Effect of SNPs on Litter Size in Swine
Abstract
1. Introduction
2. Materials and Methods
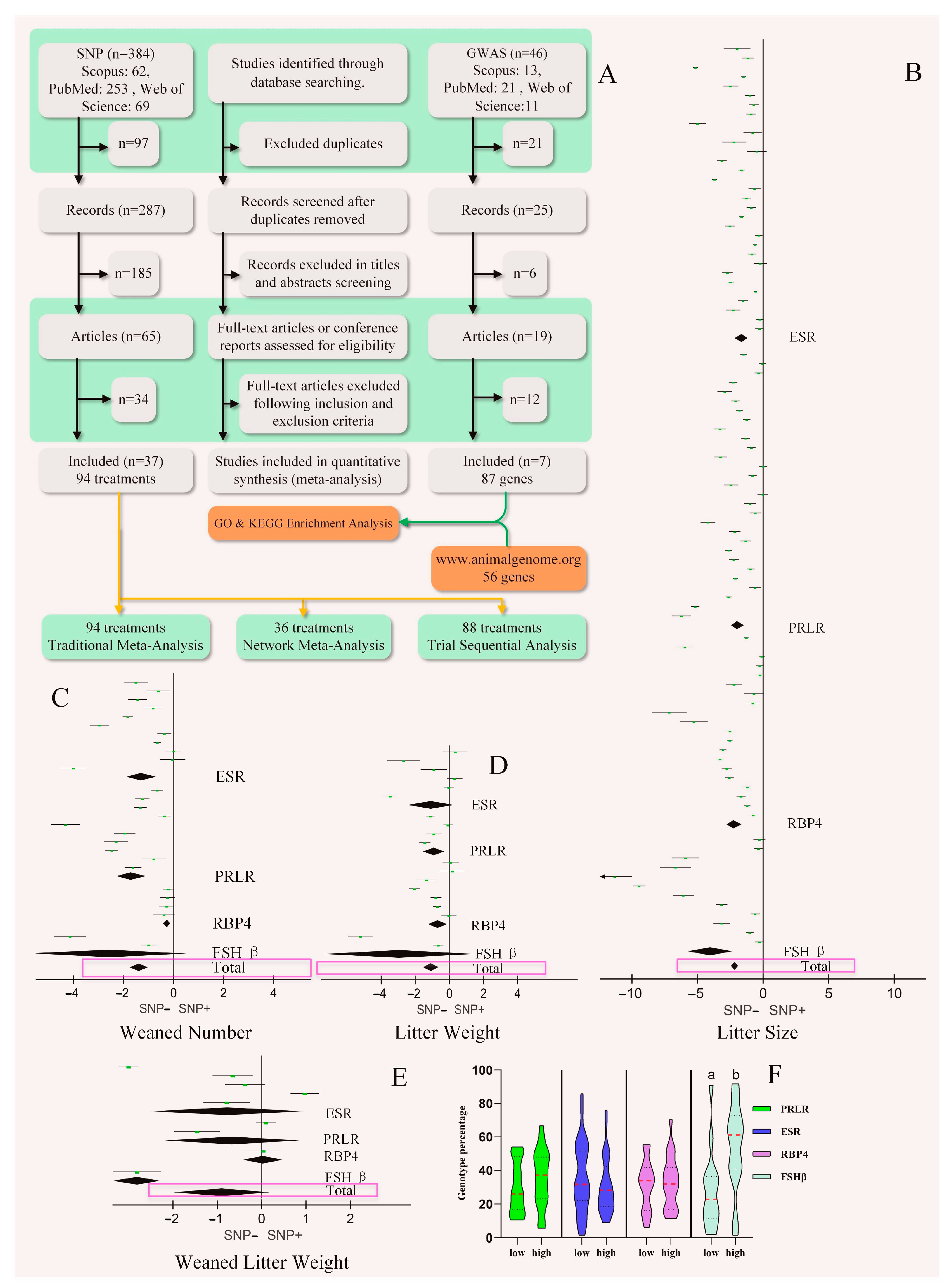
2.1. Database Search Strategy and Study Inclusion
2.2. Data Extraction
2.3. Traditional and Network Meta-Analysis
2.4. Gene Set Enrichment Analysis and PPI Network Analysis
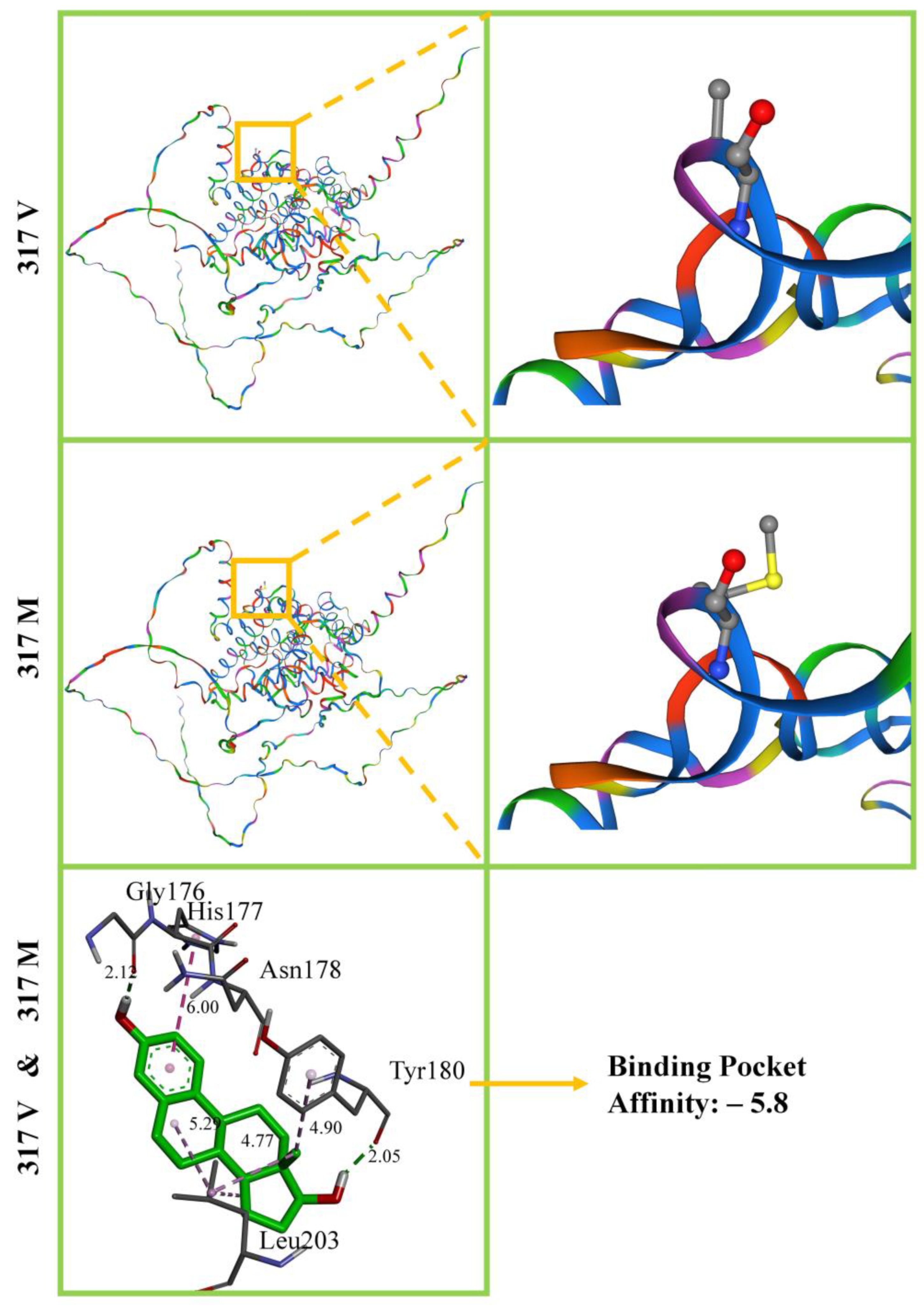
2.5. Homology Modelling of ESR 3D Structures and E2 Docking
3. Results
3.1. Traditional Meta-Analysis
3.2. Network Meta-Analysis and Trial Sequential Analysis
3.3. Gene Enrichment Analysis and Protein–Protein Interaction (PPI) Network Analysis
3.4. Homology Modelling of ESR 3D Structures and E2 Docking
4. Discussion
4.1. PCR-RFLP/SSCP Defects in Identifying SNPs
4.2. GWAS Defects in Identifying SNPs
4.3. Candidate Genes Affect Swine LS
4.4. Relationship between LS and Litter Weight
4.5. Lactation
4.6. Limitations
4.7. Effects of the Development Trend of LS on Pig Breeding
5. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Vashi, Y.; Magotra, A.; Kalita, D.; Banik, S.; Sahoo, N.R.; Gupta, S.K.; Naskar, S. Evaluation of candidate genes related to litter traits in Indian pig breeds. Reprod. Domest. Anim. 2021, 56, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, L.; Shi, L.; Zhang, P.; Li, Y.; Li, M.; Tian, J.; Wang, L.; Zhao, F. GWAS of Reproductive Traits in Large White Pigs on Chip and Imputed Whole-Genome Sequencing Data. Int. J. Mol. Sci. 2022, 23, 13338. [Google Scholar] [CrossRef]
- Hu, Z.L.; Park, C.A.; Reecy, J.M. Building a livestock genetic and genomic information knowledgebase through integrative developments of Animal QTLdb and CorrDB. Nucleic Acids Res. 2019, 47, D701–D710. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.H.; An, S.M.; Kwon, S.; Park, D.H.; Kim, T.W.; Kang, D.G.; Yu, G.E.; Kim, I.S.; Park, H.C.; Ha, J.; et al. DNA methylation patterns and gene expression associated with litter size in Berkshire pig placenta. PLoS ONE 2017, 12, e0184539. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Ning, C.; Liu, J.F.; Zhao, X. Relationship between mitochondrial DNA haplogroup and litter size in the pig. Reprod. Fertil. Dev. 2020, 32, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Deng, D.; Liu, X.; Xiao, Y.; Huang, J.; Wang, F.; Li, X.; Yu, M. A miR-18a binding-site polymorphism in CDC42 3’UTR affects CDC42 mRNA expression in placentas and is associated with litter size in pigs. Mamm. Genome 2019, 30, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Pu, Q.; Chai, J.; Chen, L.; Liu, C.; Yang, C.; Huang, Y.; Luo, J. Exosome miRNA Expression in Umbilical Cord Blood of High-Parity Sows Regulates Their Reproductive Potential. Animals 2022, 12, 2456. [Google Scholar] [CrossRef]
- Li, M.; Liu, Y.; Xie, S.; Ma, L.; Zhao, Z.; Gong, H.; Sun, Y.; Huang, T. Transcriptome analysis reveals that long noncoding RNAs contribute to developmental differences between medium-sized ovarian follicles of Meishan and Duroc sows. Sci. Rep. 2021, 11, 22510. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Zhang, H.; Li, X.; Hu, J.; Yang, G.; Sun, S. Genome-Wide Differential Expression Profiling of Ovarian circRNAs Associated with Litter Size in Pigs. Front. Genet. 2019, 10, 1010. [Google Scholar] [CrossRef] [PubMed]
- Egli, P.T.; Schupbach-Regula, G.; Nathues, H.; Ulbrich, S.E.; Grahofer, A. Influence of the farrowing process and different sow and piglet traits on uterine involution in a free farrowing system. Theriogenology 2022, 182, 1–8. [Google Scholar] [CrossRef]
- Langendijk, P.L.; Soede, N.M. Physiology and management of the peri-parturient sow in the context of changing production conditions. Reprod. Domest. Anim. 2023, 58, 84–92. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Luo Reng, Z.; Sun, S. Analysis of PRLR and BF Genotypes Associated with Litter Size in Beijing Black Pig Population. Agric. Sci. China 2008, 7, 1374–1378. [Google Scholar] [CrossRef]
- Fletcher, L.; Akhtar, N.; Zhan, X.; Jafarikia, M.; Sullivan, B.P.; Huber, L.A.; Li, J. Identification of Candidate Salivary, Urinary and Serum Metabolic Biomarkers for High Litter Size Potential in Sows (Sus scrofa). Metabolites 2022, 12, 1045. [Google Scholar] [CrossRef]
- Chen, J.; Li, F.; Yang, W.; Jiang, S.; Li, Y. Comparison of Gut Microbiota and Metabolic Status of Sows with Different Litter Sizes During Pregnancy. Front. Vet. Sci. 2021, 8, 793174. [Google Scholar] [CrossRef]
- Peltoniemi, O.; Tanskanen, T.; Kareskoski, M. One Health challenges for pig reproduction. Mol. Reprod. Dev. 2023, 90, 420–435. [Google Scholar] [CrossRef] [PubMed]
- Omelka, R.; Martiniakova, M.; Peskovicova, D.; Bauerová, M. Associations between Alu I Polymorphism in the Prolactin Receptor Gene and Reproductive Traits of Slovak Large White, White Meaty and Landrace Pigs. Asian-Australas. J. Anim. Sci. 2008, 21, 484–488. [Google Scholar] [CrossRef]
- Mihailov, N.V.; Usatov, A.V.; Getmantseva, L.V.; Bakoev, S.U. Associations between PRLR/AluI gene polymorphism with reproductive, growth, and meat traits in pigs. Cytol. Genet. 2014, 48, 323–326. [Google Scholar] [CrossRef]
