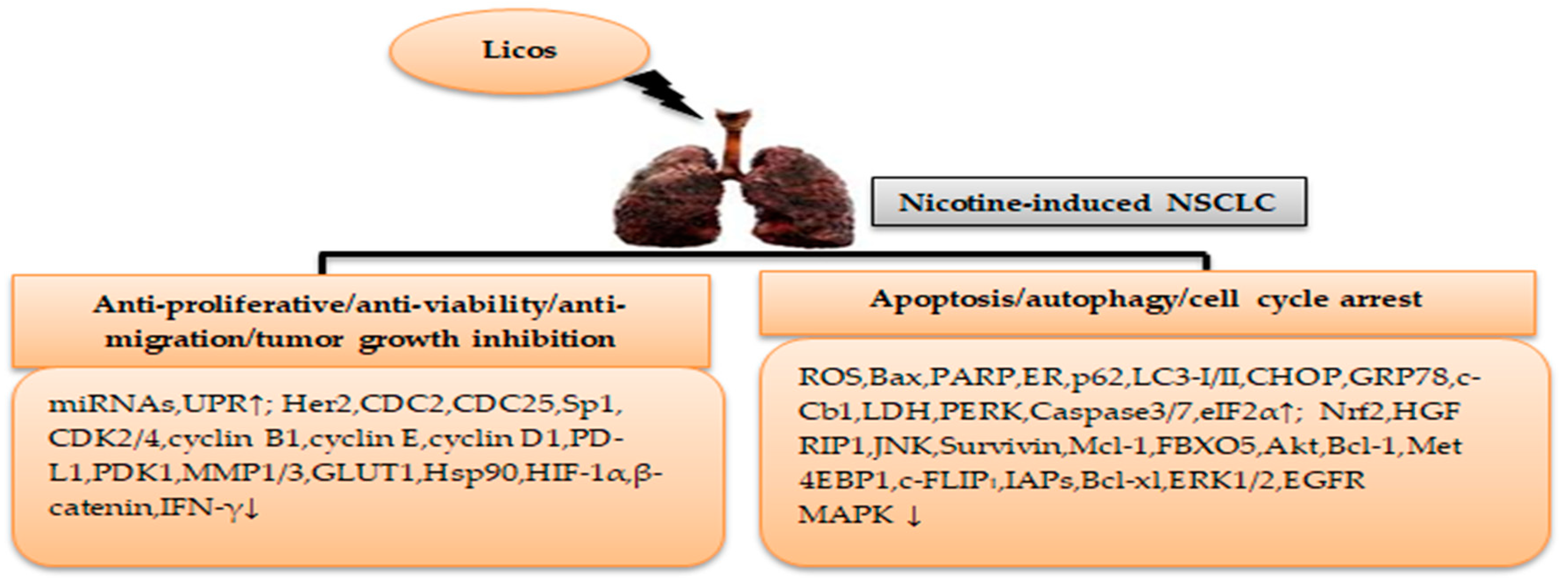
4. Licorice Licochalcone in Nicotine-Induced NSCLC Treatment
5. Licorice Echinatin in Nicotine-Induced NSCLC Treatment
6. Limitations
7. Conclusions
Funding
Conflicts of Interest
Abbreviations
| 4EBP1 | Eukaryotic translation initiation factor 4E-binding protein 1 |
| Akt | Threonine kinase |
| Aβ | Amyloid-beta |
| Bax | Bcl-2-associated X protein |
| Bcl-2 | B-cell lymphoma-2 |
| Bcl-xl | B-cell lymphoma-extra large |
| Bim | B-cell chronic lymphocytic leukemia-lymphoma like 11 gene |
| c-Cbl | c-Casitas B-lineage lymphoma |
| CD45 | Lymphocyte common antigen 45 |
| CDC2 | Cell division control protein 2 homologue |
| CDC25 | Cell division control protein 25 homologue |
| CDC42 | Cell division control protein 42 homologue |
| CDK | Cyclin-dependent kinase |
| c-FLIPL | Cellular-FLICE inhibitory protein |
| CHOP | C/EBP homologous protein |
| CHs | Chalcones |
| c-Met | Tyrosine-protein kinase Met |
| COX-2 | Cyclooxygenase-2 |
| CREP | cAMP-responsive element-binding protein |
| CS | Cigarette smoke |
| DMEM | Dulbecco’s Modified Eagle Medium |
| ECH | Echinatin |
| EGFR | Epidermal growth factor receptor |
| EGR1 | Early growth response 1 |
| eIF2α | Eukaryotic initiation factor 2α |
| EMT | Epithelial-mesenchymal transition |
| ER | Endoplasmic reticulum |
| ERK | Extracellular signal-regulated kinase |
| FAK | Focal adhesion kinase |
| FBXO5 | F-box protein 5 |
| GADD45 | Growth Arrest and DNA Damage-inducible 45 |
| GLUT1 | Glucose transporter 1 |
| GRP78 | 78-kDa glucose-regulated protein |
| GSK | Glycogen synthase kinase |
| GTPase | Guanosine triphosphatase |
| Her2 | Human epidermal growth factor receptor 2 |
| HGF | Hepatocyte growth factor |
| HIF-1α | Hypoxia inducible factor-1 |
| HPV | Human papillomavirus |
| Hsp90 | Heat shock protein 90 |
| IAPs | Inhibitor of apoptosis proteins |
| IFN-γ | Interferon-gamma |
| IGF2BP3 | Oncofetal IGF2 mRNA-binding protein 3 |
| ILTG | Isoliquiritigenin |
| JNK | c-Jun N-terminal kinase |
| LC | Lung cancer |
| LC3 | LC3-phospholipid conjugate |
| LDH | Lactate dehydrogenase |
| LEVR-1 | Lymphatic vessel endothelial receptor-1 |
| Licos | Licochalcones |
| LPS | Lipopolysaccharide |
| MAO | Monoamine oxidase |
| MAPK | Mitogen-activated protein kinase |
| MCP-1 | Monocyte chemoattractant protein-1 |
| MDM2 | Murine double minute 2 |
| Met | Mesenchymal epithelial transition factor receptor |
| mFasL | Membrane-bound Fas ligand |
| miRNAs | MicroRNAs |
| MMP | Metalloproteinase |
| mTOR | Mammalian target of rapamycin |
| NF-kB | Nuclear transcription factor-kappaB |
| NNK | 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanone |
| NNN | N-Nitrosonornicotine |
| NO | Nitric oxide |
| Nrf2 | Nuclear factor erythroid-2 related factor 2 |
| NSCLC | Non-small cell lung cancer |
| OH | Hydroxyl group |
| p21 | Protein 21 |
| p53 | Protein 53 |
| p62 | Protein 62 |
| p65 | Protein 65 |
| p70 | Protein 70 |
| PAMs | Positive allosteric modulators |
| PARP | Poly ADP ribose polymerase |
| PCNA | Proliferating cell nuclear antigen |
| PDK1 | Pyruvate dehydrogenase kinase 1 |
| PD-L1 | Programmed death-ligand 1 |
| PERK | Protein kinase RNA-like endoplasmic reticulum kinase |
| PI3K | Phosphatidylinositol-3 kinase |
| PKA | Protein kinase A |
| PPARγ | Peroxisome proliferator-activated receptor γ |
| PTEN | Phosphatase and tensin homolog |
| Raf | Retinoblastoma tumor suppressor protein-proto-oncogene |
| RIP1 | Receptor-interacting protein-1 |
| ROS | Reactive oxygen species |
| SCLC | Small cell lung cancer |
| sFasL | Soluble Fas ligand |
| Sp1 | Specificity protein 1 |
| Src | Non-receptor tyrosine kinase |
| STAT | Signal transducer and activator of transcription |
| TKI | Tyrosine kinase inhibitor |
| TNFα | Tumor necrosis factor |
