MicroRNAs as Regulators of Radiation-Induced Oxidative Stress
Abstract
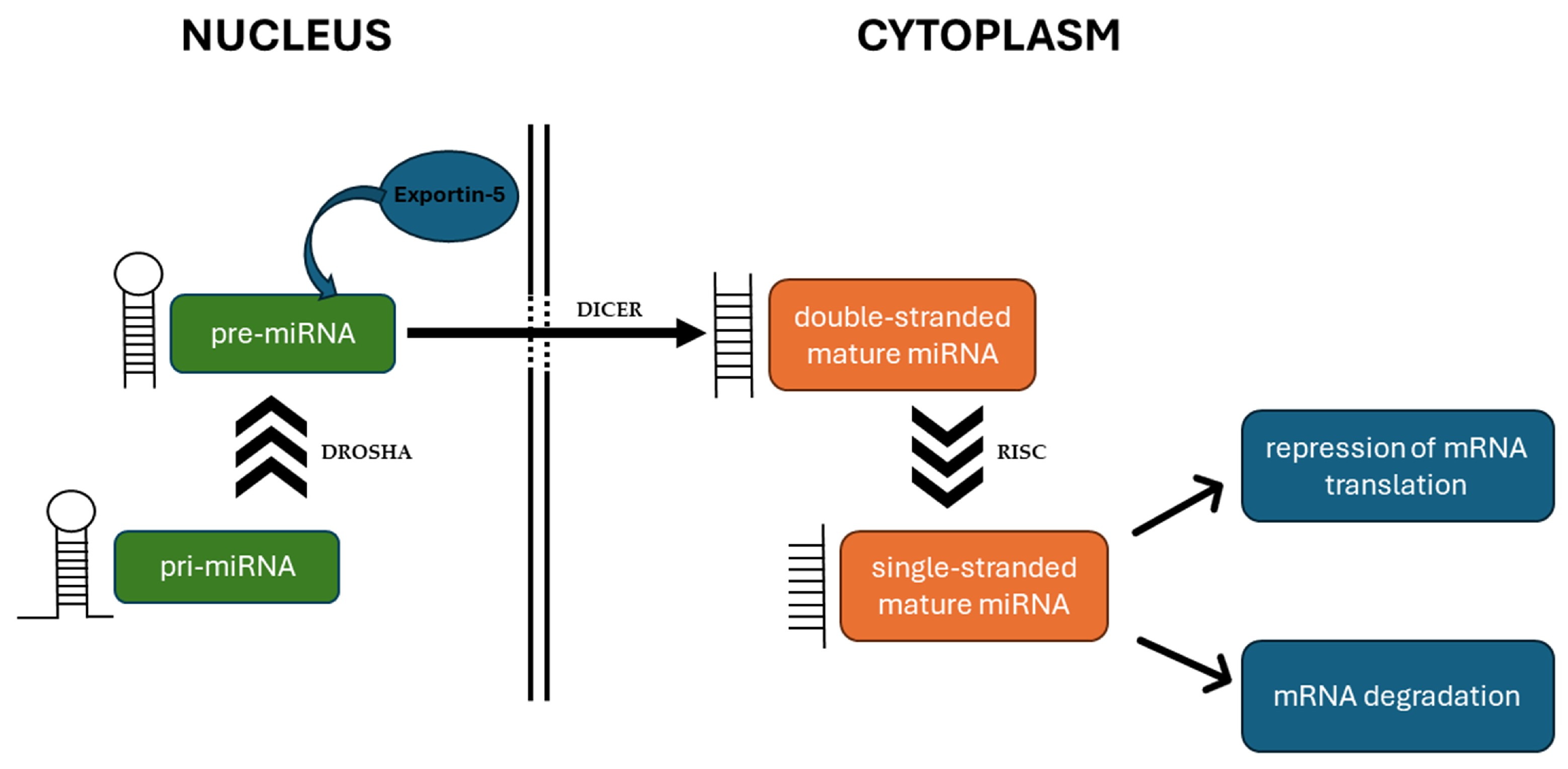
:1. Introduction
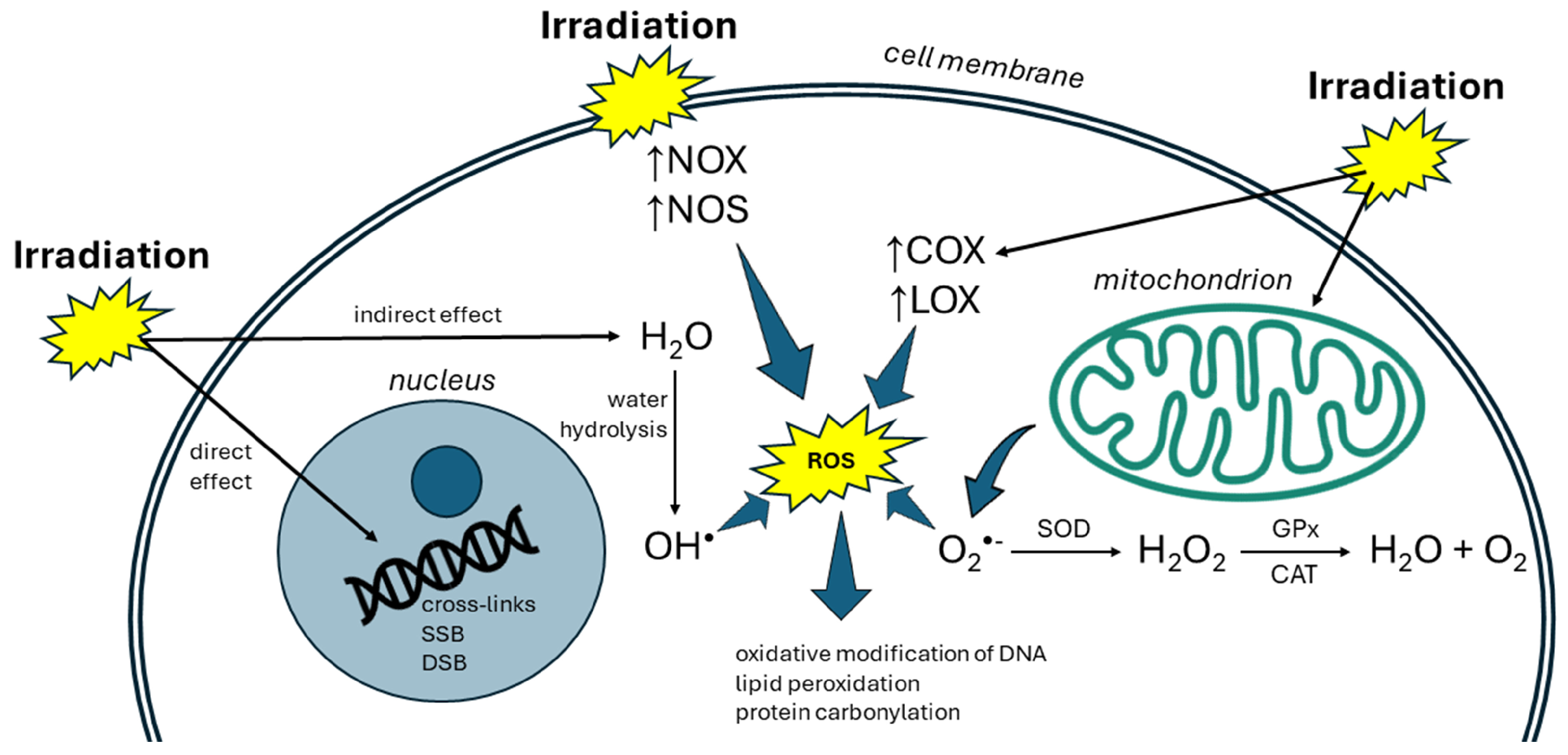
2. Radiation and Oxidative Stress
3. MiRNAs Affect ROS Production and Elimination
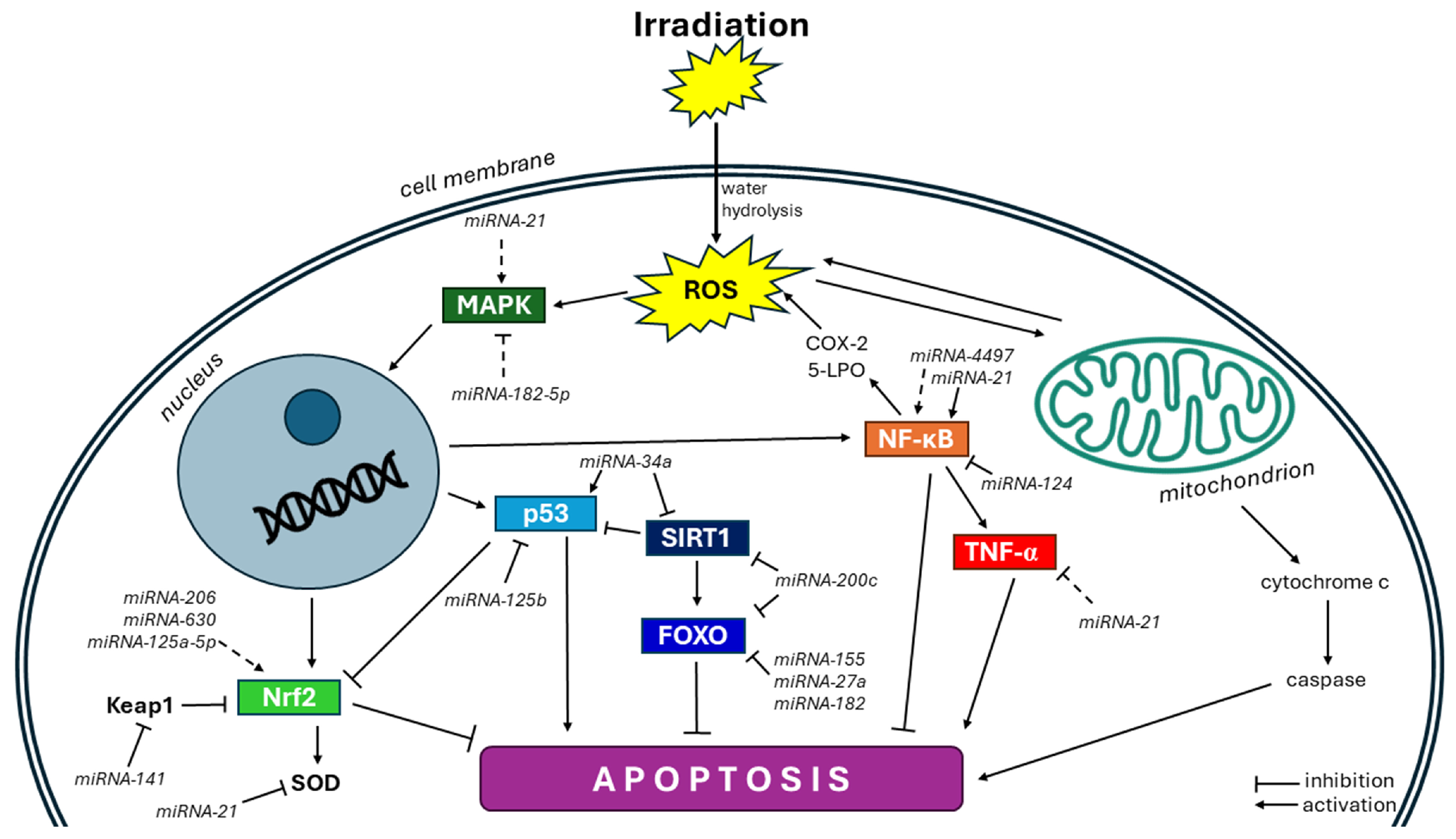
4. Evidence for miRNA Action in Radiation-Induced Oxidative Stress
4.1. Nrf2 Pathway
4.2. NF-κB Pathway
4.3. p53 Pathway
4.4. FOXO Pathway
4.5. SIRT Pathway
4.6. MAPK Pathway
5. miRNAs as Potential Therapeutics
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 4-HNE | 4-hydroxy-2-nonenal |
| 8-OHdG | 8-hydroxy-2′-deoxyguanosine |
| AKT | protein kinase B |
| AMPK | adenosine monophosphate-activated protein kinase |
| AP-1 | activator protein 1 |
| AP2a | activator protein 2a |
| Bcl-2 | B-cell lymphoma 2 |
| CAT | catalase |
| COX | cyclooxygenase |
| DSB | double-strand break |
| eNOS | endothelial nitric oxide synthase |
| ERK | extracellular-signal-regulated kinase |
| FOXO | Forkhead box O |
| GPx | glutathione peroxidase |
| GR | glutathione reductase |
| HIF-1α | hypoxia-inducible factor 1 alpha |
| HLEC | human lens epithelial cell |
| HO | heme oxygenase |
| H2O2 | hydrogen peroxide |
| HUVEC | human umbilical vein cell |
| IκBα | inhibitory protein alpha of nuclear factor kappa B |
| JNK | c-Jun N-terminal kinase |
| Keap 1 | Kelch-like ECH-associated protein 1 |
| LDH | lactate dehydrogenase |
| LOX | lipoxygenase |
| MAPK | mitogen-activated protein kinase |
| MDA | malondialdehyde |
| mRNA | messenger RNA |
| miRNA | microRNA |
| NF-κB | nuclear factor kappa B |
| NOS | nitric oxide synthase |
| NOX | NADPH oxidase |
| Nrf2 | nuclear factor erythroid 2-related factor 2 |
| O2•− | superoxide anion |
| •OH | hydroxyl radical |
| p53 | tumor suppressor protein p53 |
| PRDX | peroxiredoxin |
| pre-miRNA | precursor form of microRNA |
| pri-miRNA | primary nuclear transcript of microRNA |
| Redd1 | regulated in development and DNA damage responses 1 |
| RGC | retinal ganglion cell |
| RISC | RNA-induced silencing complex |
| RNS | reactive nitrogen species |
| ROS | reactive oxygen species |
| RPE | retinal pigment epithelium cells |
| SIRT | sirtuin |
| SOD | superoxide dismutase |
| SPRY-2 | sprouty homolog 2 |
| SSB | single-strand break |
| STAT3 | signal transducer and activator of transcription 3 |
| TXN | thioredoxin |
| TXNRD | thioredoxin reductase |
References
- Checa, J.; Aran, J.M. Reactive Oxygen Species: Drivers of Physiological and Pathological Processes. J. Inflamm. Res. 2020, 13, 1057–1073. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.P. Oxidative Stress in Health and Disease. Biomedicines 2023, 11, 2925. [Google Scholar] [CrossRef] [PubMed]
- Samet, J.M.; Wages, P.A. Oxidative Stress from Environmental Exposures. Curr. Opin. Toxicol. 2018, 7, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Azzam, E.I.; Jay-Gerin, J.-P.; Pain, D. Ionizing Radiation-Induced Metabolic Oxidative Stress and Prolonged Cell Injury. Cancer Lett. 2012, 327, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Krol, J.; Loedige, I.; Filipowicz, W. The Widespread Regulation of MicroRNA Biogenesis, Function and Decay. Nat. Rev. Genet. 2010, 11, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Marin-Muller, C.; Bharadwaj, U.; Chow, K.; Yao, Q.; Chen, C. MicroRNAs: Control and Loss of Control in Human Physiology and Disease. World J. Surg. 2009, 33, 667–684. [Google Scholar] [CrossRef] [PubMed]
- Moertl, S.; Mutschelknaus, L.; Heider, T.; Atkinson, M.J. MicroRNAs as Novel Elements in Personalized Radiotherapy. Transl. Cancer Res. 2016, 5, S1262–S1269. [Google Scholar] [CrossRef]
- Gandellini, P.; Rancati, T.; Valdagni, R.; Zaffaroni, N. MiRNAs in Tumor Radiation Response: Bystanders or Participants? Trends Mol. Med. 2014, 20, 529–539. [Google Scholar] [CrossRef]
- Kura, B.; Szeiffova Bacova, B.; Kalocayova, B.; Sykora, M.; Slezak, J. Oxidative Stress-Responsive MicroRNAs in Heart Injury. Int. J. Mol. Sci. 2020, 21, 358. [Google Scholar] [CrossRef]
- Lin, Y.-H. MicroRNA Networks Modulate Oxidative Stress in Cancer. Int. J. Mol. Sci. 2019, 20, 4497. [Google Scholar] [CrossRef]
- Sies, H. Oxidative Stress: A Concept in Redox Biology and Medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Breitenbach, M.; Eckl, P. Introduction to Oxidative Stress in Biomedical and Biological Research. Biomolecules 2015, 5, 1169–1177. [Google Scholar] [CrossRef]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.J.; Ahamed, M.; Alhadlaq, H.A.; Alshamsan, A. Mechanism of ROS Scavenging and Antioxidant Signalling by Redox Metallic and Fullerene Nanomaterials: Potential Implications in ROS Associated Degenerative Disorders. Biochim. Biophys. Acta-Gen. Subj. 2017, 1861, 802–813. [Google Scholar] [CrossRef] [PubMed]
