Association between Skin Carotenoid Levels and Cognitive Impairment Screened by Mini-Cog in Patients with Glaucoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Measurement of Skin Carotenoid Levels
2.3. Statistical Analysis
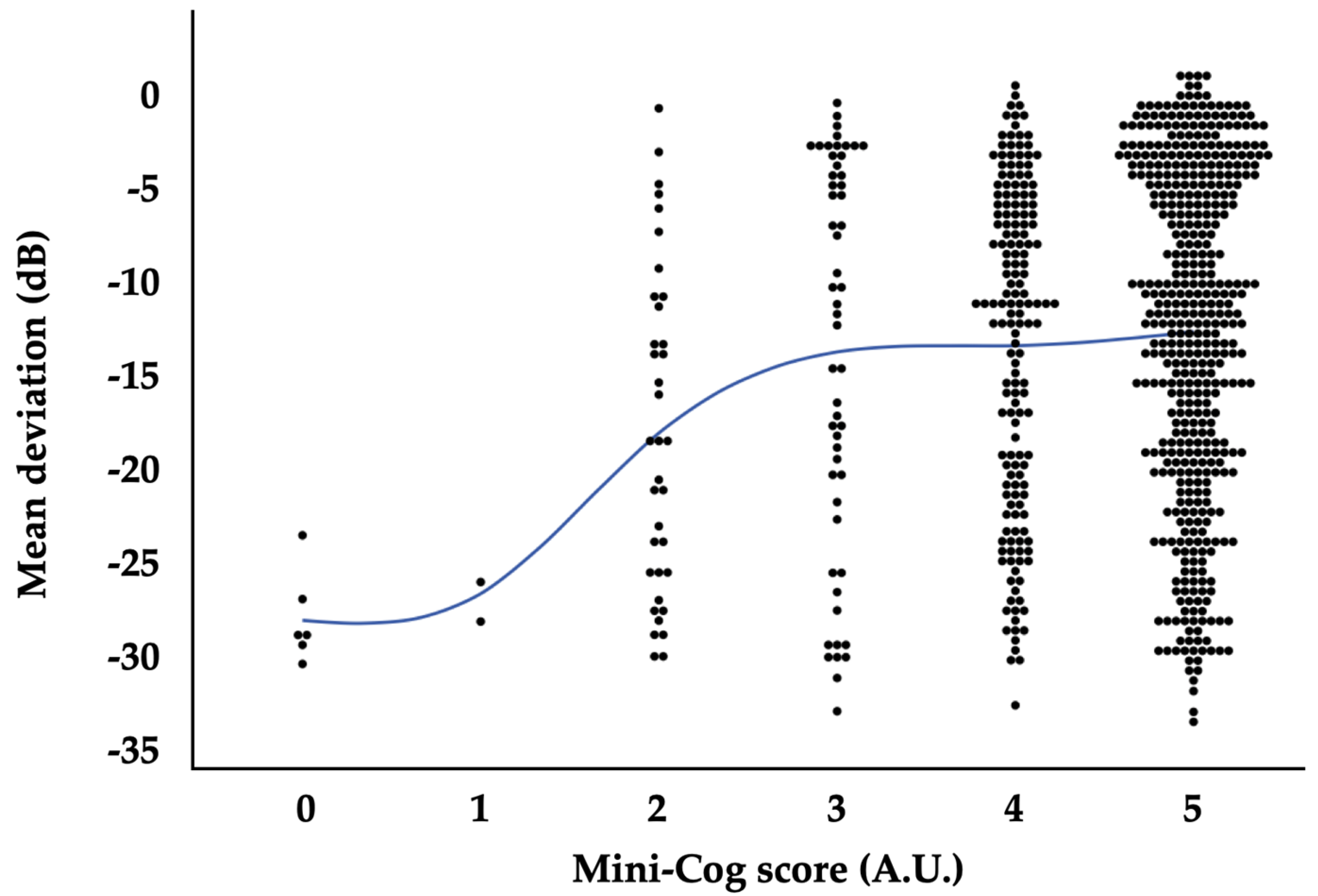
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fiedor, J.; Burda, K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef] [PubMed]
- Young, A.J.; Lowe, G.M. Antioxidant and Prooxidant Properties of Carotenoids. Arch. Biochem. Biophys. 2001, 385, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Lem, D.W.; Gierhart, D.L.; Davey, P.G. Carotenoids in the Management of Glaucoma: A Systematic Review of the Evidence. Nutrients 2021, 13, 1949. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhao, T.; Zhu, X.; Jiang, Q. Low blood carotenoid status in dementia and mild cognitive impairment: A systematic review and meta-analysis. BMC Geriatr. 2023, 23, 195. [Google Scholar] [CrossRef]
- Jonas, J.B.; Aung, T.; Bourne, R.R.; Bron, A.M.; Ritch, R.; Panda-Jonas, S. Glaucoma. Lancet 2017, 390, 2183–2193. [Google Scholar] [CrossRef] [PubMed]
- Foster, A.; Resnikoff, S. The impact of Vision 2020 on global blindness. Eye 2005, 19, 1133–1135. [Google Scholar] [CrossRef]
- Iwase, A.; Suzuki, Y.; Araie, M.; Yamamoto, T.; Abe, H.; Shirato, S.; Kuwayama, Y.; Mishima, H.K.; Shimizu, H.; Tomita, G.; et al. The prevalence of primary open-angle glaucoma in Japanese: The Tajimi Study. Ophthalmology 2004, 111, 1641–1648. [Google Scholar] [CrossRef]
- Chrysostomou, V.; Rezania, F.; Trounce, I.A.; Crowston, J.G. Oxidative stress and mitochondrial dysfunction in glaucoma. Curr. Opin. Pharmacol. 2013, 13, 12–15. [Google Scholar] [CrossRef]
- Yang, X.; Hondur, G.; Tezel, G. Antioxidant Treatment Limits Neuroinflammation in Experimental Glaucoma. Invest. Ophthalmol. Vis. Sci. 2016, 57, 2344–2354. [Google Scholar] [CrossRef]
- Dziedziak, J.; Kasarello, K.; Cudnoch-Jedrzejewska, A. Dietary Antioxidants in Age-Related Macular Degeneration and Glaucoma. Antioxidants 2021, 10, 1743. [Google Scholar] [CrossRef]
- Tezel, G. Molecular regulation of neuroinflammation in glaucoma: Current knowledge and the ongoing search for new treatment targets. Prog. Retin. Eye Res. 2022, 87, 100998. [Google Scholar] [CrossRef]
- Tanito, M.; Kaidzu, S.; Takai, Y.; Ohira, A. Correlation between Systemic Oxidative Stress and Intraocular Pressure Level. PLoS ONE. 2015, 10, e0133582. [Google Scholar] [CrossRef]