| TWIST1 | Twist family bHLH transcription factor 1 |
| Tyr | Tyrosine |
| UPR | Unfolded protein response |
| VEGF | Vascular endothelial growth factor |
| WAF1/CIP1 | Cyclin-dependent kinase inhibitor p21 |
| α7nAChR | Nicotinic acetylcholine receptor |
| β-AdrR | beta-adrenergic receptor |
References
- Ji, X.; Chen, J.; Ye, J.; Xu, S.; Lin, B.; Hou, K. Epidemiological analysis of global and regional lung cancer mortality: Based on 30-year data analysis of global burden disease database. Healthcare 2023, 11, 2920. [Google Scholar] [CrossRef] [PubMed]
- Thandra, K.C.; Barsouk, A.; Saginala, K.; Aluru, J.S.; Barsouk, A. Epidemiology of lung cancer. Contemp. Oncol. 2021, 25, 45–52. [Google Scholar]
- Schabath, M.B.; Cote, M.L. Cancer progress and priorities: Lung cancer. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1563–1579. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Yang, S.; Seng, S. Mechanisms of cancer induction by tobacco-specific NNK and NNN. Cancers 2014, 6, 1138–1156. [Google Scholar] [CrossRef]
- Warren, G.W.; Singh, A.K. Nicotine and lung cancer. J. Carcinog. 2013, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Hu, Y. α7 nicotinic acetylcholine receptors in lung cancer. Oncol. Lett. 2018, 16, 1375–1382. [Google Scholar] [CrossRef] [PubMed]
- Hajiasgharzadeh, K.; Sadigh-Eteghad, S.; Mansoori, B.; Mokhtarzadeh, A.; Shanehbandi, D.; Doustvandi, M.A.; Asadzadeh, Z.; Baradaran, B. Alpha7 nicotinic acetylcholine receptors in lung inflammation and carcinogenesis: Friends or foes? J. Cell. Physiol. 2019, 234, 14666–14679. [Google Scholar] [CrossRef]
- Rudrapal, M.; Khan, J.; Bin Dukhyil, A.Z.; Alarousy, R.M.I.I.; Attah, E.I.; Sharma, T.; Khairnar, S.J.; Bendale, A.R. Chalcone scaffolds, bioprecursors of flavonoids: Chemistry, bioactivities, and pharmacokinetics. Molecules 2021, 26, 7177. [Google Scholar] [CrossRef] [PubMed]
- Balsera, B.; Mulet, J.; Fernández-Carvajal, A.; de la Torre-Martínez, R.; Ferrer-Montiel, A.; Hernández-Jiménez, J.G.; Estévez-Herrera, J.; Borges, R.; Freitas, A.E.; López, M.G.; et al. Chalcones as positive allosteric modulators of α7 nicotinic acetylcholine receptors: A new target for a privileged structure. Eur. J. Med. Chem. 2014, 86, 724–739. [Google Scholar] [CrossRef] [PubMed]
- Jasim, H.A.; Nahar, L.; Jasim, M.A.; Moore, S.A.; Ritchie, K.J.; Sarker, S.D. Chalcones: Synthetic chemistry follows where nature leads. Biomolecules 2021, 11, 1203. [Google Scholar] [CrossRef]
- Elkanzi, N.A.A.; Hrichi, H.; Alolayan, R.A.; Derafa, W.; Zahou, F.M.; Bakr, R.B. Synthesis of chalcones derivatives and their biological activities: A Review. ACS Omega 2022, 7, 27769–27786. [Google Scholar] [CrossRef] [PubMed]
- Irfan, R.; Mousavi, S.; Alazmi, M.; Saleem, R.S.Z. A Comprehensive review of aminochalcones. Molecules 2020, 25, 5381. [Google Scholar] [CrossRef] [PubMed]
- Constantinescu, T.; Lungu, C.N. Anticancer activity of natural and synthetic chalcones. Int. J. Mol. Sci. 2021, 22, 11306. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Quispe, C.; Chamkhi, I.; El Omari, N.; Balahbib, A.; Sharifi-Rad, J.; Bouyahya, A.; Akram, M.; Iqbal, M.; Docea, A.O.; et al. Pharmacological properties of chalcones: A review of preclinical including molecular mechanisms and clinical evidence. Front. Pharmacol. 2021, 11, 592654. [Google Scholar] [CrossRef] [PubMed]
- Birsa, M.L.; Sarbu, L.G. Hydroxy chalcones and analogs with chemopreventive properties. Int. J. Mol. Sci. 2023, 24, 10667. [Google Scholar] [CrossRef] [PubMed]
- Maisto, M.; Marzocchi, A.; Keivani, N.; Piccolo, V.; Summa, V.; Tenore, G.C. Natural chalcones for the management of obesity disease. Int. J. Mol. Sci. 2023, 24, 15929. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liang, J.; Zhang, J.; Wang, Y.; Chai, X. Natural chalcones in Chinese materia medica: Licorice. Evid. Based Complement. Altern. Med. 2020, 2020, 3821248. [Google Scholar] [CrossRef] [PubMed]
