Mechanism of DAPK1 for Regulating Cancer Stem Cells in Thyroid Cancer
Abstract
:1. Introduction
2. DAPK1 Properties and Roles in Cancer
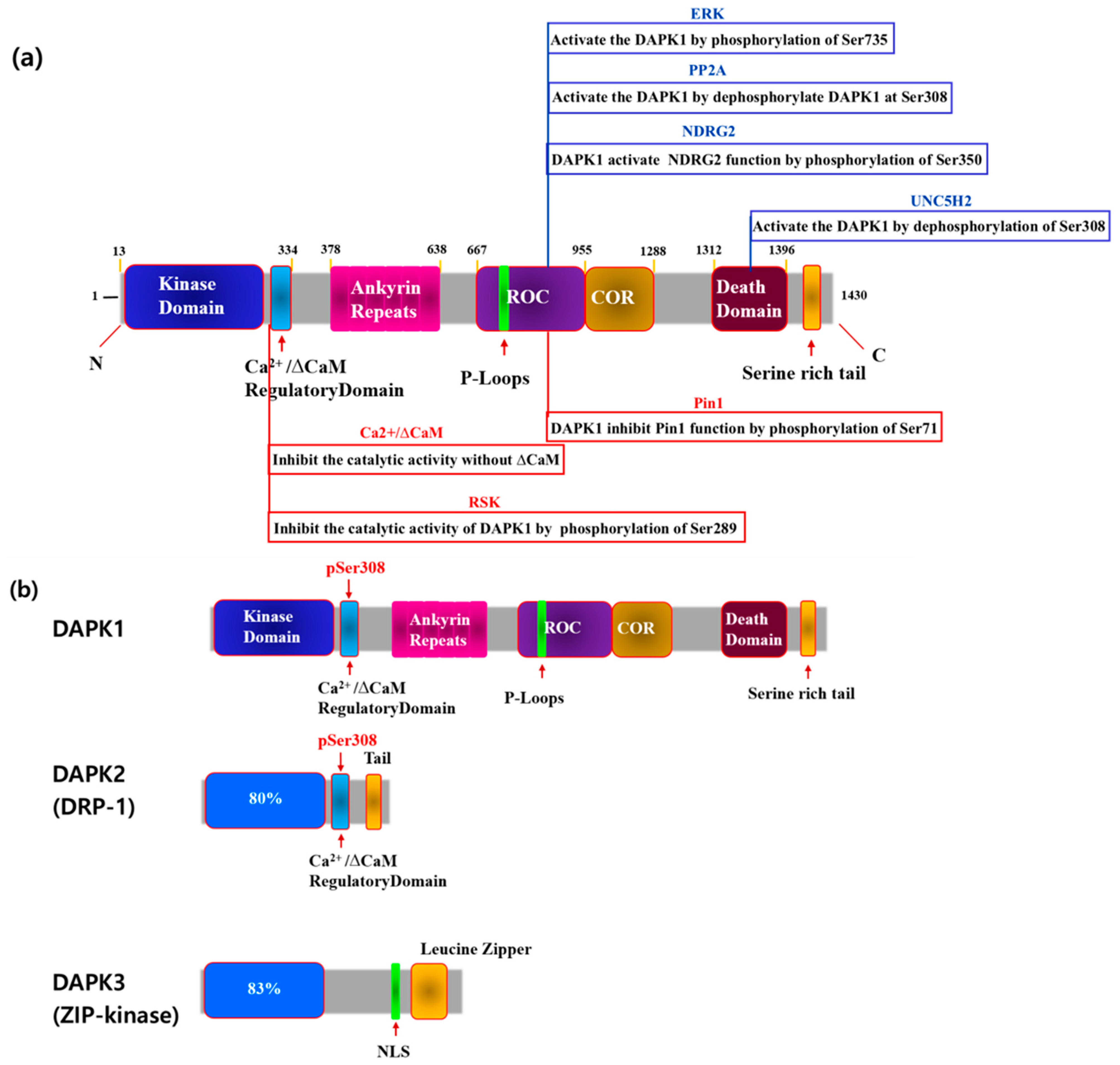
2.1. DAPK1 Structure
2.2. Death-Associated Protein Kinase Family
2.3. Phosphorylation of DAPK1
2.4. DAPK1 Is a Tumor Suppressor
2.5. Role of DAPK1 in Cancer Metastasis
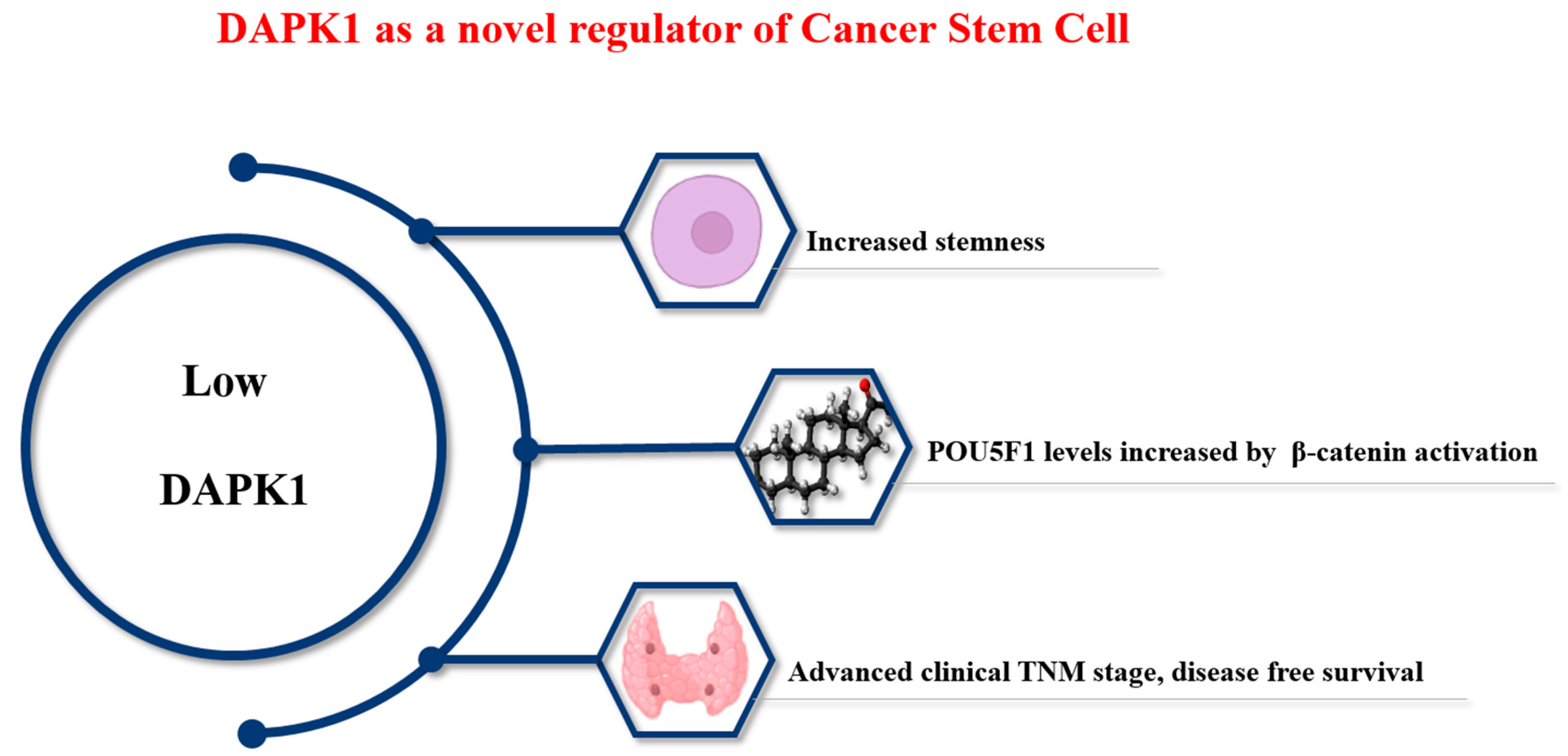
2.6. Role of DAPK1 in Thyroid Cancer
2.7. Evaluating DAPK as a Therapeutic Target
3. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Luzzi, K.J.; MacDonald, I.C.; Schmidt, E.E.; Kerkvliet, N.; Morris, V.L.; Chambers, A.F.; Groom, A.C. Multistep nature of metastatic inefficiency: Dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. Am. J. Pathol. 1998, 153, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Singh, U.P.; Grizzle, W.E.; Lillard, J.W., Jr. CXCL12-CXCR4 interactions modulate prostate cancer cell migration, metalloproteinase expression and invasion. Lab. Investig. 2004, 84, 1666–1676. [Google Scholar] [CrossRef] [PubMed]
- Maitra, A. Molecular envoys pave the way for pancreatic cancer to invade the liver. Nature 2019, 567, 181–182. [Google Scholar] [CrossRef]
- Tanabe, S.; Quader, S.; Cabral, H.; Ono, R. Interplay of EMT and CSC in Cancer and the Potential Therapeutic Strategies. Front. Pharmacol. 2020, 11, 904. [Google Scholar] [CrossRef] [PubMed]
- Ahirwar, D.K.; Nasser, M.W.; Ouseph, M.M.; Elbaz, M.; Cuitino, M.C.; Kladney, R.D.; Varikuti, S.; Kaul, K.; Satoskar, A.R.; Ramaswamy, B.; et al. Fibroblast-derived CXCL12 promotes breast cancer metastasis by facilitating tumor cell intravasation. Oncogene 2018, 37, 4428–4442. [Google Scholar] [CrossRef] [PubMed]
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular principles of metastasis: A hallmark of cancer revisited. Signal Transduct. Target. Ther. 2020, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Tam, S.Y.; Wu, V.W.C.; Law, H.K.W. Hypoxia-Induced Epithelial-Mesenchymal Transition in Cancers: HIF-1alpha and beyond. Front. Oncol. 2020, 10, 486. [Google Scholar] [CrossRef] [PubMed]
- Deiss, L.P.; Feinstein, E.; Berissi, H.; Cohen, O.; Kimchi, A. Identification of a novel serine/threonine kinase and a novel 15-kD protein as potential mediators of the gamma interferon-induced cell death. Genes. Dev. 1995, 9, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Cohen, O.; Feinstein, E.; Kimchi, A. DAP-kinase is a Ca2+/calmodulin-dependent, cytoskeletal-associated protein kinase, with cell death-inducing functions that depend on its catalytic activity. EMBO J. 1997, 16, 998–1008. [Google Scholar] [CrossRef]
