Endothelin-3 Suppresses Luteinizing Hormone Receptor Expression by Regulating the cAMP-PKA Pathway in Hen Granulosa Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Sample Collection
2.2. Construction and Identification of pcDNA3.1-EDN3 Vectors
2.3. Transfection of pcDNA3.1-EDN3 Plasmids
2.4. CCK8 Assay
2.5. EDU Assay
2.6. ELISA Assay
2.7. Quantitative Real-Time PCR Validation
2.8. RNA Sequencing and Data Analysis
2.9. Statistical Analyses
3. Results
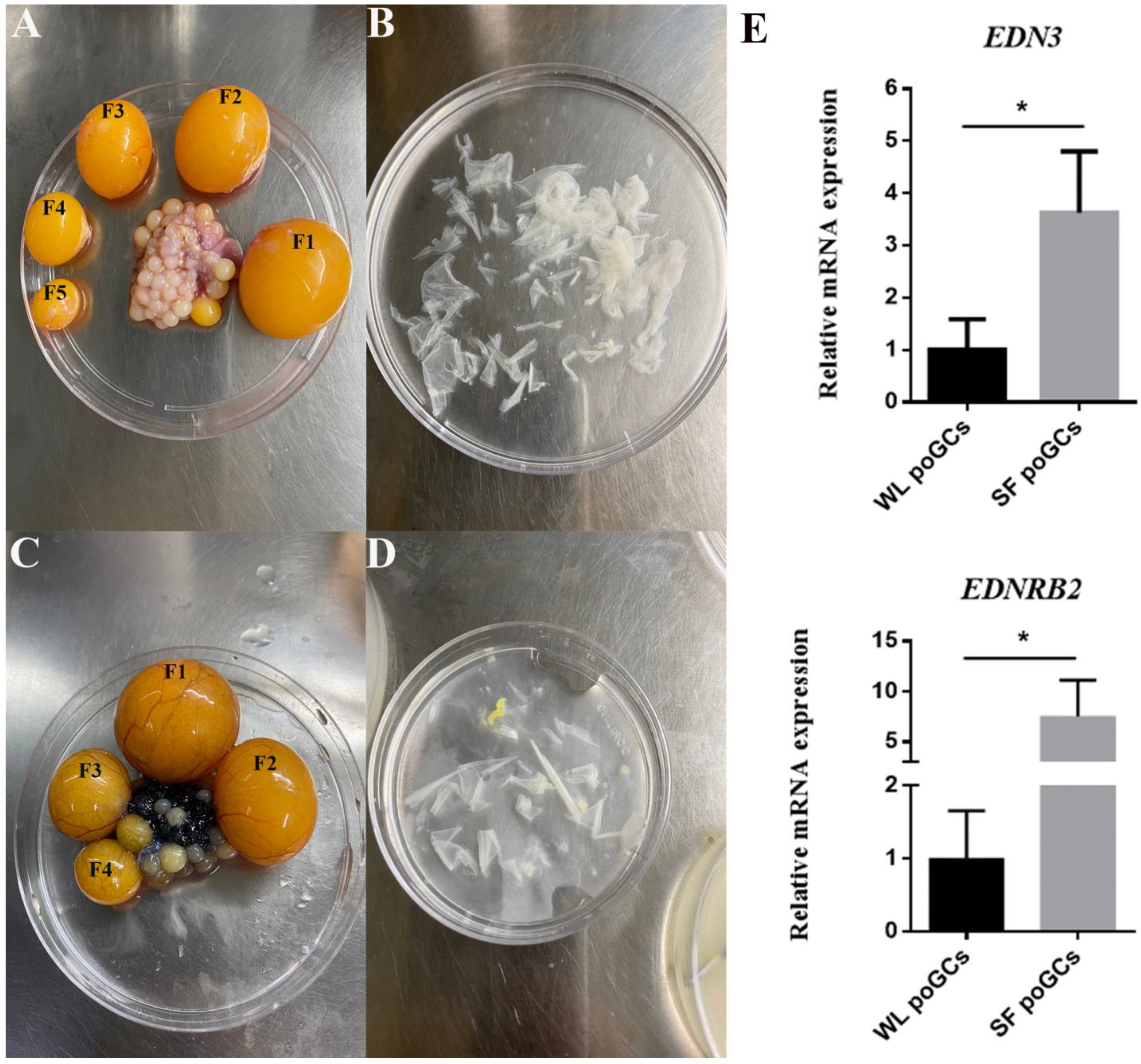
3.1. EDN3 Gene Is Highly Expressed in SF poGCs Tissue
3.2. EDN3 Overexpression in WL poGC by Constructing Recombinant Plasmid pcDNA3.1-EDN3
3.3. EDN3 Promotes the Cell Viability and DNA Replication of GCs
3.4. EDN3 Overexpression Gene Affects the Production of Sexual Hormones
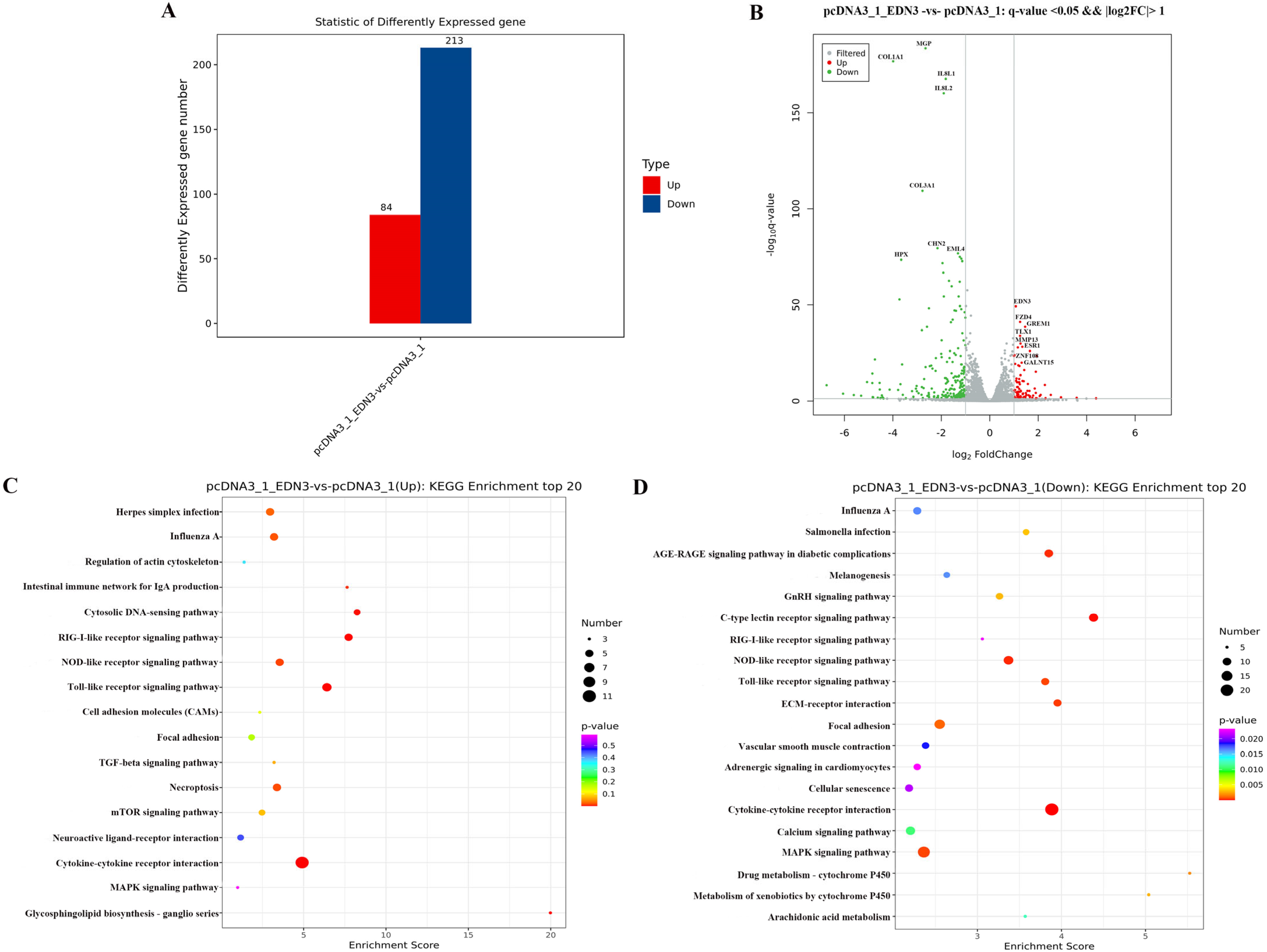
3.5. Differentially Expressed Genes (DEGs) in EDN3-Overexpressed GCs
4. Discussion
4.1. Multifaceted Role of EDN3 in Melanocyte Proliferation and Reproductive Endocrinology
4.2. EDN3 Modulates GC Viability and Hormonal Synthesis via cAMP Signaling Pathway in Reproductive Biology
4.3. EDN3 Influences Gene Expression and Granulosa Cell Differentiation in Development
4.4. Research Limitations and Prospects
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yanagisawa, M.; Kurihara, H.; Kimura, S.; Tomobe, Y.; Kobayashi, M.; Mitsui, Y.; Yazaki, Y.; Goto, K.; Masaki, T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 1988, 332, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Inoue, A.; Yanagisawa, M.; Kimura, S.; Kasuya, Y.; Miyauchi, T.; Goto, K.; Masaki, T. The human endothelin family: Three structurally and pharmacologically distinct isopeptides predicted by three separate genes. Proc. Natl. Acad. Sci. USA 1989, 86, 2863–2867. [Google Scholar] [CrossRef] [PubMed]
- Davenport, A.P.; Hyndman, K.A.; Dhaun, N.; Southan, C.; Kohan, D.E.; Pollock, J.S.; Pollock, D.M.; Webb, D.J.; Maguire, J.J. Endothelin. Pharmacol. Rev. 2016, 68, 357–418. [Google Scholar] [CrossRef] [PubMed]