- Marengo, B.; Nitti, M.; Furfaro, A.L.; Colla, R.; De Ciucis, C.; Marinari, U.M.; Pronzato, M.A.; Traverso, N.; Domenicotti, C. Redox Homeostasis and Cellular Antioxidant Systems: Crucial Players in Cancer Growth and Therapy. Oxid. Med. Cell. Longev. 2016, 2016, 6235641. [Google Scholar] [CrossRef] [PubMed]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free Radicals: Properties, Sources, Targets, and Their Implication in Various Diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Go, Y.-M.; Chandler, J.D.; Jones, D.P. The Cysteine Proteome. Free Radic. Biol. Med. 2015, 84, 227–245. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Bian, Y.; Liu, L.; Liu, L.; Liu, X.; Ma, S. Molecular Pathways Associated with Oxidative Stress and Their Potential Applications in Radiotherapy (Review). Int. J. Mol. Med. 2022, 49, 65. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.; Rudrawar, S.; Zunk, M.; Bernaitis, N.; Arora, D.; McDermott, C.; Anoopkumar-Dukie, S. Protection against Radiotherapy-Induced Toxicity. Antioxidants 2016, 5, 22. [Google Scholar] [CrossRef]
- Vorotnikova, E.; Rosenthal, R.A.; Tries, M.; Doctrow, S.R.; Braunhut, S.J. Novel Synthetic SOD/Catalase Mimetics Can Mitigate Capillary Endothelial Cell Apoptosis Caused by Ionizing Radiation. Radiat. Res. 2010, 173, 748–759. [Google Scholar] [CrossRef]
- Wang, X.-Q.; Wang, W.; Peng, M.; Zhang, X.-Z. Free Radicals for Cancer Theranostics. Biomaterials 2021, 266, 120474. [Google Scholar] [CrossRef] [PubMed]
- Jeggo, P.; Löbrich, M. Radiation-Induced DNA Damage Responses. Radiat. Prot. Dosimetry 2006, 122, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-S.; Song, M.-F.; Kasai, H.; Kawai, K. Generation and Threshold Level of 8-OHdG as Oxidative DNA Damage Elicited by Low Dose Ionizing Radiation. Genes Environ. 2013, 35, 88–92. [Google Scholar] [CrossRef]
- Reisz, J.A.; Bansal, N.; Qian, J.; Zhao, W.; Furdui, C.M. Effects of Ionizing Radiation on Biological Molecules—Mechanisms of Damage and Emerging Methods of Detection. Antioxid. Redox Signal. 2014, 21, 260–292. [Google Scholar] [CrossRef]
- Reindl, J.; Abrantes, A.M.; Ahire, V.; Azimzadeh, O.; Baatout, S.; Baeyens, A.; Baselet, B.; Chauhan, V.; Da Pieve, F.; Delbart, W.; et al. Molecular Radiation Biology. In Radiobiology Textbook; Springer International Publishing: Cham, Switzertland, 2023; pp. 83–189. [Google Scholar]
- Dong, S.; Lyu, X.; Yuan, S.; Wang, S.; Li, W.; Chen, Z.; Yu, H.; Li, F.; Jiang, Q. Oxidative Stress: A Critical Hint in Ionizing Radiation Induced Pyroptosis. Radiat. Med. Prot. 2020, 1, 179–185. [Google Scholar] [CrossRef]
- Schäfer, S.T.; Franken, L.; Adamzik, M.; Schumak, B.; Scherag, A.; Engler, A.; Schönborn, N.; Walden, J.; Koch, S.; Baba, H.A.; et al. Mitochondrial DNA. Anesthesiology 2016, 124, 923–933. [Google Scholar] [CrossRef]
- Wei, J.; Wang, B.; Wang, H.; Meng, L.; Zhao, Q.; Li, X.; Xin, Y.; Jiang, X. Radiation-Induced Normal Tissue Damage: Oxidative Stress and Epigenetic Mechanisms. Oxid. Med. Cell. Longev. 2019, 2019, 3010342. [Google Scholar] [CrossRef]
- Najafi, M.; Shirazi, A.; Motevaseli, E.; Geraily, G.; Norouzi, F.; Heidari, M.; Rezapoor, S. The Melatonin Immunomodulatory Actions in Radiotherapy. Biophys. Rev. 2017, 9, 139–148. [Google Scholar] [CrossRef]
- Carbonell, T.; Gomes, A.V. MicroRNAs in the Regulation of Cellular Redox Status and Its Implications in Myocardial Ischemia-Reperfusion Injury. Redox Biol. 2020, 36, 101607. [Google Scholar] [CrossRef]
- Minjares, M.; Wu, W.; Wang, J.-M. Oxidative Stress and MicroRNAs in Endothelial Cells under Metabolic Disorders. Cells 2023, 12, 1341. [Google Scholar] [CrossRef]
- Tonelli, C.; Chio, I.I.C.; Tuveson, D.A. Transcriptional Regulation by Nrf2. Antioxid. Redox Signal. 2018, 29, 1727–1745. [Google Scholar] [CrossRef] [PubMed]
