Targeting β-Cell Plasticity: A Promising Approach for Diabetes Treatment
Abstract
1. Introduction
2. The Main Mechanisms Involved in β-Cell Function and Identity
2.1. Inflammation
2.2. Metabolic Stress
2.3. Age-Related Dynamics of β-Cell Dedifferentiation and Transdifferentiation
2.4. Sex-Dependent Variations in Pancreatic Islet Plasticity
2.5. The Impacts of Chemicals on β-Cell Plasticity
3. Molecular Pathways Involved in β-Cell Plasticity
3.1. ER Stress
3.2. Angiotensin Receptors
3.3. mTOR Pathway
3.4. Role of Ubiquitin–Proteasome System in the Pancreatic β-Cell Plasticity
4. Transcriptional Regulation in β-Cell Plasticity
5. Translational Control
6. Epigenetic-Related Targets
6.1. Histon Modifications
6.2. Histone Acetylation
6.3. Histone Methylation
6.4. DNA Methylation
6.5. Chromatin Remodeling
6.6. MicroRNAs in Pancreatic β-Cell Function and Identity
| miRNA | Affected Genes | De/Tra/Pro/Neo | Type of Impact | References |
|---|---|---|---|---|
| miRNA-148a | Pten, Ampk | De | P | [205] |
| microRNA-483 | Aldh1a3, Socs3 | De | N | [206,207,208] |
| miR-29-a | Cdc42, Irs1, AKT | De | P | [209,210] |
| Pro | N | |||
| miR-23a | SDF-1α | Tra | N | [211] |
| miR-24 | Ire1α, MafA | De | P | [212] |
| miR-7 | Pdx1, Isl1 | Neo | P | [213,214] |
| miR-25/miR-92b | Neurod1, Mcl1 | De | P | [215] |
| miR-9 | Stxbp1 | De/Tra | P | [216] |
| miR-96 | PAK1 | β-cell dysfunction | N | [217] |
| miR-33a-5p | Abca1 | De | Positive and negative | [218] |
| miR-302s | NeuroD1 and Kat2b | De | P | [203] |
| miR-203 | Zbtb20 | De | P | [219] |
| miR-802 | NeuroD1, Fzd5 | De | P | [220] |
| miR-30d | Socs3, Glp1r, Igf1r, MafA, Pdx1, Nkx6.1 | Tra | P | [221] |
| miR-212/132 | Fbw7 | Neo | P | [222] |
| miR-690 | Sox9, Ngn3, Pdx1 | Neo | N | [223] |
| miR-223 | FoxO1, Sox6 | Pro | P | [121] |
| miR-155 | Pdx1 | De | P | [224] |
| miR-153 | SNAREs, MafA, NeuroD1, Bcl2, Ero1lb, Igf1r, Irs2 | De | P | [225] |
| miR-320a | MafF | De | P | [226] |
| miR-17-92 cluster | Pten, Cdkn1a, p57, Bcl2L11 | Pro | P | [227] |
| miR-21 | Tgfb2, Fgfr3, Smad2 | De | P | [228,229] |
| miR-184-3p | Crtc1, Slc25A2 | De and loss | P | [230] |
| miR-199b-5p | Mlk3 | Pro | P | [231] |
| miR-212-5p | Sirt2 | Insulin secretion and function | N | [232] |
| miR-375 | Motn, Mapkap 1, Pdx1, Pdpk1, Notch2, Hnf1β, Cadm1, Pax6, Crem | De | N | [233,234,235,236,237,238,239] |
| Pro | P | |||
| Neo | P | |||
| miR-200 | Zeb1 | De | P | [240] |
| miR-146a | Numb | De | N | [241] |
| miR-195 | Mfn2 | De | P | [242] |
6.7. Long Non-Coding RNAs (LncRNAs)
7. Reprogramming and Neogenesis of β-Cells
8. Mitigation of β-Cell Dedifferentiation and Transdifferentiation
8.1. Dietary Supplements
8.2. Dietary Interventions and β-Cell Function, Dedifferentiation, and Transdifferentiation
8.3. Physical Activity and β-Cell Plasticity
8.4. Antidiabetic Medications
8.4.1. Glucagon-like Peptide-1 Receptor Agonist (GLP-1 RA)
8.4.2. Sodium–Glucose Co-Transporter 2 (SGLT2) Inhibitors
8.4.3. Dipeptidyl Peptidase-4 Inhibitor (DPP-4 Inhibitor)
8.5. Hormones
8.6. Natural Products
8.7. The Role of the Gut Microbiome in β-Cell Plasticity
8.8. Other Interventions
9. Advancements in Stem Cell-Based Therapies for Diabetes Mellitus
10. β-Cell Proliferation and Regeneration
10.1. The Suppression of Dual-Specificity Tyrosine-Regulated Kinase 1A (DYRK1A)
10.2. Glucagon Receptor Antagonism in β-Cell Regeneration
10.3. The Polyamine Biosynthesis Pathway
10.4. Other Pathways Involved in β-Cell Proliferation
11. Conclusions
12. Limitations
- Lack of review on experimental models: In this study, we did not review the experimental models and the methods used to model β-cell dedifferentiation and transdifferentiation. A comprehensive examination of the various approaches to model β-cell dedifferentiation and transdifferentiation in both in vitro and in vivo settings would be highly beneficial.
- Limited scope on cell plasticity: This review primarily focused on β-cell plasticity and did not cover the plasticities of other cell types. It would be valuable to briefly explore the plasticities of other cells such as hepatocytes, hepatic stellate cells, epidermal stem cells, melanocytes, astrocytes, intestinal stem cells, cardiac fibroblasts, club cells, podocytes, and others. Investigating the plasticities of these diverse cell types could offer insights into the broader applications and implications of cellular plasticity.
- Context of cell plasticity in other diseases: While this review centered on β-cell plasticity, it would have been beneficial to study cell plasticity in the context of other diseases such as liver diseases, neurodegenerative diseases, and cancer. Understanding how cellular plasticity manifests and contributes to the pathology of these diseases could inform the development of new therapeutic strategies and enhance our knowledge of disease progression and treatment.
13. Unanswered Questions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bensellam, M.; Jonas, J.-C.; Laybutt, D.R. Mechanisms of β-cell dedifferentiation in diabetes: Recent findings and future research directions. J. Endocrinol. 2018, 236, R109–R143. [Google Scholar] [CrossRef]
- Son, J.; Accili, D. Reversing pancreatic β-cell dedifferentiation in the treatment of type 2 diabetes. Exp. Mol. Med. 2023, 55, 1652–1658. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi-Gojani, E.; Kovalchuk, I.; Kovalchuk, O. Cannabinoids and terpenes for diabetes mellitus and its complications: From mechanisms to new therapies. Trends Endocrinol. Metab. 2022, 33, 828–849. [Google Scholar] [CrossRef] [PubMed]
- Tanday, N.; Tarasov, A.I.; Moffett, R.C.; Flatt, P.R.; Irwin, N. Pancreatic islet cell plasticity: Pathogenic or therapeutically exploitable? Diabetes Obes. Metab. 2024, 26, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Honzawa, N.; Fujimoto, K. The Plasticity of Pancreatic β-Cells. Metabolites 2021, 11, 218. [Google Scholar] [CrossRef]
- Khin, P.-P.; Lee, J.-H.; Jun, H.-S. A Brief Review of the Mechanisms of β-Cell Dedifferentiation in Type 2 Diabetes. Nutrients 2021, 13, 1593. [Google Scholar] [CrossRef] [PubMed]
- Cinti, F.; Bouchi, R.; Kim-Muller, J.Y.; Ohmura, Y.; Sandoval, P.R.; Masini, M.; Marselli, L.; Suleiman, M.; Ratner, L.E.; Marchetti, P.; et al. Evidence of β-Cell Dedifferentiation in Human Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2016, 101, 1044–1054. [Google Scholar] [CrossRef]
- Gao, T.; McKenna, B.; Li, C.; Reichert, M.; Nguyen, J.; Singh, T.; Yang, C.; Pannikar, A.; Doliba, N.; Zhang, T.; et al. Pdx1 Maintains β Cell Identity and Function by Repressing an α Cell Program. Cell Metab. 2014, 19, 259–271. [Google Scholar] [CrossRef]
- Zhou, Q.; Brown, J.; Kanarek, A.; Rajagopal, J.; Melton, D.A. In vivo reprogramming of adult pancreatic exocrine cells to β-cells. Nature 2008, 455, 627–632. [Google Scholar] [CrossRef]
- Eizirik, D.L.; Sammeth, M.; Bouckenooghe, T.; Bottu, G.; Sisino, G.; Igoillo-Esteve, M.; Ortis, F.; Santin, I.; Colli, M.L.; Barthson, J.; et al. The Human Pancreatic Islet Transcriptome: Expression of Candidate Genes for Type 1 Diabetes and the Impact of Pro-Inflammatory Cytokines. PLoS Genet. 2012, 8, e1002552. [Google Scholar] [CrossRef]
- Efrat, S. Beta-cell dedifferentiation in type 2 diabetes: Concise review. Stem Cells 2019, 37, 1267–1272. [Google Scholar] [CrossRef] [PubMed]
- Chikodili, I.M.; Chioma, I.I.; Chinwendu, N.M.; IfedibaluChukwu, E.I. In-silico study for African plants with possible beta-cell regeneration effect through inhibition of DYRK1A. Sci. Phytochem. 2022, 1, 13–28. [Google Scholar] [CrossRef]
- Hædersdal, S.; Andersen, A.; Knop, F.K.; Vilsbøll, T. Revisiting the role of glucagon in health, diabetes mellitus and other metabolic diseases. Nat. Rev. Endocrinol. 2023, 19, 321–335. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Gu, L.; Yang, J.; Yang, K.; Liu, J.; Le, Y.; Lang, S.; Wang, H.; Thai, D.; Yan, H.; et al. Antagonistic Glucagon Receptor Antibody Promotes α-Cell Proliferation and Increases β-Cell Mass in Diabetic Mice. iScience 2019, 16, 326–339. [Google Scholar] [CrossRef]
- Wei, T.; Cui, X.; Jiang, Y.; Wang, K.; Wang, D.; Li, F.; Lin, X.; Gu, L.; Yang, K.; Yang, J.; et al. Glucagon Acting at the GLP-1 Receptor Contributes to β-Cell Regeneration Induced by Glucagon Receptor Antagonism in Diabetic Mice. Diabetes 2023, 72, 599–610. [Google Scholar] [CrossRef]
- Xi, Y.; Song, B.; Ngan, I.; Solloway, M.J.; Humphrey, M.; Wang, Y.; Mondal, K.; Wu, H.; Liu, W.; Lindhout, D.A.; et al. Glucagon-receptor-antagonism-mediated β-cell regeneration as an effective anti-diabetic therapy. Cell Rep. 2022, 39, 110872. [Google Scholar] [CrossRef]
- Robertson, M.A.; Padgett, L.R.; Fine, J.A.; Chopra, G.; Mastracci, T.L. Targeting polyamine biosynthesis to stimulate beta cell regeneration in zebrafish. Islets 2020, 12, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Mezza, T.; Cinti, F.; Cefalo, C.M.A.; Pontecorvi, A.; Kulkarni, R.N.; Giaccari, A. β-Cell Fate in Human Insulin Resistance and Type 2 Diabetes: A Perspective on Islet Plasticity. Diabetes 2019, 68, 1121–1129. [Google Scholar] [CrossRef]
- Zhou, Q.; Melton, D.A. Pancreas regeneration. Nature 2018, 557, 351–358. [Google Scholar] [CrossRef]
- DiMeglio, L.A.; Evans-Molina, C.; Oram, R.A. Type 1 diabetes. Lancet 2018, 391, 2449–2462. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Ferrannini, E.; Groop, L.; Henry, R.R.; Herman, W.H.; Holst, J.J.; Hu, F.B.; Kahn, C.R.; Raz, I.; Shulman, G.I. Type 2 diabetes mellitus. Nat. Rev. Dis. Prim. 2015, 1, 1–22. [Google Scholar] [CrossRef]
- Ahlqvist, E.; Prasad, R.B.; Groop, L. Subtypes of Type 2 Diabetes Determined From Clinical Parameters. Diabetes 2020, 69, 2086–2093. [Google Scholar] [CrossRef]
- McIntyre, H.D.; Catalano, P.; Zhang, C.; Desoye, G.; Mathiesen, E.R.; Damm, P. Gestational diabetes mellitus. Nat. Rev. Dis. Prim. 2019, 5, 47. [Google Scholar] [CrossRef]
- Liang, R.; Liu, N.; Wang, G.; Sun, P.; Liu, Y.; Zou, J.; Wang, L.; Ding, X.; Zhang, B.; Shen, Z.; et al. Cytohistologic analyses of β cell dedifferentiation induced by inflammation in human islets. Eur. J. Inflamm. 2021, 19. [Google Scholar] [CrossRef]
- Nordmann, T.M.; Dror, E.; Schulze, F.; Traub, S.; Berishvili, E.; Barbieux, C.; Böni-Schnetzler, M.; Donath, M.Y. The Role of Inflammation in β-cell Dedifferentiation. Sci. Rep. 2017, 7, 6285. [Google Scholar] [CrossRef]
- Urizar, A.I.; Prause, M.; Ingerslev, L.R.; Wortham, M.; Sui, Y.; Sander, M.; Williams, K.; Barrès, R.; Larsen, M.R.; Christensen, G.L.; et al. Beta cell dysfunction induced by bone morphogenetic protein (BMP)-2 is associated with histone modifications and decreased NeuroD1 chromatin binding. Cell Death Dis. 2023, 14, 399. [Google Scholar] [CrossRef]
- Wang, H.-L.; Wang, L.; Zhao, C.-Y.; Lan, H.-Y. Role of TGF-Beta Signaling in Beta Cell Proliferation and Function in Diabetes. Biomolecules 2022, 12, 373. [Google Scholar] [CrossRef]
- Blum, B.; Roose, A.N.; Barrandon, O.; Maehr, R.; Arvanites, A.C.; Davidow, L.S.; Davis, J.C.; Peterson, Q.P.; Rubin, L.L.; Melton, D.A.; et al. Reversal of β cell de-differentiation by a small molecule inhibitor of the TGFβ pathway. eLife 2014, 3, e02809. [Google Scholar] [CrossRef]
- Qian, J.; Tao, D.; Shan, X.; Xiao, X.; Chen, C. Role of angiogenesis in beta-cell epithelial–mesenchymal transition in chronic pancreatitis-induced diabetes. Mod. Pathol. 2022, 102, 290–297. [Google Scholar] [CrossRef]
- Brown, M.L.; Andrzejewski, D.; Burnside, A.; Schneyer, A.L. Activin enhances α-to β-cell transdifferentiation as a source for β-cells in male FSTL3 knockout mice. Endocrinology 2016, 157, 1043–1054. [Google Scholar] [CrossRef]
- Chen, X.Y.; Shi, Y.X.; Huang, Y.P.; Ding, M.; Shen, Q.L.; Li, C.J.; Lin, J.N. SDF-1 inhibits the dedifferentiation of islet β cells in hyperglycaemia by up-regulating FoxO1 via binding to CXCR4. J. Cell. Mol. Med. 2022, 26, 750–763. [Google Scholar] [CrossRef]
- Bosma, K.J.; Andrei, S.R.; Katz, L.S.; Smith, A.A.; Dunn, J.C.; Ricciardi, V.F.; Ramirez, M.A.; Baumel-Alterzon, S.; Pace, W.A.; Carroll, D.T.; et al. Pharmacological blockade of the EP3 prostaglandin E2 receptor in the setting of type 2 diabetes enhances β-cell proliferation and identity and relieves oxidative damage. Mol. Metab. 2021, 54, 101347. [Google Scholar] [CrossRef]
- Moin, A.S.M.; Dhawan, S.; Shieh, C.; Butler, P.C.; Cory, M.; Butler, A.E. Increased Hormone-Negative Endocrine Cells in the Pancreas in Type 1 Diabetes. J. Clin. Endocrinol. Metab. 2016, 101, 3487–3496. [Google Scholar] [CrossRef]
- Michels, A.W.; Landry, L.G.; McDaniel, K.A.; Yu, L.; Campbell-Thompson, M.; Kwok, W.W.; Jones, K.L.; Gottlieb, P.A.; Kappler, J.W.; Tang, Q.; et al. Islet-Derived CD4 T Cells Targeting Proinsulin in Human Autoimmune Diabetes. Diabetes 2016, 66, 722–734. [Google Scholar] [CrossRef]
- Diedisheim, M.; Oshima, M.; Albagli, O.; Huldt, C.W.; Ahlstedt, I.; Clausen, M.; Menon, S.; Aivazidis, A.; Andreasson, A.-C.; Haynes, W.G.; et al. Modeling human pancreatic beta cell dedifferentiation. Mol. Metab. 2018, 10, 74–86. [Google Scholar] [CrossRef]
- Oshima, M.; Knoch, K.-P.; Diedisheim, M.; Petzold, A.; Cattan, P.; Bugliani, M.; Marchetti, P.; Choudhary, P.; Huang, G.-C.; Bornstein, S.R.; et al. Virus-like infection induces human β cell dedifferentiation. J. Clin. Investig. 2018, 3, e97732. [Google Scholar] [CrossRef]
