Secondary Metabolites with Antioxidant and Antimicrobial Activities from Camellia fascicularis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Instrumentation
2.2. Chemicals and Reagents
2.3. Plant Material
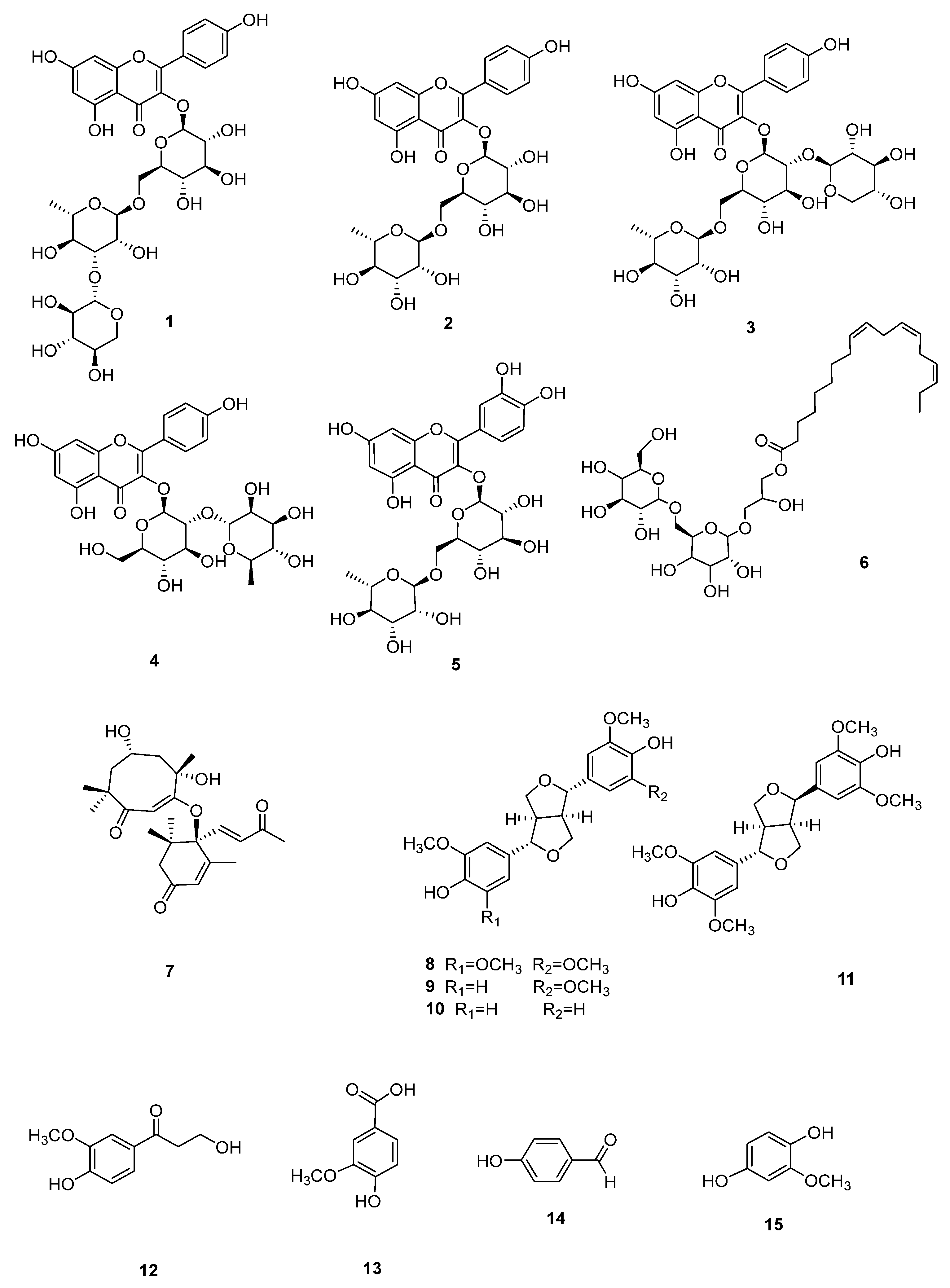
2.4. Extraction and Isolation
2.5. Chemical Structure Analysis
2.6. Antioxidant Activity
2.7. Antimicrobial Activity
3. Results and Discussion
3.1. Antioxidant Activity
3.2. Antibacterial Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Y.; Luo, X.L.; Lan, Z.Q.; Tang, J.R.; Zhao, P.; Kan, H. Ultrasonic-assisted extraction and antioxidant capacities of flavonoids from Camellia fascicularis leaves. CyTA-J. Food 2018, 16, 105–112. [Google Scholar] [CrossRef]
- Hu, X.; Tang, J.R.; Zhang, G.L.; Deng, J.; Kan, H.; Zhang, Y.J.; Zhao, P.; Liu, Y. Optimization of extraction process and antioxidant activities of saponins from Camellia fascicularis leaves. J. Food Meas. Charact. 2021, 15, 1889–1898. [Google Scholar] [CrossRef]
- Liu, Y.; Kan, H.; Fang, F.Y.; Tang, J.R.; Zhang, Y.J.; Zhao, P. Microwave-assisted extraction and antioxidant activities of polyphenols from Camellia fascicularis leaves. Curr. Top. Nutraceut. Res. 2019, 17, 164–171. [Google Scholar] [CrossRef]
- Song, L.X.; Wang, X.S.; Zheng, X.Q.; Huang, D.J. Polyphenolic antioxidant profiles of yellow Camellia. Food Chem. 2011, 129, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Yu, D.Y.; Feng, B.M.; Wang, Y.Q.; Shi, L.Y. A new acylated flavonoid glycoside from the flowers of Camellia nitidissima and its effect on the induction of apoptosis in human lymphoma U937 cells. J. Asian Nat. Prod. Res. 2012, 14, 799–804. [Google Scholar] [CrossRef]
- Gao, D.F.; Xu, M.; Zhao, P.; Zhang, X.Y.; Wang, Y.F.; Yang, C.R.; Zhang, Y.J. Kaempferol acetylated glycosides from the seed cake of Camellia oleifera. Food Chem. 2011, 124, 432–436. [Google Scholar] [CrossRef]
- Chen, J.H.; Wu, X.H. Extraction process optimization of saponin from the leaves of Camellia nitidissima Chi and its inhibition of lipase activity. Food Mach. 2020, 36, 159–165. [Google Scholar] [CrossRef]
- Peng, X.; Yu, D.Y.; Feng, B.M.; Tang, L.; Wang, Y.Q.; Shi, L.Y. Chemical constituents from the flowers of Camellia chrysantha. Guihaia 2011, 31, 550–553. [Google Scholar] [CrossRef]
- He, X.H.; Shi, Z.J.; Peng, X.W.; Kan, H.; Zhao, P.; Liu, Y. Antioxidant activities analysis of different polar solvent extracts from Camellia fascicularis H T. Chang. Chem. Ind. For. Prod. 2022, 42, 61–68. [Google Scholar] [CrossRef]
- Peng, X.W.; He, X.H.; Tang, J.R.; Xiang, J.Y.; Deng, J.; Kan, H.; Zhang, Y.J.; Zhang, G.L.; Zhao, P.; Liu, Y. Evaluation of the in vitro antioxidant and antitumor activity of extracts from Camellia fascicularis leaves. Front. Chem. 2022, 10, 1035949. [Google Scholar] [CrossRef]