- Putnova, L.; Knoll, A.; Dvořák, J.; Čepica, S. A new HpaII PCR-RFLP within the porcine prolactin receptor (PRLR) gene and study of its effect on litter size and number of teats. J. Anim. Breed. Genet. 2002, 119, 57–63. [Google Scholar] [CrossRef]
- Zhu, H.; Yang, H.; Zhao, S.; Tian, Y.; Su, Y. Associations of genetic polymorphisms with reproduction and meat quality characteristics in Chinese Hebao pigs. Can. J. Anim. Sci. 2017, 97, 183–192. [Google Scholar] [CrossRef]
- Terman, A. Effect of the polymorphism of prolactin receptor (PRLR) and leptin (LEP) genes on litter size in Polish pigs. J. Anim. Breed. Genet. 2005, 122, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Laliotis, G.P.; Marantidis, A.; Avdi, M. Association of BF, RBP4, and ESR2 Genotypes with Litter Size in an Autochthonous Pig Population. Anim. Biotechnol. 2017, 28, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Pang, P.; Li, Z.; Hu, H.; Wang, L.; Sun, H.; Mei, S.; Li, F. Genetic effect and combined genotype effect of ESR, FSHbeta, CTNNAL1 and miR-27a loci on litter size in a Large White population. Anim. Biotechnol. 2019, 30, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Sell-Kubiak, E.; Dobrzanski, J.; Derks, M.F.L.; Lopes, M.S.; Szwaczkowski, T. Meta-Analysis of SNPs Determining Litter Traits in Pigs. Genes 2022, 13, 1730. [Google Scholar] [CrossRef]
- Magotra, A.; Naskar, S.; Das, B.; Ahmad, T. A comparative study of SINE insertion together with a mutation in the first intron of follicle stimulating hormone beta gene in indigenous pigs of India. Mol. Biol. Rep. 2015, 42, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Phookan, A.; Zaman, G.U.; Das, A.; Akhtar, F.; Tamuly, S.; Choudhury, H.; Sarma, L.M. Allelic variability of estrogen receptor (ESR) gene and its effect on litter traits of Doom pigs. Trop. Anim. Health Prod. 2021, 53, 316. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Song, Y.; Zhang, W.; Xiao, T.; Peng, H. Effect of NLR family pyrin domain containing 9 gene polymorphism on litter size in large white pigs. Anim. Biotechnol. 2023, 34, 4547–4552. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Du, H.; Chen, J.; Yang, G.; Zhang, X. Porcine growth differentiation factor 9 gene polymorphisms and their associations with litter size. J. Genet. Genom. 2008, 35, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Yurina, A.; Skovorodin, E.; Dolmatova, I. Evaluation of reproductive traits and ovarian and uterine morphology of sows with different genotypes for the estrogen receptor, prolactin receptor, and follicle-stimulating hormone subunit beta genes. Can. J. Vet. Res. 2021, 85, 186–192. [Google Scholar]
- Du, Z.; D’Alessandro, E.; Asare, E.; Zheng, Y.; Wang, M.; Chen, C.; Wang, X.; Song, C. Retrotransposon Insertion Polymorphisms (RIPs) in Pig Reproductive Candidate Genes. Genes 2022, 13, 1359. [Google Scholar] [CrossRef]
- Horogh, G.; Zsolnai, A.; Komiosi, I.; Nyiri, A.; Anton, I.; Fesus, L. Oestrogen receptor genotypes and litter size in Hungarian Large White pigs. J. Anim. Breed. Genet. 2005, 122, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Goliasova, E.; Wolf, J. Impact of the ESR gene on litter size and production traits in Czech Large White pigs. Anim. Genet. 2004, 35, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Tempfli, K.; Farkas, G.; Simon, Z.; Bali Papp, A. Effects of prolactin receptor genotype on the litter size of Mangalica. Acta Vet. Hung. 2011, 59, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Horak, P.; Urban, T.; Dvorak, J. The FUT1 and ESR genes—their variability and associations with reproduction in Prestice Black-Pied sows. J. Anim. Breed. Genet. 2005, 122, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Alberto, B.S.; Haro, J.; Hori-Oshima, S.; Espinosa, A.; Ortega, M.; Perez, J.; Lemus, C.; Espinosa, A.L.; Aranguré, A.; Avila, J.G. Prolactin Receptor (PRLR) Gen Polymorphism and Associations with Reproductive Traits in Pigs. J. Anim. Vet. Adv. 2009, 8, 469–475. [Google Scholar]
- Zakova, E.; Wolf, J. Herd specific effects of the ESR gene on litter size and production traits in Czech Large White sows. Czech J. Anim. Sci. 2004, 49, 373–382. [Google Scholar] [CrossRef]
- Noguera, J.L.; Varona, L.; Gomez-Raya, L.; Sanchez, A.; Babot, D.; Estany, J.; Messer, L.; Rothschild, M.; Pérez-Enciso, M. Estrogen receptor polymorphism in Landrace pigs and its association with litter size performance. Livest. Prod. Sci. 2003, 82, 53–59. [Google Scholar] [CrossRef]
- Li, F.; Xiong, Y.; Deng, C.; Jiang, S.; Zheng, R. Frequencies, Inheritance of Porcine FSH-β Retroposon and its Association with Reproductive Traits. Asian-Australas. J. Anim. Sci. 2002, 15, 179–183. [Google Scholar] [CrossRef]
- Naha, B.; Gaur, G.; Saini, B.; Sahoo, N.; Boro, P. Polymorphism of candidate genes associated with reproductive performance in crossbred pigs. Indian J. Anim. Sci. 2020, 90, 809–812. [Google Scholar] [CrossRef]