- Tanito, M.; Kaidzu, S.; Takai, Y.; Ohira, A. Association between systemic oxidative stress and visual field damage in open-angle glaucoma. Sci. Rep. 2016, 6, 25792. [Google Scholar] [CrossRef]
- Sanz-González, S.M.; Raga-Cervera, J.; Aguirre Lipperheide, M.; Zanón-Moreno, V.; Chiner, V.; Ramírez, A.I.; Pinazo-Durán, M.D. Effect of an oral supplementation with a formula containing R-lipoic acid in glaucoma patients. Arch. Soc. Española Oftalmol. (Engl. Ed.) 2020, 95, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Medina, J.J.; Garcia-Medina, M.; Garrido-Fernandez, P.; Galvan-Espinosa, J.; Garcia-Maturana, C.; Zanon-Moreno, V.; Pinazo-Duran, M.D. A two-year follow-up of oral antioxidant supplementation in primary open-angle glaucoma: An open-label, randomized, controlled trial. Acta Ophthalmol. 2015, 93, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Romeo Villadoniga, S.; Rodriguez Garcia, E.; Sagastagoia Epelde, O.; Alvarez Diaz, M.D.; Domingo Pedrol, J.C. Effects of Oral Supplementation with Docosahexaenoic Acid (DHA) plus Antioxidants in Pseudoexfoliative Glaucoma: A 6-Month Open-Label Randomized Trial. J. Ophthalmol. 2018, 2018, 8259371. [Google Scholar] [CrossRef] [PubMed]
- Akbaraly, N.T.; Faure, H.; Gourlet, V.; Favier, A.; Berr, C. Plasma carotenoid levels and cognitive performance in an elderly population: Results of the EVA Study. J. Gerontol. A Biol. Sci. Med. Sci. 2007, 62, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Davinelli, S.; Ali, S.; Solfrizzi, V.; Scapagnini, G.; Corbi, G. Carotenoids and Cognitive Outcomes: A Meta-Analysis of Randomized Intervention Trials. Antioxidants 2021, 10, 223. [Google Scholar] [CrossRef]
- Grimmig, B.; Kim, S.H.; Nash, K.; Bickford, P.C.; Douglas Shytle, R. Neuroprotective mechanisms of astaxanthin: A potential therapeutic role in preserving cognitive function in age and neurodegeneration. Geroscience 2017, 39, 19–32. [Google Scholar] [CrossRef]
- Kalaria, R.N.; Akinyemi, R.; Ihara, M. Stroke injury, cognitive impairment and vascular dementia. Biochim. Biophys. Acta 2016, 1862, 915–925. [Google Scholar] [CrossRef]
- Wolters, F.J.; Ikram, M.A. Epidemiology of Vascular Dementia. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1542–1549. [Google Scholar] [CrossRef]