- Inbal, B.; Cohen, O.; Polak-Charcon, S.; Kopolovic, J.; Vadai, E.; Eisenbach, L.; Kimchi, A. DAP kinase links the control of apoptosis to metastasis. Nature 1997, 390, 180–184. [Google Scholar] [CrossRef]
- Chen, H.Y.; Lee, Y.R.; Chen, R.H. The functions and regulations of DAPK in cancer metastasis. Apoptosis 2014, 19, 364–370. [Google Scholar] [CrossRef] [PubMed]
- You, M.H.; Kim, B.M.; Chen, C.H.; Begley, M.J.; Cantley, L.C.; Lee, T.H. Death-associated protein kinase 1 phosphorylates NDRG2 and induces neuronal cell death. Cell Death Differ. 2017, 24, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Chen, C.H.; Suizu, F.; Huang, P.; Schiene-Fischer, C.; Daum, S.; Zhang, Y.J.; Goate, A.; Chen, R.H.; Zhou, X.Z.; et al. Death-associated protein kinase 1 phosphorylates Pin1 and inhibits its prolyl isomerase activity and cellular function. Mol. Cell 2011, 42, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Takahashi, H.; Nakamura, T.; Hioki, T.; Nagayama, S.; Ooashi, N.; Sun, X.; Ishii, T.; Kudo, Y.; Nakajima-Iijima, S.; et al. Developmental changes in distribution of death-associated protein kinase mRNAs. J. Neurosci. Res. 1999, 58, 674–683. [Google Scholar] [CrossRef]
- Buonarati, O.R.; Cook, S.G.; Goodell, D.J.; Chalmers, N.E.; Rumian, N.L.; Tullis, J.E.; Restrepo, S.; Coultrap, S.J.; Quillinan, N.; Herson, P.S.; et al. CaMKII versus DAPK1 Binding to GluN2B in Ischemic Neuronal Cell Death after Resuscitation from Cardiac Arrest. Cell Rep. 2020, 30, 1–8.e4. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.E.; Caron, N.S.; Aly, A.E.; Lemarie, F.L.; Dal Cengio, L.; Ko, Y.; Lazic, N.; Anderson, L.; Nguyen, B.; Raymond, L.A.; et al. DAPK1 Promotes Extrasynaptic GluN2B Phosphorylation and Striatal Spine Instability in the YAC128 Mouse Model of Huntington Disease. Front. Cell. Neurosci. 2020, 14, 590569. [Google Scholar] [CrossRef] [PubMed]
- Goodell, D.J.; Tullis, J.E.; Bayer, K.U. Young DAPK1 knockout mice have altered presynaptic function. J. Neurophysiol. 2021, 125, 1973–1981. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.M.; You, M.H.; Chen, C.H.; Lee, S.; Hong, Y.; Hong, Y.; Kimchi, A.; Zhou, X.Z.; Lee, T.H. Death-associated protein kinase 1 has a critical role in aberrant tau protein regulation and function. Cell Death Dis. 2014, 5, e1237. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.M.; You, M.H.; Chen, C.H.; Suh, J.; Tanzi, R.E.; Ho Lee, T. Inhibition of death-associated protein kinase 1 attenuates the phosphorylation and amyloidogenic processing of amyloid precursor protein. Hum. Mol. Genet. 2016, 25, 2498–2513. [Google Scholar] [CrossRef]