- Thorel, F.; Népote, V.; Avril, I.; Kohno, K.; Desgraz, R.; Chera, S.; Herrera, P.L. Conversion of adult pancreatic α-cells to β-cells after extreme β-cell loss. Nature 2010, 464, 1149–1154. [Google Scholar] [CrossRef]
- Brissova, M.; Haliyur, R.; Saunders, D.; Shrestha, S.; Dai, C.; Blodgett, D.M.; Bottino, R.; Campbell-Thompson, M.; Aramandla, R.; Poffenberger, G.; et al. α Cell Function and Gene Expression Are Compromised in Type 1 Diabetes. Cell Rep. 2018, 22, 2667–2676. [Google Scholar] [CrossRef]
- Furth-Lavi, J.; Hija, A.; Tornovsky-Babeay, S.; Mazouz, A.; Dahan, T.; Stolovich-Rain, M.; Klochendler, A.; Dor, Y.; Avrahami, D.; Glaser, B. Glycemic control releases regenerative potential of pancreatic beta cells blocked by severe hyperglycemia. Cell Rep. 2022, 41, 111719. [Google Scholar] [CrossRef]
- Hahn, M.; van Krieken, P.P.; Nord, C.; Alanentalo, T.; Morini, F.; Xiong, Y.; Eriksson, M.; Mayer, J.; Kostromina, E.; Ruas, J.L.; et al. Topologically selective islet vulnerability and self-sustained downregulation of markers for β-cell maturity in streptozotocin-induced diabetes. Commun. Biol. 2020, 3, 541. [Google Scholar] [CrossRef]
- Katz, L.S.; Brill, G.; Zhang, P.; Kumar, A.; Baumel-Alterzon, S.; Honig, L.B.; Gómez-Banoy, N.; Karakose, E.; Tanase, M.; Doridot, L.; et al. Maladaptive positive feedback production of ChREBPβ underlies glucotoxic β-cell failure. Nat. Commun. 2022, 13, 4423. [Google Scholar] [CrossRef]
- Oshima, M.; Pechberty, S.; Bellini, L.; Göpel, S.O.; Campana, M.; Rouch, C.; Dairou, J.; Cosentino, C.; Fantuzzi, F.; Toivonen, S.; et al. Stearoyl CoA desaturase is a gatekeeper that protects human beta cells against lipotoxicity and maintains their identity. Diabetologia 2019, 63, 395–409. [Google Scholar] [CrossRef]
- Dobosz, A.M.; Janikiewicz, J.; Krogulec, E.; Dziewulska, A.; Ajduk, A.; Szpila, M.; Nieznańska, H.; Szczepankiewicz, A.A.; Wypych, D.; Dobrzyn, A. Inhibition of stearoyl-CoA desaturase 1 in the mouse impairs pancreatic islet morphogenesis and promotes loss of β-cell identity and α-cell expansion in the mature pancreas. Mol. Metab. 2023, 67, 101659. [Google Scholar] [CrossRef]
- Leenders, F.; Groen, N.; de Graaf, N.; Engelse, M.A.; Rabelink, T.J.; de Koning, E.J.P.; Carlotti, F. Oxidative Stress Leads to β-Cell Dysfunction Through Loss of β-Cell Identity. Front. Immunol. 2021, 12, 690379. [Google Scholar] [CrossRef]
- Motomura, K.; Matsuzaka, T.; Shichino, S.; Ogawa, T.; Pan, H.; Nakajima, T.; Asano, Y.; Okayama, T.; Takeuchi, T.; Ohno, H.; et al. Single-Cell Transcriptome Profiling of Pancreatic Islets From Early Diabetic Mice Identifies Anxa10 for Ca2+ Allostasis Toward β-Cell Failure. Diabetes 2023, 73, 75–92. [Google Scholar] [CrossRef]
- Dadheech, N.; Srivastava, A.; Shah, R.G.; Shah, G.M.; Gupta, S. Role of poly(ADP-ribose) polymerase-1 in regulating human islet cell differentiation. Sci. Rep. 2022, 12, 21496. [Google Scholar] [CrossRef]
- Liu, B.; Hua, D.; Shen, L.; Li, T.; Tao, Z.; Fu, C.; Tang, Z.; Yang, J.; Zhang, L.; Nie, A.; et al. NPC1 is required for postnatal islet β cell differentiation by maintaining mitochondria turnover. Theranostics 2024, 14, 2058–2074. [Google Scholar] [CrossRef]
- Yeh, Y.-T.; Sona, C.; Yan, X.; Li, Y.; Pathak, A.; McDermott, M.I.; Xie, Z.; Liu, L.; Arunagiri, A.; Wang, Y.; et al. Restoration of PITPNA in Type 2 diabetic human islets reverses pancreatic beta-cell dysfunction. Nat. Commun. 2023, 14, 4250. [Google Scholar] [CrossRef]
- Song, J.; Ni, Q.; Sun, J.; Xie, J.; Liu, J.; Ning, G.; Wang, W.; Wang, Q. Aging Impairs Adaptive Unfolded Protein Response and Drives Beta Cell Dedifferentiation in Humans. J. Clin. Endocrinol. Metab. 2022, 107, 3231–3241. [Google Scholar] [CrossRef] [PubMed]
- Téllez, N.; Vilaseca, M.; Martí, Y.; Pla, A.; Montanya, E. β-Cell dedifferentiation, reduced duct cell plasticity, and impaired β-cell mass regeneration in middle-aged rats. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E554–E563. [Google Scholar] [CrossRef] [PubMed]
- Murao, N.; Yokoi, N.; Takahashi, H.; Hayami, T.; Minami, Y.; Seino, S. Increased glycolysis affects β-cell function and identity in aging and diabetes. Mol. Metab. 2021, 55, 101414. [Google Scholar] [CrossRef] [PubMed]
- Tanday, N.; Coulter-Parkhill, A.; Moffett, R.C.; Suruli, K.; Dubey, V.; Flatt, P.R.; Irwin, N. Sex-based impact of pancreatic islet stressors in GluCreERT2/Rosa26-eYFP mice. J. Endocrinol. 2023, 259, e230174. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.C.; Wu, S.Y.; Leung, P.S. Alcohol ingestion induces pancreatic islet dysfunction and apoptosis via mediation of FGF21 resistance. Ann. Transl. Med. 2020, 8, 310. [Google Scholar] [CrossRef] [PubMed]
- Rasineni, K.; Kubik, J.L.; Knight, K.L.; Hall, L.; Casey, C.A.; Kharbanda, K.K. Ghrelin regulates adipose tissue metabolism: Role in hepatic steatosis. Chem. Interactions 2020, 322, 109059. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Ravazzola, M.; Park, B.-H.; Bashmakov, Y.K.; Orci, L.; Unger, R.H. Metabolic mechanisms of failure of intraportally transplanted pancreatic β-cells in rats: Role of lipotoxicity and prevention by leptin. Diabetes 2007, 56, 2295–2301. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Holland, W.L.; Miller, R.A.; Wang, Z.V.; Sun, K.; Barth, B.M.; Bui, H.H.; Davis, K.E.; Bikman, B.T.; Halberg, N.; Rutkowski, J.M.; et al. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat. Med. 2011, 17, 55–63. [Google Scholar] [CrossRef]
- Dufrane, D.; van Steenberghe, M.; Guiot, Y.; Goebbels, R.-M.; Saliez, A.; Gianello, P. Streptozotocin-Induced Diabetes in Large Animals (Pigs/Primates): Role of GLUT2 Transporter and β-cell Plasticity. Transplantation 2006, 81, 36–45. [Google Scholar] [CrossRef]
- Feng, Y.; Qiu, W.-L.; Yu, X.-X.; Zhang, Y.; He, M.-Y.; Li, L.-C.; Yang, L.; Zhang, W.; Franti, M.; Ye, J. Characterizing pancreatic β-cell heterogeneity in the streptozotocin model by single-cell transcriptomic analysis. Mol. Metab. 2020, 37, 100982. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Kang, H.; Shen, J.; Hao, H.; Liu, J.; Guo, Y.; Mu, Y.; Han, W. Beta-cell regeneration from vimentin+/MafB+ cells after STZ-induced extreme beta-cell ablation. Sci. Rep. 2015, 5, 11703. [Google Scholar] [CrossRef]
- Rafacho, A.; Ortsäter, H.; Nadal, A.; Quesada, I. Glucocorticoid treatment and endocrine pancreas function: Implications for glucose homeostasis, insulin resistance and diabetes. J. Endocrinol. 2014, 223, R49–R62. [Google Scholar] [CrossRef]
- Esguerra, J.L.; Ofori, J.K.; Nagao, M.; Shuto, Y.; Karagiannopoulos, A.; Fadista, J.; Sugihara, H.; Groop, L.; Eliasson, L. Glucocorticoid induces human beta cell dysfunction by involving riborepressor GAS5 LincRNA. Mol. Metab. 2020, 32, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Beaupere, C.; Liboz, A.; Fève, B.; Blondeau, B.; Guillemain, G. Molecular Mechanisms of Glucocorticoid-Induced Insulin Resistance. Int. J. Mol. Sci. 2021, 22, 623. [Google Scholar] [CrossRef] [PubMed]
- Valtat, B.; Dupuis, C.; Zenaty, D.; Singh-Estivalet, A.; Tronche, F.; Bréant, B.; Blondeau, B. Genetic evidence of the programming of beta cell mass and function by glucocorticoids in mice. Diabetologia 2010, 54, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Pinna, J.; Sempere-Navarro, R.; Medina-Gali, R.M.; Fuentes, E.; Quesada, I.; Sargis, R.M.; Trasande, L.; Nadal, A. Endocrine disruptors in plastics alter β-cell physiology and increase the risk of diabetes mellitus. Am. J. Physiol.-Endocrinol. Metab. 2023, 324, E488–E505. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, R.S.; Medina-Gali, R.M.; Babiloni-Chust, I.; Marroqui, L.; Nadal, A. In Vitro Assays to Identify Metabolism-Disrupting Chemicals with Diabetogenic Activity in a Human Pancreatic β-Cell Model. Int. J. Mol. Sci. 2022, 23, 5040. [Google Scholar] [CrossRef] [PubMed]
- Sidarala, V.; Zhu, J.; Levi-D’ancona, E.; Pearson, G.L.; Reck, E.C.; Walker, E.M.; Kaufman, B.A.; Soleimanpour, S.A. Mitofusin 1 and 2 regulation of mitochondrial DNA content is a critical determinant of glucose homeostasis. Nat. Commun. 2022, 13, 2340. [Google Scholar] [CrossRef] [PubMed]
- Barazzuol, L.; Giamogante, F.; Calì, T. Mitochondria Associated Membranes (MAMs): Architecture and physiopathological role. Cell Calcium 2021, 94, 102343. [Google Scholar] [CrossRef] [PubMed]
- Boronat-Belda, T.; Ferrero, H.; Al-Abdulla, R.; Quesada, I.; Gustafsson, J.-A.; Nadal, Á.; Alonso-Magdalena, P. Bisphenol-A exposure during pregnancy alters pancreatic β-cell division and mass in male mice offspring: A role for ERβ. Food Chem. Toxicol. 2020, 145, 111681. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, L.F.; Masiero, M.M.; Cherkaoui, S.; Carneiro, M.H.; Barbosa Jr, F.; Zamboni, N. The alternative analog plasticizer BPS displays similar phenotypic and metabolomic responses to BPA in HepG2 and INS-1E cells. Food Chem. Toxicol. 2022, 167, 113266. [Google Scholar] [CrossRef]
- García-Arévalo, M.; Alonso-Magdalena, P.; Servitja, J.-M.; Boronat-Belda, T.; Merino, B.; Villar-Pazos, S.; Medina-Gómez, G.; Novials, A.; Quesada, I.; Nadal, A. Maternal Exposure to Bisphenol-A During Pregnancy Increases Pancreatic β-Cell Growth During Early Life in Male Mice Offspring. Endocrinology 2016, 157, 4158–4171. [Google Scholar] [CrossRef]
- Bansal, A.; Rashid, C.; Xin, F.; Li, C.; Polyak, E.; Duemler, A.; van der Meer, T.; Stefaniak, M.; Wajid, S.; Doliba, N.; et al. Sex- and Dose-Specific Effects of Maternal Bisphenol A Exposure on Pancreatic Islets of First- and Second-Generation Adult Mice Offspring. Environ. Heal. Perspect. 2017, 125, 097022. [Google Scholar] [CrossRef]
- Yang, R.; Zheng, J.; Qin, J.; Liu, S.; Liu, X.; Gu, Y.; Yang, S.; Du, J.; Li, S.; Chen, B.; et al. Dibutyl phthalate affects insulin synthesis and secretion by regulating the mitochondrial apoptotic pathway and oxidative stress in rat insulinoma cells. Ecotoxicol. Environ. Saf. 2023, 249, 114396. [Google Scholar] [CrossRef]
- Brusco, N.; Sebastiani, G.; Di Giuseppe, G.; Licata, G.; Grieco, G.E.; Fignani, D.; Nigi, L.; Formichi, C.; Aiello, E.; Auddino, S.; et al. Intra-islet insulin synthesis defects are associated with endoplasmic reticulum stress and loss of beta cell identity in human diabetes. Diabetologia 2022, 66, 354–366. [Google Scholar] [CrossRef] [PubMed]
- Rewers, M.; Ludvigsson, J. Environmental risk factors for type 1 diabetes. Lancet 2016, 387, 2340–2348. [Google Scholar] [CrossRef]
- Almanza, A.; Carlesso, A.; Chintha, C.; Creedican, S.; Doultsinos, D.; Leuzzi, B.; Luís, A.; McCarthy, N.; Montibeller, L.; More, S.; et al. Endoplasmic reticulum stress signalling—From basic mechanisms to clinical applications. FEBS J. 2018, 286, 241–278. [Google Scholar] [CrossRef]
- Chen, C.-W.; Guan, B.-J.; Alzahrani, M.R.; Gao, Z.; Gao, L.; Bracey, S.; Wu, J.; Mbow, C.A.; Jobava, R.; Haataja, L.; et al. Adaptation to chronic ER stress enforces pancreatic β-cell plasticity. Nat. Commun. 2022, 13, 4621. [Google Scholar] [CrossRef]
- Lee, H.; Lee, Y.-S.; Harenda, Q.; Pietrzak, S.; Oktay, H.Z.; Schreiber, S.; Liao, Y.; Sonthalia, S.; Ciecko, A.E.; Chen, Y.-G.; et al. Beta Cell Dedifferentiation Induced by IRE1α Deletion Prevents Type 1 Diabetes. Cell Metab. 2020, 31, 822–836.e5. [Google Scholar] [CrossRef]
- Lee, K.; Chan, J.Y.; Liang, C.; Ip, C.K.; Shi, Y.-C.; Herzog, H.; Hughes, W.E.; Bensellam, M.; Delghingaro-Augusto, V.; Koina, M.E.; et al. XBP1 maintains beta cell identity, represses beta-to-alpha cell transdifferentiation and protects against diabetic beta cell failure during metabolic stress in mice. Diabetologia 2022, 65, 984–996. [Google Scholar] [CrossRef] [PubMed]
- Kitakaze, K.; Oyadomari, M.; Zhang, J.; Hamada, Y.; Takenouchi, Y.; Tsuboi, K.; Inagaki, M.; Tachikawa, M.; Fujitani, Y.; Okamoto, Y.; et al. ATF4-mediated transcriptional regulation protects against β-cell loss during endoplasmic reticulum stress in a mouse model. Mol. Metab. 2021, 54, 101338. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Ryu, H.; Lee, H.; Yu, H.R.; Gao, Y.; Lee, K.-M.; Kim, Y.-J.; Lee, J. Endoplasmic reticulum stress in pancreatic β cells induces incretin desensitization and β-cell dysfunction via ATF4-mediated PDE4D expression. Am. J. Physiol. Endocrinol. Metab. 2023, 325, E448–E465. [Google Scholar] [CrossRef]
- Kulkarni, A.; Muralidharan, C.; May, S.C.; Tersey, S.A.; Mirmira, R.G. Inside the β Cell: Molecular Stress Response Pathways in Diabetes Pathogenesis. Endocrinology 2022, 164, bqac184. [Google Scholar] [CrossRef] [PubMed]
- Pakos-Zebrucka, K.; Koryga, I.; Mnich, K.; Ljujic, M.; Samali, A.; Gorman, A.M. The integrated stress response. EMBO Rep. 2016, 17, 1374–1395. [Google Scholar] [CrossRef] [PubMed]
- Koromilas, A.E. M(en)TORship lessons on life and death by the integrated stress response. Biochim. Biophys. Acta (BBA) Gen. Subj. 2019, 1863, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Zaborske, J.M.; Narasimhan, J.; Jiang, L.; Wek, S.A.; Dittmar, K.A.; Freimoser, F.; Pan, T.; Wek, R.C. Genome-wide Analysis of tRNA Charging and Activation of the eIF2 Kinase Gcn2p. J. Biol. Chem. 2009, 284, 25254–25267. [Google Scholar] [CrossRef]
- Kilberg, M.S.; Shan, J.; Su, N. ATF4-dependent transcription mediates signaling of amino acid limitation. Trends Endocrinol. Metab. 2009, 20, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Kanno, A.; Asahara, S.-I.; Furubayashi, A.; Masuda, K.; Yoshitomi, R.; Suzuki, E.; Takai, T.; Kimura-Koyanagi, M.; Matsuda, T.; Bartolome, A.; et al. GCN2 regulates pancreatic β cell mass by sensing intracellular amino acid levels. J. Clin. Investig. 2020, 5, e128820. [Google Scholar] [CrossRef] [PubMed]