- Calsolaro, V.; Edison, P. Neuroinflammation in Alzheimer’s disease: Current evidence and future directions. Alzheimer’s Dement. 2016, 12, 719–732. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Ji, X.; Liu, J. Neuroinflammation in Vascular Cognitive Impairment and Dementia: Current Evidence, Advances, and Prospects. Int. J. Mol. Sci. 2022, 23, 6224. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Hussain, M.D.; Yan, L.J. Microglia, neuroinflammation, and beta-amyloid protein in Alzheimer’s disease. Int. J. Neurosci. 2014, 124, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Dhapola, R.; Sarma, P.; Medhi, B.; Reddy, D.H. Neuroinflammation in Alzheimer’s Disease: Current Progress in Molecular Signaling and Therapeutics. Inflammation 2023, 46, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Mohammadzadeh Honarvar, N.; Saedisomeolia, A.; Abdolahi, M.; Shayeganrad, A.; Taheri Sangsari, G.; Hassanzadeh Rad, B.; Muench, G. Molecular Anti-inflammatory Mechanisms of Retinoids and Carotenoids in Alzheimer’s Disease: A Review of Current Evidence. J. Mol. Neurosci. 2017, 61, 289–304. [Google Scholar] [CrossRef]
- Ermakov, I.V.; Ermakova, M.; Sharifzadeh, M.; Gorusupudi, A.; Farnsworth, K.; Bernstein, P.S.; Stookey, J.; Evans, J.; Arana, T.; Tao-Lew, L.; et al. Optical assessment of skin carotenoid status as a biomarker of vegetable and fruit intake. Arch. Biochem. Biophys. 2018, 646, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Kadoh, Y.; Takayanagi, Y.; Sasaki, J.; Tanito, M. Fingertip-Measured Skin Carotenoids and Advanced Glycation End Product Levels in Glaucoma. Antioxidants 2022, 11, 1138. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, J.; Takayanagi, Y.; Kadoh, Y.; Tanito, M. Relevance of Diabetic Retinopathy with AGEs and Carotenoid Levels Assessed by Skin Sensors. Antioxidants 2022, 11, 1370. [Google Scholar] [CrossRef]
- Takayanagi, Y.; Obana, A.; Muto, S.; Asaoka, R.; Tanito, M.; Ermakov, I.V.; Bernstein, P.S.; Gellermann, W. Relationships between Skin Carotenoid Levels and Metabolic Syndrome. Antioxidants 2021, 11, 14. [Google Scholar] [CrossRef]
- Borson, S.; Scanlan, J.M.; Chen, P.; Ganguli, M. The Mini-Cog as a screen for dementia: Validation in a population-based sample. J. Am. Geriatr. Soc. 2003, 51, 1451–1454. [Google Scholar] [CrossRef] [PubMed]
- Kaulmann, A.; Bohn, T. Carotenoids, inflammation, and oxidative stress--implications of cellular signaling pathways and relation to chronic disease prevention. Nutr. Res. 2014, 34, 907–929. [Google Scholar] [CrossRef]
- Moratilla-Rivera, I.; Sánchez, M.; Valdés-González, J.A.; Gómez-Serranillos, M.P. Natural Products as Modulators of Nrf2 Signaling Pathway in Neuroprotection. Int. J. Mol. Sci. 2023, 24, 3748. [Google Scholar] [CrossRef] [PubMed]