- Peng, X.W.; Hu, X.; Zhang, Y.J.; Xu, H.; Tang, J.R.; Zhang, G.L.; Deng, J.; Kan, H.; Zhao, P.; Liu, Y. Extraction, characterization, antioxidant and anti-tumor activities of polysaccharides from Camellia fascicularis leaves. Int. J. Biol. Macromol. 2022, 222, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.Z.; Peng, X.W.; Tang, J.R.; Deng, J.; Wang, F.; Zhang, Y.J.; Zhao, P.; Kan, H.; Liu, Y. Anti-inflammatory effects of Camellia fascicularis polyphenols via attenuation of NF-κB and MAPK pathways in LPS-induced THP-1 macrophages. J. Iinflamm. Res. 2022, 15, 851–864. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.Z.; Tang, J.R.; Deng, J.; Xiang, J.Y.; Kan, H.; Zhao, P.; Zhang, Y.J.; Zhang, G.L.; Liu, Y. Evaluation of anti-inflammatory effect of Camellia fascicularis polyphenols using zebrafish model and network pharmacology. Food Sci. 2023, 44, 134–142. [Google Scholar] [CrossRef]
- Takahashi, J.A.; Gomes, D.C.; Lyra, F.H.; Santos, G.F.; Martins, L.R. The remarkable structural diversity achieved in ent-kaurane diterpenes by fungal biotransformation. Molecules 2014, 19, 1856–1886. [Google Scholar] [CrossRef]
- Sato, N.; Li, W.; Tsubaki, M.; Higai, K.; Takemoto, M.; Sasaki, T.; Onoda, T.; Suzuki, T.; Koike, K. Flavonoid glycosides from Japanese Camellia oil cakes and their inhibitory activity against advanced glycation end-products formation. J. Funct. Foods 2017, 35, 159–165. [Google Scholar] [CrossRef]
- Parveen, A.; Farooq, M.A.; Kyunn, W.W. A new oleanane type saponin from the aerial parts of Nigella sativa with anti-oxidant and anti-diabetic potential. Molecules 2020, 25, 2171. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.; Rho, T.; Yoon, K.D. Kaempferol tri-and tetrasaccharides from Camellia japonica seed cake and their inhibitory activities against matrix metalloproteinase-1 secretion using human dermal fibroblasts. Carbohyd. Res. 2020, 495, 108101. [Google Scholar] [CrossRef]
- Wu, H.; Dushenkov, S.; Ho, C.T.; Sang, S.M. Novel acetylated flavonoid glycosides from the leaves of Allium ursinum. Food Chem. 2009, 115, 592–595. [Google Scholar] [CrossRef]
- Liu, L.; Chang, X.J.; Dai, Q.J.; Wang, H.Y.; Chen, J.; Zhang, X.W. Bioactivity-guided isolation of anti-acetylcholinesterase compounds from Odontites vulgaris Moench. Med. Chem. Res. 2023, 32, 2349–2355. [Google Scholar] [CrossRef]
- Yuan, Y.; Rouzimaimait, R.; Hasan, A.; Zeng, D.; Aisa, H.A. A new anti-vitiligo unsaturated fatty glycoside from Euphorbia humifusa Willd. Nat. Prod. Res. 2023, 37, 2225–2231. [Google Scholar] [CrossRef]