- Kurinna, S.; Werner, S. NRF2 and MicroRNAs: New but Awaited Relations. Biochem. Soc. Trans. 2015, 43, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Ahmadi, Z.; Samarghandian, S.; Mohammadinejad, R.; Yaribeygi, H.; Sathyapalan, T.; Sahebkar, A. MicroRNA-Mediated Regulation of Nrf2 Signaling Pathway: Implications in Disease Therapy and Protection against Oxidative Stress. Life Sci. 2020, 244, 117329. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhao, Y.; Hou, W.; Guo, L. MiR-153 Regulates Cardiomyocyte Apoptosis by Targeting Nrf2/HO-1 Signaling. Chromosom. Res. 2019, 27, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Qi, Y.; Xu, L.; Tao, X.; Han, X.; Yin, L.; Peng, J. MicroRNA-140-5p Aggravates Doxorubicin-Induced Cardiotoxicity by Promoting Myocardial Oxidative Stress via Targeting Nrf2 and Sirt2. Redox Biol. 2018, 15, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Chen, X.; Li, H. Protective Effects of MiR-126 Specifically Regulates Nrf2 Through Ischemic Postconditioning on Renal Ischemia/Reperfusion Injury in Mice. Transplant. Proc. 2020, 52, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Baird, L.; Yamamoto, M. The Molecular Mechanisms Regulating the KEAP1-NRF2 Pathway. Mol. Cell. Biol. 2020, 40, e00099-20. [Google Scholar] [CrossRef]
- Kabaria, S.; Choi, D.C.; Chaudhuri, A.D.; Jain, M.R.; Li, H.; Junn, E. MicroRNA-7 Activates Nrf2 Pathway by Targeting Keap1 Expression. Free Radic. Biol. Med. 2015, 89, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Eades, G.; Yang, M.; Yao, Y.; Zhang, Y.; Zhou, Q. MiR-200a Regulates Nrf2 Activation by Targeting Keap1 MRNA in Breast Cancer Cells. J. Biol. Chem. 2011, 286, 40725–40733. [Google Scholar] [CrossRef]
- Ciesielska, S.; Slezak-Prochazka, I.; Bil, P.; Rzeszowska-Wolny, J. Micro RNAs in Regulation of Cellular Redox Homeostasis. Int. J. Mol. Sci. 2021, 22, 6022. [Google Scholar] [CrossRef]
- Wu, Y.; Yao, J.; Feng, K. MiR-124-5p/NOX2 Axis Modulates the ROS Production and the Inflammatory Microenvironment to Protect Against the Cerebral I/R Injury. Neurochem. Res. 2020, 45, 404–417. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-Z.; Hu, Y.-Y.; Zhao, J.; Zhao, Y.-B.; Sun, J.-D.; Yang, Y.; Ji, C.-C.; Liu, Z.-B.; Cao, W.-D.; Qu, Y.; et al. MicroRNA-34a Induces Apoptosis in the Human Glioma Cell Line, A172, through Enhanced ROS Production and NOX2 Expression. Biochem. Biophys. Res. Commun. 2014, 444, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Lu, Y.; Hu, J.; Le, H.; Yu, W.; Xu, W.; Yu, W.; Zheng, J. Mechanism of MiR-320 in Regulating Biological Characteristics of Ischemic Cerebral Neuron by Mediating Nox2/ROS Pathway. J. Mol. Neurosci. 2020, 70, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, J.; Fan, L.; He, X. MiR-423-5p Suppresses High-Glucose-Induced Podocyte Injury by Targeting Nox4. Biochem. Biophys. Res. Commun. 2018, 505, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Hong, S.; Li, W.; Wang, P.; You, J.; Zhang, X.; Tang, F.; Wang, P.; Zhang, C. MiR-99a Regulates ROS-Mediated Invasion and Migration of Lung Adenocarcinoma Cells by Targeting NOX4. Oncol. Rep. 2016, 35, 2755–2766. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-N.; Ge, M.-X.; Yuan, Z.-F. MicroRNA-182-5p Protects Human Lens Epithelial Cells against Oxidative Stress-Induced Apoptosis by Inhibiting NOX4 and P38 MAPK Signalling. BMC Ophthalmol. 2020, 20, 233. [Google Scholar] [CrossRef]
- Carlomosti, F.; D’Agostino, M.; Beji, S.; Torcinaro, A.; Rizzi, R.; Zaccagnini, G.; Maimone, B.; Di Stefano, V.; De Santa, F.; Cordisco, S.; et al. Oxidative Stress-Induced MiR-200c Disrupts the Regulatory Loop Among SIRT1, FOXO1, and ENOS. Antioxid. Redox Signal. 2017, 27, 328–344. [Google Scholar] [CrossRef] [PubMed]
- Icli, B.; Wu, W.; Ozdemir, D.; Li, H.; Cheng, H.S.; Haemmig, S.; Liu, X.; Giatsidis, G.; Avci, S.N.; Lee, N.; et al. MicroRNA-615-5p Regulates Angiogenesis and Tissue Repair by Targeting AKT/ENOS (Protein Kinase B/Endothelial Nitric Oxide Synthase) Signaling in Endothelial Cells. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1458–1474. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.-X.; Zeng, D.-Y.; Li, R.-T.; Pang, R.-P.; Yang, H.; Hu, Y.-L.; Zhang, Q.; Jiang, Y.; Huang, L.-Y.; Tang, Y.-B.; et al. Essential Role of MicroRNA-155 in Regulating Endothelium-Dependent Vasorelaxation by Targeting Endothelial Nitric Oxide Synthase. Hypertension 2012, 60, 1407–1414. [Google Scholar] [CrossRef]
- He, J.; Jiang, B.-H. Interplay Between Reactive Oxygen Species and MicroRNAs in Cancer. Curr. Pharmacol. Rep. 2016, 2, 82–90. [Google Scholar] [CrossRef]
- Zhang, X.; Ng, W.-L.; Wang, P.; Tian, L.; Werner, E.; Wang, H.; Doetsch, P.; Wang, Y. MicroRNA-21 Modulates the Levels of Reactive Oxygen Species by Targeting SOD3 and TNF α. Cancer Res. 2012, 72, 4707–4713. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Fang, F.; Zhang, J.; Josson, S.; St. Clair, W.H.; St. Clair, D.K. MiR-17* Suppresses Tumorigenicity of Prostate Cancer by Inhibiting Mitochondrial Antioxidant Enzymes. PLoS ONE 2010, 5, e14356. [Google Scholar] [CrossRef] [PubMed]
