Roles of SMAD and SMAD-Associated Signaling Pathways in Nerve Regeneration Following Peripheral Nerve Injury: A Narrative Literature Review
Abstract
1. Introduction
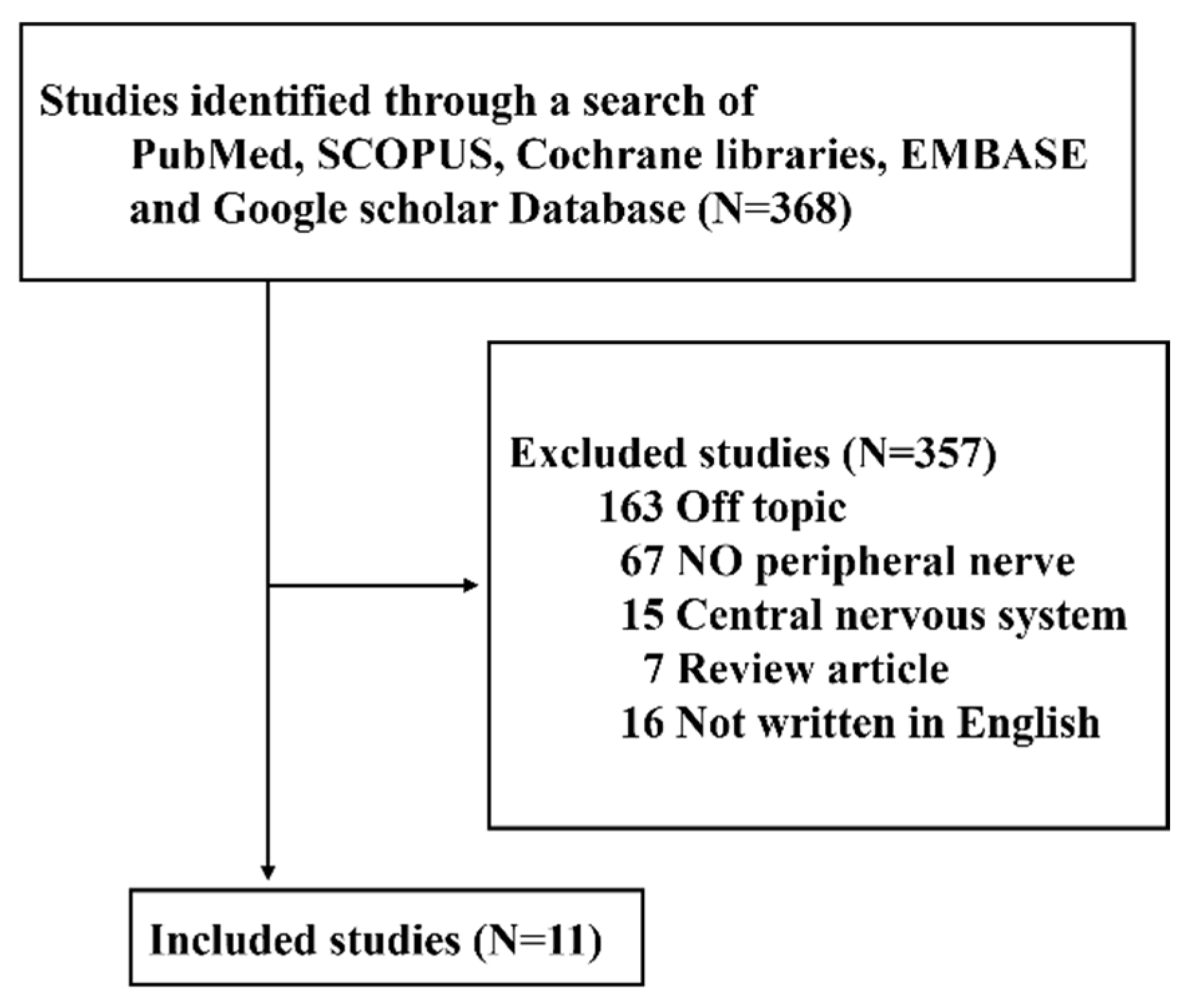
2. Methods
3. Results
4. Discussion
4.1. Expression of SMADs and Activation of SMAD-Associated Signaling Pathways in Nerve Regeneration and Degeneration after Peripheral Nerve Injury
4.2. Expression and Role of SMAD1–8 in Nerve Regeneration after Peripheral Nerve Injury (Table 1, Table 2 and Table 3)
| Nerve Injury | Increased/Decreased | SMAD Type | Functions |
|---|---|---|---|
| Transection | Increased | SMAD1 SMAD4 | Regeneration |
| SMAD2 | Degeneration | ||
| SMAD5 SMAD8 | Not yet determined | ||
| Compression | Increased | SMAD1 SMAD4 | Regeneration |
| SMAD2 | Degeneration | ||
| SMAD5 SMAD8 | Not yet determined |
| SMAD Type | Types of Target Nerves and Methods of Nerve Damage | Results |
|---|---|---|
| SMAD1 | Hypoglossal nerve transection Sciatic nerve transection Dorsal column and sciatic nerve compression Optic nerve compression Retinal ganglion cell transection Transfection of Xenopus embryos with SMAD6 | Neuroregeneration |
| SMAD2 | Hypoglossal nerve transection Dorsal column and sciatic nerve compression Sciatic nerve compression | Neuroregeneration |
| SMAD3 | No research | - |
| SMAD4 | Hypoglossal nerve transection Transfection of Xenopus embryos with SMAD6 Dorsal column and sciatic nerve compression Optic nerve compression Retinal ganglion cell transfection | Neuroregeneration |
| SMAD5 | Sciatic nerve transection Dorsal column and sciatic nerve compression Optic nerve compression Retinal ganglion cell transection | Not yet determined |
| SMAD6 | Transfection of Xenopus embryos with SMAD6 | Neurodegeneration |
| SMAD7 | No research | - |
| SMAD8 | Hypoglossal nerve transection Sciatic nerve transection Dorsal column and sciatic nerve transection Optic nerve compression Retinal ganglion cell transection | Not yet determined |
4.2.1. Hypoglossal Nerve
4.2.2. The Dorsal Root and Dorsal Column of the Spinal Cord
4.2.3. Sciatic Nerve
4.3. Expression and Role of SMAD-Associated Signaling Pathways in Nerve Regeneration after Peripheral Nerve Injury
4.3.1. BMP/SMAD1 Pathway
4.3.2. BMP/SMAD4 Pathway
4.3.3. BMP/SMAD7 Pathway
4.3.4. PI3K/GSK3/SMAD1 Pathway
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cho, Y.C. Recent Trends in Nerve Regeneration Research. Mol. Cell Biol. Newsl. 2014, 9, 1–4. [Google Scholar]
- Byun, J.Y. Facial Paralysis Disorders. Anatomy and Evaluation of Facial Nerve. Korean Soc. Otorhinolaryngol. Head Neck Surg. 2018, 3, 913–932. [Google Scholar]
- Hu, Y. Axon Injury Induced Endoplasmic Reticulum Stress and Neurodegeneration. Neural Regen. Res. 2016, 11, 1557–1559. [Google Scholar] [CrossRef] [PubMed]