- Bondurand, N.; Dufour, S.; Pingault, V. News from the endothelin-3/EDNRB signaling pathway: Role during enteric nervous system development and involvement in neural crest-associated disorders. Dev. Biol. 2018, 444 (Suppl. 1), S156–S169. [Google Scholar] [CrossRef]
- Clouthier, D.E.; Hosoda, K.; Richardson, J.A.; Williams, S.C.; Yanagisawa, H.; Kuwaki, T.; Kumada, M.; Hammer, R.E.; Yanagisawa, M. Cranial and cardiac neural crest defects in endothelin-A receptor-deficient mice. Development 1998, 125, 813–824. [Google Scholar] [CrossRef]
- Kohan, D.E.; Rossi, N.F.; Inscho, E.W.; Pollock, D.M. Regulation of blood pressure and salt homeostasis by endothelin. Physiol. Rev. 2011, 91, 1–77. [Google Scholar] [CrossRef] [PubMed]
- Schiffrin, E.L. Does Endothelin-1 Raise or Lower Blood Pressure in Humans? Nephron 2018, 139, 47–50. [Google Scholar] [CrossRef]
- Ko, C.; Gieske, M.C.; Al-Alem, L.; Hahn, Y.; Su, W.; Gong, M.C.; Iglarz, M.; Koo, Y. Endothelin-2 in ovarian follicle rupture. Endocrinology 2006, 147, 1770–1779. [Google Scholar] [CrossRef]
- Palanisamy, G.S.; Cheon, Y.P.; Kim, J.; Kannan, A.; Li, Q.; Sato, M.; Mantena, S.R.; Sitruk-Ware, R.L.; Bagchi, M.K.; Bagchi, I.C. A novel pathway involving progesterone receptor, endothelin-2, and endothelin receptor B controls ovulation in mice. Mol. Endocrinol. 2006, 20, 2784–2795. [Google Scholar] [CrossRef] [PubMed]
- Baynash, A.G.; Hosoda, K.; Giaid, A.; Richardson, J.A.; Emoto, N.; Hammer, R.E.; Yanagisawa, M. Interaction of endothelin-3 with endothelin-B receptor is essential for development of epidermal melanocytes and enteric neurons. Cell 1994, 79, 1277–1285. [Google Scholar] [CrossRef]
- Lahav, R.; Ziller, C.; Dupin, E.; Le Douarin, N.M. Endothelin 3 promotes neural crest cell proliferation and mediates a vast increase in melanocyte number in culture. Proc. Natl. Acad. Sci. USA 1996, 93, 3892–3897. [Google Scholar] [CrossRef] [PubMed]
- Lecoin, L.; Sakurai, T.; Ngo, M.-T.; Abe, Y.; Yanagisawa, M.; Le Douarin, N.M. Cloning and characterization of a novel endothelin receptor subtype in the avian class. Proc. Natl. Acad. Sci. USA 1998, 95, 3024–3029. [Google Scholar] [CrossRef] [PubMed]