- Ramdas, W.D. The relation between dietary intake and glaucoma: A systematic review. Acta Ophthalmol. 2018, 96, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Loughman, J.; Loskutova, E.; Butler, J.S.; Siah, W.F.; O’Brien, C. Macular Pigment Response to Lutein, Zeaxanthin, and Meso-zeaxanthin Supplementation in Open-Angle Glaucoma: A Randomized Controlled Trial. Ophthalmol. Sci. 2021, 1, 100039. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Z.; Zhao, J.; Li, Q.; Huang, C.; Zhu, L.; Lu, D. Neuroprotective Effect of Lutein on NMDA-Induced Retinal Ganglion Cell Injury in Rat Retina. Cell Mol. Neurobiol. 2016, 36, 531–540. [Google Scholar] [CrossRef]
- Tanito, M.; Mochiji, M.; Tsutsui, A.; Harano, A.; Ichioka, S.; Takayanagi, Y.; Kataoka, Y.; Takagi, Y.; Shii, D. Factors Associated with Topical Medication Instillation Failure in Glaucoma: VRAMS-QPiG Study. Adv. Ther. 2023, 40, 4907–4918. [Google Scholar] [CrossRef]
- Ichitani, A.; Takao, E.; Tanito, M. Roles of Cognitive Function on Visual Field Reliability Indices among Glaucoma Patients. J. Clin. Med. 2023, 12, 7119. [Google Scholar] [CrossRef]
- Sugiura, M.; Nakamura, M.; Ogawa, K.; Ikoma, Y.; Matsumoto, H.; Ando, F.; Shimokata, H.; Yano, M. Associations of serum carotenoid concentrations with the metabolic syndrome: Interaction with smoking. Br. J. Nutr. 2008, 100, 1297–1306. [Google Scholar] [CrossRef]
- Böhm, V.; Lietz, G.; Olmedilla-Alonso, B.; Phelan, D.; Reboul, E.; Bánati, D.; Borel, P.; Corte-Real, J.; de Lera, A.R.; Desmarchelier, C.; et al. From carotenoid intake to carotenoid blood and tissue concentrations—Implications for dietary intake recommendations. Nutr. Rev. 2020, 79, 544–573. [Google Scholar] [CrossRef]
- Gammone, M.A.; Pluchinotta, F.R.; Bergante, S.; Tettamanti, G.; D’Orazio, N. Prevention of cardiovascular diseases with Carotenoids. Front. Biosci. (Schol. Ed.) 2017, 9, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Giordano, P.; Scicchitano, P.; Locorotondo, M.; Mandurino, C.; Ricci, G.; Carbonara, S.; Gesualdo, M.; Zito, A.; Dachille, A.; Caputo, P.; et al. Carotenoids and cardiovascular risk. Curr. Pharm. Des. 2012, 18, 5577–5589. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Chen, X.; Geng, T.; Wan, Z.; Lu, Q.; Li, L.; Zhu, K.; Zhang, X.; Liu, Y.; Lin, X.; et al. Associations of Serum Carotenoids with Risk of Cardiovascular Mortality among Individuals with Type 2 Diabetes: Results from NHANES. Diabetes Care 2022, 45, 1453–1461. [Google Scholar] [CrossRef] [PubMed]

| N | 406 |
|---|---|
| Age (years) | |
| Mean ± SD | 69.7 ± 11.4 |
| range | 34, 92 |
| Sex | |
| Men, n (%) | 228 (56.2) |
| Women, n (%) | 178 (43.8) |
| Current smoking | |
| Yes, n (%) | 51 (12.7) |
| No, n (%) | 352 (87.3) |