- Pardal-Fernández, J.M.; García-Alvarez, G.; Jerez-García, P.; Marco-Giner, J.; Almodóvar-Alvarez, C. Peripheral Facial Paralysis. The Value of Clinical Neurophysiology. Rev. Neurol. 2003, 36, 991–996. [Google Scholar] [PubMed]
- Hussain, G.; Wang, J.; Rasul, A.; Anwar, H.; Qasim, M.; Zafar, S.; Aziz, N.; Razzaq, A.; Hussain, R.; de Aguilar, J.-L.G.; et al. Current Status of Therapeutic Approaches against Peripheral Nerve Injuries: A Detailed Story from Injury to Recovery. Int. J. Biol. Sci. 2020, 16, 116–134. [Google Scholar] [CrossRef]
- Roglio, I.; Giatti, S.; Pesaresi, M.; Bianchi, R.; Cavaletti, G.; Lauria, G.; Garcia-Segura, L.-M.; Melcangi, R.C. Neuroactive Steroids and Peripheral Neuropathy. Brain Res. Rev. 2008, 57, 460–469. [Google Scholar] [CrossRef]
- Siemionow, M.; Brzezicki, G. Chapter 8: Current Techniques and Concepts in Peripheral Nerve Repair. Int. Rev. Neurobiol. 2009, 87, 141–172. [Google Scholar] [CrossRef] [PubMed]
- Ray, W.Z.; Mackinnon, S.E. Management of Nerve Gaps: Autografts, Allografts, Nerve Transfers, and End-to-Side Neurorrhaphy. Exp. Neurol. 2010, 223, 77–85. [Google Scholar] [CrossRef]
- Samadi, P.; Saki, S.; Manoochehri, H.; Sheykhhasan, M. Therapeutic Applications of Mesenchymal Stem Cells: A Comprehensive Review. Curr. Stem Cell Res. Ther. 2021, 16, 323–353. [Google Scholar] [CrossRef]
- Lavorato, A.; Raimondo, S.; Boido, M.; Muratori, L.; Durante, G.; Cofano, F.; Vincitorio, F.; Petrone, S.; Titolo, P.; Tartara, F.; et al. Mesenchymal Stem Cell Treatment Perspectives in Peripheral Nerve Regeneration: Systematic Review. Int. J. Mol. Sci. 2021, 22, 572. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, F.-Y.; Ling, Z.-M.; Su, W.-F.; Zhao, Y.-Y.; Chen, G.; Wei, Z.-Y. The Effect of Schwann Cells/Schwann Cell-Like Cells on Cell Therapy for Peripheral Neuropathy. Front. Cell Neurosci. 2022, 16, 836931. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, N.; Kiryu-Seo, S.; Kiyama, H. Altered Expression of SMAD Family Members in Injured Motor Neurons of Rat. Brain Res. 2007, 1132, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Fagoe, N.D.; Attwell, C.L.; Kouwenhoven, D.; Verhaagen, J.; Mason, M.R.J. Overexpression of ATF3 or the Combination of ATF3, c-Jun, STAT3 and SMAD1 Promotes Regeneration of the Central Axon Branch of Sensory Neurons but without Synergistic Effects. Hum. Mol. Genet. 2015, 24, 6788–6800. [Google Scholar] [CrossRef]
- Finelli, M.J.; Wong, J.K.; Zou, H. Epigenetic Regulation of Sensory Axon Regeneration after Spinal Cord Injury. J. Neurosci. 2013, 33, 19664–19676. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Shin, J.E.; Lee, B.; Kim, H.; Jeon, Y.; Ahn, S.H.; Chi, S.W.; Cho, Y. The Stem Cell Marker Prom1 Promotes Axon Regeneration by Down-Regulating Cholesterol Synthesis via SMAD Signaling. Proc. Natl. Acad. Sci. USA 2020, 117, 15955–15966. [Google Scholar] [CrossRef]