- Iwai, M.; Hasegawa, M.; Taii, S.; Sagawa, N.; Nakao, K.; Imura, H.; Nakanishi, S.; Mori, T. Endothelins inhibit luteinization of cultured porcine granulosa cells. Endocrinology 1991, 129, 1909–1914. [Google Scholar] [CrossRef] [PubMed]
- Calogero, A.E.; Burrello, N.; Ossino, A.M. Endothelin (ET)-1 and ET-3 inhibit estrogen and cAMP production by rat granulosa cells in vitro. J. Endocrinol. 1998, 157, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Apa, R.; Miceli, F.; de Feo, D.; Mastrandrea, M.L.; Mancuso, S.; Napolitano, M.; Lanzone, A. Endothelin-1 inhibits basal and human chorionic gonadotrophin-stimulated progesterone production. Hum. Reprod. 1998, 13, 2425–2429. [Google Scholar] [CrossRef] [PubMed]
- Klipper, E.; Levit, A.; Mastich, Y.; Berisha, B.; Schams, D.; Meidan, R. Induction of endothelin-2 expression by luteinizing hormone and hypoxia: Possible role in bovine corpus luteum formation. Endocrinology 2010, 151, 1914–1922. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.-H.; Kim, E.K.; Kim, K.-H.; Lee, K.-A.; Kang, D.-W.; Kim, H.Y.; Bridges, P.; Ko, C. Expression pattern of endothelin system components and localization of smooth muscle cells in the human pre-ovulatory follicle. Hum. Reprod. 2011, 26, 1171–1180. [Google Scholar] [CrossRef] [PubMed]
- Uchide, T.; Masuda, H.; Mitsui, Y.; Saida, K. Gene expression of vasoactive intestinal contractor/endothelin-2 in ovary, uterus and embryo: Comprehensive gene expression profiles of the endothelin ligand-receptor system revealed by semi-quantitative reverse transcription-polymerase chain reaction analysis in adult mouse tissues and during late embryonic development. J. Mol. Endocrinol. 1999, 22, 161–171. [Google Scholar] [PubMed]
- Tai, Y.; Yang, X.; Han, D.; Xu, Z.; Cai, G.; Hao, J.; Zhang, B.; Deng, X. Transcriptomic diversification of granulosa cells during follicular development between White Leghorn and Silky Fowl hens. Front. Genet. 2022, 13, 965414. [Google Scholar] [CrossRef]