- Darden, C.M.; Vasu, S.; Mattke, J.; Liu, Y.; Rhodes, C.J.; Naziruddin, B.; Lawrence, M.C. Calcineurin/NFATc2 and PI3K/AKT signaling maintains β-cell identity and function during metabolic and inflammatory stress. iScience 2022, 25, 104125. [Google Scholar] [CrossRef]
- Ramalingam, L.; Sopontammarak, B.; Menikdiwela, K.R.; Moustaid-Moussa, N. Endoplasmic Reticulum (ER) Stress in Part Mediates Effects of Angiotensin II in Pancreatic Beta Cells. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 2843–2853. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, W.; Ruan, Y.; Yang, L.; Xu, N.; Chen, R.; Yang, R.; Sun, J.; Zhang, Z. Reversal of angiotensin ll-induced β-cell dedifferentiation via inhibition of NF-κb signaling. Mol. Med. 2018, 24, 43. [Google Scholar] [CrossRef]
- Li, M.-Y.; Liu, L.-Z.; Xin, Q.; Zhou, J.; Zhang, X.; Zhang, R.; Wu, Z.; Yi, J.; Dong, M. Downregulation of mTORC1 and Mcl-1 by lipid-oversupply contributes to islet β-cell apoptosis and dysfunction. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2023, 1868, 159332. [Google Scholar] [CrossRef]
- Cai, Z.; Liu, F.; Yang, Y.; Li, D.; Hu, S.; Song, L.; Yu, S.; Li, T.; Liu, B.; Luo, H. GRB10 regulates β-cell mass by inhibiting β-cell proliferation and stimulating β-cell dedifferentiation. J. Genet. Genom. 2022, 49, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Ni, Q.; Wang, Y.; Zhang, H.; Li, W.; Nie, A.; Wang, S.; Gu, Y.; Wang, Q.; Ning, G. Raptor determines β-cell identity and plasticity independent of hyperglycemia in mice. Nat. Commun. 2020, 11, 2538. [Google Scholar] [CrossRef] [PubMed]
- Ni, Q.; Sun, J.; Wang, Y.; Wang, Y.; Liu, J.; Ning, G.; Wang, W.; Wang, Q. mTORC1 is required for epigenetic silencing during β-cell functional maturation. Mol. Metab. 2022, 64, 101559. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Li, T.; Xie, Y.; Yang, J.; Fu, C.; Qiu, Y.; Shen, L.; Ni, Q.; Wang, Q.; Nie, A. Enhancing Acsl4 in absence of mTORC2/Rictor drove β-cell dedifferentiation via inhibiting FoxO1 and promoting ROS production. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2021, 1867, 166261. [Google Scholar] [CrossRef] [PubMed]
- Lupse, B.; Annamalai, K.; Ibrahim, H.; Kaur, S.; Geravandi, S.; Sarma, B.; Pal, A.; Awal, S.; Joshi, A.; Rafizadeh, S. Inhibition of PHLPP1/2 phosphatases rescues pancreatic β-cells in diabetes. Cell Rep. 2021, 36, 109490. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, P.; Zhao, Y.; Lai, K.P.; Li, R. Biomedical importance of the ubiquitin–proteasome system in diabetes and metabolic transdifferentiation of pancreatic duct epithelial cells into β-cells. Gene 2023, 858, 147191. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Francis, M.; Bhaskar, S.; Vishnuvajhala, S.; Prasanna, J. Dynamics of Ubiquitination in Differentiation and Dedifferentiation of Pancreatic β-cells: Putative Target for Diabetes. Curr. Protein Pept. Sci. 2022, 23, 602–618. [Google Scholar] [CrossRef]
- Francis, M.; Bhaskar, S.; Komanduri, S.; Sheshadri, P.; Prasanna, J.; Kumar, A. Deubiquitinase USP1 influences the dedifferentiation of mouse pancreatic β-cells. iScience 2023, 26, 106771. [Google Scholar] [CrossRef]
- Manea, T.; Nelson, J.K.; Garrone, C.M.; Hansson, K.; Evans, I.; Behrens, A.; Sancho, R. USP7 controls NGN3 stability and pancreatic endocrine lineage development. Nat. Commun. 2023, 14, 2457. [Google Scholar] [CrossRef]
- Cho, G.; Hyun, K.; Choi, J.; Shin, E.; Kim, B.; Kim, H.; Kim, J.; Han, Y.-M. Arginine 65 methylation of Neurogenin 3 by PRMT1 is required for pancreatic endocrine development of hESCs. Exp. Mol. Med. 2023, 55, 1506–1519. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, H.; Wang, T.; Wei, C.; Jiang, H.; Jiang, S.; Yang, J.; Shao, J.; Ma, L. Pax4 synergistically acts with Pdx1, Ngn3 and MafA to induce HuMSCs to differentiate into functional pancreatic β-cells. Exp. Ther. Med. 2019, 18, 2592–2598. [Google Scholar] [CrossRef]
- Lorenzo, P.I.; Juárez-Vicente, F.; Cobo-Vuilleumier, N.; García-Domínguez, M.; Gauthier, B.R. The Diabetes-Linked Transcription Factor PAX4: From Gene to Functional Consequences. Genes 2017, 8, 101. [Google Scholar] [CrossRef] [PubMed]
- Brun, T.; Franklin, I.; St-Onge, L.; Biason-Lauber, A.; Schoenle, E.J.; Wollheim, C.B.; Gauthier, B.R. The diabetes-linked transcription factor PAX4 promotes β-cell proliferation and survival in rat and human islets. J. Cell Biol. 2004, 167, 1123–1135. [Google Scholar] [CrossRef]
- Parajuli, K.R.; Zhang, Y.; Cao, A.M.; Wang, H.; Fonseca, V.A.; Wu, H. Pax4 Gene Delivery Improves Islet Transplantation Efficacy by Promoting β Cell Survival and α-to-β Cell Transdifferentiation. Cell Transplant. 2020, 29. [Google Scholar] [CrossRef]
- Auerbach, A.; Cohen, A.; Shlomai, N.O.; Weinberg-Shukron, A.; Gulsuner, S.; King, M.-C.; Hemi, R.; Levy-Lahad, E.; Abulibdeh, A.; Zangen, D. NKX2-2 Mutation Causes Congenital Diabetes and Infantile Obesity With Paradoxical Glucose-Induced Ghrelin Secretion. J. Clin. Endocrinol. Metab. 2020, 105, 3486–3495. [Google Scholar] [CrossRef]
- Doyle, M.J.; Sussel, L. Nkx2. 2 regulates β-cell function in the mature islet. Diabetes 2007, 56, 1999–2007. [Google Scholar] [CrossRef]
- Papizan, J.B.; Singer, R.A.; Tschen, S.-I.; Dhawan, S.; Friel, J.M.; Hipkens, S.B.; Magnuson, M.A.; Bhushan, A.; Sussel, L. Nkx2. 2 repressor complex regulates islet β-cell specification and prevents β-to-α-cell reprogramming. Genes Dev. 2011, 25, 2291–2305. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.R.; Torres, C.A.; Solomon, K.; Becker, T.C.; Newgard, C.B.; Wright, C.V.; Hagman, J.; Sussel, L. Cooperative Transcriptional Regulation of the Essential Pancreatic Islet Gene NeuroD1 (Beta2) by Nkx2.2 and Neurogenin 3. J. Biol. Chem. 2009, 284, 31236–31248. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, G.D.; Bender, A.S.; Cirulli, V.; Mastracci, T.L.; Kelly, S.M.; Tsirigos, A.; Kaestner, K.H.; Sussel, L. Pancreatic β cell identity requires continual repression of non–β cell programs. J. Clin. Investig. 2017, 127, 244–259. [Google Scholar] [CrossRef]
- Fontcuberta-PiSunyer, M.; García-Alamán, A.; Prades, È.; Téllez, N.; Alves-Figueiredo, H.; Ramos-Rodríguez, M.; Enrich, C.; Fernandez-Ruiz, R.; Cervantes, S.; Clua, L.; et al. Direct reprogramming of human fibroblasts into insulin-producing cells using transcription factors. Commun. Biol. 2023, 6, 256. [Google Scholar] [CrossRef]
- Pan, F.C.; Brissova, M.; Powers, A.C.; Pfaff, S.; Wright, C.V.E. Inactivating the permanent neonatal diabetes gene Mnx1 switches insulin-producing β-cells to a δ-like fate and reveals a facultative proliferative capacity in aged β-cells. Development 2015, 142, 3637–3648. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, S.E.; De Franco, E.; Allen, H.L.; Zerah, M.; Abdul-Rasoul, M.M.; Edge, J.A.; Stewart, H.; Alamiri, E.; Hussain, K.; Wallis, S.; et al. Analysis of Transcription Factors Key for Mouse Pancreatic Development Establishes NKX2-2 and MNX1 Mutations as Causes of Neonatal Diabetes in Man. Cell Metab. 2014, 19, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Arkhipova, V.; Wendik, B.; Devos, N.; Ek, O.; Peers, B.; Meyer, D. Characterization and regulation of the hb9/mnx1 beta-cell progenitor specific enhancer in zebrafish. Dev. Biol. 2012, 365, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Dalgin, G.; Ward, A.B.; Hao, L.T.; Beattie, C.E.; Nechiporuk, A.; Prince, V.E. Zebrafish mnx1 controls cell fate choice in the developing endocrine pancreas. Development 2011, 138, 4597–4608. [Google Scholar] [CrossRef] [PubMed]
- Dalgin, G.; Prince, V.E. Mnx1: A gatekeeper of β cell fate. Islets 2012, 4, 320–322. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, X.; Wan, T.; Li, Y. Role of FoxO1 in regulating autophagy in type 2 diabetes mellitus (Review). Exp. Ther. Med. 2021, 22, 707. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, Y.I.; Kitamura, T.; Kruse, J.-P.; Raum, J.C.; Stein, R.; Gu, W.; Accili, D. FoxO1 protects against pancreatic β cell failure through NeuroD and MafA induction. Cell Metab. 2005, 2, 153–163. [Google Scholar] [CrossRef]
- Casteels, T.; Zhang, Y.; Frogne, T.; Sturtzel, C.; Lardeau, C.-H.; Sen, I.; Liu, X.; Hong, S.; Pauler, F.M.; Penz, T.; et al. An inhibitor-mediated beta-cell dedifferentiation model reveals distinct roles for FoxO1 in glucagon repression and insulin maturation. Mol. Metab. 2021, 54, 101329. [Google Scholar] [CrossRef]
- Khatri, R.; Mazurek, S.; Petry, S.F.; Linn, T. Mesenchymal stem cells promote pancreatic β-cell regeneration through downregulation of FoxO1 pathway. Stem Cell Res. Ther. 2020, 11, 497. [Google Scholar] [CrossRef]
- Nie, Y.; Zhang, Y.; Liu, S.; Xu, Z.; Xia, C.; Du, L.; Yin, X.; Wang, J. Downregulation of Sirt3 contributes to β-cell dedifferentiation via FoxO1 in type 2 diabetic mellitus. Acta Diabetol. 2023, 61, 485–494. [Google Scholar] [CrossRef]
- Li, Y.; Deng, S.; Peng, J.; Wang, X.; Essandoh, K.; Mu, X.; Peng, T.; Meng, Z.-X.; Fan, G.-C. MicroRNA-223 is essential for maintaining functional β-cell mass during diabetes through inhibiting both FOXO1 and SOX6 pathways. J. Biol. Chem. 2019, 294, 10438–10448. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Xin, Y.; Yang, M.; Zhang, D.; Xu, C. Osteoprotegerin promotes islet β cell proliferation in intrauterine growth retardation rats through the PI3K/AKT/FoxO1 pathway. Int. J. Clin. Exp. Pathol. 2019, 12, 2324. [Google Scholar] [PubMed]
- Wang, K.; Cui, X.; Li, F.; Xia, L.; Wei, T.; Liu, J.; Fu, W.; Yang, J.; Hong, T.; Wei, R. Glucagon receptor blockage inhibits β-cell dedifferentiation through FoxO1. Am. J. Physiol. Metab. 2023, 324, E97–E113. [Google Scholar] [CrossRef] [PubMed]
- Ebrahim, N.; Shakirova, K.; Dashinimaev, E. PDX1 is the cornerstone of pancreatic β-cell functions and identity. Front. Mol. Biosci. 2022, 9, 1091757. [Google Scholar] [CrossRef]
- Chen, F.; Sha, M.; Wang, Y.; Wu, T.; Shan, W.; Liu, J.; Zhou, W.; Zhu, Y.; Sun, Y.; Shi, Y.; et al. Transcription factor Ets-1 links glucotoxicity to pancreatic beta cell dysfunction through inhibiting PDX-1 expression in rodent models. Diabetologia 2015, 59, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fang, X.; Wei, J.; Miao, R.; Wu, H.; Ma, K.; Tian, J. PDX-1: A Promising Therapeutic Target to Reverse Diabetes. Biomolecules 2022, 12, 1785. [Google Scholar] [CrossRef] [PubMed]
- Weidemann, B.J.; Marcheva, B.; Kobayashi, M.; Omura, C.; Newman, M.V.; Kobayashi, Y.; Waldeck, N.J.; Perelis, M.; Lantier, L.; McGuinness, O.P. Repression of latent NF-κB enhancers by PDX1 regulates β cell functional heterogeneity. Cell Metab. 2024, 36, 90–102.e107. [Google Scholar] [CrossRef] [PubMed]
- Tsuyama, T.; Sato, Y.; Yoshizawa, T.; Matsuoka, T.; Yamagata, K. Hypoxia causes pancreatic β-cell dysfunction and impairs insulin secretion by activating the transcriptional repressor BHLHE40. EMBO Rep. 2023, 24, e56227. [Google Scholar] [CrossRef]
- Vanhoose, A.M.; Samaras, S.; Artner, I.; Henderson, E.; Hang, Y.; Stein, R. MafA and MafB Regulate Pdx1 Transcription through the Area II Control Region in Pancreatic β Cells. J. Biol. Chem. 2008, 283, 22612–22619. [Google Scholar] [CrossRef]
- Aigha, I.I.; Abdelalim, E.M. NKX6. 1 transcription factor: A crucial regulator of pancreatic β cell development, identity, and proliferation. Stem Cell Res. Ther. 2020, 11, 459. [Google Scholar] [CrossRef]
- Luan, C.; Ye, Y.; Singh, T.; Barghouth, M.; Eliasson, L.; Artner, I.; Zhang, E.; Renström, E. The calcium channel subunit gamma-4 is regulated by MafA and necessary for pancreatic beta-cell specification. Commun. Biol. 2019, 2, 106. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Chirikjian, M.; Pajvani, U.B.; Bartolomé, A. MafA Regulation in β-Cells: From Transcriptional to Post-Translational Mechanisms. Biomolecules 2022, 12, 535. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, W.; Iwasa, H.; Tumurkhuu, M. Role of the Transcription Factor MAFA in the Maintenance of Pancreatic β-Cells. Int. J. Mol. Sci. 2022, 23, 4478. [Google Scholar] [CrossRef] [PubMed]
- Hang, Y.; Yamamoto, T.; Benninger, R.K.; Brissova, M.; Guo, M.; Bush, W.; Piston, D.W.; Powers, A.C.; Magnuson, M.; Thurmond, D.C.; et al. The MafA Transcription Factor Becomes Essential to Islet β-Cells Soon After Birth. Diabetes 2014, 63, 1994–2005. [Google Scholar] [CrossRef] [PubMed]
- Eto, K.; Nishimura, W.; Oishi, H.; Udagawa, H.; Kawaguchi, M.; Hiramoto, M.; Fujiwara, T.; Takahashi, S.; Yasuda, K. MafA Is Required for Postnatal Proliferation of Pancreatic β-Cells. PLoS ONE 2014, 9, e104184. [Google Scholar] [CrossRef] [PubMed]
- Gradwohl, G.; Dierich, A.; LeMeur, M.; Guillemot, F. neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc. Natl. Acad. Sci. USA 2000, 97, 1607–1611. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liu, Q.; Zhou, Z.; Ikeda, Y. PDX1, Neurogenin-3, and MAFA: Critical transcription regulators for beta cell development and regeneration. Stem Cell Res. Ther. 2017, 8, 240. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, V.; Mercier, R.; Jiménez, S.; Ye, T.; García-Sánchez, E.; Klein, A.; Meunier, A.; Ghimire, S.; Birck, C.; Jost, B.; et al. Extensive NEUROG3 occupancy in the human pancreatic endocrine gene regulatory network. Mol. Metab. 2021, 53, 101313. [Google Scholar] [CrossRef]