- Si, Y.; Cui, X.; Kim, S.; Wians, R.; Sorge, R.; Oh, S.J.; Kwan, T.; AlSharabati, M.; Lu, L.; Claussen, G.; et al. SMADs as Muscle Biomarkers in Amyotrophic Lateral Sclerosis. Ann. Clin. Transl. Neurol. 2014, 1, 778–787. [Google Scholar] [CrossRef] [PubMed]
- Hata, A.; Lagna, G.; Massagué, J.; Hemmati-Brivanlou, A. SMAD6 Inhibits BMP/SMAD1 Signaling by Specifically Competing with the SMAD4 Tumor Suppressor. Genes. Dev. 1998, 12, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Parikh, P.; Hao, Y.; Hosseinkhani, M.; Patil, S.B.; Huntley, G.W.; Tessier-Lavigne, M.; Zou, H. Regeneration of Axons in Injured Spinal Cord by Activation of Bone Morphogenetic Protein/SMAD1 Signaling Pathway in Adult Neurons. Proc. Natl. Acad. Sci. USA 2011, 108, E99–E107. [Google Scholar] [CrossRef]
- Farrukh, F.; Davies, E.; Berry, M.; Logan, A.; Ahmed, Z. BMP4/SMAD1 Signalling Promotes Spinal Dorsal Column Axon Regeneration and Functional Recovery After Injury. Mol. Neurobiol. 2019, 56, 6807–6819. [Google Scholar] [CrossRef]
- Kokubu, N.; Tsujii, M.; Akeda, K.; Iino, T.; Sudo, A. BMP-7/SMAD Expression in Dedifferentiated Schwann Cells during Axonal Regeneration and Upregulation of Endogenous BMP-7 Following Administration of PTH (1–34). J. Orthop. Surg. 2018, 26, 2309499018812953. [Google Scholar] [CrossRef]
- Saijilafu; Hur, E.-M.; Liu, C.-M.; Jiao, Z.; Xu, W.-L..; Zhou, F.-Q. PI3K-GSK3 Signalling Regulates Mammalian Axon Regeneration by Inducing the Expression of SMAD1. Nat. Commun. 2013, 4, 2690. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.; Berry, M.; Logan, A.; Ahmed, Z. Activation of the BMP4/SMAD1 Pathway Promotes Retinal Ganglion Cell Survival and Axon Regeneration. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1748–1759. [Google Scholar] [CrossRef] [PubMed]
- Burt, D.W.; Law, A.S. Evolution of the Transforming Growth Factor-β Superfamily. Prog. Growth Factor. Res. 1994, 5, 99–118. [Google Scholar] [CrossRef] [PubMed]
- Herpin, A.; Lelong, C.; Favrel, P. Transforming Growth Factor-β-Related Proteins: An Ancestral and Widespread Superfamily of Cytokines in Metazoans. Dev. Comp. Immunol. 2004, 28, 461–485. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Fang, Y.; Chen, L.; Quan, H.; Wang, Y.; Ge, R.-S. Bone Morphogenetic Protein 4 Inhibits Rat Stem/Progenitor Leydig Cell Development and Regeneration via SMAD-Dependent and SMAD-Independent Signaling. Cell Death Dis. 2022, 13, 1039. [Google Scholar] [CrossRef] [PubMed]
- Siegel, P.M.; Massagué, J. Cytostatic and Apoptotic Actions of TGF-β in Homeostasis and Cancer. Nat. Rev. Cancer 2003, 3, 807–821. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Brown, C.W.; Matzuk, M.M. Genetic Analysis of the Mammalian Transforming Growth Factor-β Superfamily. Endocr. Rev. 2002, 23, 787–823. [Google Scholar] [CrossRef] [PubMed]
- Datto, M.; Wang, X.F. The SMADs: Transcriptional Regulation and Mouse Models. Cytokine Growth Factor. Rev. 2000, 11, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Miyazono, K.; ten Dijke, P.; Heldin, C.H. TGF-β Signaling by SMAD Proteins. Adv. Immunol. 2000, 75, 115–157. [Google Scholar] [CrossRef]