- Gilbert, A.B.; Evans, A.J.; Perry, M.M.; Davidson, M.H. A method for separating the granulosa cells, the basal lamina and the theca of the preovulatory ovarian follicle of the domestic fowl (Gallus domesticus). J. Reprod. Fertil. 1977, 50, 179–181. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.; Trapnell, C.; Donaghey, J.; Rinn, J.L.; Pachter, L. Improving RNA-Seq expression estimates by correcting for fragment bias. Genome Biol. 2011, 12, R22. [Google Scholar] [CrossRef] [PubMed]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq—A Python framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- The Gene Ontology Consortium. The Gene Ontology Resource: 20 years and still GOing strong. Nucleic Acids Res. 2019, 47, D330–D338. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Araki, M.; Goto, S.; Hattori, M.; Hirakawa, M.; Itoh, M.; Katayama, T.; Kawashima, S.; Okuda, S.; Tokimatsu, T.; et al. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 2008, 36, D480–D484. [Google Scholar] [CrossRef] [PubMed]
- Laurent, C.; Marano, A.; Baldit, A.; Ferrari, M.; Perrin, J.-C.; Perroud, O.; Bianchi, A.; Kempf, H. A preliminary study exploring the mechanical properties of normal and Mgp-deficient mouse femurs during early growth. Proc. Inst. Mech. Eng. Part H 2022, 236, 1106–1117. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.-P.; Chang, H.-L.; Bamodu, O.A.; Yadav, V.K.; Huang, T.-Y.; Wu, A.T.H.; Yeh, C.-T.; Tsai, S.-H.; Lee, W.-H. Collagen 1A1 (COL1A1) Is a Reliable Biomarker and Putative Therapeutic Target for Hepatocellular Carcinogenesis and Metastasis. Cancers 2019, 11, 786. [Google Scholar] [CrossRef]
- Casado-Medrano, V.; Barrio-Real, L.; Gutiérrez-Miranda, L.; González-Sarmiento, R.; Velasco, E.A.; Kazanietz, M.G.; Caloca, M.J. Identification of a truncated beta1-chimaerin variant that inactivates nuclear Rac1. J. Biol. Chem. 2020, 295, 1300–1314. [Google Scholar] [CrossRef]
- Strakova, K.; Matricon, P.; Yokota, C.; Arthofer, E.; Bernatik, O.; Rodriguez, D.; Arenas, E.; Carlsson, J.; Bryja, V.; Schulte, G. The tyrosine Y250(2.39) in Frizzled 4 defines a conserved motif important for structural integrity of the receptor and recruitment of Disheveled. Cell. Signal. 2017, 38, 85–96. [Google Scholar] [CrossRef] [PubMed]