- Wahab, S.; Annadurai, S.; Abullais, S.S.; Das, G.; Ahmad, W.; Ahmad, M.F.; Kandasamy, G.; Vasudevan, R.; Ali, M.S.; Amir, M. Glycyrrhiza glabra (Licorice): A comprehensive review on its phytochemistry, biological activities, clinical evidence and toxicology. Plants 2021, 10, 2751. [Google Scholar] [CrossRef] [PubMed]
- Tuli, H.S.; Garg, V.K.; Mehta, J.K.; Kaur, G.; Mohapatra, R.K.; Dhama, K.; Sak, K.; Kumar, A.; Varol, M.; Aggarwal, D.; et al. Licorice (Glycyrrhiza glabra L.)-derived phytochemicals target multiple signaling pathways to confer oncopreventive and oncotherapeutic effects. Onco Targets Ther. 2022, 15, 1419–1448. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Z.; Du, Q.; Zhu, Z.; Chen, T.; Xue, Y.; Wang, Y.; Zeng, Q.; Shen, C.; Jiang, C.; et al. Pharmacological effects and underlying mechanisms of licorice-derived flavonoids. Evid. Based Complement. Altern. Med. 2022, 2022, 9523071. [Google Scholar] [CrossRef]
- Pia, G.D.M.; Sara, F.; Mario, F.; Lorenza, S. Biological effects of licochalcones. Mini Rev. Med. Chem. 2019, 19, 647–656. [Google Scholar]
- Chen, Z.; Ding, W.; Yang, X.; Lu, T.; Liu, Y. Isoliquiritigenin, a potential therapeutic agent for treatment of inflammation-associated diseases. J. Ethnopharmacol. 2024, 318 Pt B, 117059. [Google Scholar] [CrossRef]
- Cerulli, A.; Masullo, M.; Montoro, P.; Piacente, S. Licorice (Glycyrrhiza glabra, G. uralensis, and G. inflata) and their constituents as active cosmeceutical ingredients. Cosmetics 2022, 9, 7. [Google Scholar] [CrossRef]
- Fu, Y.; Chen, J.; Li, Y.-J.; Zheng, Y.-F.; Li, P. Antioxidant and anti-inflammatory activities of six flavonoids separated from licorice. Food Chem. 2013, 141, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.-L.; Yu, Y.-C.; Hsia, S.-M. Perspectives on the role of isoliquiritigenin in cancer. Cancers 2021, 13, 115. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Chin, Y.-W.; Bae, J.-K.; Seo, J.S.; Choi, Y.H. Pharmacokinetics of isoliquiritigenin and its metabolites in rats: Low bioavailability is primarily due to the hepatic and intestinal metabolism. Planta Med. 2013, 79, 1656–1665. [Google Scholar] [CrossRef]
- Choi, Y.H.; Kim, Y.-J.; Chae, H.-S.; Chin, Y.-W. In vivo gastroprotective effect along with pharmacokinetics, tissue distribution and metabolism of isoliquiritigenin in mice. Planta Med. 2015, 81, 586–593. [Google Scholar] [CrossRef]
- Qiao, H.; Zhang, X.; Wang, T.; Liang, L.; Chang, W.; Xia, H. Pharmacokinetics, biodistribution and bioavailability of isoliquiritigenin after intravenous and oral administration. Pharm. Biol. 2014, 52, 228–236. [Google Scholar] [CrossRef]
- Wang, X.-X.; Liu, G.-Y.; Yang, Y.-F.; Wu, X.-W.; Xu, W.; Yang, X.-W. Intestinal absorption of triterpenoids and flavonoids from Glycyrrhizae radix et rhizoma in the human Caco-2 monolayer cell model. Molecules 2017, 22, 1627. [Google Scholar] [CrossRef]
- Weng, Q.; Chen, L.; Ye, L.; Lu, X.; Yu, Z.; Wen, C.; Chen, Y.; Huang, G. Determination of licochalcone A in rat plasma by UPLC–MS/MS and its pharmacokinetics. Acta Chromatogr. 2019, 31, 262–265. [Google Scholar] [CrossRef]
- Li, T.; Ye, W.; Huang, B.; Lu, X.; Chen, X.; Lin, Y.; Wen, C.; Wang, X. Determination and pharmacokinetic study of echinatin by UPLC-MS/MS in rat plasma. J. Pharm. Biomed. Anal. 2019, 168, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Huang, R.; Yang, R.; Xiao, Y.; Yan, J.; Zheng, C.; Xiao, W.; Huang, C.; Wang, Y. Licorice extract inhibits growth of non-small cell lung cancer by down-regulating CDK4-cyclin D1 complex and increasing CD8+ T cell infiltration. Cancer Cell Int. 2021, 21, 529. [Google Scholar] [CrossRef] [PubMed]