- Miyatsuka, T.; Kosaka, Y.; Kim, H.; German, M.S. Neurogenin3 inhibits proliferation in endocrine progenitors by inducing Cdkn1a. Proc. Natl. Acad. Sci. USA 2010, 108, 185–190. [Google Scholar] [CrossRef]
- McDonald, E.; Li, J.; Krishnamurthy, M.; Fellows, G.F.; Goodyer, C.G.; Wang, R. SOX9 regulates endocrine cell differentiation during human fetal pancreas development. Int. J. Biochem. Cell Biol. 2012, 44, 72–83. [Google Scholar] [CrossRef]
- Dettmer, R.; Niwolik, I.; Mehmeti, I.; Jörns, A.; Naujok, O. New hPSC SOX9 and INS Reporter Cell Lines Facilitate the Observation and Optimization of Differentiation into Insulin-Producing Cells. Stem Cell Rev. Rep. 2021, 17, 2193–2209. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chen, J.; Han, J.; Yang, Z.; Yang, Y.; Li, H.; Wang, S.; Hong, Y. Sox9 is required in regeneration of pancreatic β cells following injury. Exp. Cell Res. 2023, 422, 113406. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Lin, Q.; Qi, T.; Wang, T.; Chen, C.-C.; Riggs, A.D.; Zeng, D. Growth factors and medium hyperglycemia induce Sox9+ ductal cell differentiation into β cells in mice with reversal of diabetes. Proc. Natl. Acad. Sci. USA 2016, 113, 650–655. [Google Scholar] [CrossRef] [PubMed]
- Qian, M.F.; Bevacqua, R.J.; Coykendall, V.M.; Liu, X.; Zhao, W.; Chang, C.A.; Gu, X.; Dai, X.-Q.; MacDonald, P.E.; Kim, S.K. HNF1α maintains pancreatic α and β cell functions in primary human islets. JCI Insight 2023, 8, e170884. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Rahman, M.; Haneda, M.; Tsuyama, T.; Mizumoto, T.; Yoshizawa, T.; Kitamura, T.; Gonzalez, F.J.; Yamamura, K.-I.; Yamagata, K. HNF1α controls glucagon secretion in pancreatic α-cells through modulation of SGLT1. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2020, 1866, 165898. [Google Scholar] [CrossRef] [PubMed]
- Low, B.S.J.; Lim, C.S.; Ding, S.S.L.; Tan, Y.S.; Ng, N.H.J.; Krishnan, V.G.; Ang, S.F.; Neo, C.W.Y.; Verma, C.S.; Hoon, S. Decreased GLUT2 and glucose uptake contribute to insulin secretion defects in MODY3/HNF1A hiPSC-derived mutant β cells. Nat. Commun. 2021, 12, 3133. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Jia, J.; Zhao, Q.; Zhang, Y.; Huang, B.; Wang, L.; Tian, J.; Huang, C.; Li, M.; Li, X. Novel Loss-of-Function Variant in HNF1a Induces β-Cell Dysfunction through Endoplasmic Reticulum Stress. Int. J. Mol. Sci. 2022, 23, 13022. [Google Scholar] [CrossRef] [PubMed]
- Cujba, A.-M.; Alvarez-Fallas, M.E.; Pedraza-Arevalo, S.; Laddach, A.; Shepherd, M.H.; Hattersley, A.T.; Watt, F.M.; Sancho, R. An HNF1α truncation associated with maturity-onset diabetes of the young impairs pancreatic progenitor differentiation by antagonizing HNF1β function. Cell Rep. 2022, 38, 110425. [Google Scholar] [CrossRef] [PubMed]
- Sujjitjoon, J.; Charoensuk, C.; Thanyaphon, T.; Kooptiwut, S.; Thamtarana, P.J.; Tangjittipokin, W.; Chongjaroen, N.; Chanprasert, C.; Abubakar, Z.; Lapbenjakul, S.; et al. Defective functions of HNF1A variants on BCL2L1 transactivation and beta-cell growth. Biochem. Biophys. Res. Commun. 2020, 529, 826–833. [Google Scholar] [CrossRef]
- Friedman-Mazursky, O.; Elkon, R.; Efrat, S. Redifferentiation of expanded human islet β cells by inhibition of ARX. Sci. Rep. 2016, 6, 20698. [Google Scholar] [CrossRef]
- Courtney, M.; Rabe, T.; Collombat, P.; Mansouri, A. Pax4 and Arx Represent Crucial Regulators of the Development of the Endocrine Pancreas. New J. Sci. 2014, 2014, 1–6. [Google Scholar] [CrossRef]
- Đorđević, M.; Stepper, P.; Feuerstein-Akgoz, C.; Gerhauser, C.; Paunović, V.; Tolić, A.; Rajić, J.; Dinić, S.; Uskoković, A.; Grdović, N.; et al. EpiCRISPR targeted methylation of Arx gene initiates transient switch of mouse pancreatic alpha to insulin-producing cells. Front. Endocrinol. 2023, 14, 1134478. [Google Scholar] [CrossRef] [PubMed]
- Courtney, M.; Gjernes, E.; Druelle, N.; Ravaud, C.; Vieira, A.; Ben-Othman, N.; Pfeifer, A.; Avolio, F.; Leuckx, G.; Lacas-Gervais, S.; et al. The Inactivation of Arx in Pancreatic α-Cells Triggers Their Neogenesis and Conversion into Functional β-like Cells. PLoS Genet. 2013, 9, e1003934. [Google Scholar] [CrossRef] [PubMed]
- Villamayor, L.; Rodríguez-Seguel, E.; Araujo, R.; Carrasco, M.; Bru-Tarí, E.; Mellado-Gil, J.M.; Gauthier, B.R.; Martinelli, P.; Quesada, I.; Soria, B.; et al. GATA6 Controls Insulin Biosynthesis and Secretion in Adult β-Cells. Diabetes 2017, 67, 448–460. [Google Scholar] [CrossRef]
- Chia, C.Y.; Madrigal, P.; Denil, S.L.; Martinez, I.; Garcia-Bernardo, J.; El-Khairi, R.; Chhatriwala, M.; Shepherd, M.H.; Hattersley, A.T.; Dunn, N.R. GATA6 cooperates with EOMES/SMAD2/3 to deploy the gene regulatory network governing human definitive endoderm and pancreas formation. Stem Cell Rep. 2019, 12, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Tiyaboonchai, A.; Cardenas-Diaz, F.L.; Ying, L.; Maguire, J.A.; Sim, X.; Jobaliya, C.; Gagne, A.L.; Kishore, S.; Stanescu, D.E.; Hughes, N.; et al. GATA6 Plays an Important Role in the Induction of Human Definitive Endoderm, Development of the Pancreas, and Functionality of Pancreatic β Cells. Stem Cell Rep. 2017, 8, 589–604. [Google Scholar] [CrossRef] [PubMed]
- Sanyoura, M.; Jacobsen, L.; Carmody, D.; del Gaudio, D.; Alkorta-Aranburu, G.; Arndt, K.; Hu, Y.Y.; Kobiernicki, F.; Kusmartseva, I.; Atkinson, M.A.; et al. Pancreatic Histopathology of Human Monogenic Diabetes Due to Causal Variants in KCNJ11, HNF1A, GATA6, and LMNA. J. Clin. Endocrinol. Metab. 2017, 103, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.; Luo, Y.; Wang, J.; Huang, D. Monogenic Diabetes with GATA6 Mutations: Characterization of a Novel Family and a Comprehensive Analysis of the GATA6 Clinical and Genetics Traits. Mol. Biotechnol. 2023, 66, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-S.; Kang, H.S.; Takeda, Y.; Hom, L.; Song, H.-Y.; Jensen, J.; Jetten, A.M. Glis3 Regulates Neurogenin 3 Expression in Pancreatic β-Cells and Interacts with Its Activator, Hnf6. Mol. Cells 2012, 34, 193–200. [Google Scholar] [CrossRef]
- Scoville, D.W.; Lichti-Kaiser, K.; Grimm, S.A.; Jetten, A.M. GLIS3 binds pancreatic beta cell regulatory regions alongside other islet transcription factors. J. Endocrinol. 2019, 243, 1–14. [Google Scholar] [CrossRef]
- Amin, S.; Cook, B.; Zhou, T.; Ghazizadeh, Z.; Lis, R.; Zhang, T.; Khalaj, M.; Crespo, M.; Perera, M.; Xiang, J.Z.; et al. Discovery of a drug candidate for GLIS3-associated diabetes. Nat. Commun. 2018, 9, 2681. [Google Scholar] [CrossRef] [PubMed]
- Meulebrouck, S.; Scherrer, V.; Boutry, R.; Toussaint, B.; Vaillant, E.; Dechaume, A.; Loiselle, H.; Balkau, B.; Charpentier, G.; Franc, S.; et al. Pathogenic monoallelic variants in GLIS3 increase type 2 diabetes risk and identify a subgroup of patients sensitive to sulfonylureas. Diabetologia 2023, 67, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Grieve, L.M.; Rani, A.; ZeRuth, G.T. Downregulation of Glis3 in INS1 cells exposed to chronically elevated glucose contributes to glucotoxicity-associated β cell dysfunction. Islets 2024, 16, 2344622. [Google Scholar] [CrossRef] [PubMed]
- Swisa, A.; Avrahami, D.; Eden, N.; Zhang, J.; Feleke, E.; Dahan, T.; Cohen-Tayar, Y.; Stolovich-Rain, M.; Kaestner, K.H.; Glaser, B.; et al. PAX6 maintains β cell identity by repressing genes of alternative islet cell types. J. Clin. Investig. 2016, 127, 230–243. [Google Scholar] [CrossRef] [PubMed]
- Gosmain, Y.; Katz, L.S.; Masson, M.H.; Cheyssac, C.; Poisson, C.; Philippe, J. Pax6 is crucial for β-cell function, insulin biosynthesis, and glucose-induced insulin secretion. Mol. Endocrinol. 2012, 26, 696–709. [Google Scholar] [CrossRef] [PubMed]
- Ashery-Padan, R.; Zhou, X.; Marquardt, T.; Herrera, P.; Toube, L.; Berry, A.; Gruss, P. Conditional inactivation of Pax6 in the pancreas causes early onset of diabetes. Dev. Biol. 2004, 269, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; Wang, L.; Tang, P.M.-K.; Wang, H.-L.; Li, J.-C.; Xu, B.-H.; Xue, V.W.; Tan, R.-Z.; Jin, N.; Chan, T.-F.; et al. Smad3 deficiency promotes beta cell proliferation and function in db/db mice via restoring Pax6 expression. Theranostics 2021, 11, 2845–2859. [Google Scholar] [CrossRef] [PubMed]
- Bsharat, S.; Monni, E.; Singh, T.; Johansson, J.K.; Achanta, K.; Bertonnier-Brouty, L.; Schmidt-Christensen, A.; Holmberg, D.; Kokaia, Z.; Prasad, R.B. MafB-dependent neurotransmitter signaling promotes β cell migration in the developing pancreas. Development 2023, 150, dev201009. [Google Scholar] [CrossRef] [PubMed]
- Cyphert, H.A.; Walker, E.M.; Hang, Y.; Dhawan, S.; Haliyur, R.; Bonatakis, L.; Avrahami, D.; Brissova, M.; Kaestner, K.H.; Bhushan, A.; et al. Examining How the MAFB Transcription Factor Affects Islet β-Cell Function Postnatally. Diabetes 2018, 68, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.; Tong, X.; Walker, E.M.; Dahan, T.; Cochrane, V.A.; Ashe, S.; Russell, R.; Osipovich, A.B.; Mawla, A.M.; Guo, M.; et al. Species-specific roles for the MAFA and MAFB transcription factors in regulating islet β cell identity. J. Clin. Investig. 2023, 8, e166386. [Google Scholar] [CrossRef]
- Russell, R.; Carnese, P.P.; Hennings, T.G.; Walker, E.M.; Russ, H.A.; Liu, J.S.; Giacometti, S.; Stein, R.; Hebrok, M. Loss of the transcription factor MAFB limits β-cell derivation from human PSCs. Nat. Commun. 2020, 11, 2742. [Google Scholar] [CrossRef] [PubMed]
- Bar, Y.; Russ, H.A.; Sintov, E.; Anker-Kitai, L.; Knoller, S.; Efrat, S. Redifferentiation of Expanded Human Pancreatic β-Cell-derived Cells by Inhibition of the NOTCH Pathway. J. Biol. Chem. 2012, 287, 17269–17280. [Google Scholar] [CrossRef]
- Bar, Y.; Russ, H.A.; Knoller, S.; Ouziel-Yahalom, L.; Efrat, S. HES-1 Is Involved in Adaptation of Adult Human β-Cells to Proliferation In Vitro. Diabetes 2008, 57, 2413–2420. [Google Scholar] [CrossRef] [PubMed]
- Marui, S.; Nishikawa, Y.; Shiokawa, M.; Yokode, M.; Matsumoto, S.; Muramoto, Y.; Ota, S.; Nakamura, T.; Yoshida, H.; Okada, H.; et al. Context-Dependent Roles of Hes1 in the Adult Pancreas and Pancreatic Tumor Formation. Gastroenterology 2022, 163, 1613–1629.e12. [Google Scholar] [CrossRef]
- Xu, Y.; Mao, S.; Fan, H.; Wan, J.; Wang, L.; Zhang, M.; Zhu, S.; Yuan, J.; Lu, Y.; Wang, Z.; et al. LINC MIR503HG Controls SC-β Cell Differentiation and Insulin Production by Targeting CDH1 and HES1. Adv. Sci. 2024, 11, e2305631. [Google Scholar] [CrossRef] [PubMed]
- Barth, R.; Ruoso, C.; Ferreira, S.M.; de Ramos, F.C.; Lima, F.B.; Boschero, A.C.; Dos Santos, G.J. Hepatocyte Nuclear Factor 4-α (HNF4α) controls the insulin resistance-induced pancreatic β-cell mass expansion. Life Sci. 2022, 289, 120213. [Google Scholar] [CrossRef]
- Han, E.H.; Singh, P.; Lee, I.-K.; Urrutia, R.; Chi, Y.-I. ErbB3-binding protein 1 (EBP1) represses HNF4α-mediated transcription and insulin secretion in pancreatic β-cells. J. Biol. Chem. 2019, 294, 13983–13994. [Google Scholar] [CrossRef]
- Romer, A.I.; Singer, R.A.; Sui, L.; Egli, D.; Sussel, L. Murine Perinatal β-Cell Proliferation and the Differentiation of Human Stem Cell–Derived Insulin-Expressing Cells Require NEUROD1. Diabetes 2019, 68, 2259–2271. [Google Scholar] [CrossRef]
- Bohuslavova, R.; Fabriciova, V.; Smolik, O.; Lebrón-Mora, L.; Abaffy, P.; Benesova, S.; Zucha, D.; Valihrach, L.; Berkova, Z.; Saudek, F.; et al. NEUROD1 reinforces endocrine cell fate acquisition in pancreatic development. Nat. Commun. 2023, 14, 5554. [Google Scholar] [CrossRef]
- Gu, C.; Stein, G.H.; Pan, N.; Goebbels, S.; Hörnberg, H.; Nave, K.-A.; Herrera, P.; White, P.; Kaestner, K.H.; Sussel, L.; et al. Pancreatic β Cells Require NeuroD to Achieve and Maintain Functional Maturity. Cell Metab. 2010, 11, 298–310. [Google Scholar] [CrossRef]
- Schaffer, A.E.; Taylor, B.L.; Benthuysen, J.R.; Liu, J.; Thorel, F.; Yuan, W.; Jiao, Y.; Kaestner, K.H.; Herrera, P.L.; Magnuson, M.A.; et al. Nkx6.1 Controls a Gene Regulatory Network Required for Establishing and Maintaining Pancreatic Beta Cell Identity. PLoS Genet. 2013, 9, e1003274. [Google Scholar] [CrossRef]
- Memon, B.; Younis, I.; Abubaker, F.; Abdelalim, E.M. PDX1−/NKX6. 1+ progenitors derived from human pluripotent stem cells as a novel source of insulin-secreting cells. Diabetes/Metab. Res. Rev. 2021, 37, e3400. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Kozawa, J.; Fukui, K.; Iwahashi, H.; Eguchi, H.; Shimomura, I. Increased NKX6.1 expression and decreased ARX expression in alpha cells accompany reduced beta-cell volume in human subjects. Sci. Rep. 2021, 11, 17796. [Google Scholar] [CrossRef]
- Tessem, J.S.; Moss, L.G.; Chao, L.C.; Arlotto, M.; Lu, D.; Jensen, M.V.; Stephens, S.B.; Tontonoz, P.; Hohmeier, H.E.; Newgard, C.B. Nkx6.1 regulates islet β-cell proliferation via Nr4a1 and Nr4a3 nuclear receptors. Proc. Natl. Acad. Sci. USA 2014, 111, 5242–5247. [Google Scholar] [CrossRef] [PubMed]
- Ratiu, J.; Southard, S.M.; Rust, W.L. The transcription factors ISL-1 and MAFA, but not NKX6-1 Characterize a Stem-cell Derived Population of Endocrine Pancreatic Cells Capable of Controlling blood Glucose in Rodent Models of Diabetes. Med. Res. Arch. 2023, 11. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, R.; Liu, C.; Ge, X.; Wang, Y.; Jiang, F.; Zhuang, L.; Li, T.; Zhu, Q.; Jiang, Y.; et al. Missense mutation of ISL1 (E283D) is associated with the development of type 2 diabetes. Diabetologia 2024. [Google Scholar] [CrossRef]