- Marek, K.; Dybus, A.; Arkadiusz, T. Prolactin receptor gene polymorphism and its association with litter size in Polish Landrace. Arch. Anim. Breed. 2001, 44, 547–551. [Google Scholar] [CrossRef]
- Chen, K.; Li Barr, N.; Huang, L.; Zhang, Q.; Zhang, J.; Sun, S.; Luo, M.; Wu, C. The combined genotypes effect of ESR and FSHβ genes on litter size traits in five different pig breeds. Chin. Sci. Bull. 2001, 46, 140–143. [Google Scholar] [CrossRef]
- Vega, R.; Cho, B.-W.; Castillo, R.M.; Barrientos, N.; Autriz, M.; Viña, C. Leptin (T3469C) and Estrogen Receptor (T1665G) Gene Polymorphisms and Their Associations to Backfat Thickness and Reproductive Traits of Large White Pigs (Sus scrofa L.). Philipp. J. Sci. 2018, 147, 293–300. [Google Scholar]
- Terman, A.; Polasik, D.; Korpal, A.; Wozniak, K.; Prüffer, K.; Żak, G.; Lambert, B. Association between prolactin receptor gene polymorphism (PRLR) with reproduction performance traits of Polish swine. Can. J. Anim. Sci. 2016, 97, 169–171. [Google Scholar] [CrossRef]
- Sven, M.; Vlado, V.; Mario, M.; Marija, S.; Mario, O.; Velimir, S.; Igor, S.; Marko, S.; Anamaria, E.K. Prlr-alui gene polymorphism and litter size traits in highly prolific line of topigs 20 sows. Acta Vet. 2015, 65, 463–476. [Google Scholar] [CrossRef]
- Terman, A.; Kumalska, M. The effect of a SNP in ESR gene on the reproductive performance traits in Polish sows. Russ. J. Genet. 2012, 48, 1260–1263. [Google Scholar] [CrossRef]
- Marantidis, A.; Laliotis, G.P.; Avdi, M. Association of RBP4 Genotype with Phenotypic Reproductive Traits of Sows. Genet. Res. Int. 2016, 2016, 4940532. [Google Scholar] [CrossRef] [PubMed]
- Spotter, A.; Muller, S.; Hamann, H.; Distl, O. Effect of polymorphisms in the genes for LIF and RBP4 on litter size in two German pig lines. Reprod. Domest. Anim. 2009, 44, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Rothschild, M.F.; Messer, L.; Day, A.; Wales, R.; Short, T.; Southwood, O.; Plastow, G. Investigation of the retinol-binding protein 4 (RBP4) gene as a candidate gene for increased litter size in pigs. Mamm. Genome 2000, 11, 75–77. [Google Scholar] [CrossRef] [PubMed]
- Menčik, S.; Vukovic, V.; Spehar, M.; Modric, M.; Ostović, M.; Ekert Kabalin, A. Association between ESR1 and RBP4 genes and litter size traits in a hyperprolific line of Landrace × Large White cross sows. Veterinární Medicína 2019, 64, 109–117. [Google Scholar] [CrossRef]
- Terman, A.; Kmiec, M.; Polasik, D.; Rybarczyk, A. Association Between RBP4 Gene Polymorphism and Reproductive Traits in Polish Sows. J. Anim. Vet. Adv. 2011, 10, 2639–2641. [Google Scholar]
- Niu, S.; Wang, X.; Hao, F.; Zhao, R. Effect of the polymorphism of RBP4 and OPN genes on litter size in Tibet pigs. Acta Agric. Scand. Sect. A-Anim. Sci. 2008, 58, 10–13. [Google Scholar] [CrossRef]
- Gonçalves, I.D.V.; Gonçalves, P.; Silva, J.C.; Portela, V.V., Jr.; Borges, L.F.K.; Oliveira, J.; Lovatto, P.A. Interaction between estrogen recepto and retinol-binding protein-4 polymorphisms as a tool for the selection of prolific pigs. Genet. Mol. Biol. 2008, 31, 481–486. [Google Scholar] [CrossRef]
- Lessmann, M.; Kanellopoulos, A.; Kros, J.; Orsi, F.; Bakker, M. Maximizing agricultural reuse of recycled nutrients: A spatially explicit assessment of environmental consequences and costs. J. Environ. Manag. 2023, 332, 117378. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Lv, L.; Liu, D.; Ma, H.; Radovic, C. A meta-analysis: Effect of androgens on reproduction in sows. Front. Endocrinol. 2023, 14, 1094466. [Google Scholar] [CrossRef] [PubMed]
- Klunk, J.; Vilgalys, T.P.; Demeure, C.E.; Cheng, X.; Shiratori, M.; Madej, J.; Beau, R.; Elli, D.; Patino, M.I.; Redfern, R.; et al. Evolution of immune genes is associated with the Black Death. Nature 2022, 611, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Hollmann, I.; Lingens, J.B.; Wilke, V.; Homann, C.; Teich, K.; Buch, J.; Chuppava, B.; Visscher, C. Epidemiological Study on Salmonella Prevalence in Sow Herds Using Direct and Indirect Detection Methods. Microorganisms 2022, 10, 1532. [Google Scholar] [CrossRef]
- Lillie-Jaschniski, K.; Wahner, C.; Viehmann, M.; Hauf, S.; Gale, C.; Rohde, J.; Hennig-Pauka, I. Sock and Environmental Swabs as an Efficient, Non-Invasive Tool to Assess the Salmonella Status of Sow Farms. Animals 2023, 13, 1031. [Google Scholar] [CrossRef]
- Hanrahan, J.P.; Gregan, S.M.; Mulsant, P.; Mullen, M.; Davis, G.H.; Powell, R.; Galloway, S.M. Mutations in the genes for oocyte-derived growth factors GDF9 and BMP15 are associated with both increased ovulation rate and sterility in Cambridge and Belclare sheep (Ovis aries). Biol. Reprod. 2004, 70, 900–909. [Google Scholar] [CrossRef] [PubMed]
- Nicol, L.; Bishop, S.C.; Pong-Wong, R.; Bendixen, C.; Holm, L.E.; Rhind, S.M.; McNeilly, A.S. Homozygosity for a single base-pair mutation in the oocyte-specific GDF9 gene results in sterility in Thoka sheep. Reproduction 2009, 138, 921–933. [Google Scholar] [CrossRef]