- Alsharairi, N.A. Scutellaria baicalensis and their natural flavone compounds as potential medicinal drugs for the treatment of nicotine-induced non-small-cell lung cancer and asthma. Int. J. Environ. Res. Public Health 2021, 18, 5243. [Google Scholar] [CrossRef] [PubMed]
- Alsharairi, N.A. Insights into the mechanisms of action of proanthocyanidins and anthocyanins in the treatment of nicotine-induced non-small cell lung cancer. Int. J. Mol. Sci. 2022, 23, 7905. [Google Scholar] [CrossRef]
- Alsharairi, N.A. Quercetin derivatives as potential therapeutic agents: An updated perspective on the treatment of nicotine-induced non-small cell lung cancer. Int. J. Mol. Sci. 2023, 24, 15208. [Google Scholar] [CrossRef] [PubMed]
- Blaskovic, S.; Donati, Y.; Ruchonnet-Metrailler, S.; Avila, Y.; Schittny, D.; Schlepütz, C.M.; Schittny, J.C.; Barazzone-Argiroffo, C. Early life exposure to nicotine modifies lung gene response after elastase-induced emphysema. Respir. Res. 2022, 23, 44. [Google Scholar] [CrossRef] [PubMed]
- d’Adesky, N.D.; de Rivero Vaccari, J.P.; Bhattacharya, P.; Schatz, M.; Perez-Pinzon, M.A.; Bramlett, H.M.; Raval, A.P. Nicotine alters estrogen receptor-beta-regulated inflammasome activity and exacerbates ischemic brain damage in female rats. Int. J. Mol. Sci. 2018, 19, 1330. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, Z.-Y.; Li, Y.-P.; Li, D.; Huang, S.-L.; Gu, L.-Q.; Xu, J.; Huang, Z.-S. Syntheses and evaluation of novel isoliquiritigenin derivatives as potential dual inhibitors for amyloid-beta aggregation and 5-lipoxygenase. Eur. J. Med. Chem. 2013, 66, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Jia, J. Isoliquiritigenin confers neuroprotection and alleviates amyloid-β42-induced neuroinflammation in microglia by regulating the Nrf2/NF-κB signaling. Front. Neurosci. 2021, 15, 638772. [Google Scholar] [CrossRef]
- Dorandish, S.; Williams, A.; Atali, S.; Sendo, S.; Price, D.; Thompson, C.; Guthrie, J.; Heyl, D.; Evans, H.G. Regulation of amyloid-β levels by matrix metalloproteinase-2/9 (MMP2/9) in the media of lung cancer cells. Sci. Rep. 2021, 11, 9708. [Google Scholar] [CrossRef]
- Prajapati, R.; Seong, S.H.; Park, S.E.; Paudel, P.; Jung, H.A.; Choi, J.S. Isoliquiritigenin, a potent human monoamine oxidase inhibitor, modulates dopamine D1, D3, and vasopressin V1A receptors. Sci. Rep. 2021, 11, 23528. [Google Scholar] [CrossRef]
- Dhabal, S.; Das, P.; Biswas, P.; Kumari, P.; Yakubenko, V.P.; Kundu, S.; Cathcart, M.K.; Kundu, M.; Biswas, K.; Bhattacharjee, A. Regulation of monoamine oxidase A (MAO-A) expression, activity, and function in IL-13-stimulated monocytes and A549 lung carcinoma cells. J. Biol. Chem. 2018, 293, 14040–14064. [Google Scholar] [CrossRef]
- Huang, B.; Zhou, Z.; Liu, J.; Wu, X.; Li, X.; He, Q.; Zhang, P.; Tang, X. The role of monoamine oxidase A in HPV-16 E7-induced epithelial-mesenchymal transition and HIF-1α protein accumulation in non-small cell lung cancer cells. Int. J. Biol. Sci. 2020, 16, 2692–2703. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, D.; Li, Y.; Li, Y.; Cui, W.; Li, Y.; Li, H.; Li, X.; Wang, D. Potential monoamine oxidase A inhibitor suppressing paclitaxel-resistant non-small cell lung cancer metastasis and growth. Thorac. Cancer 2020, 11, 2858–2866. [Google Scholar] [CrossRef]
- Yang, X.-G.; Li, Y.-Y.; Zhao, D.-X.; Cui, W.; Li, H.; Li, X.-Y.; Li, Y.-X.; Wang, D. Repurposing of a monoamine oxidase A inhibitor-heptamethine carbocyanine dye conjugate for paclitaxel-resistant non-small cell lung cancer. Oncol. Rep. 2021, 45, 306–1314. [Google Scholar] [CrossRef]
- Tian, T.; Sun, J.; Wang, J.; Liu, Y.; Liu, H. Isoliquiritigenin inhibits cell proliferation and migration through the PI3K/AKT signaling pathway in A549 lung cancer cells. Oncol. Lett. 2018, 16, 6133–6139. [Google Scholar] [CrossRef]