- Zhang, D.W.; Yang, Y.; Yao, F.; Yu, Q.Y.; Dai, S.J. Solalyratins A and B, new anti-inflammatory metabolites from Solanum lyratum. J. Nat. Med. 2012, 66, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.L.; Xu, Y.J.; Shi, H.X.; Liu, Y.; Xu, T.H. Lignanoids from Solanum lyratum. J. Chin. Pharm. Sci. 2018, 27, 289–294. [Google Scholar] [CrossRef]
- Aung, H.T.; Saw, K.T.; Latt, M.M.; Vidari, G.; Komori, Y.; Takaya, Y. Lignans and coumarins from the stem bark of Alyxia fascicularis (Wall. ex G. Don) Benth. ex Hook. f. Nat. Prod. Res. 2024, 38, 1616–1623. [Google Scholar] [CrossRef]
- Xu, W.Z.; Jin, H.Z.; Zhang, W.D.; Fu, J.J.; Hu, X.J.; Zhang, W.; Yan, S.K.; Shen, Y.H. Studies on the chemical constituents of Daphne pedunculata. Chem. Nat. Compd. 2008, 44, 771–772. [Google Scholar] [CrossRef]
- Hu, B.; Khutsishvili, M.; Fayvush, G.; Atha, D.; Borris, R.P. Phytochemical investigations and antimicrobial activities of Anchusa azurea. Chem. Nat. Compd. 2020, 56, 119–121. [Google Scholar] [CrossRef]
- Shataer, D.; Abdulla, R.; Ma, Q.L.; Liu, G.Y.; Aisa, H.A. Chemical composition of extract of Corylus avellana Shells. Chem. Nat. Compd. 2020, 56, 338–340. [Google Scholar] [CrossRef]
- Xie, G.Q.; Zhao, G.S.; Pei, S.C.; Zheng, Y.P.; Zang, H.; Li, L. Isolation and identification of active ingredients and biological activity of Dioscorea nipponica makino. BMC Complement. Med. 2023, 23, 240. [Google Scholar] [CrossRef]
- Ge, L.L.; Xie, Q.J.; Wei, X.F.; Li, Y.F.; Shen, W.Y.; Hu, Y.G.; Yao, J.; Wang, S.L.; Du, X.; Zeng, X.B. Five undescribed plant-derived bisphenols from Artemisia capillaris aerial parts: Structure elucidation, anti-hepatoma activities and plausible biogenetic pathway. Arab. J. Chem. 2023, 16, 104580. [Google Scholar] [CrossRef]
- Zhao, Y.; Tao, M.H.; Chen, Y.C.; Yu, J.P.; Zhang, W.M. Isolation and identification of secondary metabolites from the solid culture of endophytic fungal strain Botryosphaeria rhodina A13 from Aquilaria sinensis. Nat. Prod. Res. Dev. 2015, 27, 799–803. [Google Scholar] [CrossRef]
- Sridhar, K.; Charles, A.L. In vitro antioxidant activity of Kyoho grape extracts in DPPH and ABTS assays: Estimation methods for EC50 using advanced statistical programs. Food Chem. 2019, 275, 41–49. [Google Scholar] [CrossRef]