- Long, B.; Gan, T.-Y.; Zhang, R.-C.; Zhang, Y.-H. MiR-23a Regulates Cardiomyocyte Apoptosis by Targeting Manganese Superoxide Dismutase. Mol. Cells 2017, 40, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Haque, R.; Chun, E.; Howell, J.C.; Sengupta, T.; Chen, D.; Kim, H. MicroRNA-30b-Mediated Regulation of Catalase Expression in Human ARPE-19 Cells. PLoS ONE 2012, 7, e42542. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wells, A.; Padilla, M.T.; Kato, K.; Kim, K.C.; Lin, Y. A Signaling Pathway Consisting of MiR-551b, Catalase and MUC1 Contributes to Acquired Apoptosis Resistance and Chemoresistance. Carcinogenesis 2014, 35, 2457–2466. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Zhao, L.; Zheng, J.; Wang, K.; Deng, H.; Liu, P.; Chen, L.; Mu, H. MicroRNA-144 Modulates Oxidative Stress Tolerance in SH-SY5Y Cells by Regulating Nuclear Factor Erythroid 2-Related Factor 2-Glutathione Axis. Neurosci. Lett. 2017, 655, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Huang, H.; Fan, Y.; Kong, B.; Hu, H.; Hu, K.; Guo, J.; Mei, Y.; Liu, W.-L. Effects of Downregulation of MicroRNA-181a on H2O2-Induced H9c2 Cell Apoptosis via the Mitochondrial Apoptotic Pathway. Oxid. Med. Cell. Longev. 2014, 2014, 960362. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Liu, H.; Chen, F.; Li, D.; Zhao, Y. MiR-214 Promotes the Alcohol-Induced Oxidative Stress via Down-Regulation of Glutathione Reductase and Cytochrome P450 Oxidoreductase in Liver Cells. Alcohol. Clin. Exp. Res. 2014, 38, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Xu, Y. P53, Oxidative Stress, and Aging. Antioxid. Redox Signal. 2011, 15, 1669–1678. [Google Scholar] [CrossRef]
- Li, J.; Aung, L.H.H.; Long, B.; Qin, D.; An, S.; Li, P. MiR-23a Binds to P53 and Enhances Its Association with MiR-128 Promoter. Sci. Rep. 2015, 5, 16422. [Google Scholar] [CrossRef]
- Lu, B.; Christensen, I.; Ma, L.; Wang, X.; Jiang, L.; Wang, C.; Feng, L.; Zhang, J.; Yan, Q. MiR-24-P53 Pathway Evoked by Oxidative Stress Promotes Lens Epithelial Cell Apoptosis in Age-Related Cataracts. Mol. Med. Rep. 2018, 17, 5021–5028. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Pan, Z.; Yang, Z.; Zhang, D.; Chen, Q. Overexpression of MicroRNA-122 Resists Oxidative Stress-Induced Human Umbilical Vascular Endothelial Cell Injury by Inhibition of P53. Biomed Res. Int. 2020, 2020, 9791608. [Google Scholar] [CrossRef]
- Lingappan, K. NF-ΚB in Oxidative Stress. Curr. Opin. Toxicol. 2018, 7, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Ren, X.; Zhang, X.; Luo, Y.; Wang, G.; Huang, K.; Feng, S.; Bao, X.; Huang, K.; He, X.; et al. Selective Killing of Lung Cancer Cells by MiRNA-506 Molecule through Inhibiting NF-ΚB P65 to Evoke Reactive Oxygen Species Generation and P53 Activation. Oncogene 2015, 34, 691–703. [Google Scholar] [CrossRef] [PubMed]
- Gu, X. MicroRNA-124 Prevents H2O2-Induced Apoptosis and Oxidative Stress in Human Lens Epithelial Cells via Inhibition of the NF-ΚB Signaling Pathway. Pharmacology 2018, 102, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Bian, J.; Ge, W.; Jiang, Z. MiR-26a-5p Attenuates Oxidative Stress and Inflammation in Diabetic Retinopathy through the USP14/NF-ΚB Signaling Pathway. J. Ophthalmol. 2024, 2024, 1470898. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.K.; Chhabra, G.; Ndiaye, M.A.; Garcia-Peterson, L.M.; Mack, N.J.; Ahmad, N. The Role of Sirtuins in Antioxidant and Redox Signaling. Antioxid. Redox Signal. 2018, 28, 643–661. [Google Scholar] [CrossRef] [PubMed]
- Rane, S.; He, M.; Sayed, D.; Vashistha, H.; Malhotra, A.; Sadoshima, J.; Vatner, D.E.; Vatner, S.F.; Abdellatif, M. Downregulation of MiR-199a Derepresses Hypoxia-Inducible Factor-1α and Sirtuin 1 and Recapitulates Hypoxia Preconditioning in Cardiac Myocytes. Circ. Res. 2009, 104, 879–886. [Google Scholar] [CrossRef]
- Baker, J.R.; Vuppusetty, C.; Colley, T.; Papaioannou, A.I.; Fenwick, P.; Donnelly, L.; Ito, K.; Barnes, P.J. Oxidative Stress Dependent MicroRNA-34a Activation via PI3Kα Reduces the Expression of Sirtuin-1 and Sirtuin-6 in Epithelial Cells. Sci. Rep. 2016, 6, 35871. [Google Scholar] [CrossRef]
- Hou, M.; Zuo, X.; Li, C.; Zhang, Y.; Teng, Y. Mir-29b Regulates Oxidative Stress by Targeting SIRT1 in Ovarian Cancer Cells. Cell. Physiol. Biochem. 2017, 43, 1767–1776. [Google Scholar] [CrossRef]