- Hsu, Y.; Kuo, P.; Chiang, L.; Lin, C. Isoliquiritigenin inhibits the proliferation and induces the apoptosis of human non-small cell lung cancer A549 cells. Clin. Exp. Pharmacol. Physiol. 2004, 31, 414–418. [Google Scholar] [CrossRef]
- Li, T.; Satomi, Y.; Katoh, D.; Shimada, J.; Baba, M.; Okuyama, T.; Nishino, H.; Kitamura, N. Induction of cell cycle arrest and p21(CIP1/WAF1) expression in human lung cancer cells by isoliquiritigenin. Cancer Lett. 2004, 207, 27–35. [Google Scholar]
- Zhou, Y.; Ho, W.S. Combination of liquiritin, isoliquiritin and isoliquirigenin induce apoptotic cell death through upregulating p53 and p21 in the A549 non-small cell lung cancer cells. Oncol. Rep. 2014, 31, 298–304. [Google Scholar] [CrossRef]
- Heng, W.S.; Cheah, S. Identification of phytochemical-based β-catenin nuclear localization inhibitor in NSCLC: Differential targeting population from member of isothiocyanates. Molecules 2021, 26, 399. [Google Scholar] [CrossRef]
- Cui, Y.; Wu, Y.; Wang, C.; Wang, Z.; Li, Y.; Jiang, Z.; Zhao, W.; Pan, Z. Isoliquiritigenin inhibits non-small cell lung cancer progression via m6A/IGF2BP3-dependent TWIST1 mRNA stabilization. Phytomedicine 2022, 104, 154299. [Google Scholar] [CrossRef]
- Qiao, F.; Zhao, Y.; Mai, Y.; Guo, J.; Dong, L.; Zhang, W.; Yang, J. Isoliquiritigenin Nanosuspension enhances cytostatic effects in A549 lung cancer cells. Planta Med. 2020, 86, 538–547. [Google Scholar] [CrossRef]
- Chen, C.; Shenoy, A.K.; Padia, R.; Fang, D.; Jing, Q.; Yang, P.; Su, S.; Huang, S. Suppression of lung cancer progression by isoliquiritigenin through its metabolite 2, 4, 2’, 4’-Tetrahydroxychalcone. J. Exp. Clin. Cancer Res. 2018, 37, 243. [Google Scholar] [CrossRef]
- Jung, S.K.; Lee, M.; Lim, D.Y.; Kim, J.E.; Singh, P.; Lee, S.; Jeong, C.; Lim, T.; Chen, H.; Chi, Y.; et al. Isoliquiritigenin induces apopto-sis and inhibits xenograft tumor growth of human lung cancer cells by targeting both wild type and L858R/T790M mutant EGFR. J. Biol. Chem. 2014, 289, 35839–35848. [Google Scholar] [CrossRef]
- Deng, N.; Qiao, M.; Li, Y.; Liang, F.; Li, J.; Liu, Y. Anticancer effects of licochalcones: A review of the mechanisms. Front. Pharmacol. 2023, 14, 1074506. [Google Scholar] [CrossRef]
- Kwon, S.J.; Park, S.Y.; Kwon, G.T.; Lee, K.W.; Kang, W.-H.; Choi, M.-S.; Yun, J.W.; Jeon, J.-H.; Jun, J.G.; Park, J.H.Y. Licochalcone E present in licorice suppresses lung metastasis in the 4T1 mammary orthotopic cancer model. Cancer Prev. Res. 2013, 6, 603–613. [Google Scholar] [CrossRef]
- Furusawa, J.-I.; Funakoshi-Tago, M.; Tago, K.; Mashino, T.; Inoue, H.; Sonoda, Y.; Kasahara, T. Licochalcone A significantly suppresses LPS signaling pathway through the inhibition of NF-kappaB p65 phosphorylation at serine 276. Cell Signal 2009, 21, 778–785. [Google Scholar] [CrossRef]
- Furusawa, J.-I.; Funakoshi-Tago, M.; Mashino, T.; Tago, K.; Inoue, H.; Sonoda, Y.; Kasahara, T. Glycyrrhiza inflata-derived chalcones, Licochalcone A, Licochalcone B and Licochalcone D, inhibit phosphorylation of NF-kappaB p65 in LPS signaling pathway. Int. Immunopharmacol. 2009, 9, 499–507. [Google Scholar] [CrossRef]
- Dong, M.; Yang, Z.; Gao, Q.; Deng, Q.; Li, L.; Chen, H. Protective effects of isoliquiritigenin and licochalcone B on the immunotoxicity of BDE-47: Antioxidant effects based on the activation of the Nrf2 pathway and inhibition of the NF-κB pathway. Antioxidants 2024, 13, 445. [Google Scholar] [CrossRef]
- Fang, M.; Su, K.; Wang, X.; Guan, P.; Hu, X. Study on molecular mechanisms of destabilizing Aβ(1–42) protofibrils by licochalcone A and licochalcone B using molecular dynamics simulations. J. Mol. Graph. Model. 2023, 122, 108500. [Google Scholar] [CrossRef]