- Wang, S.; Shi, X.; Li, H.; Pang, P.; Pei, L.; Shen, H.; Lu, Y. DAPK1 Signaling Pathways in Stroke: From Mechanisms to Therapies. Mol. Neurobiol. 2017, 54, 4716–4722. [Google Scholar] [CrossRef]
- Su, Y.; Deng, M.F.; Xiong, W.; Xie, A.J.; Guo, J.; Liang, Z.H.; Hu, B.; Chen, J.G.; Zhu, X.; Man, H.Y.; et al. MicroRNA-26a/Death-Associated Protein Kinase 1 Signaling Induces Synucleinopathy and Dopaminergic Neuron Degeneration in Parkinson’s Disease. Biol. Psychiatry 2019, 85, 769–781. [Google Scholar] [CrossRef]
- Lu, Y.; Gong, Z.; Jin, X.; Zhao, P.; Zhang, Y.; Wang, Z. LncRNA MALAT1 targeting miR-124-3p regulates DAPK1 expression contributes to cell apoptosis in Parkinson’s Disease. J. Cell. Biochem. 2020, 121, 4838–4848. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Wang, B.; Koikawa, K.; Nezu, Y.; Qiu, C.; Lee, T.H.; Zhou, X.Z. Inhibition of death-associated protein kinase 1 attenuates cis P-tau and neurodegeneration in traumatic brain injury. Prog. Neurobiol. 2021, 203, 102072. [Google Scholar] [CrossRef] [PubMed]
- Noori, T.; Shirooie, S.; Sureda, A.; Sobarzo-Sanchez, E.; Dehpour, A.R.; Saldias, M.; Akkol, E.K. Regulation of DAPK1 by Natural Products: An Important Target in Treatment of Stroke. Neurochem. Res. 2022, 47, 2142–2157. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Cui, W.; Wang, Q.; Zhou, J.; Wu, X.; Wang, J.; Zhang, S.; Hu, Q.; Han, L.; Du, Y.; et al. MicroRNA-124/Death-Associated Protein Kinase 1 Signaling Regulates Neuronal Apoptosis in Traumatic Brain Injury via Phosphorylating NR2B. Front. Cell. Neurosci. 2022, 16, 892197. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.; Xu, X.; Peng, L.; Zhong, X.; Zhang, W.; Soundarapandian, M.M.; Balel, C.; Wang, M.; Jia, N.; Zhang, W.; et al. DAPK1 interaction with NMDA receptor NR2B subunits mediates brain damage in stroke. Cell 2010, 140, 222–234. [Google Scholar] [CrossRef] [PubMed]
- You, M.H.; Lee, W.K.; Jin, M.; Song, D.E.; Cheng, S.Y.; Kim, T.Y.; Kim, W.B.; Jeon, M.J.; Kim, W.G. Death-Associated Protein Kinase 1 Inhibits Progression of Thyroid Cancer by Regulating Stem Cell Markers. Cells 2021, 10, 2994. [Google Scholar] [CrossRef] [PubMed]
- Shiloh, R.; Bialik, S.; Kimchi, A. The DAPK family: A structure-function analysis. Apoptosis 2014, 19, 286–297. [Google Scholar] [CrossRef]