- Huang, B.; Sun, X.; Xu, A. MiR-217 Inhibition Relieves Oxidative Stress-Induced Melanocyte Damage by Targeting Sirtuin 1. Biotechnol. Biotechnol. Equip. 2020, 34, 182–190. [Google Scholar] [CrossRef]
- Son, Y.; Cheong, Y.-K.; Kim, N.-H.; Chung, H.-T.; Kang, D.G.; Pae, H.-O. Mitogen-Activated Protein Kinases and Reactive Oxygen Species: How Can ROS Activate MAPK Pathways? J. Signal Transduct. 2011, 2011, 792639. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhang, J.; Sun, X.; Ma, G.; Luo, G.; Miao, Z.; Song, L. MiR-132 Improves the Cognitive Function of Rats with Alzheimer’s Disease by Inhibiting the MAPK1 Signal Pathway. Exp. Ther. Med. 2020, 20, 159. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Liu, J.; Wang, X.; Feng, B.; Ren, Y.; Zheng, J.; Yu, K.; Yu, H.; Li, K.; Zhu, F.; et al. ROS-Mediated MAPK Activation Aggravates Hyperoxia-Induced Acute Lung Injury by Promoting Apoptosis of Type II Alveolar Epithelial Cells via the STAT3/MiR-21–5p Axis. Mol. Immunol. 2023, 163, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Zhan-qiang, H.; Hai-hua, Q.; Chi, Z.; Miao, W.; Cui, Z.; Zi-yin, L.; Jing, H.; Yi-wei, W. MiR-146a Aggravates Cognitive Impairment and Alzheimer Disease-like Pathology by Triggering Oxidative Stress through MAPK Signaling. Neurologia 2023, 38, 486–494. [Google Scholar] [CrossRef]
- Fu, X.; Shen, Y.; Wang, W.; Li, X. MiR-30a-5p Ameliorates Spinal Cord Injury-induced Inflammatory Responses and Oxidative Stress by Targeting Neurod 1 through MAPK/ERK Signalling. Clin. Exp. Pharmacol. Physiol. 2018, 45, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Simone, N.L.; Soule, B.P.; Ly, D.; Saleh, A.D.; Savage, J.E.; DeGraff, W.; Cook, J.; Harris, C.C.; Gius, D.; Mitchell, J.B. Ionizing Radiation-Induced Oxidative Stress Alters MiRNA Expression. PLoS ONE 2009, 4, e6377. [Google Scholar] [CrossRef]
- Chakraborty, N.; Gautam, A.; Holmes-Hampton, G.P.; Kumar, V.P.; Biswas, S.; Kumar, R.; Hamad, D.; Dimitrov, G.; Olabisi, A.O.; Hammamieh, R.; et al. MicroRNA and Metabolite Signatures Linked to Early Consequences of Lethal Radiation. Sci. Rep. 2020, 10, 5424. [Google Scholar] [CrossRef]
- Kwon, J.-E.; Kim, B.-Y.; Kwak, S.-Y.; Bae, I.-H.; Han, Y.-H. Ionizing Radiation-Inducible MicroRNA MiR-193a-3p Induces Apoptosis by Directly Targeting Mcl-1. Apoptosis 2013, 18, 896–909. [Google Scholar] [CrossRef]
- Dickey, J.S.; Zemp, F.J.; Martin, O.A.; Kovalchuk, O. The Role of MiRNA in the Direct and Indirect Effects of Ionizing Radiation. Radiat. Environ. Biophys. 2011, 50, 491–499. [Google Scholar] [CrossRef]
- Rzeszowska-Wolny, J.; Hudy, D.; Biernacki, K.; Ciesielska, S.; Jaksik, R. Involvement of MiRNAs in Cellular Responses to Radiation. Int. J. Radiat. Biol. 2022, 98, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Wen, P.; Fu, Y.; Gao, Y.; Qi, X.; Chen, B.; Tao, Y.; Wu, L.; Xu, A.; Lu, H.; et al. Radiation Induces Apoptosis Primarily through the Intrinsic Pathway in Mammalian Cells. Cell. Signal. 2019, 62, 109337. [Google Scholar] [CrossRef] [PubMed]
- Trachootham, D.; Lu, W.; Ogasawara, M.A.; Valle, N.R.-D.; Huang, P. Redox Regulation of Cell Survival. Antioxid. Redox Signal. 2008, 10, 1343–1374. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-Y.; Wang, M.; Zuo, C.-Y.; Mao, M.-X.; Peng, X.-C.; Cai, J. Nrf-2 as a Novel Target in Radiation Induced Lung Injury. Heliyon 2024, 10, e29492. [Google Scholar] [CrossRef] [PubMed]
- Pecorelli, A.; Valacchi, G. Oxidative-Stress-Sensitive MicroRNAs in UV-Promoted Development of Melanoma. Cancers 2022, 14, 3224. [Google Scholar] [CrossRef] [PubMed]
- You, G.-R.; Cheng, A.-J.; Shen, E.Y.-L.; Fan, K.-H.; Huang, Y.-F.; Huang, Y.-C.; Chang, K.-P.; Chang, J.T. MiR-630 Promotes Radioresistance by Induction of Anti-Apoptotic Effect via Nrf2–GPX2 Molecular Axis in Head–Neck Cancer. Cells 2023, 12, 2853. [Google Scholar] [CrossRef]
- Qiu, J.; Fang, Y.; Xiao, S.; Zeng, F. AP2a-Mediated Upregulation of MiR-125a-5p Ameliorates Radiation-Induced Oxidative Stress Injury via BRD4/Nrf2/HO-1 Signaling. Radiat. Res. 2022, 199, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.-B.; Li, K.; Yi, N.; Li, X.; Wang, F.; Xue, B.; Pan, Y.; Yao, J.; Jiang, Q.; Wu, Z. MiRNA-141 Attenuates UV-Induced Oxidative Stress via Activating Keap1-Nrf2 Signaling in Human Retinal Pigment Epithelium Cells and Retinal Ganglion Cells. Oncotarget 2017, 8, 13186–13194. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Gupta, D.; Arora, R. NF-ΚB as a Key Player in Regulation of Cellular Radiation Responses and Identification of Radiation Countermeasures. Discoveries 2015, 3, e35. [Google Scholar] [CrossRef]