- Goumans, M.J.; Valdimarsdottir, G.; Itoh, S.; Lebrin, F.; Larsson, J.; Mummery, C.; Karlsson, S.; ten Dijke, P. Activin Receptor-like Kinase (ALK)1 Is an Antagonistic Mediator of Lateral TGFβ/ALK5 Signaling. Mol. Cell 2003, 12, 817–828. [Google Scholar] [CrossRef]
- Hata, A.; Shi, Y.; Massagué, J. TGF-β Signaling and Cancer: Structural and Functional Consequences of Mutations in SMADs. Mol. Med. Today 1998, 4, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Souchelnytskyi, S.; Tamaki, K.; Engström, U.; Wernstedt, C.; ten Dijke, P.; Heldin, C.H. Phosphorylation of Ser465 and Ser467 in the C Terminus of SMAD2 Mediates Interaction with SMAD4 and Is Required for Transforming Growth Factor-β Signaling. J. Biol. Chem. 1997, 272, 28107–28115. [Google Scholar] [CrossRef] [PubMed]
- Gélabert, C.; Papoutsoglou, P.; Golán, I.; Ahlström, E.; Ameur, A.; Heldin, C.-H.; Caja, L.; Moustakas, A. The Long Non-Coding RNA LINC00707 Interacts with SMAD Proteins to Regulate TGFβ Signaling and Cancer Cell Invasion. Cell Commun. Signal 2023, 21, 271. [Google Scholar] [CrossRef]
- Xin, X.; Cheng, X.; Zeng, F.; Xu, Q.; Hou, L. The Role of TGF-β/SMAD Signaling in Hepatocellular Carcinoma: From Mechanism to Therapy and Prognosis. Int. J. Biol. Sci. 2024, 20, 1436–1451. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Lyu, C.; Chen, D.; Cai, W.; Kou, F.; Li, Q.; Wei, H.; Zhang, H. Gallic Acid Treats Hypertrophic Scar in Rabbit Ears via the TGF-β/SMAD and TRPC3 Signaling Pathways. Pharmaceuticals 2023, 16, 1514. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Prenger, M.S.; Norton, D.D.; Mei, L.; Kusiak, J.W.; Bai, G. Nerve Growth Factor Uses Ras/ERK and Phosphatidylinositol 3-Kinase Cascades to up-Regulate the N-Methyl-D-Aspartate Receptor 1 Promoter. J. Biol. Chem. 2001, 276, 45372–45379. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-Y.; Ju, W.-K. CAMP/PKA Pathway and Mitochondrial Protection in Oxidative Stress-Induced Optic Nerve Head Astrocytes. Neural Regen. Res. 2021, 16, 108–109. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Song, L.-J.; Ding, Z.-B.; Chai, Z.; Yu, J.-Z.; Xiao, B.-G.; Ma, C.-G. Advantages of Rho-Associated Kinases and Their Inhibitor Fasudil for the Treatment of Neurodegenerative Diseases. Neural Regen. Res. 2022, 17, 2623–2631. [Google Scholar] [CrossRef] [PubMed]
- Berardo, A.; Bacaglio, C.R.; Báez, B.B.; Sambuelli, R.; Sheikh, K.A.; Lopez, P.H.H. Blockade of Rho-Associated Kinase Prevents Inhibition of Axon Regeneration of Peripheral Nerves Induced by Anti-Ganglioside Antibodies. Neural Regen. Res. 2024, 19, 895–899. [Google Scholar] [CrossRef]
- Byun, Y.S.; Youn, H.J.; Lee, S.U. The Present of Peripheral Nerve Regeneration. J. Korean Orthop. Assoc. 2023, 58, 9–17. [Google Scholar] [CrossRef]