- Liu, G.; Liu, H.; Tian, W.; Liu, C.; Yang, H.; Wang, H.; Gao, L.; Huang, Y. Dietary nucleotides influences intestinal barrier function, immune responses and microbiota in 3-day-old weaned piglets. Int. Immunopharmacol. 2023, 117, 109888. [Google Scholar] [CrossRef]
- Zhang, D.; Deng, Y.; Kukanja, P.; Agirre, E.; Bartosovic, M.; Dong, M.; Ma, C.; Ma, S.; Su, G.; Bao, S.; et al. Spatial epigenome-transcriptome co-profiling of mammalian tissues. Nature 2023, 616, 113–122. [Google Scholar] [CrossRef]
- Kamimoto, K.; Stringa, B.; Hoffmann, C.M.; Jindal, K.; Solnica-Krezel, L.; Morris, S.A. Dissecting cell identity via network inference and in silico gene perturbation. Nature 2023, 614, 742–751. [Google Scholar] [CrossRef] [PubMed]
- Tarazi, S.; Aguilera-Castrejon, A.; Joubran, C.; Ghanem, N.; Ashouokhi, S.; Roncato, F.; Wildschutz, E.; Haddad, M.; Oldak, B.; Gomez-Cesar, E.; et al. Post-gastrulation synthetic embryos generated ex utero from mouse naive ESCs. Cell 2022, 185, 3290–3306.e25. [Google Scholar] [CrossRef] [PubMed]
- Hamazaki, N.; Kyogoku, H.; Araki, H.; Miura, F.; Horikawa, C.; Hamada, N.; Shimamoto, S.; Hikabe, O.; Nakashima, K.; Kitajima, T.S.; et al. Reconstitution of the oocyte transcriptional network with transcription factors. Nature 2021, 589, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Ishikura, Y.; Ohta, H.; Sato, T.; Murase, Y.; Yabuta, Y.; Kojima, Y.; Yamashiro, C.; Nakamura, T.; Yamamoto, T.; Ogawa, T.; et al. In vitro reconstitution of the whole male germ-cell development from mouse pluripotent stem cells. Cell Stem Cell 2021, 28, 2167–2179.e9. [Google Scholar] [CrossRef] [PubMed]
- Nami, Y.; Haghshenas, B.; Javanmard, A.; Samari, M.; Mohammadi, N.; Oroojalian, F.; Mokhtarzadeh, A. A critical review of the recent concept of artificial mechanical uterus design in relation to the maternal microbiome: An Update to past researches. J. Reprod. Immunol. 2023, 156, 103828. [Google Scholar] [CrossRef] [PubMed]
- Brinke, I.; Große-Brinkhaus, C.; Roth, K.; Pröll-Cornelissen, M.J.; Henne, H.; Schellander, K.; Tholen, E. Genomic background and genetic relationships between boar taint and fertility traits in German Landrace and Large White. BMC Genet. 2020, 21, 61. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-Y.; Freitas, P.H.; Oliveira, H.; Lazaro, S.; Huang, Y.; Howard, J.; Gu, Y.; Schinckel, A.; Brito, L. Genotype-by-environment interactions for reproduction, body composition, and growth traits in maternal-line pigs based on single-step genomic reaction norms. Genet. Sel. Evol. 2021, 53, 51. [Google Scholar] [CrossRef] [PubMed]
- Sell-Kubiak, E.; Knol, E.; Lopes, M. Evaluation of the phenotypic and genomic background of variability based on litter size of Large White pigs. Genet. Sel. Evol. 2022, 54, 1. [Google Scholar] [CrossRef] [PubMed]
- Suwannasing, R.; Duangjinda, M.; Boonkum, W.; Taharnklaew, R.; Tuangsithtanon, K. The identification of novel regions for reproduction trait in Landrace and Large White pigs using a single step genome-wide association study. Asian-Australas. J. Anim. Sci. 2018, 31, 1852–1862. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ding, X.; Tan, Z.; Xing, K.; Yang, T.; Wang, Y.; Sun, D.; Wang, C. Genome-wide association study for reproductive traits in a Large White pig population. Anim. Genet. 2018, 49, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wang, Y.; Huang, X.; Wu, S.; Bao, W. A genome-wide association study of important reproduction traits in Large White pigs. Gene 2022, 838, 146702. [Google Scholar] [CrossRef]
- An, S.; Hwang, J.; Kwon, S.; Yu, G.; Park, D.; Kang, D.; Kim, T.; Park, H.; Ha, J.; Kim, C. Effect of Single Nucleotide Polymorphisms in IGFBP2 and IGFBP3 Genes on Litter Size Traits in Berkshire Pigs. Anim. Biotechnol. 2017, 29, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Balcells, I.; Castelló, A.; Mercadé, A.; Noguera, J.L.; Fernández-Rodríguez, A.; Sànchez, A.; Tomàs, A. Analysis of porcine MUC4 gene as a candidate gene for prolificacy QTL on SSC13 in an Iberian × Meishan F2 population. BMC Genet. 2011, 12, 93. [Google Scholar] [CrossRef] [PubMed]
- Balcells, I.; Castelló, A.; Noguera, J.L.; Fernández-Rodríguez, A.; Sánchez, A.; Tomás, A. Sequencing and gene expression of the porcine ITIH SSC13 cluster and its effect on litter size in an Iberian × Meishan F2 population. Anim. Reprod. Sci. 2011, 128, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Buske, B.; Brunsch, C.; Zeller, K.; Reinecke, P.; Brockmann, G. Analysis of properdin (BF) genotypes associated with litter size in a commercial pig cross population. J. Anim. Breed. Genet. 2005, 122, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Buske, B.; Sternstein, I.; Reißmann, M.; Brockmann, G. Detection of novel single-nucleotide polymorphisms (SNPs) in the CYP21 gene and association analysis of two SNPs for CYP21 and ESR2 with litter size in a commercial sow population. J. Anim. Breed. Genet. 2006, 123, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Chang, T.; Su, H.Y. Characterization of porcine leptin receptor polymorphisms and their association with reproduction and production traits. Anim. Biotechnol. 2004, 15, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Coster, A.; Madsen, O.; Heuven, H.; Dibbits, B.; Groenen, M.; Arendonk, J.; Bovenhuis, H. The Imprinted Gene DIO3 Is a Candidate Gene for Litter Size in Pigs. PLoS ONE 2012, 7, e31825. [Google Scholar] [CrossRef] [PubMed]