- Melnik, B.C.; John, S.M.; Carrera-Bastos, P.; Schmitz, G. MicroRNA-21-Enriched Exosomes as Epigenetic Regulators in Melanomagenesis and Melanoma Progression: The Impact of Western Lifestyle Factors. Cancers 2020, 12, 2111. [Google Scholar] [CrossRef]
- Cooper, S.; Bowden, G. Ultraviolet B Regulation of Transcription Factor Families: Roles of Nuclear Factor-Kappa B (NF-KB) and Activator Protein-1 (AP-1) in UVB-Induced Skin Carcinogenesis. Curr. Cancer Drug Targets 2007, 7, 325–334. [Google Scholar] [CrossRef]
- Yang, L.; Hu, Z.; Jin, Y.; Huang, N.; Xu, S. MiR-4497 Mediates Oxidative Stress and Inflammatory Injury in Keratinocytes Induced by Ultraviolet B Radiation through Regulating NF-ΚB Expression. Ital. J. Dermatol. Venereol. 2022, 157, 84–91. [Google Scholar] [CrossRef]
- Zhang, M.; Qian, J.; Chen, M.; Kong, S.; Lawrence, T.S. 361. Radiation-Induced Lung Apoptosis Is Mediated by TNF-Alpha Action. Mol. Ther. 2005, 11, S141. [Google Scholar] [CrossRef]
- Kura, B.; Kalocayova, B.; LeBaron, T.W.; Frimmel, K.; Buday, J.; Surovy, J.; Slezak, J. Regulation of MicroRNAs by Molecular Hydrogen Contributes to the Prevention of Radiation-Induced Damage in the Rat Myocardium. Mol. Cell. Biochem. 2019, 457, 61–72. [Google Scholar] [CrossRef]
- Kim, W.; Lee, S.; Seo, D.; Kim, D.; Kim, K.; Kim, E.; Kang, J.; Seong, K.M.; Youn, H.; Youn, B. Cellular Stress Responses in Radiotherapy. Cells 2019, 8, 1105. [Google Scholar] [CrossRef]
- Faraonio, R.; Vergara, P.; Di Marzo, D.; Pierantoni, M.G.; Napolitano, M.; Russo, T.; Cimino, F. P53 Suppresses the Nrf2-Dependent Transcription of Antioxidant Response Genes. J. Biol. Chem. 2006, 281, 39776–39784. [Google Scholar] [CrossRef]
- Qin, Y.; Zhao, J.; Min, X.; Wang, M.; Luo, W.; Wu, D.; Yan, Q.; Li, J.; Wu, X.; Zhang, J. MicroRNA-125b Inhibits Lens Epithelial Cell Apoptosis by Targeting P53 in Age-Related Cataract. Biochim. Biophys. Acta—Mol. Basis Dis. 2014, 1842, 2439–2447. [Google Scholar] [CrossRef]
- Liu, C.; Zhou, C.; Gao, F.; Cai, S.; Zhang, C.; Zhao, L.; Zhao, F.; Cao, F.; Lin, J.; Yang, Y.; et al. MiR-34a in Age and Tissue Related Radio-Sensitivity and Serum MiR-34a as a Novel Indicator of Radiation Injury. Int. J. Biol. Sci. 2011, 7, 221–233. [Google Scholar] [CrossRef]
- Klotz, L.-O.; Sánchez-Ramos, C.; Prieto-Arroyo, I.; Urbánek, P.; Steinbrenner, H.; Monsalve, M. Redox Regulation of FoxO Transcription Factors. Redox Biol. 2015, 6, 51–72. [Google Scholar] [CrossRef]
- Tseng, A.H.-H.; Wu, L.-H.; Shieh, S.-S.; Wang, D.L. SIRT3 Interactions with FOXO3 Acetylation, Phosphorylation and Ubiquitinylation Mediate Endothelial Cell Responses to Hypoxia. Biochem. J. 2014, 464, 157–168. [Google Scholar] [CrossRef]
- Guan, L.; Zhang, L.; Gong, Z.; Hou, X.; Xu, Y.; Feng, X.; Wang, H.; You, H. FoxO3 Inactivation Promotes Human Cholangiocarcinoma Tumorigenesis and Chemoresistance through Keap1-Nrf2 Signaling. Hepatology 2016, 63, 1914–1927. [Google Scholar] [CrossRef]
- Ambrogini, E.; Almeida, M.; Martin-Millan, M.; Paik, J.-H.; DePinho, R.A.; Han, L.; Goellner, J.; Weinstein, R.S.; Jilka, R.L.; O’Brien, C.A.; et al. FoxO-Mediated Defense against Oxidative Stress in Osteoblasts Is Indispensable for Skeletal Homeostasis in Mice. Cell Metab. 2010, 11, 136–146. [Google Scholar] [CrossRef]
- Zhu, L.; Xue, F.; Cui, Y.; Liu, S.; Li, G.; Li, J.; Guan, B.; Zeng, H.; Bian, W.; Yang, C.; et al. MiR-155-5p and MiR-760 Mediate Radiation Therapy Suppressed Malignancy of Non-small Cell Lung Cancer Cells. BioFactors 2019, 45, 393–400. [Google Scholar] [CrossRef]
- Wang, P.; Zhu, C.; Ma, M.; Chen, G.; Song, M.; Zeng, Z.; Lu, W.; Yang, J.; Wen, S.; Chiao, P.J.; et al. Micro-RNA-155 Is Induced by K-Ras Oncogenic Signal and Promotes ROS Stress in Pancreatic Cancer. Oncotarget 2015, 6, 21148–21158. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, X.; Dai, Y.; Niu, X.; Zhou, M. MiR-27a Downregulation Promotes Cutaneous Squamous Cell Carcinoma Progression via Targeting EGFR. Front. Oncol. 2020, 9, 1565. [Google Scholar] [CrossRef]
- Guttilla, I.K.; White, B.A. Coordinate Regulation of FOXO1 by MiR-27a, MiR-96, and MiR-182 in Breast Cancer Cells. J. Biol. Chem. 2009, 284, 23204–23216. [Google Scholar] [CrossRef]
- Chen, G.; Yu, L.; Dong, H.; Liu, Z.; Sun, Y. MiR-182 Enhances Radioresistance in Non-small Cell Lung Cancer Cells by Regulating FOXO3. Clin. Exp. Pharmacol. Physiol. 2019, 46, 137–143. [Google Scholar] [CrossRef]