- Bohuslavova, R.; Fabriciova, V.; Lebrón-Mora, L.; Malfatti, J.; Smolik, O.; Valihrach, L.; Benesova, S.; Zucha, D.; Berkova, Z.; Saudek, F.; et al. ISL1 controls pancreatic alpha cell fate and beta cell maturation. Cell Biosci. 2023, 13, 53. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Wang, W.; Zhang, H.; Liu, Y.; Chen, P.; Ma, K.; Zhou, C. ISL1 Promotes Pancreatic Islet Cell Proliferation. PLoS ONE 2011, 6, e22387. [Google Scholar] [CrossRef]
- Connors, C.T.; Villaca, C.B.; Anderson-Baucum, E.K.; Rosario, S.R.; Rutan, C.D.; Childress, P.J.; Padgett, L.R.; Robertson, M.A.; Mastracci, T.L. A Translational Regulatory Mechanism Mediated by Hypusinated Eukaryotic Initiation Factor 5A Facilitates β-Cell Identity and Function. Diabetes 2023, 73, 461–473. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, C. Targeting β-cell dedifferentiation and transdifferentiation: Opportunities and challenges. Endocr. Connect. 2021, 10, R213–R228. [Google Scholar] [CrossRef]
- Wu, Q.-J.; Zhang, T.-N.; Chen, H.-H.; Yu, X.-F.; Lv, J.-L.; Liu, Y.-Y.; Liu, Y.-S.; Zheng, G.; Zhao, J.-Q.; Wei, Y.-F. The sirtuin family in health and disease. Signal Transduct. Target. Ther. 2022, 7, 402. [Google Scholar] [PubMed]
- Cao, H.; Chung, A.C.; Ming, X.; Mao, D.; Lee, H.M.; Cao, X.; Rutter, G.A.; Chan, J.C.; Tian, X.Y.; Kong, A.P. Autotaxin signaling facilitates β cell dedifferentiation and dysfunction induced by Sirtuin 3 deficiency. Mol. Metab. 2022, 60, 101493. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Sheng, C.; Zhou, F.; Zhu, K.; Wang, S.; Liu, Q.; Yuan, M.; Xu, Z.; Liu, Y.; Lu, J.; et al. CBP/p300 HAT maintains the gene network critical for β cell identity and functional maturity. Cell Death Dis. 2021, 12, 476. [Google Scholar] [CrossRef] [PubMed]
- Vanderkruk, B.; Maeshima, N.; Pasula, D.J.; An, M.; McDonald, C.L.; Suresh, P.; Luciani, D.S.; Lynn, F.C.; Hoffman, B.G. Methylation of histone H3 lysine 4 is required for maintenance of beta cell function in adult mice. Diabetologia 2023, 66, 1097–1115. [Google Scholar] [CrossRef] [PubMed]
- Al-Hasani, K.; Marikar, S.N.; Kaipananickal, H.; Maxwell, S.; Okabe, J.; Khurana, I.; Karagiannis, T.; Liang, J.J.; Mariana, L.; Loudovaris, T.; et al. EZH2 inhibitors promote β-like cell regeneration in young and adult type 1 diabetes donors. Signal Transduct. Target. Ther. 2024, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Parveen, N.; Dhawan, S. DNA Methylation Patterning and the Regulation of Beta Cell Homeostasis. Front. Endocrinol. 2021, 12, 651258. [Google Scholar] [CrossRef]
- Parveen, N.; Wang, J.K.; Bhattacharya, S.; Cuala, J.; Rajkumar, M.S.; Butler, A.E.; Wu, X.; Shih, H.-P.; Georgia, S.K.; Dhawan, S. DNA Methylation-Dependent Restriction of Tyrosine Hydroxylase Contributes to Pancreatic β-Cell Heterogeneity. Diabetes 2023, 72, 575–589. [Google Scholar] [CrossRef] [PubMed]
- Celen, C.; Chuang, J.-C.; Shen, S.; Li, L.; Maggiore, G.; Jia, Y.; Luo, X.; Moore, A.; Wang, Y.; Otto, J.E.; et al. Arid1a loss potentiates pancreatic β-cell regeneration through activation of EGF signaling. Cell Rep. 2022, 41, 111581. [Google Scholar] [CrossRef] [PubMed]
- Spaeth, J.M.; Liu, J.-H.; Peters, D.; Guo, M.; Osipovich, A.B.; Mohammadi, F.; Roy, N.; Bhushan, A.; Magnuson, M.A.; Hebrok, M.; et al. The Pdx1-Bound Swi/Snf Chromatin Remodeling Complex Regulates Pancreatic Progenitor Cell Proliferation and Mature Islet β-Cell Function. Diabetes 2019, 68, 1806–1818. [Google Scholar] [CrossRef] [PubMed]
- Davidson, R.K.; Kanojia, S.; Wu, W.; Kono, T.; Xu, J.; Osmulski, M.; Bone, R.N.; Casey, N.; Evans-Molina, C.; Sims, E.K.; et al. The Chd4 Helicase Regulates Chromatin Accessibility and Gene Expression Critical for β-Cell Function In Vivo. Diabetes 2023, 72, 746–757. [Google Scholar] [CrossRef]
- Davidson, R.K.; Weaver, S.A.; Casey, N.; Kanojia, S.; Hogarth, E.; Aguirre, R.S.; Sims, E.K.; Evans-Molina, C.; Spaeth, J.M. The Chd4 subunit of the NuRD complex regulates Pdx1-controlled genes involved in β-cell function. J. Mol. Endocrinol. 2022, 69, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Soltani, A.; Jafarian, A.; Allameh, A. The Predominant microRNAs in β-cell Clusters for Insulin Regulation and Diabetic Control. Curr. Drug Targets 2020, 21, 722–734. [Google Scholar] [CrossRef] [PubMed]
- Sebastiani, G.; Grieco, G.E.; Brusco, N.; Ventriglia, G.; Formichi, C.; Marselli, L.; Marchetti, P.; Dotta, F. MicroRNA Expression Analysis of In Vitro Dedifferentiated Human Pancreatic Islet Cells Reveals the Activation of the Pluripotency-Related MicroRNA Cluster miR-302s. Int. J. Mol. Sci. 2018, 19, 1170. [Google Scholar] [CrossRef]
- Melnik, B.C.; Schmitz, G. Milk Exosomal microRNAs: Postnatal Promoters of β Cell Proliferation but Potential Inducers of β Cell De-Differentiation in Adult Life. Int. J. Mol. Sci. 2022, 23, 11503. [Google Scholar] [CrossRef]
- Melnik, B.C. Milk exosomal miRNAs: Potential drivers of AMPK-to-mTORC1 switching in β-cell de-differentiation of type 2 diabetes mellitus. Nutr. Metab. 2019, 16, 85. [Google Scholar] [CrossRef]
- Wang, Z.; Mohan, R.; Chen, X.; Matson, K.; Waugh, J.; Mao, Y.; Zhang, S.; Li, W.; Tang, X.; Satin, L.S.; et al. microRNA-483 Protects Pancreatic β-Cells by Targeting ALDH1A3. Endocrinology 2021, 162, bqab031. [Google Scholar] [CrossRef]
- Mohan, R.; Mao, Y.; Zhang, S.; Zhang, Y.-W.; Xu, C.-R.; Gradwohl, G.; Tang, X. Differentially Expressed MicroRNA-483 Confers Distinct Functions in Pancreatic β- and α-Cells. J. Biol. Chem. 2015, 290, 19955–19966. [Google Scholar] [CrossRef]
- Gannon, M. Micro-RNA-mediated Maintenance of Beta-cell Identity Reveals Targets for Reversing Beta-cell Dedifferentiation. Endocrinology 2021, 162, bqab067. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, Y.; Shi, Y.; Zhang, Y.; Liu, K.; Liang, R.; Sun, P.; Chang, X.; Tang, W.; Zhang, Y.; et al. Expression of miRNA-29 in Pancreatic β Cells Promotes Inflammation and Diabetes via TRAF3. Cell Rep. 2021, 34, 108576. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, Y.; Ye, Y.; Li, D.; Liu, Y.; Lee, E.; Zhang, M.; Dai, X.; Zhang, X.; Wang, S. Pancreatic β cells control glucose homeostasis via the secretion of exosomal miR-29 family. J. Extracell. Vesicles 2021, 10, e12055. [Google Scholar] [CrossRef]
- Lang, H.; Lin, N.; Chen, X.; Xiang, J.; Zhang, X.; Kang, C. Repressing miR-23a promotes the transdifferentiation of pancreatic α cells to β cells via negatively regulating the expression of SDF-1α. PLoS ONE 2024, 19, e0299821. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Sun, Y.; Zhou, Y.; Zhang, Y.; Zhang, T.; Li, Y.; You, W.; Chang, X.; Yuan, L.; Han, X. MicroRNA-24 promotes pancreatic beta cells toward dedifferentiation to avoid endoplasmic reticulum stress-induced apoptosis. J. Mol. Cell Biol. 2019, 11, 747–760. [Google Scholar] [CrossRef] [PubMed]
- de Jesus, D.S.; Mak, T.C.; Wang, Y.-F.; von Ohlen, Y.; Bai, Y.; Kane, E.; Chabosseau, P.; Chahrour, C.M.; Distaso, W.; Salem, V. Dysregulation of the Pdx1/Ovol2/Zeb2 axis in dedifferentiated β-cells triggers the induction of genes associated with epithelial–mesenchymal transition in diabetes. Mol. Metab. 2021, 53, 101248. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, D.; Zhou, Y.; Cui, S. MicroRNA-7a inhibits Isl1 expression to regulate insulin secretion by targeting Raf1 and Mapkap1 in NIT-1 cells. Vitr. Cell. Dev. Biol. Anim. 2021, 57, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Yu, Y.; Yang, Y.; Xiao, X.; Sun, T.; Chang, X.; Tang, W.; Zhu, Y.; Han, X. miR-25 and miR-92b regulate insulin biosynthesis and pancreatic β-cell apoptosis. Endocrine 2022, 76, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Wang, Y.; Zhang, H.; Kong, D. Identification of miR-9 as a negative factor of insulin secretion from beta cells. J. Physiol. Biochem. 2018, 74, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, S.; Li, H.; Wan, J.; Zhou, Q.; Zhou, Y.; Zhang, C. microRNA-96 protects pancreatic β-cell function by targeting PAK1 in gestational diabetes mellitus. Biofactors 2018, 44, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Qu, X.; Chen, Y.; Feng, Q.; Zhang, Y.; Hu, J.; Li, X. MicroRNA-33a-5p sponges to inhibit pancreatic β-cell function in gestational diabetes mellitus LncRNA DANCR. Reprod. Biol. Endocrinol. 2020, 18, 1–9. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Y.; Sun, Y.; He, L.; Liu, K.; Zhang, Y.; Zhou, Y.; Chang, X.; Liang, R.; Wang, S. The miR-203/ZBTB20/MAFA Axis Orchestrates Pancreatic β-Cell Maturation and Identity During Weaning and Diabetes. SSRN 2023, 4467623. [Google Scholar] [CrossRef]
- Zhang, F.; Ma, D.; Zhao, W.; Wang, D.; Liu, T.; Liu, Y.; Yang, Y.; Liu, Y.; Mu, J.; Li, B.; et al. Obesity-induced overexpression of miR-802 impairs insulin transcription and secretion. Nat. Commun. 2020, 11, 1822. [Google Scholar] [CrossRef]
- Mao, Y.; Schoenborn, J.; Wang, Z.; Chen, X.; Matson, K.; Mohan, R.; Zhang, S.; Tang, X.; Arunagiri, A.; Arvan, P.; et al. Transgenic overexpression of microRNA-30d in pancreatic beta-cells progressively regulates beta-cell function and identity. Sci. Rep. 2022, 12, 11969. [Google Scholar] [CrossRef] [PubMed]
- Bai, C.; Ren, Q.; Liu, H.; Li, X.; Guan, W.; Gao, Y. miR-212/132-enriched extracellular vesicles promote differentiation of induced pluripotent stem cells into pancreatic beta cells. Front. Cell Dev. Biol. 2021, 9, 673231. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Huang, Y.; Guo, Y.; Xiong, Y.; Zhu, S.; Xu, L.; Lu, J.; Li, X.; Wan, J.; Lu, Y.; et al. microRNA-690 regulates induced pluripotent stem cells (iPSCs) differentiation into insulin-producing cells by targeting Sox9. Stem Cell Res. Ther. 2019, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cong, R.; Lv, T.; Liu, K.; Chang, X.; Li, Y.; Han, X.; Zhu, Y. Islet-resident macrophage-derived miR-155 promotes β cell decompensation via targeting PDX1. iScience 2024, 27, 109540. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhou, S.; Shi, Y.; Zhou, Y.; Zhang, Y.; Liu, K.; Zhu, Y.; Han, X. Inhibition of miR-153, an IL-1β-responsive miRNA, prevents beta cell failure and inflammation-associated diabetes. Metabolism 2020, 111, 154335. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Yin, Z.; Zhao, Y.; Li, H.; Dai, B.; Fan, J.; He, M.; Nie, X.; Wang, C.-Y.; Wang, D.W.; et al. miR-320a induces pancreatic β cells dysfunction in diabetes by inhibiting MafF. Mol. Ther. Nucleic Acids 2021, 26, 444–457. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Zhang, J.; Chen, X.; Lang, J.; Li, L.; Chen, F.; Tian, L.; Meng, Y.; Yu, X. MicroRNA-17-92 Regulates Beta-Cell Restoration After Streptozotocin Treatment. Front. Endocrinol. 2020, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.M.; Mirmira, R.; Anderson, R.; Sims, E. MiR-21 Contributes to Cytokine-Induced Beta Cell Dysfunction via Inhibition of mRNAs Regulating Beta Cell Identity. FASEB J. 2019, 33, 694.13. [Google Scholar] [CrossRef]
- Ibrahim, S. miR-21 Exacerbates Cytokine Induced Beta Cell Dysfunction via Inhibition of mRNAs Regulating Beta Cell Identity. Ph.D. Thesis, Indiana University-Purdue University Indianapolis, Indianapolis, IN, USA, 2020. [Google Scholar]
- Grieco, G.E.; Brusco, N.; Fignani, D.; Nigi, L.; Formichi, C.; Licata, G.; Marselli, L.; Marchetti, P.; Salvini, L.; Tinti, L.; et al. Reduced miR-184-3p expression protects pancreatic β-cells from lipotoxic and proinflammatory apoptosis in type 2 diabetes via CRTC1 upregulation. Cell Death Discov. 2022, 8, 340. [Google Scholar] [CrossRef]
- Sato-Kunisada, R.; Yoshida, N.; Nakamura, S.; Uchiyama, H.; Matsumoto, H. Enhanced Expression of miR-199b-5p Promotes Proliferation of Pancreatic.β-Cells by Down-Regulation of MLK3. MicroRNA 2016, 5, 57–65. [Google Scholar] [CrossRef]
- Qian, B.; Yang, Y.; Tang, N.; Wang, J.; Sun, P.; Yang, N.; Chen, F.; Wu, T.; Sun, T.; Li, Y.; et al. M1 macrophage-derived exosomes impair beta cell insulin secretion via miR-212-5p by targeting SIRT2 and inhibiting Akt/GSK-3β/β-catenin pathway in mice. Diabetologia 2021, 64, 2037–2051. [Google Scholar] [CrossRef] [PubMed]
- Eliasson, L. The small RNA miR-375—A pancreatic islet abundant miRNA with multiple roles in endocrine beta cell function. Mol. Cell. Endocrinol. 2017, 456, 95–101. [Google Scholar] [CrossRef]
- Li, Y.; Xu, X.; Liang, Y.; Liu, S.; Xiao, H.; Li, F.; Cheng, H.; Fu, Z. miR-375 enhances palmitate-induced lipoapoptosis in insulin-secreting NIT-1 cells by repressing myotrophin (V1) protein expression. Int. J. Clin. Exp. Pathol. 2010, 3, 254–264. [Google Scholar]
- Guo, C.; Sun, Y.-Q.; Li, Q.; Zhang, J.-C. MiR-7, miR-9 and miR-375 contribute to effect of Exendin-4 on pancreatic β-cells in high-fat-diet-fed mice. Clin. Investig. Med. 2018, 41, E16–E24. [Google Scholar] [CrossRef]
- Lin, X.; Cheng, L.; Wan, Y.; Yan, Y.; Zhang, Z.; Li, X.; Wu, J.; Wang, X.; Xu, M. Ang II Controls the Expression of Mapkap1 by miR-375 and Affects the Function of Islet β Cells. Endocrine, Metab. Immune Disord. Drug Targets 2023, 23, 1186–1200. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, J.; Wang, X.; Li, Z.; Niu, D.; Wang, M.; Xiang, J.; Yue, Y.; Xia, Y.; Li, X. miR-375 Promotes Pancreatic Differentiation In Vitro by Affecting Different Target Genes at Different Stages. Stem Cells Int. 2021, 2021, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Keller, D.M.; Perez, I.G. Dual regulation of miR-375 and CREM genes in pancreatic beta cells. Islets 2022, 14, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Nathan, G.; Kredo-Russo, S.; Geiger, T.; Lenz, A.; Kaspi, H.; Hornstein, E.; Efrat, S. MiR-375 Promotes Redifferentiation of Adult Human β Cells Expanded In Vitro. PLoS ONE 2015, 10, e0122108. [Google Scholar] [CrossRef]