| BMI (kg/m2) | |
| Mean ± SD | 23.2 ± 3.5 |
| range | 12.3, 37.8 |
| Mean BP (mmHg) | |
| Mean ± SD | 101.9 ± 14.1 |
| range | 70.0, 148.3 |
| HR (bpm) | |
| Mean ± SD | 74.1 ± 12.2 |
| range | 49, 117 |
| Mini-Cog score | |
| 5, n (%) | 254 (62.6) |
| 4, n (%) | 93 (22.9) |
| 3, n (%) | 31 (7.6) |
| 2, n (%) | 21 (5.2) |
| 1, n (%) | 2 (0.5) |
| 0, n (%) | 5 (1.2) |
| positive, n (%) | 28 (6.9) |
| negative, n (%) | 378 (93.1) |
| Skin carotenoid (A.U.) | |
| Mean ± SD | 325.1 ± 119.3 |
| range | 78.0, 803.5 |
| Positive | Negative | p-Value a | |
|---|---|---|---|
| N | 28 | 378 | |
| Age (years) | |||
| Mean ± SD | 79.5 ± 7.6 | 69.0 ± 11.3 | <0.0001 ** |
| range | 64, 90 | 34, 92 | |
| Sex | |||
| Men, n (%) | 17 (60.7) | 211 (55.8) | 0.7 |
| Women, n (%) | 11 (39.3) | 167 (44.2) | |
| Current smoking | |||
| Yes, n (%) | 1 (3.6) | 50 (13.3) | 0.23 |
| No, n (%) | 27 (96.4) | 320 (86.7) | |
| BMI (kg/m2) | |||
| Mean ± SD | 23.5 ± 3.4 | 23.2 ± 3.5 | 0.6 |
| range | 17.4, 29.6 | 12.3, 37.8 | |
| Mean BP (mmHg) | |||
| Mean ± SD | 91.9 ± 30.4 | 100.8 ± 18.1 | 0.1 |
| range | 70.7, 132.7 | 70.0, 148.3 | |
| HR (bpm) | |||
| Mean ± SD | 74.3 ± 14.5 | 74.0 ± 12.0 | 0.9 |
| range | 49, 105 | 50, 117 | |
| Skin carotenoid (A.U.) | |||
| Mean ± SD | 269.5 ± 86.4 | 329.2 ± 120.4 | 0.01 * |
| range | 136.5, 527.5 | 78.0, 803.5 |
| Positive | Negative | p-Value a | |
|---|---|---|---|
| N | 56 | 756 | |
| VA (LogMAR) | |||
| Mean ± SD | 0.61 ± 0.86 | 0.24 ± 0.54 | <0.0001 ** |
| range | −0.08, 2.89 | −0.08, 2.89 | |
| IOP (mmHg) | |||
| Mean ± SD | 24.0 ± 12.0 | 21.4 ± 8.9 | 0.04 * |
| range | 9, 59 | 6, 76 | |
| Lens status | |||
| Phakia | 17 (30.4) | 401 (53.0) | 0.001 ** |
| Pseudophakia/aphakia | 39 (69.6) | 355 (47.0) | |
| Pseudoexfoliation | |||
| Yes, n (%) | 17 (30.4) | 144 (19.1) | 0.054 |
| No, n (%) | 39 (69.6) | 612 (80.9) | |
| Antiglaucoma medications (n) | |||
| Mean ± SD | 2.4 ± 1.4 | 2.6 ± 1.4 | 0.4 |
| range | 0, 5 | 0, 6 | |
| MD (dB) | |||
| Mean ± SD | −19.64 ± 9.07 | −12.46 ± 9.28 | <0.0001 ** |
| range | −30.67, 0.25 | −33.89, 1.84 | |
| Types of glaucoma | |||
| POAG, n (%) | 30 (53.6) | 438 (57.9) | 0.1 |
| EXG, n (%) | 16 (28.6) | 135 (17.9) | |
| Others, n (%) | 10 (17.9) | 183 (24.2) | |
| Estimate | Lower CI | Upper CI | p Value a | |
|---|---|---|---|---|
| Age (/year) | 0.29 | −0.60 | 1.175 | 0.5 |
| Women (/men) | 23.08 | 14.12 | 32.05 | <0.0001 ** |
| Current smoking (/no) | −25.90 | −39.21 | −12.60 | 0.0001 ** |
| BMI (/kg/m2) | −0.20 | −2.91 | 2.51 | 0.9 |
| Mean BP (/mmHg) | −0.37 | −1.06 | 0.33 | 0.3 |