- Kim, N.; Chen, D.; Zhou, X.Z.; Lee, T.H. Death-Associated Protein Kinase 1 Phosphorylation in Neuronal Cell Death and Neurodegenerative Disease. Int. J. Mol. Sci. 2019, 20, 3131. [Google Scholar] [CrossRef]
- Chen, C.H.; Wang, W.J.; Kuo, J.C.; Tsai, H.C.; Lin, J.R.; Chang, Z.F.; Chen, R.H. Bidirectional signals transduced by DAPK-ERK interaction promote the apoptotic effect of DAPK. EMBO J. 2005, 24, 294–304. [Google Scholar] [CrossRef]
- Chen, D.; Zhou, X.Z.; Lee, T.H. Death-Associated Protein Kinase 1 as a Promising Drug Target in Cancer and Alzheimer’s Disease. Recent. Pat. Anticancer. Drug Discov. 2019, 14, 144–157. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Luo, W.; Zeng, C.; Zhang, Y.; Wang, L.; Yao, W.; Nie, C. PP2A mediates apoptosis or autophagic cell death in multiple myeloma cell lines. Oncotarget 2017, 8, 80770–80789. [Google Scholar] [CrossRef]
- Singh, P.; Ravanan, P.; Talwar, P. Death Associated Protein Kinase 1 (DAPK1): A Regulator of Apoptosis and Autophagy. Front. Mol. Neurosci. 2016, 9, 46. [Google Scholar] [CrossRef] [PubMed]
- Steinmann, S.; Kunze, P.; Hampel, C.; Eckstein, M.; Bertram Bramsen, J.; Muenzner, J.K.; Carle, B.; Ndreshkjana, B.; Kemenes, S.; Gasparini, P.; et al. DAPK1 loss triggers tumor invasion in colorectal tumor cells. Cell Death Dis. 2019, 10, 895. [Google Scholar] [CrossRef] [PubMed]
- Marsolier, J.; Perichon, M.; Weitzman, J.B.; Medjkane, S. Secreted parasite Pin1 isomerase stabilizes host PKM2 to reprogram host cell metabolism. Commun. Biol. 2019, 2, 152. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Henderson, P.; Pettersson, S.; Satsangi, J.; Hupp, T.; Stevens, C. Tuberous sclerosis-2 (TSC2) regulates the stability of death-associated protein kinase-1 (DAPK) through a lysosome-dependent degradation pathway. FEBS J. 2011, 278, 354–370. [Google Scholar] [CrossRef] [PubMed]
- Llambi, F.; Lourenco, F.C.; Gozuacik, D.; Guix, C.; Pays, L.; Del Rio, G.; Kimchi, A.; Mehlen, P. The dependence receptor UNC5H2 mediates apoptosis through DAP-kinase. EMBO J. 2005, 24, 1192–1201. [Google Scholar] [CrossRef]
- Fitamant, J.; Guenebeaud, C.; Coissieux, M.M.; Guix, C.; Treilleux, I.; Scoazec, J.Y.; Bachelot, T.; Bernet, A.; Mehlen, P. Netrin-1 expression confers a selective advantage for tumor cell survival in metastatic breast cancer. Proc. Natl. Acad. Sci. USA 2008, 105, 4850–4855. [Google Scholar] [CrossRef]