- Pan, Z.; Dong, H.; Huang, N.; Fang, J. Oxidative Stress and Inflammation Regulation of Sirtuins: New Insights into Common Oral Diseases. Front. Physiol. 2022, 13, 953078. [Google Scholar] [CrossRef]
- Joo, H.-Y.; Woo, S.R.; Shen, Y.-N.; Yun, M.Y.; Shin, H.-J.; Park, E.-R.; Kim, S.-H.; Park, J.-E.; Ju, Y.-J.; Hong, S.H.; et al. SIRT1 Interacts with and Protects Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH) from Nuclear Translocation: Implications for Cell Survival after Irradiation. Biochem. Biophys. Res. Commun. 2012, 424, 681–686. [Google Scholar] [CrossRef]
- Lacombe, J.; Zenhausern, F. Emergence of MiR-34a in Radiation Therapy. Crit. Rev. Oncol. Hematol. 2017, 109, 69–78. [Google Scholar] [CrossRef]
- Feng, Z.; Zhang, C.; Wu, R.; Hu, W. Tumor Suppressor P53 Meets MicroRNAs. J. Mol. Cell Biol. 2011, 3, 44–50. [Google Scholar] [CrossRef]
- Nowicka, Z.; Tomasik, B.; Kozono, D.; Stawiski, K.; Johnson, T.; Haas-Kogan, D.; Ussowicz, M.; Chowdhury, D.; Fendler, W. Serum MiRNA-Based Signature Indicates Radiation Exposure and Dose in Humans: A Multicenter Diagnostic Biomarker Study. Radiother. Oncol. 2023, 185, 109731. [Google Scholar] [CrossRef]
- Sun, G.; Huang, D.; Li, K.; Jiang, Q. MicroRNA-4532 Inhibition Protects Human Lens Epithelial Cells from Ultra-Violet-Induced Oxidative Injury via Activating SIRT6-Nrf2 Signaling. Biochem. Biophys. Res. Commun. 2019, 514, 777–784. [Google Scholar] [CrossRef]
- Munshi, A.; Ramesh, R. Mitogen-Activated Protein Kinases and Their Role in Radiation Response. Genes Cancer 2013, 4, 401–408. [Google Scholar] [CrossRef]
- Mei, Y.; Bian, C.; Li, J.; Du, Z.; Zhou, H.; Yang, Z.; Zhao, R.C.H. MiR-21 Modulates the ERK–MAPK Signaling Pathway by Regulating SPRY2 Expression during Human Mesenchymal Stem Cell Differentiation. J. Cell. Biochem. 2013, 114, 1374–1384. [Google Scholar] [CrossRef]
- Mahmoudi, R.; Saidijam, M.; Nikzad, S.; Tapak, L.; Alvandi, M.; Afshar, S. Human Exposure to Low Dose Ionizing Radiation Affects MiR-21 and MiR-625 Expression Levels. Mol. Biol. Rep. 2022, 49, 1321–1327. [Google Scholar] [CrossRef]
- Lin, M.-Y.; Chang, Y.-C.; Wang, S.-Y.; Yang, M.-H.; Chang, C.-H.; Hsiao, M.; Kitsis, R.N.; Lee, Y.-J. OncomiR MiR-182-5p Enhances Radiosensitivity by Inhibiting the Radiation-Induced Antioxidant Effect through SESN2 in Head and Neck Cancer. Antioxidants 2021, 10, 1808. [Google Scholar] [CrossRef]
- Liu, Z.; Li, T.; Zhu, F.; Deng, S.; Li, X.; He, Y. Regulatory Roles of MiR-22/Redd1-Mediated Mitochondrial ROS and Cellular Autophagy in Ionizing Radiation-Induced BMSC Injury. Cell Death Dis. 2019, 10, 227. [Google Scholar] [CrossRef]
- Zhang, D.; Deng, J.; Wu, Y.; Tang, T.; Xiong, L.; Zheng, Y.; Xu, X. MicroRNA-223-3p Protect Against Radiation-Induced Cardiac Toxicity by Alleviating Myocardial Oxidative Stress and Programmed Cell Death via Targeting the AMPK Pathway. Front. Cell Dev. Biol. 2022, 9, 801661. [Google Scholar] [CrossRef]
- de Souza, M.G.; de Jesus, S.F.; Santos, E.M.; Gomes, E.S.B.; de Paulo Santiago Filho, A.; Santos, E.M.S.; da Silveira, L.H.; Santos, S.H.S.; de Paula, A.M.B.; Farias, L.C.; et al. Radiation Therapy Reduced Blood Levels of LDH, HIF-1α, and MiR-210 in OSCC. Pathol. Oncol. Res. 2020, 26, 433–442. [Google Scholar] [CrossRef] [PubMed]
- van Rooij, E.; Kauppinen, S. Development of MicroRNA Therapeutics Is Coming of Age. EMBO Mol. Med. 2014, 6, 851–864. [Google Scholar] [CrossRef]
- Iacomino, G. MiRNAs: The Road from Bench to Bedside. Genes 2023, 14, 314. [Google Scholar] [CrossRef]
- Bernardo, B.C.; Gregorevic, P.; Ritchie, R.H.; McMullen, J.R. Generation of MicroRNA-34 Sponges and Tough Decoys for the Heart: Developments and Challenges. Front. Pharmacol. 2018, 9, 1090. [Google Scholar] [CrossRef]
- Diener, C.; Keller, A.; Meese, E. Emerging Concepts of MiRNA Therapeutics: From Cells to Clinic. Trends Genet. 2022, 38, 613–626. [Google Scholar] [CrossRef]
- Dasgupta, I.; Chatterjee, A. Recent Advances in MiRNA Delivery Systems. Methods Protoc. 2021, 4, 10. [Google Scholar] [CrossRef]
- Hong, D.S.; Kang, Y.-K.; Borad, M.; Sachdev, J.; Ejadi, S.; Lim, H.Y.; Brenner, A.J.; Park, K.; Lee, J.-L.; Kim, T.-Y.; et al. Phase 1 Study of MRX34, a Liposomal MiR-34a Mimic, in Patients with Advanced Solid Tumours. Br. J. Cancer 2020, 122, 1630–1637. [Google Scholar] [CrossRef]
- Hanna, J.; Hossain, G.S.; Kocerha, J. The Potential for MicroRNA Therapeutics and Clinical Research. Front. Genet. 2019, 10, 478. [Google Scholar] [CrossRef]