- Dall’Olio, S.; Fontanesi, L.; Tognazzi, L.; Russo, V. Genetic structure of candidate genes for litter size in Italian Large White pigs. Vet. Res. Commun. 2010, 34, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Dan, L.; Yonggang, L. Molecular characteristics and association analysis with litter size trait for porcine KLK7 gene. Anim. Biotechnol. 2020, 31, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Depuydt, J.; De Smet, S.L.A.X.; Grijspeerdt, K.; Herman, L. Association study of an AvaI and PvuII polymorphism at the porcine estrogen receptor (ESR) gene, with litter size. Arch. Tierz. Arch. Anim. Breed. 1999, 42, 172–174. [Google Scholar]
- Ding, N.-S.; Ren, D.-R.; Guo, Y.-M.; Ren, J.; Yan, Y.; Ma, J.-W.; Chen, K.-F.; Huang, L.-S. Genetic variation of porcine prostaglandin-endoperoxide synthase 2 (PTGS2) gene and its association with reproductive traits in an Erhualian x Duroc F2 population. Yi Chuan Xue Bao 2006, 33, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Drogemuller, C.; Hamann, H.; Distl, O. Candidate gene markers for litter size in different German pig lines1. J. Anim. Sci. 2001, 79, 2565–2570. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Chen, J.; Cui, J.; Wang, X.; Zhang, X. Polymorphisms on SSC15q21-q26 Containing QTL for reproduction in Swine and its association with litter size. Genet. Mol. Biol. 2009, 32, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.X.; Huang, Y.S.; Ye, J.; Zhang, W.; Li, Y.; Nie, Q.H. Identification and characterization of RFRP gene in pigs and its association with reproductive traits. Genet. Mol. Res. 2014, 13, 1661–1671. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Xie, S.-Y.; Zhou, J.-S.; Sun, G.-R.; Lu, P.; Li, M. Polymorphisms of the bone morphogenetic protein 7 gene (BMP7) and association analysis with sow productive traits. Anim. Reprod. Sci. 2013, 142, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Rodríguez, A.; Rodríguez, C.; Varona, L.; Balcells, I.; Noguera, J.L.; Óvilo, C.; Fernández, A.I. Analysis of candidate genes underlying two epistatic quantitative trait loci on SSC12 affecting litter size in pig. Anim. Genet. 2010, 41, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Franco, M.; Antunes, R.; Silva, H.; Goulart, L. Association of PIT1, GH and GHRH polymorphisms with performance and carcass traits in Landrace pigs. J. Appl. Genet. 2005, 46, 195–200. [Google Scholar]
- Gibson, J.P.; Jiang, Z.H.; Robinson, J.A.B.; Archibald, A.L.; Haley, C.S. No detectable association of the ESR PvuII mutation with sow productivity in a Meishan × Large White F2 population. Anim. Genet. 2002, 33, 448–450. [Google Scholar] [CrossRef]
- He, L.C.; Li, P.H.; Ma, X.; Sui, S.P.; Gao, S.; Kim, S.W.; Gu, Y.Q.; Huang, Y.; Ding, N.S.; Huang, R.H. Identification of new single nucleotide polymorphisms affecting total number born and candidate genes related to ovulation rate in Chinese Erhualian pigs. Anim. Genet. 2017, 48, 48–54. [Google Scholar] [CrossRef]
- He, Y.; Zhou, X.; Zheng, R.; Jiang, Y.; Yao, Z.; Wang, X.; Zhang, Z.; Zhang, H.; Li, J.; Yuan, X. The Association of an SNP in the EXOC4 Gene and Reproductive Traits Suggests Its Use as a Breeding Marker in Pigs. Animals 2021, 11, 521. [Google Scholar] [CrossRef]
- Houde, A.A.; Murphy, B.D.; Mathieu, O.; Bordignon, V.; Palin, M.F. Characterization of swine adiponectin and adiponectin receptor polymorphisms and their association with reproductive traits. Anim. Genet. 2008, 39, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Hunyadi-Bagi, Á.; Balogh, P.; Nagy, K.; Kusza, S. Association and polymorphism study of seven candidate genes with reproductive traits in three pig breeds in Hungary. Acta Biochim. Pol. 2016, 63, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.H.; An, S.M.; Yu, G.E.; Park, D.H.; Kang, D.G.; Kim, T.W.; Park, H.C.; Ha, J.; Kim, C.W. Association of single-nucleotide polymorphisms in NAT9 and MAP3K3 genes with litter size traits in Berkshire pigs. Arch. Anim. Breed. 2018, 61, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Jafarikia, M.; Méthot, S.; Maignel, L.; Fortin, F.; Wyss, S.; Sullivan, B.; Palin, M.-F. Association of adiponectin and adiponectin receptor genes with sow productivity estimated breeding values. Mol. Biol. Rep. 2015, 42, 1391–1401. [Google Scholar] [CrossRef] [PubMed]
- Jiugang, Z.; Jing, L.; Yonggang, L. Characterization, expression profile, polymorphism and association of porcine NAT9 gene. Mol. Biol. Rep. 2012, 39, 3137–3142. [Google Scholar] [CrossRef] [PubMed]