- Castets, M.; Coissieux, M.M.; Delloye-Bourgeois, C.; Bernard, L.; Delcros, J.G.; Bernet, A.; Laudet, V.; Mehlen, P. Inhibition of endothelial cell apoptosis by netrin-1 during angiogenesis. Dev. Cell 2009, 16, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Lin, Y.M.; Chung, H.C.; Lang, Y.D.; Lin, C.J.; Huang, J.; Wang, W.C.; Lin, F.M.; Chen, Z.; Huang, H.D.; et al. miR-103/107 promote metastasis of colorectal cancer by targeting the metastasis suppressors DAPK and KLF4. Cancer Res. 2012, 72, 3631–3641. [Google Scholar] [CrossRef] [PubMed]
- Bagci, B.; Sari, M.; Karadayi, K.; Turan, M.; Ozdemir, O.; Bagci, G. KRAS, BRAF oncogene mutations and tissue specific promoter hypermethylation of tumor suppressor SFRP2, DAPK1, MGMT, HIC1 and p16 genes in colorectal cancer patients. Cancer Biomark. 2016, 17, 133–143. [Google Scholar] [CrossRef]
- Matsumoto, H.; Nagao, M.; Ogawa, S.; Kanehiro, H.; Hisanaga, M.; Ko, S.; Ikeda, N.; Fujii, H.; Koyama, F.; Mukogawa, T.; et al. Prognostic significance of death-associated protein-kinase expression in hepatocellular carcinomas. Anticancer. Res. 2003, 23, 1333–1341. [Google Scholar] [PubMed]
- Catto, J.W.; Azzouzi, A.R.; Rehman, I.; Feeley, K.M.; Cross, S.S.; Amira, N.; Fromont, G.; Sibony, M.; Cussenot, O.; Meuth, M.; et al. Promoter hypermethylation is associated with tumor location, stage, and subsequent progression in transitional cell carcinoma. J. Clin. Oncol. 2005, 23, 2903–2910. [Google Scholar] [CrossRef] [PubMed]
- Gozuacik, D.; Kimchi, A. DAPk protein family and cancer. Autophagy 2006, 2, 74–79. [Google Scholar] [CrossRef]
- Wang, L.C.; Lo, A.; Scholler, J.; Sun, J.; Majumdar, R.S.; Kapoor, V.; Antzis, M.; Cotner, C.E.; Johnson, L.A.; Durham, A.C.; et al. Targeting fibroblast activation protein in tumor stroma with chimeric antigen receptor T cells can inhibit tumor growth and augment host immunity without severe toxicity. Cancer Immunol. Res. 2014, 2, 154–166. [Google Scholar] [CrossRef]
- Ye, X.; Weinberg, R.A. Epithelial-Mesenchymal Plasticity: A Central Regulator of Cancer Progression. Trends Cell Biol. 2015, 25, 675–686. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, C.; Li, K.; Ye, Y.; Shen, A.; Guo, L.; Chen, P.; Meng, C.; Wang, Q.; Yang, X.; et al. Death-associated protein kinase 1 suppresses hepatocellular carcinoma cell migration and invasion by upregulation of DEAD-box helicase 20. Cancer Sci. 2020, 111, 2803–2813. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Chen, J.; Shu, Y.; Liu, S.; Wu, L.; Ji, J.; Liu, Z.; Tang, Q.; Zhou, Z.; Cheng, Y.; et al. Correlation of DAPK1 methylation and the risk of gastrointestinal cancer: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0184959. [Google Scholar] [CrossRef]