- Hegarty, S.V.; O’Keeffe, G.W.; Sullivan, A.M. BMP-SMAD 1/5/8 Signalling in the Development of the Nervous System. Prog. Neurobiol. 2013, 109, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, H.; Chen, L.; Shen, Y.; Cao, Y.; Li, X.; Yao, J. Indirubin Alleviates Retinal Neurodegeneration through the Regulation of PI3K/AKT Signaling. J. Biomed. Res. 2024, 38, 256–268. [Google Scholar] [CrossRef] [PubMed]
- Gámez, B.; Rodríguez-Carballo, E.; Graupera, M.; Rosa, J.L.; Ventura, F. Class I PI-3-Kinase Signaling Is Critical for Bone Formation Through Regulation of SMAD1 Activity in Osteoblasts. J. Bone Min. Res. 2016, 31, 1617–1630. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, C.-Y.; Lee, W.-S.; Liu, K.-F.; Tsou, H.-K.; Chen, C.-J.; Peng, W.-H.; Tsai, J.-C. Allantoin Ameliorates Amyloid β-Peptide-Induced Memory Impairment by Regulating the PI3K/Akt/GSK-3β Signaling Pathway in Rats. Biomed. Pharmacother. 2022, 153, 113389. [Google Scholar] [CrossRef]
| Author/ Year/ Reference | Study Design | Species and/or Sample | Nerve/Injury Method | Detection Method | Target Substance(s) Associated with Autophagy | Results: Conclusions |
|---|---|---|---|---|---|---|
| Okuyama N et al., 2007 [12] | Animal study | Wistar rats | Hypoglossal nerve transection | RT-PCR, in situ hybridization, immunohistochemistry, Western blot analysis | SMAD1, -2, -3, -4, -5, -6, -7, -8 | The expression of Mad1, -2, and -4 mRNAs was significantly upregulated in injured motor neurons, whereas SMAD8 mRNA was downregulated. SMAD5–7 mRNA showed no significant alterations. : SMAD-mediated signaling might have an important role during nerve regeneration. |
| Fagoe ND et al., 2015 [13] | Animal study | Fisher 344 rats | Dorsal root transection injury | Immunohistochemistry, histological analysis, functional tests | ATF3, c-Jun, SMAD1, STAT3 | Overexpression of ATF3, c-Jun, STAT3 and SMAD1 led to an increase in the rate of regeneration of injured dorsal root axons. : ATF3, c-Jun, STAT3 and SMAD1 individually contribute to regenerative axon growth of injured DRG neurons. |
| Finelli MJ et al., 2013 [14] | Animal study, in vivo | C57BL/6 mice, CD1 mice | Transection of sciatic nerve and ascending sensory fibers of spinal cord | DRG culture and neurite outgrowth assay, immunohistochemistry, chromatin immunoprecipitation, qRT-PCR, coimmunoprecipitation, Western blot, promoter sequence analysis | AcH4, SMAD1 | AcH4 enrichment and pSMAD1 nuclear accumulation occurred predominantly in neurons and not glial cells in DRGs after a peripheral axotomy. : During the epigenetic reprogramming process, histone-modifying enzymes work together with SMAD1 to facilitate transcriptional regulation of RAGs. |
| Lee J et al., 2020 [15] | Animal study | Prom1 KO mice | Sciatic nerve crush injury | Adult DRG cell culture, embryonic DRG cell culture, repleting assay | Prom1, SMAD2, cholesterol | PROM1 interacted with the TGF-βRI receptor, ALK4, to synergistically induce phosphorylation of SMAD2. : SMAD signaling is responsible for the enhanced axon regeneration induced by PROM1 overexpression. |