- Kumchoo, T.; Mekchay, S. Association of NR4A1 and GNB2L1 genes with reproductive traits in commercial pig breeds. Genet. Mol. Res. 2015, 14, 16276–16284. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.; Zhao, J.; Liu, Y. Molecular cloning, sequence characterization, polymorphism and association analysis of porcine ROPN1 gene. Mol. Biol. Rep. 2012, 39, 2739–2743. [Google Scholar] [CrossRef] [PubMed]
- Laplana, M.; Estany, J.; Fraile, L.J.; Pena, R.N. Resilience Effects of SGK1 and TAP1 DNA Markers during PRRSV Outbreaks in Reproductive Sows. Animals 2020, 10, 902. [Google Scholar] [CrossRef]
- Lei, Y.; Yonggang, L. Cloning, sequence, and association analysis of porcine OSAP gene. Appl. Biochem. Biotechnol. 2012, 168, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.L.; Jennen, D.G.J.; Ponsuksili, S.; Tholen, E.; Tesfaye, D.; Schellander, K.; Wimmers, K. Haplotype analysis of β-actin gene for its association with sperm quality and boar fertility. J. Anim. Breed. Genet. 2006, 123, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.C.; Liu, G.F.; Wang, A.G.; Kong, L.J.; Wang, X.F.; Fu, J.L. Effect of polymorphism in the leukemia inhibitory factor gene on litter size in Large White pigs. Mol. Biol. Rep. 2009, 36, 1833–1838. [Google Scholar] [CrossRef] [PubMed]
- Linville, R.C.; Pomp, D.; Johnson, R.K.; Rothschild, M.F. Candidate gene analysis for loci affecting litter size and ovulation rate in swine. J. Anim. Sci. 2001, 79, 60–67. [Google Scholar] [CrossRef]
- Liu, C.; Ran, X.; Niu, X.; Li, S.; Wang, J.; Zhang, Q. Insertion of 275-bp SINE into first intron of PDIA4 gene is associated with litter size in Xiang pigs. Anim. Reprod. Sci. 2018, 195, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, W.; Yang, D.; Liu, Y. Molecular Characterization, Polymorphism, and Association of Porcine GADD45G Gene. Anim. Biotechnol. 2015, 26, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.Q.; Li, F.E.; Deng, C.Y. Short Communication: Molecular cloning and expression pattern of the porcine 5-aminolevulinate synthase 1 (ALAS1) gene and its association with reproductive traits. Genet. Mol. Res. 2016, 15. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.Q.; Li, F.E.; Deng, C.Y.; Xiong, Y.Z. Molecular cloning, tissue expression and association of porcine NR4A1 gene with reproductive traits. Mol. Biol. Rep. 2011, 38, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.Q.; Li, F.E.; Deng, C.Y.; Zuo, B.; Zheng, R.; Xiong, Y.Z. Polymorphism of the pig 17beta-hydroxysteroid dehydrogenase type1 (HSD17B1) gene and its association with reproductive traits. Anim. Reprod. Sci. 2009, 114, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, Y.; Li, W.; Zhang, Y.; Yang, Y.; Li, H.; Geng, Z.; Ao, H.; Zhou, R.; Li, K. NRDR inhibits estradiol synthesis and is associated with changes in reproductive traits in pigs. Mol. Reprod. Dev. 2019, 86, 63–74. [Google Scholar] [CrossRef]
- Marantidis, A.; Papadopoulos, A.I.; Michailidis, G.; Avdi, M. Association of BF gene polymorphism with litter size in a commercial pig cross population. Anim. Reprod. Sci. 2013, 141, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Menčik, S.; Vuković, V.; Jiang, Z.; Ostović, M.; Sušić, V.; Žura Žaja, I.; Samardžija, M.; Ekert Kabalin, A. Effect of RNF4-SacII gene polymorphism on reproductive traits of Landrace × Large White crossbred sows. Reprod. Domest. Anim. Zuchthyg. 2020, 55, 1286–1293. [Google Scholar] [CrossRef] [PubMed]
- Mucha, A.; Ropka-Molik, K.; Piórkowska, K.; Tyra, M.; Oczkowicz, M. Effect of EGF, AREG and LIF genes polymorphisms on reproductive traits in pigs. Anim. Reprod. Sci. 2013, 137, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, G.; Ovilo, C.; Estellé, J.; Silió, L.; Fernández, A.; Rodriguez, C. Association with litter size of new polymorphisms on ESR1 and ESR2 genes in a Chinese-European pig line. Genet. Sel. Evol. 2007, 39, 195–206. [Google Scholar] [CrossRef]
- Muñoz, M.; Fernández, A.I.; Ovilo, C.; Muñoz, G.; Rodriguez, C.; Fernández, A.; Alves, E.; Silió, L. Non-additive effects of RBP4, ESR1 and IGF2 polymorphisms on litter size at different parities in a Chinese-European porcine line. Genet. Sel. Evol. 2010, 42, 23. [Google Scholar] [CrossRef] [PubMed]
- Niu, B.; Li, F.; Xiong, Y.; Wang, X. Characterization and association analysis with litter size traits of porcine matrix metalloproteinase-9 gene (pMMP-9). Appl. Biochem. Biotechnol. 2013, 171, 786–794. [Google Scholar] [CrossRef] [PubMed]
- Norseeda, W.; Liu, G.; Teltathum, T.; Supakankul, P.; Sringarm, K.; Naraballobh, W.; Khamlor, T.; Chomdej, S.; Nganvongpanit, K.; Krutmuang, P.; et al. Association of IL-4 and IL-4R Polymorphisms with Litter Size Traits in Pigs. Animals 2021, 11, 1154. [Google Scholar] [CrossRef]