- Qian, J.; Wang, Y.L.; Lin, J.; Yao, D.M.; Xu, W.R.; Wu, C.Y. Aberrant methylation of the death-associated protein kinase 1 (DAPK1) CpG island in chronic myeloid leukemia. Eur. J. Haematol. 2009, 82, 119–123. [Google Scholar] [CrossRef]
- Martello, G.; Rosato, A.; Ferrari, F.; Manfrin, A.; Cordenonsi, M.; Dupont, S.; Enzo, E.; Guzzardo, V.; Rondina, M.; Spruce, T.; et al. A MicroRNA targeting dicer for metastasis control. Cell 2010, 141, 1195–1207. [Google Scholar] [CrossRef]
- Tur, M.K.; Daramola, A.K.; Gattenlohner, S.; Herling, M.; Chetty, S.; Barth, S. Restoration of DAP Kinase Tumor Suppressor Function: A Therapeutic Strategy to Selectively Induce Apoptosis in Cancer Cells Using Immunokinase Fusion Proteins. Biomedicines 2017, 5, 59. [Google Scholar] [CrossRef] [PubMed]
- Gade, P.; Kimball, A.S.; DiNardo, A.C.; Gangwal, P.; Ross, D.D.; Boswell, H.S.; Keay, S.K.; Kalvakolanu, D.V. Death-associated Protein Kinase-1 Expression and Autophagy in Chronic Lymphocytic Leukemia Are Dependent on Activating Transcription Factor-6 and CCAAT/Enhancer-binding Protein-beta. J. Biol. Chem. 2016, 291, 22030–22042. [Google Scholar] [CrossRef] [PubMed]
- Miyazono, K. Transforming growth factor-beta signaling in epithelial-mesenchymal transition and progression of cancer. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2009, 85, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Nong, S.; Wei, Z.; Wang, Z.; Ma, L.; Guan, Y.; Ni, J. Reduced DAPK1 Expression Promotes Stem Cell-Like Characteristics of Prostate Cancer Cells by Activating ZEB1 via Hippo/YAP Signaling Pathway. Stem Cells Dev. 2021, 30, 934–945. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Sims-Mourtada, J.; Izzo, J.G.; Apisarnthanarax, S.; Wu, T.T.; Malhotra, U.; Luthra, R.; Liao, Z.; Komaki, R.; van der Kogel, A.; Ajani, J.; et al. Hedgehog: An attribute to tumor regrowth after chemoradiotherapy and a target to improve radiation response. Clin. Cancer Res. 2006, 12, 6565–6572. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, L.; De Sousa, E.M.F.; van der Heijden, M.; Cameron, K.; de Jong, J.H.; Borovski, T.; Tuynman, J.B.; Todaro, M.; Merz, C.; Rodermond, H.; et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat. Cell Biol. 2010, 12, 468–476. [Google Scholar] [CrossRef]
- Gu, J.W.; Rizzo, P.; Pannuti, A.; Golde, T.; Osborne, B.; Miele, L. Notch signals in the endothelium and cancer “stem-like” cells: Opportunities for cancer therapy. Vasc. Cell 2012, 4, 7. [Google Scholar] [CrossRef]