- KKobayashi, H.; Gieniec, K.A.; Wright, J.A.; Wang, T.; Asai, N.; Mizutani, Y.; Lida, T.; Ando, R.; Suzuki, N.; Lannagan, T.R.; et al. The Balance of Stromal BMP Signaling Mediated by GREM1 and ISLR Drives Colorectal Carcinogenesis. Gastroenterology 2021, 160, 1224–1239.e30. [Google Scholar] [CrossRef]
- Cheng, L.; Arata, A.; Mizuguchi, R.; Qian, Y.; Karunaratne, A.; A Gray, P.; Arata, S.; Shirasawa, S.; Bouchard, M.; Luo, P.; et al. Tlx3 and Tlx1 are post-mitotic selector genes determining glutamatergic over GABAergic cell fates. Nat. Neurosci. 2004, 7, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Dorshorst, B.; Molin, A.-M.; Rubin, C.-J.; Johansson, A.M.; Strömstedt, L.; Pham, M.-H.; Chen, C.-F.; Hallböök, F.; Ashwell, C.; Andersson, L. A complex genomic rearrangement involving the endothelin 3 locus causes dermal hyperpigmentation in the chicken. PLoS Genet. 2011, 7, e1002412. [Google Scholar] [CrossRef] [PubMed]
- Hallet, M.M.; Ferrand, R. Quail melanoblast migration in two breeds of fowl and in their hybrids: Evidence for a dominant genic control of the mesodermal pigment cell pattern through the tissue environment. J. Exp. Zool. 1984, 230, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Nataf, V.; Amemiya, A.; Yanagisawa, M.; Le Douarin, N.M. The expression pattern of endothelin 3 in the avian embryo. Mech. Dev. 1998, 73, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Li, J.; Zhang, Y.; Han, D.; Hua, G.; Wang, J.; Deng, X.; Wu, C. Genomic Analysis Reveals Pleiotropic Alleles at EDN3 and BMP7 Involved in Chicken Comb Color and Egg Production. Front. Genet. 2019, 10, 612. [Google Scholar] [CrossRef] [PubMed]
- Son, W.Y.; Das, M.; Shalom-Paz, E.; Holzer, H. Mechanisms of follicle selection and development. Minerva Ginecol. 2011, 63, 89–102. [Google Scholar] [PubMed]
- Lonergan, P. Influence of progesterone on oocyte quality and embryo development in cows. Theriogenology 2011, 76, 1594–1601. [Google Scholar] [CrossRef]