- Panasiewicz, G.; Bieniek-Kobuszewska, M.; Lipka, A.; Majewska, M.; Jedryczko, R.; Szafranska, B. Novel effects of identified SNPs within the porcine Pregnancy-Associated Glycoprotein gene family (pPAGs) on the major reproductive traits in Hirschmann hybrid-line sows. Res. Vet. Sci. 2017, 114, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, M.; Pal, A.; Samanta, A.K.; Banerjee, S.; Samanta, R. Mutations in cytochrome B gene effects female reproduction of Ghungroo pig. Theriogenology 2018, 119, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Rempel, L.A.; Nonneman, D.J.; Wise, T.H.; Erkens, T.; Peelman, L.; Rohrer, G.A. Association analyses of candidate single nucleotide polymorphisms on reproductive traits in swine. J. Anim. Sci. 2010, 88, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Rothschild, M.; Jacobson, C.; Vaske, D.; Tuggle, C.; Wang, L.; Short, T.; Eckardt, G.; Sasaki, S.; Vincent, A.; McLaren, D.; et al. The estrogen receptor locus is associated with a major gene influencing litter size in pigs. Proc. Natl. Acad. Sci. USA 1996, 93, 201–205. [Google Scholar] [CrossRef]
- Santana, B.; Biase, F.; Antunes, R.; Borges, M.; Franco, M.; Goulart, L. Association of the estrogen receptor gene Pvu II restriction polymorphism with expected progeny differences for reproductive and performance traits in swine herds in Brazil. Genet. Mol. Biol. 2006, 29, 273–277. [Google Scholar] [CrossRef][Green Version]
- Shifei, P.; Yonggang, L. Molecular characterization, polymorphism and association of porcine IBP4 gene. Folia Biol. 2012, 58, 30–36. [Google Scholar]
- Sironen, A.I.; Uimari, P.; Serenius, T.; Mote, B.; Rothschild, M.; Vilkki, J. Effect of polymorphisms in candidate genes on reproduction traits in Finnish pig populations. J. Anim. Sci. 2010, 88, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Spötter, A.; Drögemüller, C.; Hamann, H.; Distl, O. Evidence of a new leukemia inhibitory factor-associated genetic marker for litter size in a synthetic pig line1. J. Anim. Sci. 2005, 83, 2264–2270. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Mei, S.; Sun, X.; Peng, X.; Zhang, X.; Ma, C.; Wang, L.; Hua, L.; Li, F. Associations of TCF12, CTNNAL1 and WNT10B gene polymorphisms with litter size in pigs. Anim. Reprod. Sci. 2013, 140, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Tomás, A.; Casellas, J.; Ramírez, O.; Muñoz, G.; Noguera, J.L.; Sánchez, A. High amino acid variation in the intracellular domain of the pig prolactin receptor (PRLR) and its relation to ovulation rate and piglet survival traits1. J. Anim. Sci. 2006, 84, 1991–1998. [Google Scholar] [CrossRef] [PubMed]
- Vallet, J.L.; Freking, B.A.; Leymaster, K.A.; Christenson, R.K. Allelic variation in the erythropoietin receptor gene is associated with uterine capacity and litter size in swine. Anim. Genet. 2005, 36, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Vallet, J.L.; Freking, B.A.; Leymaster, K.A.; Christenson, R.K. Allelic variation in the secreted folate binding protein gene is associated with uterine capacity in swine. J. Anim. Sci. 2005, 83, 1860–1867. [Google Scholar] [CrossRef]
- Van Rens, B.T.T.M.; Evans, G.J.; Lende, T.v.d. Components of litter size in gilts with different prolactin receptor genotypes. Theriogenology 2003, 59, 915–926. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; Lan, J.; Zhao, J.; Chen, L.; Liu, Y. Molecular characterization, polymorphism and association of porcine SPATA19 gene. Mol. Biol. Rep. 2012, 39, 9741–9746. [Google Scholar] [CrossRef]
- Wu, F.; Zhang, W.; Song, Q.-Q.; Li, H.-H.; Xu, M.-S.; Liu, G.-L.; Zhang, J.-Z. Association analysis of polymorphisms of G protein-coupled receptor 54 gene exons with reproductive traits in Jiaxing Black sows. Asian-Australas. J. Anim. Sci. 2019, 32, 1104–1111. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.P.; Wang, A.G.; Li, N.; Fu, J.L.; Zhao, X.B. Association with TGF-β1 Gene Polymorphisms and Reproductive Performance of Large White Pig. Reprod. Domest. Anim. 2010, 45, 1028–1032. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Jinluan, F.; Aiguo, W. Effect of VNTR polymorphism of the Muc1 gene on litter size of pigs. Mol. Biol. Rep. 2012, 39, 6251–6258. [Google Scholar] [CrossRef]
- Xudong, W.; Guiying, Z.; Guowen, F.; Yonggang, L. Molecular Cloning of the Porcine HTRA3 Gene and Association of a SNP with Litter Size Traits. Folia Biol. 2017, 63, 217–221. [Google Scholar] [CrossRef]
- Yin, H.; Du, X.; Li, Q.; Pan, Z.; Wu, W.; Liu, H.; Li, Q. Variants in BMP7 and BMP15 3′-UTRs Associated with Reproductive Traits in a Large White Pig Population. Animals 2019, 9, 905. [Google Scholar] [CrossRef]
- Yong, W.J.; Jing, L.; Jiugang, Z.; Lei, C.; Yonggang, L. Molecular characterization, polymorphism and association of porcine MYST2 gene. Mol. Biol. Rep. 2012, 39, 7711–7716. [Google Scholar] [CrossRef]