- Levy, D.; Plu-Bureau, G.; Decroix, Y.; Hugol, D.; Rostene, W.; Kimchi, A.; Gompel, A. Death-associated protein kinase loss of expression is a new marker for breast cancer prognosis. Clin. Cancer Res. 2004, 10, 3124–3130. [Google Scholar] [CrossRef]
- Wu, Q.; He, Y.; Liu, X.; Luo, F.; Jiang, Y.; Xiang, M.; Zhao, R. Cancer stem cell-like cells-derived exosomal CDKN2B-AS1 stabilizes CDKN2B to promote the growth and metastasis of thyroid cancer via TGF-beta1/Smad2/3 signaling. Exp. Cell. Res. 2022, 419, 113268. [Google Scholar] [CrossRef]
- Yang, L.; Shi, P.; Zhao, G.; Xu, J.; Peng, W.; Zhang, J.; Zhang, G.; Wang, X.; Dong, Z.; Chen, F.; et al. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct. Target Ther. 2020, 5, 8. [Google Scholar] [CrossRef]
- Kong, W.J.; Zhang, S.; Guo, C.K.; Wang, Y.J.; Chen, X.; Zhang, S.L.; Zhang, D.; Liu, Z.; Kong, W. Effect of methylation-associated silencing of the death-associated protein kinase gene on nasopharyngeal carcinoma. Anticancer Drugs 2006, 17, 251–259. [Google Scholar] [CrossRef]
- Wu, B.; Yao, H.; Wang, S.; Xu, R. DAPK1 modulates a curcumin-induced G2/M arrest and apoptosis by regulating STAT3, NF-kappaB, and caspase-3 activation. Biochem. Biophys. Res. Commun. 2013, 434, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Li, F.; Shi, K.; Yang, Y.; Xu, C. Sodium selenite-induced activation of DAPK promotes autophagy in human leukemia HL60 cells. BMB Rep. 2012, 45, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Gandesiri, M.; Chakilam, S.; Ivanovska, J.; Benderska, N.; Ocker, M.; Di Fazio, P.; Feoktistova, M.; Gali-Muhtasib, H.; Rave-Frank, M.; Prante, O.; et al. DAPK plays an important role in panobinostat-induced autophagy and commits cells to apoptosis under autophagy deficient conditions. Apoptosis 2012, 17, 1300–1315. [Google Scholar] [CrossRef]
- Wu, J.; Hu, C.P.; Gu, Q.H.; Li, Y.P.; Song, M. Trichostatin A sensitizes cisplatin-resistant A549 cells to apoptosis by up-regulating death-associated protein kinase. Acta Pharmacol. Sin. 2010, 31, 93–101. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
You, M.-H. Mechanism of DAPK1 for Regulating Cancer Stem Cells in Thyroid Cancer. Curr. Issues Mol. Biol. 2024, 46, 7086-7096. https://doi.org/10.3390/cimb46070422
You M-H. Mechanism of DAPK1 for Regulating Cancer Stem Cells in Thyroid Cancer. Current Issues in Molecular Biology. 2024; 46(7):7086-7096. https://doi.org/10.3390/cimb46070422
Chicago/Turabian StyleYou, Mi-Hyeon. 2024. "Mechanism of DAPK1 for Regulating Cancer Stem Cells in Thyroid Cancer" Current Issues in Molecular Biology 46, no. 7: 7086-7096. https://doi.org/10.3390/cimb46070422