| HR (/bpm) | −1.53 | −2.33 | −0.73 | 0.0002 ** |
| Pseudoexfoliation (/no) | 0.01 | −0.01 | 0.01 | >0.9 |
| Phakia (/pseudophakia or aphakia) | −0.01 | −0.01 | 0.01 | >0.9 |
| Post intraocular surgery (/no) | −0.01 | −0.01 | 0.01 | >0.9 |
| VA (/LogMAR) | −0.01 | −0.01 | 0.01 | >0.9 |
| IOP (/mmHg) | 0.01 | −0.01 | 0.01 | >0.9 |
| Antiglaucoma medications (/number) | 0.01 | −0.01 | 0.01 | >0.9 |
| MD (/dB) | 0.01 | −0.01 | 0.01 | >0.9 |
| Mini-Cog (/negative) | −32.07 | −50.40 | −13.74 | 0.0006 ** |
| Estimate | Lower CI | Upper CI | p Value a | |
|---|---|---|---|---|
| Age (/year) | 0.042 | −0.03 | 0.11 | 0.2 |
| Women (/men) | 0.38 | −0.34 | 1.10 | 0.3 |
| Current smoke (/no) | −0.24 | −1.31 | 0.83 | 0.7 |
| BMI (/kg/m2) | 0.01 | −0.20 | 0.20 | >0.9 |
| Mean BP (/mmHg) | 0.03 | −0.02 | 0.08 | 0.3 |
| HR (/bpm) | 0.03 | −0.03 | 0.09 | 0.3 |
| Pseudoexfoliation (/no) | −0.33 | −1.17 | 0.51 | 0.4 |
| Phakia (/pseudophakia or aphakia) | −1.67 | −2.41 | −0.92 | <0.0001 ** |
| Post intraocular surgery (/no) | −1.10 | −2.13 | −0.06 | 0.04 * |
| VA (/LogMAR) | −8.23 | −9.53 | −6.94 | <0.0001 ** |
| IOP (/mmHg) | −0.10 | −0.17 | −0.02 | 0.009 * |
| Antiglaucoma medications (/number) | −1.29 | −1.76 | −0.82 | <0.0001 ** |
| Mini-Cog (/negative) | −2.14 | −3.60 | −0.66 | 0.005 ** |
| Skin carotenoid (/A.U.) | 0.01 | −0.01 | 0.01 | 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takayanagi, Y.; Kadoh, Y.; Sasaki, J.; Obana, A.; Tanito, M. Association between Skin Carotenoid Levels and Cognitive Impairment Screened by Mini-Cog in Patients with Glaucoma. Curr. Issues Mol. Biol. 2024, 46, 6940-6950. https://doi.org/10.3390/cimb46070413
Takayanagi Y, Kadoh Y, Sasaki J, Obana A, Tanito M. Association between Skin Carotenoid Levels and Cognitive Impairment Screened by Mini-Cog in Patients with Glaucoma. Current Issues in Molecular Biology. 2024; 46(7):6940-6950. https://doi.org/10.3390/cimb46070413
Chicago/Turabian StyleTakayanagi, Yuji, Yoichi Kadoh, Junichi Sasaki, Akira Obana, and Masaki Tanito. 2024. "Association between Skin Carotenoid Levels and Cognitive Impairment Screened by Mini-Cog in Patients with Glaucoma" Current Issues in Molecular Biology 46, no. 7: 6940-6950. https://doi.org/10.3390/cimb46070413
APA StyleTakayanagi, Y., Kadoh, Y., Sasaki, J., Obana, A., & Tanito, M. (2024). Association between Skin Carotenoid Levels and Cognitive Impairment Screened by Mini-Cog in Patients with Glaucoma. Current Issues in Molecular Biology, 46(7), 6940-6950. https://doi.org/10.3390/cimb46070413