- Muto, E.; Okada, T.; Yamanaka, T.; Uchino, H.; Inazu, M. Licochalcone E, a β-Amyloid aggregation inhibitor, regulates microglial M1/M2 polarization via inhibition of CTL1-mediated choline uptake. Biomolecules 2023, 13, 191. [Google Scholar] [CrossRef]
- Chen, G.; Ma, Y.; Jiang, Z.; Feng, Y.; Han, Y.; Tang, Y.; Zhang, J.; Ni, H.; Li, X.; Li, N. Lico A causes ER stress and apoptosis via up-regulating miR-144-3p in human lung cancer cell line H292. Front. Pharmacol. 2018, 9, 837. [Google Scholar] [CrossRef]
- Huang, H.-C.; Tsai, L.-L.; Tsai, J.-P.; Hsieh, S.-C.; Yang, S.-F.; Hsueh, J.-T.; Hsieh, Y.-H. Licochalcone A inhibits the migration and invasion of human lung cancer cells via inactivation of the Akt signaling pathway with downregulation of MMP-1/-3 expression. Tumour Biol. 2014, 35, 12139–12149. [Google Scholar] [CrossRef]
- Lin, Y.-J.; Liang, W.-M.; Chen, C.-J.; Tsang, H.; Chiou, J.-S.; Liu, X.; Cheng, C.-F.; Lin, T.-H.; Liao, C.-C.; Huang, S.-M.; et al. Network analysis and mechanisms of action of Chinese herb-related natural compounds in lung cancer cells. Phytomedicine 2019, 58, 152893. [Google Scholar] [CrossRef]
- Luo, W.; Sun, R.; Chen, X.; Li, J.; Jiang, J.; He, Y.; Shi, S.; Wen, H. ERK activation-mediated autophagy induction resists licochalcone A-induced anticancer activities in lung cancer cells in vitro. Onco Targets Ther. 2021, 13, 13437–13450. [Google Scholar] [CrossRef]
- Park, M.K.; Ji, J.; Haam, K.; Han, T.-H.; Lim, S.; Kang, M.-J.; Lim, S.S.; Ban, H.S. Licochalcone A inhibits hypoxia-inducible factor-1α accumulation by suppressing mitochondrial respiration in hypoxic cancer cells. Biomed. Pharmacother. 2021, 133, 111082. [Google Scholar] [CrossRef]
- Qiu, C.; Zhang, T.; Zhang, W.; Zhou, L.; Yu, B.; Wang, W.; Yang, Z.; Liu, Z.; Zou, P.; Liang, G. Licochalcone A inhibits the proliferation of human lung cancer cell lines A549 and H460 by inducing G2/M cell cycle arrest and ER stress. Int. J. Mol. Sci. 2017, 18, 1761. [Google Scholar] [CrossRef]
- Seo, Y.H. Discovery of licochalcone A as a natural product inhibitor of Hsp90 and its effect on gefitinib resistance in non-small cell lung cancer (NSCLC). Bull. Korean Chem. Soc. 2013, 34, 1917. [Google Scholar] [CrossRef][Green Version]
- Tang, Z.H.; Chen, X.; Wang, Z.-Y.; Chai, K.; Wang, Y.-F.; Xu, X.-H.; Wang, X.-W.; Lu, J.-H.; Wang, Y.-T.; Chen, X.-P.; et al. Induction of C/EBP homologous protein-mediated apoptosis and autophagy by licochalcone A in non-small cell lung cancer cells. Sci. Rep. 2016, 6, 26241. [Google Scholar] [CrossRef]
- Yuan, L.-W.; Jiang, X.-M.; Xu, Y.-L.; Huang, M.-Y.; Chen, Y.-C.; Yu, W.-P.; Su, M.-X.; Ye, Z.-H.; Chen, X.; Wang, Y.; et al. Licochalcone A inhibits interferon-gamma-induced programmed death-ligand 1 in lung cancer cells. Phytomedicine 2021, 80, 153394. [Google Scholar] [CrossRef]
- Han, S.; Li, X.; Gan, Y.; Li, W. Licochalcone A promotes the ubiquitination of c-Met to abrogate gefitinib resistance. Biomed. Res. Int. 2022, 2022, 5687832. [Google Scholar] [CrossRef]
- Fan, X.; Guan, G.; Wang, J.; Jin, M.; Wang, L.; Duan, X. Licochalcone A induces cell cycle arrest and apoptosis via suppressing MAPK signaling pathway and the expression of FBXO5 in lung squamous cell cancer. Oncol. Rep. 2023, 50, 214. [Google Scholar] [CrossRef]
- Gao, F.; Li, M.; Yu, X.; Liu, W.; Zhou, L.; Li, W. Licochalcone A inhibits EGFR signalling and translationally suppresses survivin expression in human cancer cells. J. Cell Mol. Med. 2021, 25, 813–826. [Google Scholar] [CrossRef]
- Oh, H.-N.; Lee, M.-H.; Kim, E.; Yoon, G.; Chae, J.-I.; Shim, J.-H. Licochalcone B inhibits growth and induces apoptosis of human non-small-cell lung cancer cells by dual targeting of EGFR and MET. Phytomedicine 2019, 63, 153014. [Google Scholar] [CrossRef]