- van der Ree, M.H.; de Vree, J.M.; Stelma, F.; Willemse, S.; van der Valk, M.; Rietdijk, S.; Molenkamp, R.; Schinkel, J.; van Nuenen, A.C.; Beuers, U.; et al. Safety, Tolerability, and Antiviral Effect of RG-101 in Patients with Chronic Hepatitis C: A Phase 1B, Double-Blind, Randomised Controlled Trial. Lancet 2017, 389, 709–717. [Google Scholar] [CrossRef]
- Gomez, I.G.; MacKenna, D.A.; Johnson, B.G.; Kaimal, V.; Roach, A.M.; Ren, S.; Nakagawa, N.; Xin, C.; Newitt, R.; Pandya, S.; et al. Anti–MicroRNA-21 Oligonucleotides Prevent Alport Nephropathy Progression by Stimulating Metabolic Pathways. J. Clin. Investig. 2015, 125, 141–156. [Google Scholar] [CrossRef]
- Gallant-Behm, C.L.; Piper, J.; Lynch, J.M.; Seto, A.G.; Hong, S.J.; Mustoe, T.A.; Maari, C.; Pestano, L.A.; Dalby, C.M.; Jackson, A.L.; et al. A MicroRNA-29 Mimic (Remlarsen) Represses Extracellular Matrix Expression and Fibroplasia in the Skin. J. Investig. Dermatol. 2019, 139, 1073–1081. [Google Scholar] [CrossRef]
- Anastasiadou, E.; Seto, A.G.; Beatty, X.; Hermreck, M.; Gilles, M.-E.; Stroopinsky, D.; Pinter-Brown, L.C.; Pestano, L.; Marchese, C.; Avigan, D.; et al. Cobomarsen, an Oligonucleotide Inhibitor of MiR-155, Slows DLBCL Tumor Cell Growth In Vitro and In Vivo. Clin. Cancer Res. 2021, 27, 1139–1149. [Google Scholar] [CrossRef]
- Täubel, J.; Hauke, W.; Rump, S.; Viereck, J.; Batkai, S.; Poetzsch, J.; Rode, L.; Weigt, H.; Genschel, C.; Lorch, U.; et al. Novel Antisense Therapy Targeting MicroRNA-132 in Patients with Heart Failure: Results of a First-in-Human Phase 1b Randomized, Double-Blind, Placebo-Controlled Study. Eur. Heart J. 2021, 42, 178–188. [Google Scholar] [CrossRef]
- van Zandwijk, N.; Pavlakis, N.; Kao, S.C.; Linton, A.; Boyer, M.J.; Clarke, S.; Huynh, Y.; Chrzanowska, A.; Fulham, M.J.; Bailey, D.L.; et al. Safety and Activity of MicroRNA-Loaded Minicells in Patients with Recurrent Malignant Pleural Mesothelioma: A First-in-Man, Phase 1, Open-Label, Dose-Escalation Study. Lancet Oncol. 2017, 18, 1386–1396. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, F. Comment on: “MicroRNA Mimics or Inhibitors as Antiviral Therapeutic Approaches Against COVID-19”. Drugs 2021, 81, 1691–1692. [Google Scholar] [CrossRef]
- Pan, W.; Chai, B.; Li, L.; Lu, Z.; Ma, Z. P53/MicroRNA-34 Axis in Cancer and Beyond. Heliyon 2023, 9, e15155. [Google Scholar] [CrossRef]
- Monahan, P.E.; Négrier, C.; Tarantino, M.; Valentino, L.A.; Mingozzi, F. Emerging Immunogenicity and Genotoxicity Considerations of Adeno-Associated Virus Vector Gene Therapy for Hemophilia. J. Clin. Med. 2021, 10, 2471. [Google Scholar] [CrossRef]
| miRNA | ROS-Producing Enzyme | Experimental Model | Reference |
|---|---|---|---|
| miRNA-124-5p | NOX2 | rats cerebral I/R injury | [42] |
| miRNA-34a | NOX2 | human glioma cells | [43] |
| miRNA-320 | NOX2 | ischemic cerebral neurons | [44] |
| miRNA-423-5p | NOX4 | mouse podocyte cells | [45] |
| miRNA-99a | NOX4 | lung adenocarcinoma cells | [46] |
| miRNA-182-5p | NOX4 | human lens epithelial cells | [47] |
| miRNA-200c | eNOS | HUVEC cells | [48] |
| miRNA-615-5p | eNOS | HUVEC cells | [49] |
| miRNA-155 | eNOS | HUVEC cells | [50] |
| miRNA | Antioxidant Enzyme | Experimental Model | Reference |
|---|---|---|---|
| miRNA-21 | SOD | human bronchial epithelial cells | [52] |
| miRNA-17-3p | SOD, GPx, TXNRD | prostate cancer cells | [53] |
| miRNA-23a | SOD | cardiomyocytes | [54] |
| miRNA-30b | CAT | human retinal pigment epithelial cell line | [55] |
| miRNA-551b | CAT | human lung cancer cells | [56] |
| miRNA-144 | GPx, GR | human neuroblastoma SH-SY5Y cells | [57] |
| miRNA-181a | GPx | cardiomyocytes | [58] |
| miRNA-214 | GR | liver cells | [59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kura, B.; Pavelkova, P.; Kalocayova, B.; Pobijakova, M.; Slezak, J. MicroRNAs as Regulators of Radiation-Induced Oxidative Stress. Curr. Issues Mol. Biol. 2024, 46, 7097-7113. https://doi.org/10.3390/cimb46070423
Kura B, Pavelkova P, Kalocayova B, Pobijakova M, Slezak J. MicroRNAs as Regulators of Radiation-Induced Oxidative Stress. Current Issues in Molecular Biology. 2024; 46(7):7097-7113. https://doi.org/10.3390/cimb46070423
Chicago/Turabian StyleKura, Branislav, Patricia Pavelkova, Barbora Kalocayova, Margita Pobijakova, and Jan Slezak. 2024. "MicroRNAs as Regulators of Radiation-Induced Oxidative Stress" Current Issues in Molecular Biology 46, no. 7: 7097-7113. https://doi.org/10.3390/cimb46070423