- Title, A.C.; Silva, P.N.; Godbersen, S.; Hasenöhrl, L.; Stoffel, M. The miR-200–Zeb1 axis regulates key aspects of β-cell function and survival in vivo. Mol. Metab. 2021, 53, 101267. [Google Scholar] [CrossRef]
- He, Q.; Song, J.; Cui, C.; Wang, J.; Hu, H.; Guo, X.; Yang, M.; Wang, L.; Yan, F.; Liang, K. Mesenchymal stem cell-derived exosomal miR-146a reverses diabetic β-cell dedifferentiation. Stem Cell Res. Ther. 2021, 12, 1–16. [Google Scholar] [CrossRef]
- Xu, Y.; Tang, Z.; Dai, H.; Hou, J.; Li, F.; Tang, Z.; Zhang, D. MiR-195 promotes pancreatic β-cell dedifferentiation by targeting Mfn2 and impairing Pi3k/Akt signaling in type 2 diabetes. Obesity 2022, 30, 447–459. [Google Scholar] [CrossRef]
- Sisino, G.; Zhou, A.-X.; Dahr, N.; Sabirsh, A.; Soundarapandian, M.M.; Perera, R.; Larsson-Lekholm, E.; Magnone, M.C.; Althage, M.; Tyrberg, B. Long noncoding RNAs are dynamically regulated during β-cell mass expansion in mouse pregnancy and control β-cell proliferation in vitro. PLoS ONE 2017, 12, e0182371. [Google Scholar] [CrossRef]
- López–Noriega, L.; Rutter, G.A. Long Non-Coding RNAs as Key Modulators of Pancreatic β-Cell Mass and Function. Front. Endocrinol. 2021, 11, 610213. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.-L.; Wang, F.-F.; Shu, J.; Tian, S.; Jiang, Y.; Zhang, D.; Wang, N.; Luo, Q.; Zhang, Y.; Jin, F. Transgenerational glucose intolerance with Igf2/H19 epigenetic alterations in mouse islet induced by intrauterine hyperglycemia. Diabetes 2012, 61, 1133–1142. [Google Scholar] [CrossRef]
- Sanchez-Parra, C.; Jacovetti, C.; Dumortier, O.; Lee, K.; Peyot, M.-L.; Guay, C.; Prentki, M.; Laybutt, D.R.; Van Obberghen, E.; Regazzi, R. Contribution of the Long Noncoding RNA H19 to β-Cell Mass Expansion in Neonatal and Adult Rodents. Diabetes 2018, 67, 2254–2267. [Google Scholar] [CrossRef]
- Alfaifi, M.; Verma, A.K.; Alshahrani, M.Y.; Joshi, P.C.; Alkhathami, A.G.; Ahmad, I.; Hakami, A.R.; Beg, M.M.A. Assessment of Cell-Free Long Non-Coding RNA-H19 and miRNA-29a, miRNA-29b Expression and Severity of Diabetes. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 3727–3737. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.-X.; Mondal, T.; Tabish, A.M.; Abadpour, S.; Ericson, E.; Smith, D.M.; Knöll, R.; Scholz, H.; Kanduri, C.; Tyrberg, B.; et al. The long noncoding RNA TUNAR modulates Wnt signaling and regulates human β-cell proliferation. Am. J. Physiol. Metab. 2021, 320, E846–E857. [Google Scholar] [CrossRef]
- You, L.; Wang, N.; Yin, D.; Wang, L.; Jin, F.; Zhu, Y.; Yuan, Q.; De, W. Downregulation of Long Noncoding RNA Meg3 Affects Insulin Synthesis and Secretion in Mouse Pancreatic Beta Cells. J. Cell. Physiol. 2015, 231, 852–862. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhu, Y.; Xie, M.; Wang, L.; Jin, F.; Li, Y.; Yuan, Q.; De, W. Long Noncoding RNA Meg3 Regulates Mafa Expression in Mouse Beta Cells by Inactivating Rad21, Smc3 or Sin3α. Cell. Physiol. Biochem. 2018, 45, 2031–2043. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Yang, Y.; Chen, X.; Liu, Y.; Hu, Q.; Huang, B.; Liu, Y.; Pan, Y.; Zhang, Y.; Liu, D.; et al. The long non-coding RNA βFaar regulates islet β-cell function and survival during obesity in mice. Nat. Commun. 2021, 12, 3997. [Google Scholar] [CrossRef]
- Rashidmayvan, M.; Sahebi, R.; Ghayour-Mobarhan, M. Long non-coding RNAs: A valuable biomarker for metabolic syndrome. Mol. Genet. Genom. 2022, 297, 1169–1183. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Xu, Y.; Lu, Y.; Zhu, S.; Guo, Y.; Sun, C.; Xu, L.; Chen, X.; Zhao, Y.; Yu, B. lncRNA Gm10451 regulates PTIP to facilitate iPSCs-derived β-like cell differentiation by targeting miR-338-3p as a ceRNA. Biomaterials 2019, 216, 119266. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.F.; Liu, Y.H.; Wang, D.W.; Liu, T.S.; Yang, Y.; Guo, J.M.; Pan, Y.; Zhang, Y.F.; Du, H.; Li, L.; et al. Obesity-induced reduced expression of the lncRNA ROIT impairs insulin transcription by downregulation of Nkx6.1 methylation. Diabetologia 2020, 63, 811–824. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.; Lin, N.; Ma, Q.; Chen, R.; Zhang, Z.; Wen, W.; Chen, H.; Sun, J. Circulating LncRNAs Analysis in Patients with Type 2 Diabetes Reveals Novel Genes Influencing Glucose Metabolism and Islet β-Cell Function. Cell. Physiol. Biochem. 2018, 46, 335–350. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Yao, F.; Lang, Y.; Feng, X. Elevated Circulating LINC-P21 Serves as a Diagnostic Biomarker of Type 2 Diabetes Mellitus and Regulates Pancreatic β-cell Function by Sponging miR-766-3p to Upregulate NR3C2. Exp. Clin. Endocrinol. Diabetes 2020, 130, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, L.; Wang, X.; Geng, Z.; Wan, M.; Hao, J.; Liu, H.; Fan, Y.; Xu, T.; Li, Z. Long non-coding RNA LncCplx2 regulates glucose homeostasis and pancreatic β cell function. Mol. Metab. 2024, 80, 101878. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Wang, F.; Shi, X.; Ma, H.; Du, Y.; Hou, L.; Xing, N. LncRNA MALAT1 induces the dysfunction of β cells via reducing the histone acetylation of the PDX-1 promoter in type 1 diabetes. Exp. Mol. Pathol. 2020, 114, 104432. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Guo, J.; Xu, P.; Gui, R. Long non-coding RNA GAS5 maintains insulin secretion by regulating multiple miRNAs in INS-1 832/13 cells. Front. Mol. Biosci. 2020, 7, 559267. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.; Wang, N.; Zhu, Y.; You, L.; Wang, L.; De, W.; Tang, W. Downregulation of Long Noncoding RNA Gas5 Affects Cell Cycle and Insulin Secretion in Mouse Pancreatic β Cells. Cell. Physiol. Biochem. 2017, 43, 2062–2073. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.; Zhang, X. LncRNA LINC01018 Screens Type 2 Diabetes Mellitus and Regulates β Cell Function Through Modulating miR-499a-5p. Horm. Metab. Res. 2023, 55, 642–648. [Google Scholar] [CrossRef]
- Pardo, C.A.C.; Massoz, L.; Dupont, M.A.; Bergemann, D.; Bourdouxhe, J.; Lavergne, A.; Tarifeño-Saldivia, E.; Helker, C.S.; Stainier, D.Y.; Peers, B.; et al. A δ-cell subpopulation with a pro-β-cell identity contributes to efficient age-independent recovery in a zebrafish model of diabetes. eLife 2022, 11, e67576. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Chawla, P.; Hnatiuk, A.; Kamel, M.; Silva, L.D.; Spanjaard, B.; Eski, S.E.; Janjuha, S.; Olivares-Chauvet, P.; Kayisoglu, O. A single-cell atlas of de novo β-cell regeneration reveals the contribution of hybrid β/δ-cells to diabetes recovery in zebrafish. Development 2022, 149, dev199853. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Huang, X.; Liu, Z.; Pu, W.; Lv, Z.; He, L.; Li, Y.; Zhou, Q.; Lui, K.O.; Zhou, B. Pre-existing beta cells but not progenitors contribute to new beta cells in the adult pancreas. Nat. Metab. 2021, 3, 352–365. [Google Scholar] [CrossRef]
- Piran, R.; Lee, S.-H.; Li, C.-R.; Charbono, A.; Bradley, L.M.; Levine, F. Pharmacological induction of pancreatic islet cell transdifferentiation: Relevance to type I diabetes. Cell Death Dis. 2014, 5, e1357. [Google Scholar] [CrossRef] [PubMed]
- Chera, S.; Baronnier, D.; Ghila, L.; Cigliola, V.; Jensen, J.N.; Gu, G.; Furuyama, K.; Thorel, F.; Gribble, F.M.; Reimann, F.; et al. Diabetes recovery by age-dependent conversion of pancreatic δ-cells into insulin producers. Nature 2014, 514, 503–507. [Google Scholar] [CrossRef]
- Bru-Tari, E.; Cobo-Vuilleumier, N.; Alonso-Magdalena, P.; Dos Santos, R.S.; Marroqui, L.; Nadal, A.; Gauthier, B.R.; Quesada, I. Pancreatic alpha-cell mass in the early-onset and advanced stage of a mouse model of experimental autoimmune diabetes. Sci. Rep. 2019, 9, 9515. [Google Scholar] [CrossRef] [PubMed]
- Hang, Y.; Stein, R. MafA and MafB activity in pancreatic β cells. Trends Endocrinol. Metab. 2011, 22, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Furuyama, K.; Chera, S.; van Gurp, L.; Oropeza, D.; Ghila, L.; Damond, N.; Vethe, H.; Paulo, J.A.; Joosten, A.M.; Berney, T. Diabetes relief in mice by glucose-sensing insulin-secreting human α-cells. Nature 2019, 567, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Ma, J.; Li, Y.; Zhou, Y.; Luo, L.; Yang, Y. Pax4-Ghrelin mediates the conversion of pancreatic ε-cells to β-cells after extreme β-cell loss in zebrafish. Development 2023, 150, dev201306. [Google Scholar] [CrossRef]
- Sasaki, S.; Lee, M.Y.Y.; Wakabayashi, Y.; Suzuki, L.; Winata, H.; Himuro, M.; Matsuoka, T.-A.; Shimomura, I.; Watada, H.; Lynn, F.C.; et al. Spatial and transcriptional heterogeneity of pancreatic beta cell neogenesis revealed by a time-resolved reporter system. Diabetologia 2022, 65, 811–828. [Google Scholar] [CrossRef]
- Huang, H.; Yang, K.; Wang, R.; Han, W.H.; Kuny, S.; Zelmanovitz, P.H.; Sauvé, Y.; Chan, C.B. β-Cell compensation concomitant with adaptive endoplasmic reticulum stress and β-cell neogenesis in a diet-induced type 2 diabetes model. Appl. Physiol. Nutr. Metab. 2019, 44, 1355–1366. [Google Scholar] [CrossRef]
- Gribben, C.; Lambert, C.; Messal, H.A.; Hubber, E.-L.; Rackham, C.; Evans, I.; Heimberg, H.; Jones, P.; Sancho, R.; Behrens, A. Ductal Ngn3-expressing progenitors contribute to adult β cell neogenesis in the pancreas. Cell Stem Cell 2021, 28, 2000–2008.e4. [Google Scholar] [CrossRef] [PubMed]
- Karampelias, C.; Watt, K.; Mattsson, C.L.; Ruiz, F.; Rezanejad, H.; Mi, J.; Liu, X.; Chu, L.; Locasale, J.W.; Korbutt, G.S.; et al. MNK2 deficiency potentiates β-cell regeneration via translational regulation. Nat. Chem. Biol. 2022, 18, 942–953. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; An, H.; Ni, K.; Chen, B.; Li, H.; Li, Y.; Sheng, G.; Zhou, C.; Xie, M.; Chen, S.; et al. Glutathione prevents chronic oscillating glucose intake-induced β-cell dedifferentiation and failure. Cell Death Dis. 2019, 10, 321. [Google Scholar] [CrossRef]
- John, A.N.; Iqbal, Z.; Colley, S.; Morahan, G.; Makishima, M.; Jiang, F.-X. Vitamin D receptor-targeted treatment to prevent pathological dedifferentiation of pancreatic β cells under hyperglycaemic stress. Diabetes Metab. 2018, 44, 269–280. [Google Scholar] [CrossRef]
- Xing, C.; Tang, M.; Yang, J.; Wang, S.; Xu, Q.; Feng, W.; Mu, Y.; Li, F.; Zhao, A.Z. Eicosapentaenoic acid metabolites promotes the trans-differentiation of pancreatic α cells to β cells. Biochem. Pharmacol. 2023, 216, 115775. [Google Scholar] [CrossRef] [PubMed]
- Karampelias, C.; Rezanejad, H.; Rosko, M.; Duan, L.; Lu, J.; Pazzagli, L.; Bertolino, P.; Cesta, C.E.; Liu, X.; Korbutt, G.S.; et al. Reinforcing one-carbon metabolism via folic acid/Folr1 promotes β-cell differentiation. Nat. Commun. 2021, 12, 3362. [Google Scholar] [CrossRef]
- Kaya-Dagistanli, F.; Ozturk, M. Transdifferentiation of both intra- and extra-islet cells into beta cells in nicotinamide treated neonatal diabetic rats: An in situ hybridization and double immunohistochemical study. Acta Histochem. 2020, 122, 151612. [Google Scholar] [CrossRef] [PubMed]
- Cristelo, C.; Nunes, R.; Pinto, S.; Marques, J.M.; Gama, F.M.; Sarmento, B. Targeting β Cells with Cathelicidin Nanomedicines Improves Insulin Function and Pancreas Regeneration in Type 1 Diabetic Rats. ACS Pharmacol. Transl. Sci. 2023, 6, 1544–1560. [Google Scholar] [CrossRef]
- Patel, S.; Yan, Z.; Remedi, M.S. Intermittent fasting protects β-cell identity and function in a type-2 diabetes model. Metabolism 2024, 153, 155813. [Google Scholar] [CrossRef]
- Li, C.; Zhu, J.; Wei, S.; Ye, X.; Yang, L.; Wang, Z.; Chen, Y. Intermittent protein restriction improves glucose homeostasis in Zucker diabetic fatty rats and single-cell sequencing reveals distinct changes in β cells. J. Nutr. Biochem. 2023, 114, 109275. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, Y.; Sun, S.; Zhang, S.; Yuan, L.; Xu, Y.; Li, X.; Chen, G.; Wei, X.; Liu, C. Ketogenic diet alleviates β-cell dedifferentiation but aggravates hepatic lipid accumulation in db/db mice. Nutrition 2024, 119, 112284. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Zhou, J.; Yang, X.; Wu, R.; Liu, H.; Shao, H.; Huang, B.; Kang, X.; Yang, L.; Liu, D. A Chinese medical nutrition therapy diet accompanied by intermittent energy restriction alleviates type 2 diabetes by enhancing pancreatic islet function and regulating gut microbiota composition. Food Res. Int. 2022, 161, 111744. [Google Scholar] [CrossRef]
- Lv, C.; Sun, Y.; Zhang, Z.Y.; Aboelela, Z.; Qiu, X.; Meng, Z.-X. β-cell dynamics in type 2 diabetes and in dietary and exercise interventions. J. Mol. Cell Biol. 2022, 14, mjac046. [Google Scholar] [CrossRef]
- Lyngbaek, M.P.P.; Legaard, G.E.; Bennetsen, S.L.; Feineis, C.S.; Rasmussen, V.; Moegelberg, N.; Brinkløv, C.F.; Nielsen, A.B.; Kofoed, K.S.; Lauridsen, C.A.; et al. The effects of different doses of exercise on pancreatic β-cell function in patients with newly diagnosed type 2 diabetes: Study protocol for and rationale behind the "DOSE-EX" multi-arm parallel-group randomised clinical trial. Trials 2021, 22, 244. [Google Scholar] [CrossRef]
- Chow, L.S.; Gerszten, R.E.; Taylor, J.M.; Pedersen, B.K.; Van Praag, H.; Trappe, S.; Febbraio, M.A.; Galis, Z.S.; Gao, Y.; Haus, J.M. Exerkines in health, resilience and disease. Nat. Rev. Endocrinol. 2022, 18, 273–289. [Google Scholar] [CrossRef] [PubMed]