- Oh, H.-N.; Lee, M.-H.; Kim, E.; Kwak, A.-W.; Yoon, G.; Cho, S.-S.; Liu, K.; Chae, J.-I.; Shim, J.-H. Licochalcone D induces ROS-dependent apoptosis in gefitinib-sensitive or resistant lung cancer cells by targeting EGFR and MET. Biomolecules 2020, 10, 297. [Google Scholar] [CrossRef]
- Liang, M.; Li, X.; Ouyang, X.; Xie, H.; Chen, D. Antioxidant mechanisms of echinatin and licochalcone A. Molecules 2018, 24, 3. [Google Scholar] [CrossRef]
- Oh, H.-N.; Lee, M.-H.; Kim, E.; Kwak, A.-W.; Seo, J.-H.; Yoon, G.; Cho, S.-S.; Choi, J.-S.; Lee, S.-M.; Seo, K.-S.; et al. Dual inhibition of EGFR and MET by Echinatin retards cell growth and induces apoptosis of lung cancer cells sensitive or resistant to gefitinib. Phytother. Res. 2020, 34, 388–400. [Google Scholar] [CrossRef]
| Experimental Models | NSCLC Cell Line | Dose/Administration | Therapeutic Effects | Ref. |
|---|---|---|---|---|
| In vitro | A549 | A549 cells were treated with ILTG at 20 μM concentration for 24–72 h incubation at 37 °C | Anti-proliferative, anti-invasion, anti-migration, apoptosis | [46] |
| In vitro | A549 | A549 cells were treated with ILTG at 0, 2, 10, 20, and 40 μmol/L concentrations for 12–72 h of incubation at 37 °C | Anti-proliferative, apoptosis, G1 phase cell cycle arrest | [47] |
| In vitro | A549 | A549 cells were treated with ILTG at 10, 20, 30, 40, and 40 μM concentrations for 24–72 h of incubation at 42 and 70 °C | Anti-proliferative, G2/M phase cell cycle arrest | [48] |
| In vitro | A549 | A549 cells were treated with ILTG and flavonoid glycosides (liquiritin, isoliquiritin) at 1.95–500 μg/mL concentrations for 24–72 h of incubation | Apoptosis, G2/M phase cell cycle arrest | [49] |
| In vitro | SK-LU-1 | SK-LU-1cells were treated with ILTG at 1.56, 3.13, 6.25, 12.5, 25, and 50 μg/mL concentrations for 24–72 h of incubation | Inhibition of cell growth | [50] |
| In vitro | A549, H1299 | NSCLC cells were treated with ILTG at 6.25, 12.5, and 25 μM concentrations for 24–72 h of incubation at 37 °C | Anti-proliferative, anti-invasion, anti-migration | [51] |
| In vitro | A549 | A549 cells were treated with pure ILTG and ILTG nanosuspensions at 0.03, 0.06, 0.09, 0.12, 0.15, and 0.15 μM concentrations for 24–72 h incubation at 37 °C | Apoptosis | [52] |
| In vitro/vivo | A549, H1299, H1975 | NSCLC cells were treated with ILTG at 0, 3, and 10 μM concentrations for 24 h of incubation at 37 °C Athymic nude mice were injected with a mixture of DMEM and Matrigel in a 1:1 ratio and then classified into 4 groups, in which each group received a vehicle: ILTG (25 mg/kg), tetrahydroxychalcone (25 mg/kg), or AZD0530 (20 mg/kg) | Anti-migration, inhibition of cell growth, and tumorigenesis | [53] |
| In vitro/vivo | A549, HCC827GR, H1975, H1650, and HCC827 | NSCLC cells were treated with ILTG at 0, 10, 20, and 40 μM concentrations for 24 h of incubation at 37 °C Athymic nude mice were divided into 4 groups: vehicle, ILTG (1 mg/kg), ILTG (5 mg/kg), and gefitinib (5 mg/kg) | Apoptosis | [54] |
| Experimental Models | Lico Classification | NSCLC Cell Line | Dose/Administration | Therapeutic Effects | Ref. |
|---|---|---|---|---|---|
| In vitro | LicoA | SK-LU-1 | SK-LU-1cells were treated with licoA at 1.56, 3.13, 6.25, 12.5, 25, and 50 μg/mL concentrations for 24–72 h of incubation | Inhibition of tumor growth | [50] |
| In vitro | LicoA | H292 | H292 cells were treated with licoA at 0, 1, 10, 20, 40, and 80 μM concentrations for 24–96 h of incubation at 37 °C | Anti-proliferative, apoptosis, and autophagy | [62] |
| In vitro | LicoA | A549, H460 | NSCLC cells were treated with licoA at 0, 2, 5, 10, and 20 μM concentrations for 24 and 48 h of incubation at 37 °C | Anti-migration, anti-invasion | [63] |
| In vitro | LicoA | A549 | A549 cells were treated with licoA at 20, 40, and 80 μM concentrations for 24 h of incubation at 37 °C | Apoptosis, S and G2/M phase cell cycle arrest, autophagy | [64] |