- Weedon-Fekjaer, M.S.; Tasken, K. Review: Spatiotemporal dynamics of hCG/cAMP signaling and regulation of placental function. Placenta 2012, 33 (Suppl.), S87–S91. [Google Scholar] [CrossRef]
- Manna, P.R.; Eubank, D.W.; Lalli, E.; Sassone-Corsi, P.; Stocco, D.M. Transcriptional regulation of the mouse steroidogenic acute regulatory protein gene by the cAMP response-element binding protein and steroidogenic factor 1. J. Mol. Endocrinol. 2003, 30, 381–397. [Google Scholar] [CrossRef] [PubMed]
- Square, T.A.; Jandzik, D.; Massey, J.L.; Romášek, M.; Stein, H.P.; Hansen, A.W.; Purkayastha, A.; Cattell, M.V.; Medeiros, D.M. Evolution of the endothelin pathway drove neural crest cell diversification. Nature 2020, 585, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Dagamajalu, S.; Rex, D.; Gopalakrishnan, L.; Karthikkeyan, G.; Gurtoo, S.; Modi, P.K.; Mohanty, V.; Mujeeburahiman, M.; Soman, S.; Raju, R.; et al. A network map of endothelin mediated signaling pathway. J. Cell Commun. Signal. 2021, 15, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Johnson, A.L. Differentiation of the granulosa layer from hen prehierarchal follicles associated with follicle-stimulating hormone receptor signaling. Mol. Reprod. Dev. 2018, 85, 729–737. [Google Scholar] [CrossRef]
- Ruijtenberg, S.; van den Heuvel, S. Coordinating cell proliferation and differentiation: Antagonism between cell cycle regulators and cell type-specific gene expression. Cell Cycle 2016, 15, 196–212. [Google Scholar] [CrossRef]



| Primer Name | Primer Sequence | Size (bp) | Temperature |
|---|---|---|---|
| EDN3-F | CTATAGGGAGAGACCCAAGCTGGGCCACCATGGAGCTGGGCTTATTG | 522 | 57 °C |
| EDN3-R | CCAAGCTTAAGTTTAAACGCTAGGCCTGAGCGAGCGTGTGCTCC |
| Genes | Primer Sequence (5′-3′) | Annealing Temperature | Size (bp) |
|---|---|---|---|
| EDN3 | F-TCAACACCCCAGAGAGGACT R-AGAGCACCGAAATGAAGGCT | 59 °C | 116 |
| EDNRB2 | F-CCTCATTGCTCTGCCCATCA R-GCCACTGCTCGATACCTGTC | 58 °C | 159 |
| LHCGR | F- ACGAATCGCTGACACTCAAAC R-CTCTCAGGGCATCGTTGTGT | 60 °C | 138 |