- Rivero-Cruz, J.F.; Granados-Pineda, J.; Pedraza-Chaverri, J.; Perez-Rojas, J.M.; Kumar-Passari, A.; Diaz-Ruiz, G.; Rivero-Cruz, B.E. Phytochemical constituents, antioxidant, cytotoxic, and antimicrobial activities of the ethanolic extract of Mexican Brown Propolis. Antioxidants 2020, 9, 70. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Huang, J.M.; Wen, C.; Zhou, Z.S.; Feng, C.C.; Hu, C.H.; Zhou, P.F.; Yin, G.P. Chemical constituents from whole herb of Hedyotis scandens. China J. Chin. Mater. Med. 2023, 48, 6082–6087. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Yu, S.L.; Lin, C.X.; Dong, D.J.; Xiao, J.B.; Ye, Y.B.; Wang, M.F. Roles of flavonoids in ischemic heart disease: Cardioprotective effects and mechanisms against myocardial ischemia and reperfusion injury. Phytomedicine 2024, 126, 155409. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Lin, J.; Bai, B.; Bo, T.; He, Y.; Fan, S.; Zhang, J. Study on purification, identification and antioxidant of flavonoids extracted from Perilla leaves. Molecules 2023, 28, 7273. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.; Yan, L.L. Optimization of pressure-enhanced solid-liquid extraction of flavonoids from Flos Sophorae and evaluation of their antioxidant activity. Sep. Purif. Technol. 2017, 175, 170–176. [Google Scholar] [CrossRef]
- Lachowicz, S.; Seliga, Ł.; Pluta, S. Distribution of phytochemicals and antioxidative potency in fruit peel, flesh, and seeds of Saskatoon berry. Food Chem. 2020, 305, 125430. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.J.; Chen, M.L.; Liang, D. Lignans and terpenoids from Gaultheria leucocarpa var. yunnanensis and their anti-inflammatory and antioxidant activities. Fitoterapia 2022, 162, 105293. [Google Scholar] [CrossRef] [PubMed]
- Jang, W.Y.; Kim, M.-Y.; Cho, J.Y. Antioxidant, anti-inflammatory, anti-menopausal, and anti-cancer effects of lignans and their metabolites. Int. J. Mol. Sci. 2022, 23, 15482. [Google Scholar] [CrossRef]
- Ul’yanovskii, N.V.; Onuchina, A.A.; Faleva, A.V.; Gorbova, N.S.; Kosyakov, D.S. Comprehensive characterization of chemical composition and antioxidant activity of lignan-rich coniferous knotwood extractives. Antioxidants 2022, 11, 2338. [Google Scholar] [CrossRef]
- Vek, V.; Keržič, E.; Poljanšek, I.; Eklund, P.; Humar, M.; Oven, P. Wood extractives of silver fir and their antioxidant and antifungal properties. Molecules 2021, 26, 6412. [Google Scholar] [CrossRef]
- Zhang, M.; Shuai, X.X.; Wei, Z.; Dai, T.T.; Wei, C.B.; Li, Y.; He, J.J.; Du, L.Q. Characterization, antioxidant and antitumor activities of phenolic compounds from Amomum villosum Lour. Front. Nutr. 2024, 11, 1327164. [Google Scholar] [CrossRef]
- Li, K.; Fan, H.; Yin, P.P.; Yang, L.G.; Xue, Q.; Li, X.; Sun, L.W.; Liu, Y.J. Structure-activity relationship of eight high content flavonoids analyzed with a preliminary assign-score method and their contribution to antioxidant ability of flavonoids-rich extract from Scutellaria baicalensis shoots. Arab. J. Chem. 2018, 11, 159–170. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, W.B.; Liu, J.C.; Liu, H.; Zhang, C.L.; Chen, D.L.; Jian, Z.G. Identification of six flavonoids as novel cellular antioxidants and their structure-activity relationship. Oxid. Med. Cell. Longev. 2020, 2020, 4150897. [Google Scholar] [CrossRef]