- Wedell-Neergaard, A.S.; Lehrskov, L.L.; Christensen, R.H.; Legaard, G.E.; Dorph, E.; Larsen, M.K.; Launbo, N.; Fagerlind, S.R.; Seide, S.K.; Nymand, S.; et al. Exercise-induced changes in visceral adipose tissue mass are regulated by IL-6 signaling: A randomized controlled trial. Yearb. Paediatr. Endocrinol. 2019, 29, 844–855. [Google Scholar] [CrossRef]
- Lehrskov, L.L.; Lyngbaek, M.P.; Soederlund, L.; Legaard, G.E.; Ehses, J.A.; Heywood, S.E.; Albrechtsen, N.J.W.; Holst, J.J.; Karstoft, K.; Pedersen, B.K.; et al. Interleukin-6 Delays Gastric Emptying in Humans with Direct Effects on Glycemic Control. Cell Metab. 2018, 27, 1201–1211.e3. [Google Scholar] [CrossRef]
- Rutti, S.; Dusaulcy, R.; Hansen, J.S.; Howald, C.; Dermitzakis, E.T.; Pedersen, B.K.; Pinget, M.; Plomgaard, P.; Bouzakri, K. Angiogenin and Osteoprotegerin are type II muscle specific myokines protecting pancreatic beta-cells against proinflammatory cytokines. Sci. Rep. 2018, 8, 10072. [Google Scholar] [CrossRef] [PubMed]
- Becic, T.; Studenik, C.; Hoffmann, G. Exercise Increases Adiponectin and Reduces Leptin Levels in Prediabetic and Diabetic Individuals: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Med. Sci. 2018, 6, 97. [Google Scholar] [CrossRef]
- Ohashi, K.; Parker, J.L.; Ouchi, N.; Higuchi, A.; Vita, J.A.; Gokce, N.; Pedersen, A.A.; Kalthoff, C.; Tullin, S.; Sams, A.; et al. Adiponectin Promotes Macrophage Polarization toward an Anti-inflammatory Phenotype. J. Biol. Chem. 2010, 285, 6153–6160. [Google Scholar] [CrossRef] [PubMed]
- Fulgenzi, G.; Hong, Z.; Tomassoni-Ardori, F.; Barella, L.F.; Becker, J.; Barrick, C.; Swing, D.; Yanpallewar, S.; Croix, B.S.; Wess, J.; et al. Novel metabolic role for BDNF in pancreatic β-cell insulin secretion. Nat. Commun. 2020, 11, 1950. [Google Scholar] [CrossRef] [PubMed]
- Strömberg, A.; Olsson, K.; Dijksterhuis, J.P.; Rullman, E.; Schulte, G.; Gustafsson, T. CX3CL1—A macrophage chemoattractant induced by a single bout of exercise in human skeletal muscle. Am. J. Physiol. Integr. Comp. Physiol. 2016, 310, R297–R304. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Du, F.; Li, X.; Wang, M.; Duan, R.; Zhang, J.; Wu, Y.; Zhang, Q. Effects and underlying mechanisms of irisin on the proliferation and apoptosis of pancreatic β cells. PLoS ONE 2017, 12, e0175498. [Google Scholar] [CrossRef] [PubMed]
- Natalicchio, A.; Marrano, N.; Biondi, G.; Spagnuolo, R.; Labarbuta, R.; Porreca, I.; Cignarelli, A.; Bugliani, M.; Marchetti, P.; Perrini, S.; et al. The Myokine Irisin Is Released in Response to Saturated Fatty Acids and Promotes Pancreatic β-Cell Survival and Insulin Secretion. Diabetes 2017, 66, 2849–2856. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Hanna, T.; Suda, N.; Karsenty, G.; Ducy, P. Osteocalcin Promotes β-Cell Proliferation During Development and Adulthood Through Gprc6a. Diabetes 2014, 63, 1021–1031. [Google Scholar] [CrossRef] [PubMed]
- Kover, K.; Yan, Y.; Tong, P.Y.; Watkins, D.; Li, X.; Tasch, J.; Hager, M.; Clements, M.; Moore, W.V. Osteocalcin protects pancreatic beta cell function and survival under high glucose conditions. Biochem. Biophys. Res. Commun. 2015, 462, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Feng, J.; Wei, T.; Zhang, L.; Lang, S.; Yang, K.; Yang, J.; Liu, J.; Sterr, M.; Lickert, H.; et al. Pancreatic alpha cell glucagon–liver FGF21 axis regulates beta cell regeneration in a mouse model of type 2 diabetes. Diabetologia 2022, 66, 535–550. [Google Scholar] [CrossRef] [PubMed]
- Davey, J.R.; Estevez, E.; Thomson, R.E.; Whitham, M.; Watt, K.I.; Hagg, A.; Qian, H.; Henstridge, D.C.; Ludlow, H.; Hedger, M.P.; et al. Intravascular Follistatin gene delivery improves glycemic control in a mouse model of type 2 diabetes. FASEB J. 2020, 34, 5697–5714. [Google Scholar] [CrossRef]
- Curran, M.; Drayson, M.T.; Andrews, R.C.; Zoppi, C.; Barlow, J.P.; Solomon, T.P.; Narendran, P. The benefits of physical exercise for the health of the pancreatic β-cell: A review of the evidence. Exp. Physiol. 2020, 105, 579–589. [Google Scholar] [CrossRef]
- Tanday, N.; Irwin, N.; Moffett, R.C.; Flatt, P.R.; O’Harte, F.P.M. Beneficial actions of a long-acting apelin analogue in diabetes are related to positive effects on islet cell turnover and transdifferentiation. Diabetes Obes. Metab. 2020, 22, 2468–2478. [Google Scholar] [CrossRef]
- Li, H.-Q.; Spitzer, N.C. Exercise enhances motor skill learning by neurotransmitter switching in the adult midbrain. Nat. Commun. 2020, 11, 2195. [Google Scholar] [CrossRef] [PubMed]
- Ben-Othman, N.; Vieira, A.; Courtney, M.; Record, F.; Gjernes, E.; Avolio, F.; Hadzic, B.; Druelle, N.; Napolitano, T.; Navarro-Sanz, S.; et al. Long-Term GABA Administration Induces Alpha Cell-Mediated Beta-like Cell Neogenesis. Cell 2016, 168, 73–85.e11. [Google Scholar] [CrossRef]
- Li, J.; Casteels, T.; Frogne, T.; Ingvorsen, C.; Honoré, C.; Courtney, M.; Huber, K.V.; Schmitner, N.; Kimmel, R.A.; Romanov, R.A.; et al. Artemisinins Target GABAA Receptor Signaling and Impair α Cell Identity. Cell 2016, 168, 86–100.e15. [Google Scholar] [CrossRef]
- Maykish, A.; Sikalidis, A.K. Utilization of Hydroxyl-Methyl Butyrate, Leucine, Glutamine and Arginine Supplementation in Nutritional Management of Sarcopenia—Implications and Clinical Considerations for Type 2 Diabetes Mellitus Risk Modulation. J. Pers. Med. 2020, 10, 19. [Google Scholar] [CrossRef]
- Tanday, N.; Flatt, P.R.; Irwin, N.; Moffett, R.C. Liraglutide and sitagliptin counter beta- to alpha-cell transdifferentiation in diabetes. J. Endocrinol. 2020, 245, 53–64. [Google Scholar] [CrossRef]
- Tanday, N.; Irwin, N.; Flatt, P.R.; Moffett, R.C. Dapagliflozin exerts positive effects on beta cells, decreases glucagon and does not alter beta-to alpha-cell transdifferentiation in mouse models of diabetes and insulin resistance. Biochem. Pharmacol. 2020, 177, 114009. [Google Scholar] [CrossRef]
- Takahashi, K.; Nakamura, A.; Miyoshi, H.; Nomoto, H.; Kitao, N.; Omori, K.; Yamamoto, K.; Cho, K.Y.; Terauchi, Y.; Atsumi, T. Effect of the sodium–glucose cotransporter 2 inhibitor luseogliflozin on pancreatic beta cell mass in db/db mice of different ages. Sci. Rep. 2018, 8, 6864. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Parmar, N.; Rathwa, N.; Palit, S.P.; Li, Y.; Garcia-Ocaña, A.; Begum, R. A novel therapeutic combination of sitagliptin and melatonin regenerates pancreatic β-cells in mouse and human islets. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2022, 1869, 119263. [Google Scholar] [CrossRef]
- Moin, A.S.M.; Butler, A.E. Alterations in Beta Cell Identity in Type 1 and Type 2 Diabetes. Curr. Diabetes Rep. 2019, 19, 83. [Google Scholar] [CrossRef] [PubMed]
- Téllez, N.; Montanya, E. Gastrin induces ductal cell dedifferentiation and b-cell neogenesis after 90% pancreatectomy. J. Endocrinol. 2014, 223, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Kucukbas, G.N.; Komuroglu, A.U.; Dirik, D.; Korpe, B.; Kose, C.; Karaaslan, O.; Dirik, Y.; Sahin, H.G. Maternal serum xenin-25 levels in gestational diabetes mellitus. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 9902–9907. [Google Scholar] [CrossRef] [PubMed]
- Michele, L.A.; Mantzouratou, P.; Cerullo, D.; Sangalli, F.; Corna, D.; Zoja, C.; Remuzzi, G.; Xinaris, C. Thyroid hormone signalling regulates beta-cell pathological growth, dedifferentiation and transdifferentiation in diabetic pancreas. Endocr. Abstr. 2023, 92, OP13-03. [Google Scholar] [CrossRef]
- Guo, D.; He, J.; Guo, H.; Song, G.; Peng, L.; An, M.; Wang, C. Angiotensin (1–7) reverses glucose-induced islet β cell dedifferentiation by Wnt/β-catenin/FoxO1 signalling pathway. Endokrynol. Pol. 2023, 74, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Banoy, N.; Guseh, J.S.; Li, G.; Rubio-Navarro, A.; Chen, T.; Poirier, B.; Putzel, G.; Rosselot, C.; Pabón, M.A.; Camporez, J.P.; et al. Adipsin preserves beta cells in diabetic mice and associates with protection from type 2 diabetes in humans. Nat. Med. 2019, 25, 1739–1747. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Huan, Y.; Liu, Q.; Li, C.; Cao, H.; Ji, W.; Gao, X.; Fu, Y.; Li, P.; Zhang, R. Morus alba L.(Sangzhi) alkaloids promote insulin secretion, restore diabetic β-cell function by preventing dedifferentiation and apoptosis. Front. Pharmacol. 2022, 13, 841981. [Google Scholar] [CrossRef] [PubMed]
- Gojani, E.G.; Wang, B.; Li, D.-P.; Kovalchuk, O.; Kovalchuk, I. Anti-Inflammatory Properties of Eugenol in Lipopolysaccharide-Induced Macrophages and Its Role in Preventing β-Cell Dedifferentiation and Loss Induced by High Glucose-High Lipid Conditions. Molecules 2023, 28, 7619. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhou, J.; Hu, R.; Zou, L.; Ji, L.; Jiang, G. Piperine protects against pancreatic β-cell dysfunction by alleviating macrophage inflammation in obese mice. Life Sci. 2021, 274, 119312. [Google Scholar] [CrossRef] [PubMed]
- Gojani, E.G.; Wang, B.; Li, D.-P.; Kovalchuk, O.; Kovalchuk, I. The Impact of Psilocybin on High Glucose/Lipid-Induced Changes in INS-1 Cell Viability and Dedifferentiation. Genes 2024, 15, 183. [Google Scholar] [CrossRef]
- Gojani, E.G.; Wang, B.; Li, D.-P.; Kovalchuk, O.; Kovalchuk, I. Effect of THC, CBD, THCV, CBC and CBN Cannabinoids on β-Cells Exposed to High Glucose—High Lipids. Preprints 2023, 2023090973. [Google Scholar] [CrossRef]
- Kannan, P.; Raghunathan, M.; Mohan, T.; Palanivelu, S.; Periandavan, K. Gymnemic Acid Ameliorates Pancreatic β-Cell Dysfunction by Modulating Pdx1 Expression: A Possible Strategy for β-Cell Regeneration. Tissue Eng. Regen. Med. 2022, 19, 603–616. [Google Scholar] [CrossRef]
- Moosavi, A.; Yazdanparast, R. Beta cell regeneration upon magainin and growth hormone treatment as a possible alternative to insulin therapy. FEBS Open Bio. 2023, 13, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Yin, J.; Wang, K.; Chen, X.; Wang, H.; Hu, X.; Wang, W.; Wang, L.; Bei, W.; Guo, J. FTZ promotes islet β-cell regeneration in T1DM mice via the regulation of nuclear proliferation factors. J. Ethnopharmacol. 2023, 315, 116564. [Google Scholar] [CrossRef]
- Martínez-Montoro, J.I.; Damas-Fuentes, M.; Fernández-García, J.C.; Tinahones, F.J. Role of the Gut Microbiome in Beta Cell and Adipose Tissue Crosstalk: A Review. Front. Endocrinol. 2022, 13, 869951. [Google Scholar] [CrossRef]
- Wang, S.; Yuan, M.; Zhang, L.; Zhu, K.; Sheng, C.; Zhou, F.; Xu, Z.; Liu, Q.; Liu, Y.; Lu, J.; et al. Sodium butyrate potentiates insulin secretion from rat islets at the expense of compromised expression of β cell identity genes. Cell Death Dis. 2022, 13, 67. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, S.S.; Prause, M.; Williams, K.; Barrès, R.; Billestrup, N. Butyrate inhibits IL-1β-induced inflammatory gene expression by suppression of NF-κB activity in pancreatic beta cells. J. Biol. Chem. 2022, 298, 102312. [Google Scholar] [CrossRef]
- Vandenbempt, V.; Eski, S.E.; Brahma, M.K.; Li, A.; Negueruela, J.; Bruggeman, Y.; Demine, S.; Xiao, P.; Cardozo, A.K.; Baeyens, N.; et al. HAMSAB diet ameliorates dysfunctional signaling in pancreatic islets in autoimmune diabetes. iScience 2024, 27, 108694. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Widjaja, J.; Sun, J.; Li, M.; Qiao, Z.; Cao, T.; Wang, Y.; Zhang, X.; Zhang, Z.; Gu, Y.; et al. Bariatric surgery induces pancreatic cell transdifferentiation as indicated by single-cell transcriptomics in Zucker diabetic rats. J. Diabetes 2023. [Google Scholar] [CrossRef]
- Koduru, S.V.; Leberfinger, A.N.; Ozbolat, I.T.; Ravnic, D.J. Navigating the Genomic Landscape of Human Adipose Stem Cell-Derived β-Cells. Stem Cells Dev. 2021, 30, 1153–1170. [Google Scholar] [CrossRef]
- Kamal, M.M.; Ammar, R.A.; Kassem, D.H. Silencing of forkhead box protein O-1 (FOXO-1) enhances insulin-producing cell generation from adipose mesenchymal stem cells for diabetes therapy. Life Sci. 2024, 344, 122579. [Google Scholar] [CrossRef]
- Zaeifi, D.; Azarnia, M. Promoting β-cells function by the recapitulation of in vivo microenvironmental differentiation signals. Cell Tissue Res. 2023, 393, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Russ, H.A.; Wang, X.; Zhang, M.; Ma, T.; Xu, T.; Tang, S.; Hebrok, M.; Ding, S. Human pancreatic beta-like cells converted from fibroblasts. Nat. Commun. 2016, 7, 10080. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, T.; Liang, R.; Wang, G.; Liu, Y.; Zou, J.; Liu, N.; Zhang, B.; Liu, Y.; Ding, X.; et al. Mesenchymal stem cells ameliorate β cell dysfunction of human type 2 diabetic islets by reversing β cell dedifferentiation. EBioMedicine 2020, 51, 102615. [Google Scholar] [CrossRef]
- Isildar, B.; Ozkan, S.; Ercin, M.; Gezginci-Oktayoglu, S.; Oncul, M.; Koyuturk, M. 2D and 3D cultured human umbilical cord-derived mesenchymal stem cell-conditioned medium has a dual effect in type 1 diabetes model in rats: Immunomodulation and beta-cell regeneration. Inflamm. Regen. 2022, 42, 55. [Google Scholar] [CrossRef] [PubMed]
- Goode, R.A.; Hum, J.M.; Kalwat, M.A. Therapeutic Strategies Targeting Pancreatic Islet β-Cell Proliferation, Regeneration, and Replacement. Endocrinology 2023, 164, bqac193. [Google Scholar] [CrossRef] [PubMed]