| Ying Si et al., 2013 [16] | Animal study, human ALS | G39A superoxide dismutase (SOD)1 mice, Human ALS muscle | Sciatic nerve transection | Behavioral assessment, Western blot, immunohistochemistry, statistical analysis, NGS, qRT-PCR | SMAD1/5/8 | SMAD8, and to a lesser extent, SMAD1 and -5, mRNAs were significantly elevated in human ALS muscle samples. SMAD8 showed a substantially greater fold-change following sciatic injury compared with controls (up to 17-fold at end stage) relative to SMAD1 and -5 (up to 5-fold at end stage). : SMAD1, -5, -8 mRNA, and protein levels, as well as SMAD phosphorylation, are elevated in ALS muscle and could potentially serve as markers of disease progression. |
| Hata A et al., 1998 [17] | In vivo, in vitro | Xenopus embryos and in mammalian cells | Transfection with reporter plasmid and different amounts of SMAD6/CS2 | In vivo phosphate labeling, yeast two-hybrid system, transcriptional assay, xenopus injections and animal cap assay, cloning of human and Xenopus SMAD6 | SMAD6, BMP/SMAD1 pathway | BMP receptor signaling induced phosphorylation of SMAD1, which then associated with SMAD4. SMAD6 significantly blocked signaling by the BMP/SMAD1 pathway without interfering with receptor-mediated phosphorylation of SMAD1. : SMAD6 competes with SMAD4 for binding to receptor-activated SMAD1, yielding an inactive SMAD1–SMAD6 complex. |
| Parikh P et al., 2011 [18] | Animal study | C57BL/6 mice | Dorsal column transection, right sciatic nerve transection | AAV and intrathecal injection. Labeling of ascending sensory axons in the fasciculus gracilis | BMP/SMAD pathway | SMAD1 is developmentally regulated in DRG neurons and governs axon growth potential. BMP/SMAD1 signaling is essential for the conditioning effect in adult DRG neurons. Post-injury AAV-BMP4 injection promotes sensory axon regeneration. : Activation of SMAD1 promotes axonal regeneration following sciatic nerve injury. |
| Farrukh F et al., 2019 [19] | Animal study | Sprague Dawley rats | DC and SN crush injury | Microarray analysis, qRT-PCR, immunohistochemistry, BMP4 enzyme-linked immunosorbent assay, electrophysiology, functional testing | BMP4/SMAD1 pathway | The levels of SMAD1, SMAD2, SMAD4, SMAD5, SMAD8 and Bmp4 mRNA were up-regulated 2.0- to 6.7-fold compared with sham controls, whereas Noggin was downregulated 2-fold. : Activation of the BMP4/SMAD1 pathway is a potential therapeutic strategy for promoting axon regenerative signaling in the CNS. |