- Yang, T.; Zhang, Z.; Ning, F.; Yuan, L.; Yang, X.; Luo, L. New theoretical perspective for easy evaluation of the antioxidant properties of typical flavonoids. Microchem. J. 2024, 197, 109786. [Google Scholar] [CrossRef]
- Tao, Y.; Zhang, H.; Wang, Y. Revealing and predicting the relationship between the molecular structure and antioxidant activity of flavonoids. LWT Food Sci. Technol. 2023, 174, 114433. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, J.K.; Wang, N.L.; Su, Y.B.; Yao, X.S. Antioxidant phenanthrenes and lignans from Denderobium nobile. J. Chin. Pharm. Sci. 2008, 17, 314–318. [Google Scholar]
- Chen, J.X.; Yang, J.; Ma, L.L.; Li, J.; Shahzad, N.; Kim, C.K. Structure-antioxidant activity relationship of methoxy, phenolic hydroxyl, and carboxylic acid groups of phenolic acids. Sci. Rep. 2020, 10, 2611. [Google Scholar] [CrossRef] [PubMed]
- Soltan, O.I.A.; Gazwi, H.S.S.; Ragab, A.E.; Aljohani, A.S.M.; El-Ashmawy, I.M.; Batiha, G.E.S.; Hafiz, A.A.; Abdel-Hameed, S.M. Assessment of bioactive phytochemicals and utilization of Rosa canina fruit extract as a novel natural antioxidant for mayonnaise. Molecules 2023, 28, 3350. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Su, R.Q.; Zhang, W.T.; Yao, G.L.; Chen, J. Antibacterial activity of tea saponin from Camellia oleifera shell by novel extraction method. Ind. Crop. Prod. 2020, 153, 112604. [Google Scholar] [CrossRef]
- Goulas, V.; Exarchou, V.; Kanetis, L.; Gerothanassis, I.P. Evaluation of the phytochemical content, antioxidant activity and antimicrobial properties of mountain tea (Sideritis syriaca) decoction. J. Funct. Foods 2014, 6, 248–258. [Google Scholar] [CrossRef]
- Keller, A.C.; Weir, L.T.; Broeckling, D.C.; Ryan, E.P. Antibacterial activity and phytochemical profile of fermented Camellia sinensis (fuzhuan tea). Food Res. Int. 2013, 53, 945–949. [Google Scholar] [CrossRef]
- Morikawa, T.; Ninomiya, K.; Miyake, S.; Miki, Y.; Okamoto, M.; Yoshikawa, M.; Muraoka, O. Flavonol glycosides with lipid accumulation inhibitory activity and simultaneous quantitative analysis of 15 polyphenols and caffeine in the flower buds of Camellia sinensis from different regions by LCMS. Food Chem. 2013, 140, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Liu, H.; Yang, J.; Gupta, V.K.; Jiang, Y. New insights on bioactivities and biosynthesis of flavonoid glycosides. Trends Food Sci. Tech. 2018, 79, 116–124. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Yamaguchi, S.; Kunimi, K.; Matuda, H.; Yamahara, J. Stomachic principles in Ginger. III. An anti-ulcer principle, 6-gingesulfonic acid, and three monoacyldigalactosylglycerols, gingerglycolipids A, B, and C, from Zingiberis Rhizoma originating in Taiwan. Chem. Pharm. Bull. 1994, 42, 1226–1230. [Google Scholar] [CrossRef] [PubMed]
- Guevara, L.; Domínguez-Anaya, M.Á.; Ortigosa, A.; González-Gordo, S.; Díaz, C.; Vicente, F.; Corpas, F.J.; Pérez del Palacio, J.; Palma, J.M. Identification of Compounds with Potential Therapeutic uses from sweet pepper (Capsicum annuum L.) fruits and their modulation by nitric oxide (NO). Int. J. Mol. Sci. 2021, 22, 4476. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Luo, J.; Liu, L.; Peng, X. Hypoglycemic effect of edible fungi polysaccharides depends on their metabolites from the fermentation of human fecal microbiota. Foods 2024, 13, 97. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.G.; Park, D.H.; Vu, N.K.; Muvva, C.; Hwang, H.; Song, S.; Lee, H.S.; Kim, T.J.; Kwon, H.C.; Park, K.; et al. Glycolipids derived from the korean endemic plant Aruncus aethusifoliu inducing glucose uptake in mouse skeletal muscle C2C12 cells. Plants 2024, 13, 608. [Google Scholar] [CrossRef]
- Wang, H.; Cong, W.L.; Fu, Z.L.; Chen, D.F.; Wang, Q. Anti-complementary constituents of Viola kunawarensis. Nat. Prod. Res. 2017, 31, 2312–2315. [Google Scholar] [CrossRef]
- Szakiel, A.; Voutquenne-Nazabadioko, L.; Henry, M. Separation and structural analysis of lignan glycoside isolated from Vaccinium myrtillus L. rhizomes. Planta Med. 2008, 74, PB117. [Google Scholar] [CrossRef]
- Wu, H.Y.; Ke, J.P.; Wang, W.; Kong, Y.S.; Zhang, P.; Ling, T.J.; Bao, G.H. Discovery of neolignan glycosides with acetylcolinesterase inhibitory activity from Huangjinya green tea guided by ultra performance liquid chromatography–tandem mass spectrometry data and Global Natural Product Social molecular networking. J. Agric. Food Chem. 2019, 67, 11986–11993. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.; Sousa-Baptista, J.; Lenha-Silva, A.F.; Calheiros, D.; Correia, E.; Figueirinha, A.; Salgueiro, L.; Gonçalves, T. Azorean black tea (Camellia sinensis) antidermatophytic and fungicidal properties. Molecules 2023, 28, 7775. [Google Scholar] [CrossRef]
- Rahman, M.J.; Camargo, A.C.; Shahidi, F. Phenolic profiles and antioxidant activity of defatted camelina and sophia seeds. Food Chem. 2018, 240, 917–925. [Google Scholar] [CrossRef]

| Fractions (g) | ||||||||
|---|---|---|---|---|---|---|---|---|