- Yonggang, L.; Xueshan, X. Molecular characterization, tissue expression, polymorphism and association of porcine LCK gene. Mol. Biol. Rep. 2012, 39, 4023–4028. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.F.; Jafer, O.; Affara, N.A.; Gong, Y.Z.; Yang, L.G.; Liu, J.; Moaeen-ud-Din, M.; Li, W.M.; Zhang, S.J. Association of four new single-nucleotide polymorphisms in follicle-stimulating hormone receptor and zona pellucida glycoprotein with reproductive traits in pigs. Animal 2007, 1, 1249–1253. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yuan, J.F.; Moaeen-ud-Din, M.; Gong, Y.Z.; Peng, X.L.; Yang, L.G.; Feng, Y.P.; Liu, J.; Hu, B.; Affara, N.A.; Jafer, O.; et al. Identification of mutations of zona pellucida glycoprotein (ZP3) and its association with pig reproductive traits. J. Anim. Breed. Genet. 2007, 124, 144–149. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, L.; Li, Y.; Li, W.; Yan, H.; Liu, X.; Zhao, K.; Wang, L. A substitution within erythropoietin receptor gene D1 domain associated with litter size in Beijing Black pig, Sus scrofa. Anim. Sci. J. 2011, 82, 627–632. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, R.; Ma, X.; Jiang, N.; Zhou, W.; Gao, C.; Zhao, M.; Niu, P.; Zhang, Z.; Li, Q.; et al. Association of Rs339939442 in the AHR Gene with Litter Size are Inconsistent among Chinese Indigenous Pigs and Western Commercial Pigs. Animals 2019, 10, 11. [Google Scholar] [CrossRef]
- Zhang, W.; Fang, M.; Li, Y.; Nie, Q.; Zhang, X. Identification of TDRP1 gene and its association with pig reproduction traits. DNA Cell Biol. 2012, 31, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.D.; Zhu, H.Y.; Zhou, J.; Wang, N.; Zhou, N.; Huang, L.; Wu, T.; Feng, Y.F.; Ding, Y.Y.; Yin, Z.J. Relationship between polymorphisms in exon 10 of FSHR gene and litter size in swine. Genet. Mol. Res. 2015, 14, 8252–8261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.; Mei, S.Q.; Peng, X.W.; Niu, B.Y.; Ren, Z.Q.; Zuo, B.; Xu, D.Q.; Lei, M.G.; Zheng, R.; Jiang, S.W.; et al. Molecular characterization and SNPs analysis of the porcine Deleted in AZoospermia Like (pDAZL) gene. Anim. Reprod. Sci. 2009, 112, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.; Mei, S.Q.; Peng, X.W.; Zuo, B.; Lei, M.G.; Xiong, Y.Z.; Li, F.E. Molecular cloning and polymorphism of the porcine H2AFZ gene. Animal 2009, 3, 779–782. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, N.; Xiao, L.; Cao, G.; Chen, Y.; Zhang, S.; Chen, Y.; Wu, C.; Zhang, J.; Sun, S.; et al. FSHB subunit gene is associated with major gene controlling litter size in commercial pig breeds. Sci. China Ser. C Life Sci. 1998, 41, 664–668. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Liu, Y. Molecular Cloning of Porcine SUN5 Gene and Association between a SNP with Litter Size Trait. Anim. Biotechnol. 2017, 28, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.J.; Yu, M.; Liu, B.; Zhu, Z.Z.; Xiong, T.A.; Fan, B.; Xu, S.P.; Du, Y.Q.; Peng, Z.Z.; Li, K. Genetic status of ESR locus and other unidentified genes associated with litter size in Chinese indigenous Tongcheng pig breed after a long time selection. Asian-Australas. J. Anim. Sci. 2004, 17, 598–602. [Google Scholar] [CrossRef]
- Ziółkowska, A.; Bogdzińska, M.; Biegniewski, J. Polymorphism of prolactin receptor gene (PRLR) in the polish landrace and polish large white swine population and reproductive traits. J. Cent. Eur. Agric. 2010, 11, 443–448. [Google Scholar] [CrossRef]



| Reference | Chip | Number of LW | Available SNPs | Detected Genes | Threshold −log10 (p-Value) | Study and Year |
|---|---|---|---|---|---|---|
| [52] | Illumina Porcine SNP60 Bead Chip | 2410 sows 3122 boars | 39,450 SNPs | PCIF1, LRRC27, CFAP46, KNDC1, ADAM8, CALY, ECHS1, MTG1, CYP2E1 | 0.05 | Brinke, 2020 |
| [53] | Bead Chips | 7017 sows 1669 boars | 55,375 SNPs | TRRAP, MID1, ACOT9, TRPC5, MAMLD1 | 0.05 | Chen, 2021 |
| [54] | GeneSeek Custom 50K SNP Chip, GeneSeek Custom 80K SNP Chip, Porcine SNP60 Bead Chip | 11,451 sows 781 boars | 11,230 SNPs | GANC, VPS39, NCR2, NRN1, CUL9, LMBR1, KLF14, UBE2H, AHCYL2, SMO, NCR2, FOXP4, MDFI, TRERF1, ADGRF1, SH3GL3, FSD2, AP3B2, PSTPIP1, ARID3B, FLT1, KATNAL1, USPL1, FRY | ≤6 | Sell-Kubiak, 2022 |
| [55] | Illumina Porcine SNP 60k Bead Chip | 175 LW | 47,865 SNPs | ALDH1A3, LRRK1, TRPC5, RTL4, LHFPL1, SLITRK2, ALDH1A3, LRRK1, EPHB6, TRPC5, RTL4, LHFPL1 | NM | Suwannasing, 2018 |
| [2] | 50K chip | 2655 LW | 43,549 SNPs | TTLL11, UNC93B1, TBX10, CDC14A, COL2A1, SENP1, CCDC184, KANSL2, SLC41A2, GSC, KLF3, PLXDC2, RIPK4, MMADHC, LYPD6, KIF5C, EPC2, ORC4 | 0.05 | Wang, 2022 |
| [56] | Porcine SNP80 Bead Chip | 1207 LW | 51,443 SNPs | IFITM2, IL1B2, GCK | ≤6 | Wang, 2018 |
| [57] | Affymetrix Porcine 55 K SNP Chip | 695 LW | 64,812 SNPs | STT3B, THRB, TUSC3 | ≤5 | Wu, 2022 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Z.; Lv, L.; Liu, D.; Ma, H.; Radović, Č. Effect of SNPs on Litter Size in Swine. Curr. Issues Mol. Biol. 2024, 46, 6328-6345. https://doi.org/10.3390/cimb46070378
Guo Z, Lv L, Liu D, Ma H, Radović Č. Effect of SNPs on Litter Size in Swine. Current Issues in Molecular Biology. 2024; 46(7):6328-6345. https://doi.org/10.3390/cimb46070378
Chicago/Turabian StyleGuo, Zhenhua, Lei Lv, Di Liu, Hong Ma, and Čedomir Radović. 2024. "Effect of SNPs on Litter Size in Swine" Current Issues in Molecular Biology 46, no. 7: 6328-6345. https://doi.org/10.3390/cimb46070378
APA StyleGuo, Z., Lv, L., Liu, D., Ma, H., & Radović, Č. (2024). Effect of SNPs on Litter Size in Swine. Current Issues in Molecular Biology, 46(7), 6328-6345. https://doi.org/10.3390/cimb46070378