| PGR | F-TAACGCAAAGGCTGTCC R-CCGAAATCCTGGTAGCA | 55 °C | 215 |
| ER1 | F-TATTGATGATCGGCTTAGTCTGGC R-CGAGCAGCAGTAGCCAGTAGCA | 63 °C | 145 |
| STAR | F-CCATCAGCCAGGAGCTCAG R-ATCTCGCTGAAGGGCTTCTC | 58 °C | 124 |
| CYP11A1 | F-TACCGTGACTACCGCAACAA R-AAAAAGTCCTGGCTCACCTGG | 60 °C | 152 |
| HSD3B1 | F-GCCAGAGGATCGTTCGCTTA R-CATCTCGGATGTCCCCTTCC | 59 °C | 153 |
| HSD11B2 | F-CACACCAATGGCACAGGTCTC R-GTGCGGAAGTTGCCCAATG | 61 °C | 98 |
| ADCY3 | F-CGAGCTGGACAAGTGTGGAT R-AGCTCGTGGCGAGAGAAGTA | 60 °C | 102 |
| ADCY6 | F-GCCCGACAGGATTCAGGTAA R-CACCTTAATGACGCCTCGGT | 60 °C | 88 |
| ADCY8 | F-CTGGTGGAATTCCTGGGAGA R-CGTTCTTTGCCATGCCCTTC | 59 °C | 94 |
| ADCY9 | F-CTGCGATGTCAGCATAGACGA R-TCCTCCTGGAGCCTTCGTA | 60 °C | 82 |
| PRKACB | F-CGAGATCGAGAGCGGCATTCA R-CAAGTTCAGAGACGTGATGGT | 60 °C | 85 |
| NFATC1 | F-GCGGCGGAGGAAGAACACTA R-GACAATGAGGAATGCGCCAC | 60 °C | 83 |
| CREB3L1 | F-TGAAATGACTCCAGCACCTGT R-CTGCACCAGGTCATCTGC | 58 °C | 85 |
| CREB5 | F-AACCTTCCCTCAGTCCAACG R-CTTTGATTCCTCATAAATCGCG | 58 °C | 123 |
| CREB3L2 | F-ATGGAGGTGTGCGAGAGC R-AATTCTGTGAAGTGCGTGTGG | 59 °C | 119 |
| GAPDH | F-TCGGAGTCAACGGATTTGGC R-ACAGTGCCCTTGAAGTGTCC | 60 °C | 163 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tai, Y.; Han, D.; Yang, X.; Cai, G.; Li, H.; Li, J.; Deng, X. Endothelin-3 Suppresses Luteinizing Hormone Receptor Expression by Regulating the cAMP-PKA Pathway in Hen Granulosa Cells. Curr. Issues Mol. Biol. 2024, 46, 7832-7845. https://doi.org/10.3390/cimb46080464
Tai Y, Han D, Yang X, Cai G, Li H, Li J, Deng X. Endothelin-3 Suppresses Luteinizing Hormone Receptor Expression by Regulating the cAMP-PKA Pathway in Hen Granulosa Cells. Current Issues in Molecular Biology. 2024; 46(8):7832-7845. https://doi.org/10.3390/cimb46080464
Chicago/Turabian StyleTai, Yurong, Deping Han, Xue Yang, Ganxian Cai, Huaiyu Li, Junying Li, and Xuemei Deng. 2024. "Endothelin-3 Suppresses Luteinizing Hormone Receptor Expression by Regulating the cAMP-PKA Pathway in Hen Granulosa Cells" Current Issues in Molecular Biology 46, no. 8: 7832-7845. https://doi.org/10.3390/cimb46080464