- Basile, G.; Qadir, M.; Mauvais-Jarvis, F.; Vetere, A.; Shoba, V.; Modell, A.; Pastori, R.; Russ, H.; Wagner, B.; Dominguez-Bendala, J. Emerging diabetes therapies: Bringing back the β-cells. Mol. Metab. 2022, 60, 101477. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Karakose, E.; Argmann, C.; Wang, H.; Balev, M.; Brody, R.I.; Rivas, H.G.; Liu, X.; Wood, O.; Liu, H.; et al. Disrupting the DREAM complex enables proliferation of adult human pancreatic β cells. J. Clin. Investig. 2022, 132, e157086. [Google Scholar] [CrossRef] [PubMed]
- Barzowska, A.; Pucelik, B.; Pustelny, K.; Matsuda, A.; Martyniak, A.; Stępniewski, J.; Maksymiuk, A.; Dawidowski, M.; Rothweiler, U.; Dulak, J.; et al. DYRK1A Kinase Inhibitors Promote β-Cell Survival and Insulin Homeostasis. Cells 2021, 10, 2263. [Google Scholar] [CrossRef]
- Guo, Y.; Li, L.; Yao, Y.; Li, H. Regeneration of Pancreatic β-Cells for Diabetes Therapeutics by Natural DYRK1A Inhibitors. Metabolites 2022, 13, 51. [Google Scholar] [CrossRef]
- Czarna, A.; Wang, J.; Zelencova, D.; Liu, Y.; Deng, X.; Choi, H.G.; Zhang, T.; Zhou, W.; Chang, J.W.; Kildalsen, H.; et al. Novel Scaffolds for Dual Specificity Tyrosine-Phosphorylation-Regulated Kinase (DYRK1A) Inhibitors. J. Med. Chem. 2018, 61, 7560–7572. [Google Scholar] [CrossRef]
- Ackeifi, C.; Wang, P.; Karakose, E.; Fox, J.E.M.; González, B.J.; Liu, H.; Wilson, J.; Swartz, E.; Berrouet, C.; Li, Y.; et al. GLP-1 receptor agonists synergize with DYRK1A inhibitors to potentiate functional human β cell regeneration. Sci. Transl. Med. 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Kimani, C.N.; Reuter, H.; Kotzé, S.H.; Venter, P.; Ramharack, P.; Muller, C.J.F. Pancreatic beta cell regenerative potential of Zanthoxylum chalybeum Engl. Aqueous stem bark extract. J. Ethnopharmacol. 2024, 320, 117374. [Google Scholar] [CrossRef]
- Gu, L.; Cui, X.; Lang, S.; Wang, H.; Hong, T.; Wei, R. Glucagon receptor antagonism increases mouse pancreatic δ-cell mass through cell proliferation and duct-derived neogenesis. Biochem. Biophys. Res. Commun. 2019, 512, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Feng, J.; Wei, T.; Gu, L.; Wang, D.; Lang, S.; Yang, K.; Yang, J.; Yan, H.; Wei, R.; et al. Pro-α-cell-derived β-cells contribute to β-cell neogenesis induced by antagonistic glucagon receptor antibody in type 2 diabetic mice. iScience 2022, 25, 104567. [Google Scholar] [CrossRef]
- Wang, M.-Y.; Dean, E.D.; Quittner-Strom, E.; Zhu, Y.; Chowdhury, K.H.; Zhang, Z.; Zhao, S.; Li, N.; Ye, R.; Lee, Y.; et al. Glucagon blockade restores functional β-cell mass in type 1 diabetic mice and enhances function of human islets. Proc. Natl. Acad. Sci. USA 2021, 118, e2022142118. [Google Scholar] [CrossRef]
- Lang, S.; Yang, J.; Yang, K.; Gu, L.; Cui, X.; Wei, T.; Liu, J.; Le, Y.; Wang, H.; Wei, R.; et al. Glucagon receptor antagonist upregulates circulating GLP-1 level by promoting intestinal L-cell proliferation and GLP-1 production in type 2 diabetes. BMJ Open Diabetes Res. Care 2020, 8, e001025. [Google Scholar] [CrossRef]
- Lang, S.; Wei, R.; Wei, T.; Gu, L.; Feng, J.; Yan, H.; Yang, J.; Hong, T. Glucagon receptor antagonism promotes the production of gut proglucagon-derived peptides in diabetic mice. Peptides 2020, 131, 170349. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, Y.; Xu, N.; Zhou, W.; Yang, L.; Chen, R.; Yang, R.; Sun, J.; Chen, H. A New Way for Beta Cell Neogenesis: Transdifferentiation from Alpha Cells Induced by Glucagon-Like Peptide 1. J. Diabetes Res. 2019, 2019, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Wang, D.; Cui, X.; Wei, T.; Yang, K.; Yang, J.; Wei, R.; Hong, T. Combination of GLP-1 Receptor Activation and Glucagon Blockage Promotes Pancreatic β-Cell Regeneration In Situ in Type 1 Diabetic Mice. J. Diabetes Res. 2021, 2021, 1–10. [Google Scholar] [CrossRef]
- Wei, R.; Cui, X.; Feng, J.; Gu, L.; Lang, S.; Wei, T.; Yang, J.; Liu, J.; Le, Y.; Wang, H.; et al. Dapagliflozin promotes beta cell regeneration by inducing pancreatic endocrine cell phenotype conversion in type 2 diabetic mice. Metabolism 2020, 111, 154324. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, J.; Xia, L.; Wei, T.; Cui, X.; Wang, D.; Jin, Z.; Lin, X.; Li, F.; Yang, K. Gut microbiota–tryptophan metabolism–GLP-1 axis participates in β-cell regeneration induced by dapagliflozin. Diabetes 2024, 73, 926–940. [Google Scholar] [CrossRef] [PubMed]
- Sachs, S.; Bastidas-Ponce, A.; Tritschler, S.; Bakhti, M.; Böttcher, A.; Sánchez-Garrido, M.A.; Tarquis-Medina, M.; Kleinert, M.; Fischer, K.; Jall, S.; et al. Targeted pharmacological therapy restores β-cell function for diabetes remission. Nat. Metab. 2020, 2, 192–209. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Cui, X.; Lin, X.; Yang, J.; Wei, R.; Hong, T.; Yang, K. γ-aminobutyric acid modulates α-cell hyperplasia but not β-cell regeneration induced by glucagon receptor antagonism in type 1 diabetic mice. Acta Diabetol. 2022, 60, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Karacay, C.; Prietl, B.; Harer, C.; Ehall, B.; Haudum, C.W.; Bounab, K.; Franz, J.; Eisenberg, T.; Madeo, F.; Kolb, D.; et al. The effect of spermidine on autoimmunity and beta cell function in NOD mice. Sci. Rep. 2022, 12, 4502. [Google Scholar] [CrossRef] [PubMed]
- Sims, E.K.; Kulkarni, A.; Hull, A.; Woerner, S.E.; Cabrera, S.; Mastrandrea, L.D.; Hammoud, B.; Sarkar, S.; Nakayasu, E.S.; Mastracci, T.L.; et al. Inhibition of polyamine biosynthesis preserves β cell function in type 1 diabetes. Cell Rep. Med. 2023, 4, 101261. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Jia, Y.-F.; Tapadar, S.; Weaver, J.D.; Raji, I.O.; Pithadia, D.J.; Javeed, N.; García, A.J.; Choi, D.-S.; Matveyenko, A.V. Inhibition of TBK1/IKKε promotes regeneration of pancreatic β-cells. Sci. Rep. 2018, 8, 15587. [Google Scholar] [CrossRef]
- Charbord, J.; Ren, L.; Sharma, R.B.; Johansson, A.; Ågren, R.; Chu, L.; Tworus, D.; Schulz, N.; Charbord, P.; Stewart, A.F.; et al. In vivo screen identifies a SIK inhibitor that induces β cell proliferation through a transient UPR. Nat. Metab. 2021, 3, 682–700. [Google Scholar] [CrossRef]
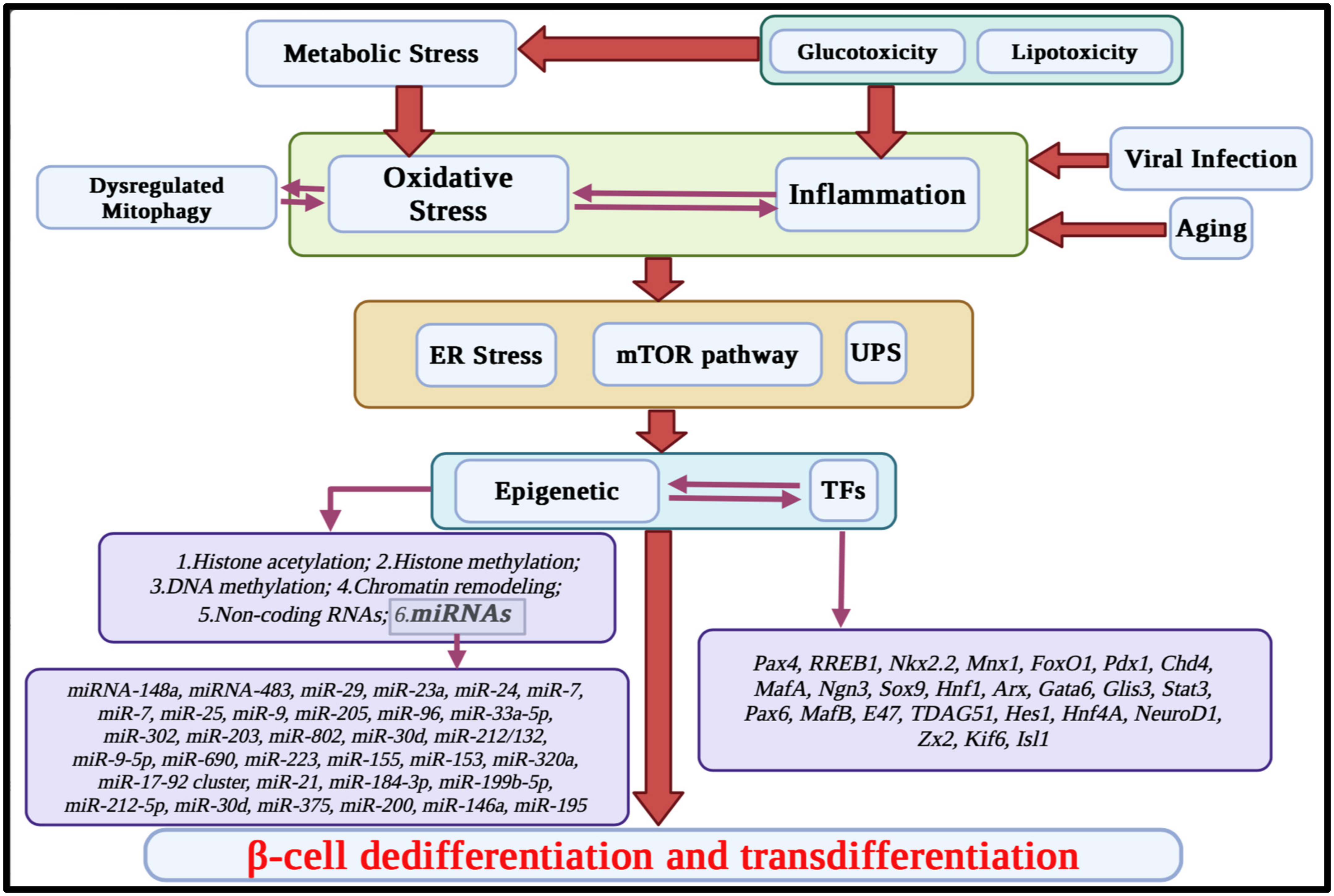
| Transcription Factor | Full Name | Targets Genes | Impact on the Genes | De/Tra/Neo/Pro | Impact on β-Cell Plasticity/Regeneration | References |
|---|---|---|---|---|---|---|
| Pax4 | Paired Box 4 | GCG | R | De | N | [101,102,103,104] |
| Arx | R | |||||
| Ins | I | Tra | N | |||
| Bcl-xL | I | |||||
| Gherlin | R | Neo | P | |||
| SST | R | |||||
| IAPP | R | Pro | P | |||
| C-myc | I | |||||
| Nkx2.2 | NK2 homeobox 2 | Ins | I | De | N | [105,106,107,108,109,110] |
| Pax6 | I | |||||
| Nkx6-1 | I | Tra | N | |||
| MafA | I | |||||
| NeuroD1 | I | Neo | P | |||
| Arx | R | |||||
| SST | R | Pro | NM | |||
| Gherlin | R | |||||
| Mnx1 (also known as HLXB9 or HB9) | Motor neuron and pancreas homeobox 1 | Nkx6.1 | I | De | N | [111,112,113,114,115] |
| Tra | N | |||||
| Ins | I | Neo | NM | |||
| Pro | P | |||||
| FoxO1 | Forkhead box O1 | Ins1 | R/I | De | N and P | [116,117,118,119,120,121,122,123] |
| Pdx1 | R | Tra | N and P | |||
| NeuroD | I | |||||
| Superoxide dismutase 1 (Sod1) | I | Neo | N | |||
| Catalase (Cat) | I | |||||
| MafA | I | Pro | P | |||
| Glutathione peroxidase 1 | I | |||||
| Pdx1 | Pancreatic and Duodenal Homeobox 1 | Ins | I | De | N | [124,125,126,127,128] |
| Glut2 | I | |||||
| Gck | I | Tra | N | |||
| IAPP | I | |||||
| SST | I | Neo | P | |||
| Pdx1 | I | |||||
| Ngn3 | I | Pro | P | |||
| NFκB | R | |||||
| MafA | I | |||||
| MafA | Musculoaponeurotic Fibrosarcoma Oncogene Family, Protein A | Ins | I | De | N | [129,130,131,132,133,134,135] |
| Glut2 | I | |||||
| Gck | I | Tra | N | |||
| Cacng4 | I | |||||
| Nanog | R | Neo | P | |||
| Pdx1 | I | |||||
| NeuroD1 | I | Pro | P | |||
| Nkx6.1 | I | |||||
| Glis3 | I | |||||
| Ngn3 | Neurogenin 3 | NeuroD1 | I | De | N | [99,136,137,138,139] |
| Pax4 | I | |||||
| Pax6 | I | Tra | N | |||
| Isl1 | I | |||||
| Cdkn1a | I | Neo | P | |||
| Nkx2-2 | I | |||||
| Insm1 | I | Pro | N | |||
| Kcnj11 | I | |||||
| Gck | I | |||||
| Slc30a8 | I | |||||
| Slc18a2 | I | |||||
| SOX9 | Sex-determining region Y (SRY)-box 9 | Ngn3 | I | De | N | [140,141,142,143] |
| Nkx6.1 | I | Tra | N | |||
| Pax6 | I | Neo | P | |||
| Ins | I | Pro | P | |||
| HNF1A | Hepatocyte Nuclear Factor 1 A | HNF4A | I | De | N | [144,145,146,147,148,149] |
| AS1 | I | |||||
| Tmem27 | I | |||||
| Kcnj11 | I | |||||
| Glut2 | I | Tra | N | |||
| Ins | I | Ne | P | |||
| NeuroDI | I | Pro | P | |||
| Gck | I | |||||
| MafB | I | |||||
| Gipr | I | |||||
| Glpr1 | I | |||||
| G6pc2 | I | |||||
| Slc5a1 | I | |||||
| Pdx1 | I | |||||
| Bcl2li(Bcl-xL) | I | |||||
| ARX | Aristaless-Related Homeobox | Gcg | I | De | p | [150,151,152,153] |
| Pax4 | R | |||||
| SST | R | Tra | p | |||
| Neo | N | |||||
| Pro | ||||||
| GATA6 | GATA Binding Protein 6 | iNOS | R | De | N | [154,155,156,157,158] |
| Ins | I | |||||
| Glut2 | I | Tra | N | |||
| Abcc8 | I | |||||
| Snap-25 | I | Neo | P | |||
| Pdx1 | I | |||||
| Nkx6.1 | I | Pro | P | |||
| MafA | I | |||||
| GLIS3 | GLI Similar 3 | Ins | I | De | N | [159,160,161,162,163] |
| Ngn3 | I | Tra | N | |||
| MafA | I | Neo | P | |||
| Glut2 | I | Pro | P | |||
| PAX6 | Paired Box 6 | Ins | I | De | N/P | [164,165,166,167] |
| GCG | R | |||||
| Glut2 | I | |||||
| Gipr | I | |||||
| Nkx2.2 | I | |||||
| PC1/3 | I | |||||
| Glp1r | I | |||||
| SST | R | Tra | N/P | |||
| Gherlin | R | |||||
| Isi | R | Neo | P | |||
| Foxa2 | R/I | |||||
| Ngn3 | R/I | Pro | P | |||
| Pdx1 | R/I | |||||
| NeuroD1 | R/I | |||||
| MafA | R/I | |||||
| Nkx6.1 | R/I | |||||
| MafB | Musculoaponeurotic Fibrosarcoma Oncogene Homolog B | GAST | R | De | N | [168,169,170,171] |
| SST | R | |||||
| P2ry1 | I | Tra | N | |||
| Adra2A | I | |||||
| Tph1 | I | Neo | P | |||
| Ins | I | |||||
| ChrnA3, ChrnA4, ChrnB4 | I | Pro | P | |||
| Robo1, Robo2, Nrp1, Nrp2 | I | |||||
| Hes1 | Hairy and Enhancer of Split-1 | Ngn3 | R | De | P | [172,173,174,175] |
| NeuroD | R | Tra | P | |||
| Ptf1a | R | Neo | N | |||
| p27Kip1 and p57Kip2 | R | Pro | P | |||
| HNF4 A | Hepatocyte Nuclear Factor 4 A | Aldolase B | I | De | N | [176,177] |
| Glut2 | I | |||||
| Gck | I | Tra | N | |||
| L-pyruvate kinase | I | |||||
| Kir6.2 | I | Neo | P | |||
| Ins | I | |||||
| Pdx1 | I | Pro | P | |||
| Pax4 | I | |||||
| Ngn3 | I | |||||
| Ki67 | I | |||||
| NeuroD1 | Neurogenic Differentiation 1 | Ins | I | De | N | [178,179,180] |
| Gck | I | |||||
| Abcc8/Sur1, Kcnj11 | I | |||||
| Cacna1a | I | Tra | N | |||
| Mafa | I | |||||
| Nkx6-1 | I | |||||
| Pdx1 | I | Neo | P | |||
| Nkx2-2 | I | |||||
| Insm1 | I | |||||
| Isl1 | I | Pro | P | |||
| Pax6 | I | |||||
| Rfx6 | I | |||||
| Cdh4 | R | |||||
| Ocln | R | |||||
| Mecom | R | |||||
| ldha | R | |||||
| Nkx6.1 | NK6 homeobox 1 | Glut2 | I | De | N | [130,181,182,183,184] |
| G6pc2 | I | |||||
| Arx | R | Tra | N | |||
| Pdx1 | I | |||||
| MafA | I | Neo | P | |||
| Ero1lb | I | Pro | P | |||
| Slc30a8 | I | |||||
| Nr4a1 and Nr4a3 | I | |||||
| ISL1 | ISL LIM homeobox 1 | MafA | I | De | N | [185,186,187,188] |
| Pdx1 | I | |||||
| Glut2 | I | |||||
| Ins | I | |||||
| NeuroD1 | I | Tra | N | |||
| Pax6 | I | |||||
| MafB, | I | |||||
| Nkx6.2 | I | |||||
| Insm1 | I | Neo | P | |||
| Sox9 | I | |||||
| Arx | I | |||||
| Foxa2 | I | |||||
| Nkx6.1 | I | Pro | P | |||
| GCG | I | |||||
| SST | I | |||||
| G6pc2 | I |
| LncRNAs | Direct and Indirect Targets | De/Tra/Pro/Neo | Type of Effect | References |
|---|---|---|---|---|
| Gm16308 | Pro | P | [243] | |
| DANCR | miR-33a-5p | Pro | P | [218,244] |
| H19 | miRNA let-7- miRNA-29a | Pro | P | [245,246,247] |
| TUNAR | EZH2 | Pro | P | [248] |
| Meg3 | EZH2, Pdx1, MafA | De | N | [249,250] |
| βFaar | miR-138-5p | De | N | [251] |
| PLUTO | Pdx1 | De | N | [124] |
| HILNC25 | Glis3 | De | N | [252] |
| Gm10451 | miR-338-3p | De | N | [253] |
| 810019D21Rik (RIOT) | Nkx6.1 | De | N | [254] |
| p3134 | Pdx1 | De | N | [124,255] |
| linc-p21 | miR-766-3p | Pro | N | [256] |
| HOTAIR | Pdx1 | De | N | [124] |
| LncCplx2 | EZH2 | Pro | N | [257] |
| MALAT1 | Pdx1 | De | P | [258] |
| MIR503HG | CtBP1 | Neo | N | [175] |
| GAS5 | miR-29a-3p, miR-96-3p, miR-208a-3p | De | N | [259,260] |
| Pro | P | |||
| LINC01018 | miR-499a-5p | De | P | [261] |
| Pro | N |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghasemi Gojani, E.; Rai, S.; Norouzkhani, F.; Shujat, S.; Wang, B.; Li, D.; Kovalchuk, O.; Kovalchuk, I. Targeting β-Cell Plasticity: A Promising Approach for Diabetes Treatment. Curr. Issues Mol. Biol. 2024, 46, 7621-7667. https://doi.org/10.3390/cimb46070453
Ghasemi Gojani E, Rai S, Norouzkhani F, Shujat S, Wang B, Li D, Kovalchuk O, Kovalchuk I. Targeting β-Cell Plasticity: A Promising Approach for Diabetes Treatment. Current Issues in Molecular Biology. 2024; 46(7):7621-7667. https://doi.org/10.3390/cimb46070453
Chicago/Turabian StyleGhasemi Gojani, Esmaeel, Sweta Rai, Farzaneh Norouzkhani, Salma Shujat, Bo Wang, Dongping Li, Olga Kovalchuk, and Igor Kovalchuk. 2024. "Targeting β-Cell Plasticity: A Promising Approach for Diabetes Treatment" Current Issues in Molecular Biology 46, no. 7: 7621-7667. https://doi.org/10.3390/cimb46070453
APA StyleGhasemi Gojani, E., Rai, S., Norouzkhani, F., Shujat, S., Wang, B., Li, D., Kovalchuk, O., & Kovalchuk, I. (2024). Targeting β-Cell Plasticity: A Promising Approach for Diabetes Treatment. Current Issues in Molecular Biology, 46(7), 7621-7667. https://doi.org/10.3390/cimb46070453