| Kokubu N et al., 2018 [20] | Animal study, in vitro | Sprague-DawleySprague Dawley rats | Sciatic nerve crush injury | Functional assessment, immunohistochemical analysis, fluorescent double immunostaining, Western blot analysis, RT-PCR, SC culture, MTS cell proliferation assay | BMP-7, Noggin, SMAD, PTH (I-34) | BMP-7 and SMAD protein and mRNA were significantly upregulated in axon SCsSCs, and this increase was maintained for 4 weeks. Application of PTH(I-34) also upregulated BMP-7 on SCs. : Axonal regeneration can be induced by upregulating endogenous BMP-7 on SCs through PTH (I-34) administration. |
| Saijilafu et al., 2013 [21] | Animal study | CF-1 mice, bax−/− mice | Sciatic nerve crush and transection, cultured DRG neurons | qRT-PCR, in vivo electroporation, statistics, immunohistochemistry, fluorescence imaging | PI3K/GSK3-SMAD1 | Acute depletion of the transcription factor SMAD1, which is induced by PI3K/GSK3 signaling, prevented axon regeneration in vivo in adult mice. : PI3K/GSK3/SMAD1 signaling is a central module for promoting sensory axon regeneration in the mammalian nervous system. |
| Thompson A et al., 2019 [22] | Animal study | Sprague Dawley rats, Fischer rats | Optic nerve crush injury, laser capture microdissection of retinal ganglion cell (RGC) | Immunocytochemistry, qRT-PCR, quantification of RGC survival and Muller cell activation | BMP4/SMAD1 pathway | Bmp4, SMAD1, SMAD4, SMAD5, SMAD8, Smif, and Msg1 mRNAs were upregulated 3.2-, 4.5-, 5.5-, 2-, 3-, 3-, and 2-fold, respectively (p < 0.05), in RGCs with neurites compared with RGCs without neurites. : Activation of the BMP4/SMAD1 pathway promotes survival and axon regeneration independent of mTOR, and therefore may be of therapeutic interest. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Yon, D.K.; Choi, Y.S.; Lee, J.; Yeo, J.H.; Kim, S.S.; Lee, J.M.; Yeo, S.G. Roles of SMAD and SMAD-Associated Signaling Pathways in Nerve Regeneration Following Peripheral Nerve Injury: A Narrative Literature Review. Curr. Issues Mol. Biol. 2024, 46, 7769-7781. https://doi.org/10.3390/cimb46070460
Lee J, Yon DK, Choi YS, Lee J, Yeo JH, Kim SS, Lee JM, Yeo SG. Roles of SMAD and SMAD-Associated Signaling Pathways in Nerve Regeneration Following Peripheral Nerve Injury: A Narrative Literature Review. Current Issues in Molecular Biology. 2024; 46(7):7769-7781. https://doi.org/10.3390/cimb46070460
Chicago/Turabian StyleLee, Jeongmin, Dong Keon Yon, Yong Sung Choi, Jinseok Lee, Joon Hyung Yeo, Sung Soo Kim, Jae Min Lee, and Seung Geun Yeo. 2024. "Roles of SMAD and SMAD-Associated Signaling Pathways in Nerve Regeneration Following Peripheral Nerve Injury: A Narrative Literature Review" Current Issues in Molecular Biology 46, no. 7: 7769-7781. https://doi.org/10.3390/cimb46070460
APA StyleLee, J., Yon, D. K., Choi, Y. S., Lee, J., Yeo, J. H., Kim, S. S., Lee, J. M., & Yeo, S. G. (2024). Roles of SMAD and SMAD-Associated Signaling Pathways in Nerve Regeneration Following Peripheral Nerve Injury: A Narrative Literature Review. Current Issues in Molecular Biology, 46(7), 7769-7781. https://doi.org/10.3390/cimb46070460