| In vitro | LicoA | A549, H23, H460, H1299, SPC-A1 | NSCLC cells were treated with licoA at 0, 5, 10, 20, 30, and 40 μM concentrations for 48 h of incubation at 37 °C for 7 days | Apoptosis, autophagy | [65] |
| In vitro | LicoA | HCT116, H1299, H322 | NSCLC cells were treated with licoA at 5, 10, 15, and 20 μM concentrations for 6 h of incubation at 37 °C | Anti-viability | [66] |
| In vitro | LicoA | A549, H460 | NSCLC cells were treated with licoA at 20, 40, and 60 μM concentrations for 4 and 12 h of incubation at 37 °C | Anti-proliferative, apoptosis, G2/M phase cell cycle arrest | [67] |
| In vitro | LicoA | H1975 | H1975 cells were treated with licoA at 0, 10, 30, 50, and 70 μM concentrations for 24–72 h of incubation at 37 °C | Anti-proliferative | [68] |
| In vitro | LicoA | A549, H1299 | NSCLC cells were treated with licoA at 0, 5, 10, 15, and 20 μM concentrations for 24 h of incubation at 37 °C | Apoptosis, sub-G1 phase cell cycle arrest, autophagy | [69] |
| In vitro | LicoA | A549, H1299, H1650 | NSCLC cells were treated with licoA at 5, 10, 15, and 20 μM concentrations for 24 and 48 h of incubation at 37 °C | Anti-proliferative, apoptosis | [70] |
| In vivo | LicoA | HCC827, PC-9 | Female athymic nude mice were injected with 20 mg/kg licoA for a period of six weeks | Anti-viability, apoptosis | [71] |
| In vitro/vivo | LicoA | H226, H1703 | NSCLC cells were treated with licoA at 0, 10, 20, and 40 μM concentrations for 24–72 h incubation at 37 °C BALB/c-nu mice (male) were divided into 4 groups: control (saline containing 20% SBE-β-CD), licoA (7.5 mg/kg), licoA (15 mg/kg), and cisplatin (2 mg/kg) | Anti-proliferative, inhibition of tumor growth, apoptosis, G1 phase cell cycle arrest | [72] |
| In vitro/vivo | LicoA | A549, HCC827, H1975, and H3255 | NSCLC cells were treated with licoA at 0, 5, 10, 20, 40, 80, and 200 μM concentrations for 24–96 h of incubation at 37 °C Athymic nude mice (female) were injected with 10 mg/kg licoA | Inhibition tumor growth, apoptosis | [73] |
| In vitro | LicoB | HCC827, HCC827GR | NSCLC cells were treated with licoB at 5, 10, 15, and 20 μM concentrations for 48 h of incubation at 37 °C | Anti-proliferative, apoptosis, G2/M phase cell cycle arrest | [74] |
| In vitro | LicoD | HCC827, HCC827GR | NSCLC cells were treated with licoD at 5, 10, 15, and 20 μM concentrations for 48 h of incubation at 37 °C | Anti-proliferative, apoptosis, G2/M phase cell cycle arrest | [75] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsharairi, N.A. The Role of Licorice Chalcones as Molecular Genes and Signaling Pathways Modulator—A Review of Experimental Implications for Nicotine-Induced Non-Small Cell Lung Cancer Treatment. Curr. Issues Mol. Biol. 2024, 46, 5894-5908. https://doi.org/10.3390/cimb46060352
Alsharairi NA. The Role of Licorice Chalcones as Molecular Genes and Signaling Pathways Modulator—A Review of Experimental Implications for Nicotine-Induced Non-Small Cell Lung Cancer Treatment. Current Issues in Molecular Biology. 2024; 46(6):5894-5908. https://doi.org/10.3390/cimb46060352
Chicago/Turabian StyleAlsharairi, Naser A. 2024. "The Role of Licorice Chalcones as Molecular Genes and Signaling Pathways Modulator—A Review of Experimental Implications for Nicotine-Induced Non-Small Cell Lung Cancer Treatment" Current Issues in Molecular Biology 46, no. 6: 5894-5908. https://doi.org/10.3390/cimb46060352
APA StyleAlsharairi, N. A. (2024). The Role of Licorice Chalcones as Molecular Genes and Signaling Pathways Modulator—A Review of Experimental Implications for Nicotine-Induced Non-Small Cell Lung Cancer Treatment. Current Issues in Molecular Biology, 46(6), 5894-5908. https://doi.org/10.3390/cimb46060352