| A | 1 B* | D | E | F | G | H | I | Fr. II–III |
| 6.16 | 2.61 | 4.52 | 7.16 | 5.63 | 9.53 | 2.81 | 5.73 | 44.15/45.00 |
| Compound | ABTS+ B Assay (%) | |||
|---|---|---|---|---|
| 500 µg/mL | 100 µg/mL | 50 µg/mL | 10 µg/mL | |
| 1 | 49.84 ± 1.88 e | - | - | - |
| 2 | 43.23 ± 2.54 f | - | - | - |
| 3 | 42.73 ± 1.81 f | - | - | - |
| 4 | 66.40 ± 1.17 d | - | - | - |
| 5 | 99.07 ± 0.49 a | 98.75 ± 0.22 a | 85.90 ± 1.0 b | 15.59 ± 3.21 f |
| 6 | 20.12 ± 1.09 i | - | - | - |
| 7 | 90.81 ± 0.90 b | 70.08 ± 3.86 b | 39.61 ± 2.73 e | - |
| 8 | 99.85 ± 0.65 a | 99.89 ± 0.14 a | 98.31 ± 1.22 a | 21.59 ± 0.14 cde |
| 9 | 98.66 ± 0.84 a | 99.20 ± 0.74 a | 97.88 ± 1.11 a | 36.61 ± 0.42 a |
| 10 | 99.48 ± 0.46 a | 99.82 ± 0.24 a | 86.74 ± 1.54 b | 26.25 ± 0.53 b |
| 11 | 99.40 ± 0.61 a | 99.32 ± 0.27 a | 98.36 ± 0.34 a | 22.28 ± 2.72 cd |
| 12 | 100.16 ± 0.90 a | 99.47 ± 0.76 a | 87.82 ± 1.38 b | 19.91 ± 1.54 de |
| 13 | 87.22 ± 1.23 c | 63.87 ± 2.13 c | 54.63 ± 2.14 d | - |
| 14 | 40.18 ± 0.69 g | - | - | - |
| 15 | 97.69 ± 3.54 a | 58.11 ± 2.46 d | 44.02 ± 4.27 e | 18.54 ± 1.44 ef |
| A ascorbic acid | - | 99.85 ± 0.03 a | 61.78 ± 0.69 c | 25.00 ± 2.06 bc |
| Compound | MIC b µg/mL | ||
|---|---|---|---|
| E. coli | S. aureus | P. aeruginosa | |
| 1 | 250.00 | - | - |
| 2 | 250.00 | - | - |
| 3 | 250.00 | 250.00 | - |
| 4 | 250.00 | - | - |
| 5 | 250.00 | 250.00 | 62.50 |
| 6 | 250.00 | 250.00 | - |
| 7 | 125.00 | 250.00 | 62.50 |
| 8 | 250.00 | 250.00 | - |
| 9 | 250.00 | 250.00 | 62.50 |
| 10 | 250.00 | - | - |
| 11 | 250.00 | 125.00 | 62.50 |
| 12 | 250.00 | - | - |
| 13 | 250.00 | - | 62.50 |
| 14 | 250.00 | 250.00 | - |
| 15 | 250.00 | 250.00 | 125.00 |
| a Penicillin | 31.25 | 31.25 | 125.00 |
| a Tetracycline | 7.81 | 15.63 | 62.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, J.; Li, R.; Wu, B.; Tang, J.; Kan, H.; Zhao, P.; Zhang, Y.; Wang, W.; Liu, Y. Secondary Metabolites with Antioxidant and Antimicrobial Activities from Camellia fascicularis. Curr. Issues Mol. Biol. 2024, 46, 6769-6782. https://doi.org/10.3390/cimb46070404
Tang J, Li R, Wu B, Tang J, Kan H, Zhao P, Zhang Y, Wang W, Liu Y. Secondary Metabolites with Antioxidant and Antimicrobial Activities from Camellia fascicularis. Current Issues in Molecular Biology. 2024; 46(7):6769-6782. https://doi.org/10.3390/cimb46070404
Chicago/Turabian StyleTang, Jiandong, Ruonan Li, Boxiao Wu, Junrong Tang, Huan Kan, Ping Zhao, Yingjun Zhang, Weihua Wang, and Yun Liu. 2024. "Secondary Metabolites with Antioxidant and Antimicrobial Activities from Camellia fascicularis" Current Issues in Molecular Biology 46, no. 7: 6769-6782. https://doi.org/10.3390/cimb46070404





